Abstract
Backgrounds
The incidence of nonalcoholic fatty liver disease (NAFLD) is rapidly increasing due to the prevalence of obesity. NAFLD is a major risk factor of hepatocellular carcinoma (HCC). Even with successful surgical removal, the presence of NAFLD is associated with an increased recurrence of HCC. Despite the extensive study of NAFLD, its underlying mechanism(s) remains essentially unknown and there are no FDA-approved drugs for its treatment. Alterations in microRNA (miR) expression have been observed in human fatty livers. However, regulatory mechanism(s) of miRNA biogenesis and their role in regulating the development of NAFLD is poorly described.
Methods
We used immunohistochemistry, luciferase assays and immunoblotting to study the regulatory mechanism of miR-378 biogenesis. Wild-type mice kept on a high fat diet (HFD) were injected with miR-378 inhibitors or a mini-circle expression system containing miR-378 to study loss and gain-of functions of miR-378.
Results
miR-378 was significantly increased in fatty livers of dietary obese mice and human hepatoma HepG2 cells with accumulated lipid. Further studies identified NRF1 (Nuclear receptor factor 1), a key regulator of fatty acid oxidation (FAO), as a direct target of miR-378. Overexpression of miR-378 impaired FAO and promoted lipid accumulation in murine hepatoma Hepa1–6 cells. In contrast, knockdown of miR-378 using its ASO (anti-sense oligo) improved FAO and reduced intracellular lipid content in Hepa1–6 cells. Liver-specific expression of miR-378 impaired FAO, which subsequently promoted the development of hepatosteatosis. Antagonizing miR-378 via injecting miR-378-ASO into HFD-treated mice led to increased expression of Nrf1, improved FAO and decreased hepatosteatosis. Additional knockdown of up-regulated Nrf1 offset the effects of miR-378-ASO, suggesting that Nrf1 mediated the inhibitory effect of miR-378-ASO on hepatosteatosis. Furthermore, Nrf1 was identified as a transcriptional repressor of miR-378. Ablation of Nrf1 using its shRNA in livers led to increased miR-378, which subsequently resulted in reduced FAO and elevated hepatic lipid content.
Conclusions
These findings identified a negative feedback loop between miR-378 and Nrf1 that promotes the pathogenesis of hepatosteatosis, and suggests the use of miR-378 as a potential therapeutic target for NAFLD.
Keywords: microRNA, hepatosteatosis, fatty acid oxidation, transcription regulation
1. Introduction
The incidence of non-alcoholic fatty liver disease (NAFLD) is estimated to be 20–45% in the general population of the Western world due to the growing prevalence of obesity [1]. Although NAFLD carries a relatively benign prognosis, a significant proportion of patients will progress to non-alcoholic steatohepatitis (NASH) and later cirrhosis with risk of developing hepatocellular carcinoma (HCC) [2]. It is estimated that NASH-related HCC accounts for more than 13% of HCC cases in US [3]. Although the pathogenesis of NAFLD has been studied extensively, the precise mechanism(s) is still under investigation.
It is widely considered that hepatic insulin resistance is closely linked to NAFLD [4]. The principal function of insulin in the liver is to suppress glucose production when blood glucose concentrations increase. Normally, insulin induces de novo lipogenesis by modulating Srebps (Sterol regulatory element-binding proteins) at multiple levels [5]. In a state of insulin resistance, compensatory hyperinsulinemia is postulated to persistently activate Srebp1c transcription and cleavage, which increases expression of lipogenic genes and accelerates hepatic triglyceride accumulation [6]. Another major factor to increase hepatosteatosis is impaired fatty acid oxidation (FAO). Hepatic free fatty acids (FFAs) provide energy for the liver via mitochondrial β-oxidation. When FFA oxidation is unable to utilize the overloaded FFAs, excessive FFAs are esterified into triglycerides, resulting in hepatosteatosis. Decreased triglyceride secretion also contributes to hepatosteatosis [7].
The discovery of a class of naturally-occurring small non-coding RNAs, termed microRNAs (miRNAs) [8], has stimulated a new field of research on the pathogenesis of NAFLD. It is now well-established that alterations in miRNA expression do occur in patients with NAFLD/NASH [9, 10]. miRNAs are shown to be important regulators of lipid and energy homeostasis [10]. Our interest in miR-378 initially arose from miRNA profiling studies in the livers of dietary obese mice. We observed that a high fat diet (HFD) significantly induced expression of miR-378 in livers of mice. Most notably, miR-378 is embedded in the first intron of the Ppargc1β gene [11], which encodes peroxisome proliferator-activated receptor γ coactivator 1 (PGC1β), a key regulator of mitochondrial biogenesis, thermogenesis, and glucose and fatty acid metabolism [12]. Although the role of PGC1β in regulating energy and lipid homeostasis has been studied extensively, the role of miR-378 in hepatosteatosis and its dysregulation in fatty livers is not well described.
Our database mining revealed that NRF1 (Nuclear receptor factor 1) is a potential target of miR-378. Nrf1 is a member of the cap-n-collar vertebrate transcription factor family that commonly contains a unique basic-leucine zipper domain [13]. Nrf1 functions as a transcription factor which activates the expression of some key metabolic genes regulating cellular growth and nuclear genes required for respiration, heme biosynthesis, and mitochondrial DNA transcription and replication [13]. Mice deficient in Nrf1 die during development [14]; and it has been reported that hepatocyte-specific knockout of Nrf1 causes a liver pathology resembling human NASH [13]. Further analysis revealed a potential binding site of Nrf1 within the promoter of miR-378. All these findings led to our hypothesis that a negative feedback loop between miR- 378 and Nrf1 promotes the pathogenesis of hepatosteatosis. This study was designed to investigate the regulatory mechanism of increased miR-378 in fatty lives and elucidate how miR-378 promotes hepatic lipid accumulation in an established mouse model of obesity.
2. Material and Methods
2.1 Establishment of dietary obese mice
Eight-week-old wild-type male C57Bl/6 mice (Jackson Laboratory, n=6) were maintained on either a standard diet (SD) (Open Source D12450B: 10% Kcal fat) or a high fat diet (HFD) (Open Source D12492: 60% Kcal fat) for 8 weeks as described by Vickers et al [15]. Mice were not subjected to fasting and re-feeding. After such time, livers were collected for miRNA and gene expression analysis.
2.2 Preparation of mini-circle expression vectors for miR-378 and shRNA of Nrf1
Mini-circle vectors were purchased from System Biosciences (Cat. MN511A-1). Specifically, we inserted the murine genomic region containing the precursor of miR-378 into the mini-circle parental plasmid. A transthyretin gene (TTR) promoter was inserted into the upstream of miR-378 precursor to ensure liver-specific expression of miR-378 [16]. This new construct was referred to as MC-TTR-miR-378. To rule out a non-specific effect of the plasmid, we generated a miR-378 mis-matched-expression vector by mutating the seed region of miR-378, termed MC-TTR-miR-378-MM. To obtain an expression vector for Nrf1 shRNA, we inserted the verified shRNA of Nrf1 into the mini circle vectors and the TTR promoter was used to ensure hepatic expression. This vector was referred as to MC-TTR-Nrf1-shRNA. To prepare the mini-circle, parental MC-TTR-miR-378 or MC-TTR-Nrf1-shRNA vector was transformed into a special host E. coli bacterial strain ZYCY10P3S2T (System Biosciences, Cat: MN900A-1). Mini-circles were made based on the manufacturer’s instructions.
2.3 MC-TTR-miR-378 treatment of dietary obese mice
The male C57Bl/6 mice kept on HFD for 8 weeks were divided into two groups; one group (n=10) was treated with MC-TTR-miR-378 and the other with MC-TTR-miR-378-MM (control, n=10). Mice received a dose of 1.5 µg/g MC-TTR-miR-378 or MC-TTR-miR-378-MM complexed with in vivo-jetPEI (Polyplus Transfection, Strasbourg, France) weekly for 8 weeks via tail vein injection. At that time point, the mice were anesthetized, and blood was collected by way of cardiac puncture. Subsequently, the livers were harvested and immediately frozen in liquid nitrogen for gene expression and histological analysis. Mice were not subjected to fasting and re-feeding. All procedures involving mice were approved by the Institutional Animal Care Committee at the University of Minnesota.
2.4 Generation of miR-378-ASO
The miR-378-ASO was produced by Exiqon. To prevent toxicity and facilitate efficient cellular uptake, a short (< 16mer) oligonucleotide design was chosen that carried a fully phosphorothioate-modified backbone. High target affinity was ensured by LNA modifications. The oligonucleotide was purified by reverse phase high-performance liquid chromatography and lyophilized.
2.5 Intravenous injection of miR-378-ASO
Lyophilized miR-378-ASO was re-suspended in NaCl 0.9% to a final concentration of 30 µg/µl. A dose of 25 µg/g body weight miR-378-ASO in a total volume of 100 µl NaCl 0.9% was injected via the tail vein into male C57BL/6 mice (Jackson Laboratory).
To examine whether Nrf1 mediates the inhibitory effect of miR-378 on hepatosteatosis, 8 week old wild-type C57Bl/6 mice were maintained on HFD for 8 weeks. At 16 weeks of age, mice were divided into three groups: Group I (control group, n=10) were injected with the scramble control (25 µg/g); Group II (n=10) was injected with 25 µg/g miR-378-ASO. This group was used to determine loss-of function of miR-378 for hepatosteatosis; Group III (n=10) received a combination of 25 µg/g miR-378-ASO and 1.5 µg/g MC-TTR-Nrf1-shRNA. This group was used to determine whether additional knockdown of Nrf1 can recover the development of hepatosteatosis inhibited by miR-378-ASO. Mice were not subjected to fasting and re-feeding. Mice were housed, fed, and monitored in accordance with protocols approved by the committee for animal research at the University of Minnesota.
2.6 Fatty acid treatment of HepG2 cells
Sodium oleate was obtained from Sigma-Aldrich and dissolved in DMEM medium with 1% fatty acid free bovine serum albumin (BSA) (Sigma). Oleate treatment of HepG2 cells was carried out as previously described with minor revision [17]. Specifically, HepG2 cells, maintained in our laboratory, were plated in 4-well chamber slides with DMEM medium supplemented with 10% FBS (Invitrogen). After 24 hours, HepG2 cells were treated with either control medium (DMEM supplemented with 1% fatty acid free BSA), or medium containing oleate (0.5 mM). The cells were cultured for another 24 hours, then lipid accumulation and miR-378 expression were determined by Oil-Red Staining (Sigma-Aldrich) and qRT-PCR, respectively.
2.7 Fatty acid oxidation (FAO) Assay
Cells were seeded in XF24 Cell Culture Microplates (Seahorse Bioscience, Hepa1–6 at 25,000 cells/well, mouse primary hepatocytes at 40, 000 cells/well) and cultured overnight in growth medium. The following day, medium was replaced with substrate-limited media and the cells incubated for an additional 24 hours. One hour before the assay, cells were equilibrated with KHB buffer (pH 7.4, 110 mmol/L NaCl, 4.7 mmol/L KCl, 2 mmol/L MgSO4, 1.2 mmol/L Na2HPO4, 2.5 mmol/L glucose, and 0.5 mmol/L carnitine) and incubated at 37°C for 1 h without CO2. For induction of FAO, palmitate-BSA complex was injected at a final concentration of 100 µmol/L into XF-24 cartridge (Seahorse Bioscience). FAO capacity was represented as increased oxygen consumption rate (OCR) in response to the palmitate-BSA complex.
2.8 Chromatin immunoprecipitation (ChIP) assay
Approximately 130 mg of livers from mice treated with HFD or SD was processed using the ChIP Assay Kit (Abcam, ab500) based on the manufacture’s protocol. Nrf1 Antibody was purchased from Abcam (ab34682). The binding region was detected in PCR reactions. A 10 kb region downstream from the binding site was used as a negative control and chromatin solution was reserved for input control. Primers flanking the binding site of Nrf1 within the murine miR-378 promoter were: forward, 5′-CTGGATGAAGAGCTCTCGTCCTTC -3′ and reverse, 5′-CTACCTGCGGGAGGAATTGTAGT -3′. The negative control primers were: forward, 5′-CTTCTATATGAAGAGACAGAGTAC -3′ and reverse, 5′-TGGAGTCCCTGCTATGTAGAGCCAG -3′. qPCR was used to determine the percentage of DNA precipitated by Nrf1 antibody relative to the amount of input DNA (% input).
3. Results
3.1 miR-378 is robustly induced in fatty livers of obese mice and in human HepG2 cells exposed to high levels of fatty acid
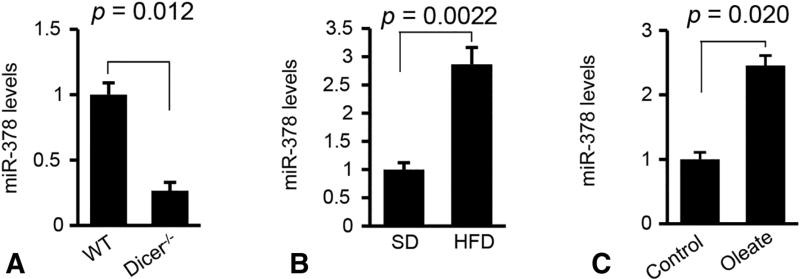
To identify miRNAs essential for the pathogenesis of NAFLD, we attempted to identify miRNAs that are highly and specifically expressed in hepatocytes by comparing miRNA profiles of livers of hepatocyte-specific Dicer1 knockout and wild-type (WT) mice [17]. Dicer1 encodes a critical enzyme of miRNA maturation [8]. Among 88 miRNAs that were reduced after Dicer1 knockout [17], miR-378 was reduced ≥ 3 fold in Dicer1 knockout mice, suggesting that hepatocytes represent a major source of miR-378 expression (Figure 1A). To study the role of miR-378 in NAFLD, we treated wild-type C57Bl/6 mice with a HFD (Supplementary Figure 1A–C). As expected, miR-378 expression was robustly increased in fatty livers of HFD-treated mice (Figure 1B) [11]. Oleic acids (OA) are the most abundant unsaturated fatty acids in liver triglycerides in human individuals [18]. Human HepG2 cells were used for our in vitro studies due to their increased sensitivity to fat accumulation. OA treatment increased intracellular lipids in HepG2 cells (Supplementary Figure 1D), which were also associated with increased miR-378 (Figure 1C). Together, hepatocytes represent the major source of miR-378 expression in the liver. Expression of miR-378 is reduced in fatty livers of HFD-treated mice and human HepG2 cells with accumulated lipid, leading us to focus on miR-378.
Figure 1. Hepatic lipid accumulation increased expression of miR-378.
(A) miR-378 levels in livers of Dicer1−/− (n=6) and WT mice (n=6). (B) Levels of miR-378 in livers of HFD-fed mice (n=6) compared to SD-fed mice (n=6). (C) miR-378 levels in HepG2 cells treated with oleate. HepG2 cells treated with DMEM medium without oleate served as control. Student’s t test was used for statistical analysis. Data represent mean ± SEM. P values are indicated.
3.2 Nrf1 is a direct target of miR-378
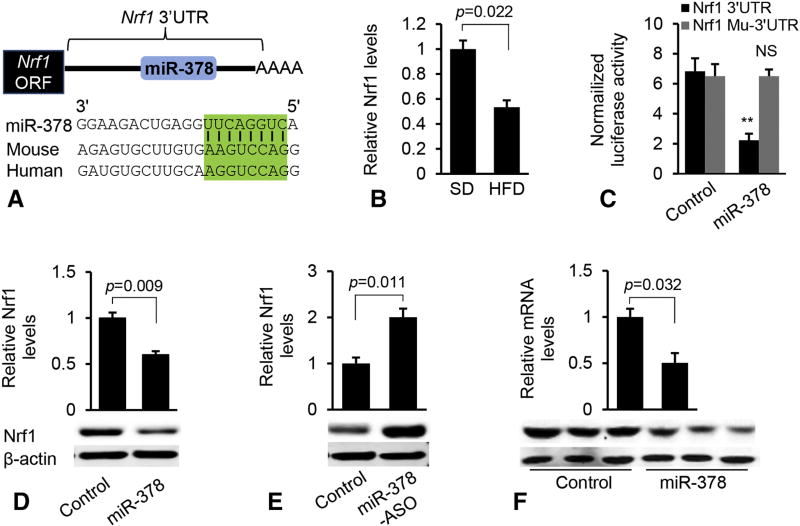
Potential effects of miR-378 on lipid homeostasis prompted us to explore the downstream effectors of miR-378. Combining bioinformatics prediction [19, 20] and Ago HITS-CLIP (high-throughput sequencing of RNAs isolated by crosslinking immunoprecipitation (HITS-CLIP) from Argonaute protein complex) [21], we identified Nrf1, a critical regulator of FAO and liver injury [22], as a potential target of miR-378 (Figure 2A). Consistent with increased miR-378, expression of Nrf1 was significantly reduced in fatty livers (Figure 2B).
Figure 2. Nrf1 is a direct target of miR-378.
(A) Graphic representation of the conserved miR-378 binding motifs within the 3’UTRs of Nrf1 between human and mouse. Complementary sequences to the seed region of miR-378 within the 3’UTRs are conserved among two species (highlighted in green). (B) mRNA levels of Nrf1 in livers of mice fed with SD (n=6) or HFD (n=6). (C) Luciferase activity of the reporter constructs containing either the WT or mutated 3’UTR of murine Nrf1 after treatment with miR-378 mimics. Hepa1–6 cells transfected with the empty luciferase reporter vector and scramble served as control. NS: no significance. (D) qRT-PCR and immunoblot analysis of Nrf1 after MC-TTR-miR-378 or MC-TTR-miR-378-MM (control) transfection into Hepa1–6 cells. (E) qRT-PCR and Western blot analysis of Nrf1 after miR-378-ASO transfection into Hepa1–6 cells (20 nM). Hepa1–6 cells that received scramble served as control. (F) Reduced protein and mRNA levels of Nrf1 after MC-TTR-miR-378 injection into dietary obese mice. Control mice were treated with the same dose of MC-TTR-miR-378-MM. Student’s t test was used for statistical analysis. The data shown are representative of experiments repeated three times and conducted in triplicate. Data represent mean ± SEM. P values are indicated.
To investigate whether miR-378 suppresses Nrf1 directly via a putative binding site(s) in its 3’UTR (3’ untranslated region), we generated a luciferase reporter construct in which the 3’UTR with wild-type or mutated miR-378 binding site was embedded downstream of the luciferase. Indeed, luciferase expression was repressed by miR-378 in Hepa1–6 cells (Figure 2C) [23], whereas its expression with the mutated 3’UTR was not altered significantly (Figure 2C) [23], suggesting the direct repression of miR-378 on Nrf1. MiR-378 also inhibited expression of endogenous Nrf1 (Figure 2D). Moreover, an increase in Nrf1 expression occurred in Hepa1–6 cells incubated with an miR-378 inhibitor (Figure 2E). Delivery of miR-378 into livers of mice also repressed mRNA and protein levels of both Nrf1 (Figure 2F). In summary, Nrf1 is a direct target of miR-378.
3.3 miR-378 impairs FAO by modulating expression of Nrf1
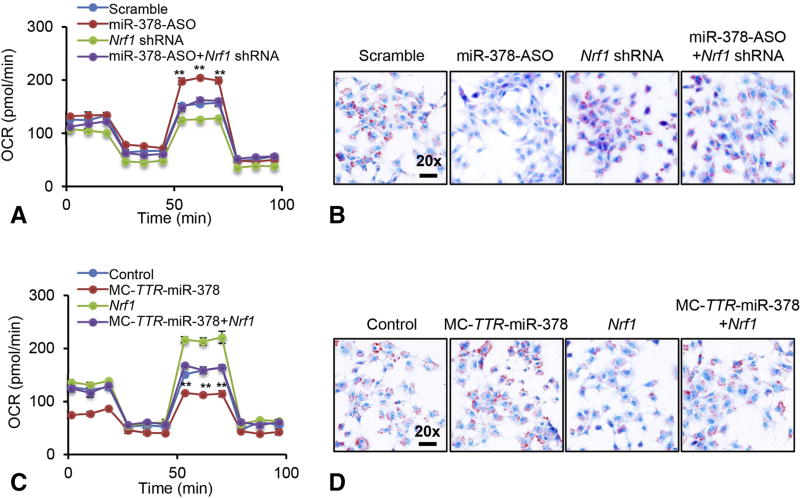
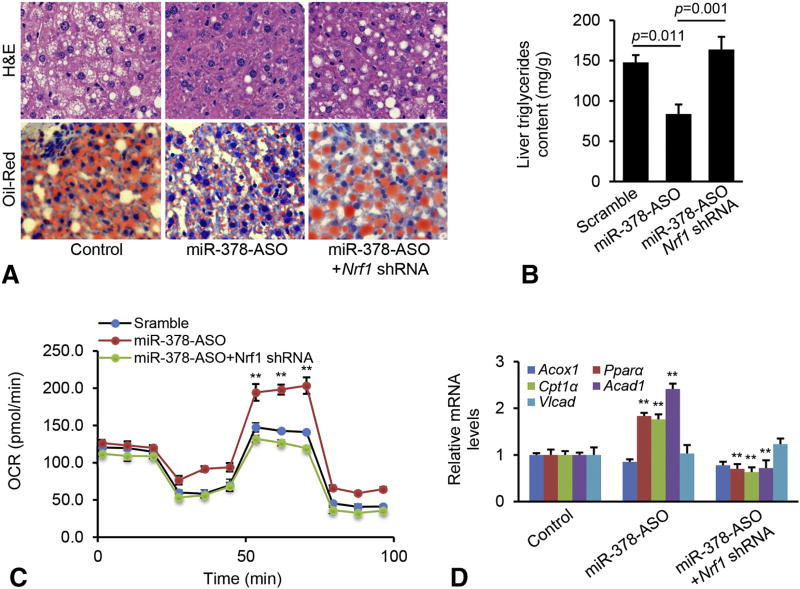
Nrf1 is a pivotal modulator of nuclear-encoded mitochondrial proteins and mitochondrial biogenesis [22], suggesting that miR-378 regulates FAO by targeting Nrf1. To confirm this, Hepa1–6 cells incubated with oleate were transfected with scramble, Nrf1 shRNA, miR-378-ASO, or a combination of miR-378-ASO and Nrf1 shRNA (Supplementary Figure 2A). Such design allowed us to determine whether Nrf1 was required for miR-378 to impair FAO. As expected, miR-378 knockdown improved FAO, and an additional knockdown of increased Nrf1 offset the effect of miR-378-ASO (Figure 3A). A single treatment of Nrf1 shRNA also impaired FAO rate, which was lower than that in Hepa1–6 cells treated with a combination of miR-378-ASO and Nrf1 shRNA (Figure 3A). Consistent with the improved FAO, miR-378-ASO significantly reduced intracellular lipid content in Hepa1–6 cells, while inhibiting upregulated Nrf1 was associated with recovered levels of lipid accumulation (Figure 3B). Lipid content was higher in Hepa1–6 cells treated with Nrf1 shRNA than the combination of Nrf1 shRNA and miR-378-ASO (Figure 3B).
Figure 3. miR-378 impaired FAO via targeting Nrf1.
(A–B) FAO rate and Oil-Red staining of three groups of Hepa1–6 cells treated with scramble (control), MC-TTR-Nrf1-shRNA, miR-378-ASO or a combination of miR-378-ASO and MC-TTR-Nrf1-shRNA. Hepa1–6 cells were maintained on DMEM medium containing 0.5 mM oleate. OCR: oxygen consumption rate. (C–D) FAO rate and Oil-Red staining of three groups of Hepa1–6 cells treated with the empty vector (Control), MC-TTR-miR-378, MC-TTR-Nrf1 or a combination of MC-TTR-miR-378 and MC-TTR-Nrf1. Student’s t test was used for statistical analysis. The data shown are representative of experiments repeated three times and conducted in triplicate. Data represent mean ± SEM. **p < 0.01
Gain-of function studies further confirmed our speculation that miR-378 impaired FAO by targeting Nrf1. Hepa1–6 cells under oleate treatment were transfected with either empty vector, MC-TTR-Nrf1, MC-TTR-miR-378 or a combination of MC-TTR-Nrf1 and MC-TTR-miR-378. Overexpression of miR-378 dramatically impaired the ability of Hepa1–6 cell to oxidize fatty acid and facilitated lipid accumulation (Figure 3C–D). MC-TTR-Nrf1 expressed the Nrf1 open reading frame (ORF), and was resistant to miR-378 suppression due to lack of a miRNA binding site. Indeed, re-introduction of Nrf1 partially rescued the ability of Hepa1–6 cells to oxidize fatty acid, which subsequently decreased intracellular lipid content (Figure 3C–D). A single treatment of MC-TTR-Nrf1 improved FAO rate and reduced intracellular lipid content, which was higher than that in Hepa1–6 cells treated with a combination of MC-TTR-miR-378 and MC-TTR-Nrf1.
Based on these observations, our attention was drawn to genes controlling FAO and lipogenesis. MiR-378 impaired expression of FAO genes including Cpt1a (Carnitine palmitoyltransferase 1A), Acox1 (Acyl-coenzyme A oxidase 1), Pparα (Peroxisome proliferator-activated receptor alpha), Acad1 (Acyl-coenzyme A dehydrogenase 1), and Vlcad (Acyl-CoA dehydrogenase, very long chain) [24, 25], and expression of these genes recovered after additional Nrf1 was introduced in Hepa1–6 cells (Supplementary Figure 2C). Furthermore, loss-of function of miR-378 facilitated expression of the genes controlling FAO and antagonizing upregulated Nrf1 impaired their expression (Supplementary Figure 2D). Interestingly, both overexpression and knockdown of miR-378 had no significant effects on expression of lipogenic genes including Srebp1c, Scd1 (Stearoyl-CoA desaturase-1), Fasn (Fatty acid synthase) and Gpat (Glycerol-3-phosphate acyltransferase) (Supplementary Figure 2E–F). Together, these data indicated that miR-378 impaired FAO, which was mediated primarily via suppression of Nrf1.
3.4 Liver-specific expression of miR-378 impairs FAO and promotes hepatosteatosis
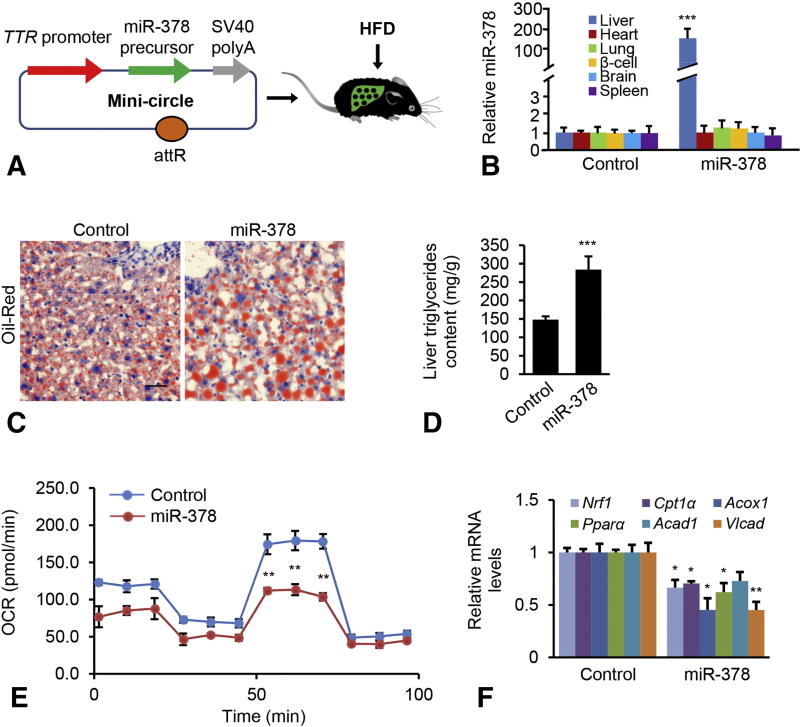
We next examined whether miR-378 is able to induce hepatic lipid accumulation in mice. For this purpose, we generated a miR-378 in vivo expression vector using a mini-circle, an episomal DNA vector that are produced as circular expression cassettes devoid of any bacterial plasmid DNA [26]. A transthyretin gene (TTR) promoter was used to ensure liver-specific expression of miR-378 [17]. This construct was referred to as MC-TTR-miR-378 (Figure 4A). To rule out non-specific effects of the plasmid, we generated a miR-378 mis-matched-expression vector, termed MC-TTR-miR-378-MM. Injection of MC-TTR-miR-378 into dietary obese mice led to increased miR-378 in liver, but no significant change in other tissues (Figure 4B). C57Bl6 mice, which had been on a HFD for 8 weeks, were injected with either MC-TTR-miR-378-MM (control) or MC-TTR-miR-378 for an additional 8 weeks. MiR-378 treatment led to a significant increase in hepatic lipid content (Figure 4C–D). Consistent with the aggravated hepatosteatosis, liver-specific expression of miR-378 dramatically reduced expression of Nrf1 (Supplementary Figure 3A), which subsequently impaired the ability of hepatocytes to oxidize fatty acid (Figure 4E). MiR-378-treated mice also exhibited reduced mRNA levels of Cpt1α, Acox1, Pparα, Acad1 and Vlcad [24, 25] (Figure 4F). In contrast, no significant changes in levels of Srebp1c, Scd1, Fasn and Gpat were detected in livers of mice treated with MC-TTR-miR-378 (Supplementary Figure 3B), indicating that impaired FAO rather than lipogenesis is a major contributor to hepatosteatosis promoted by miR-378.
Figure 4. miR-378 exacerbated hepatosteatosis.
(A) Diagram of hepatic-specific miR-378 expression vector construction (MC-TTR-miR-378). (B) Organ distribution of miR-378 after a single intravenous injection of MC-TTR-miR-378 (n=6). Control mice received MC-TTR-miR-378-MM (n=6). (C–D) Oil-Red staining of livers and hepatic lipid content from two groups of mice treated with MC-TTR-miR-378 (n=10) or MC-TTR-miR-378-MM (n=10). Eight-week-old wild-type male C57Bl/6 mice were maintained on a HFD (Open Source D12492: 60% Kcal fat) for 8 weeks. After 8 weeks of HFD administration, mice were injected with either MC-TTR-miR-378 or MC-TTR-miR-378-MM (control) for another 8 weeks. (E) Reduced FAO in hepatocytes of mice treated with MC-TTR-miR-378. (F) MC-TTR-miR-378 treatment reduced mRNA levels of Nrf1, Cpt1a, Ppara, Acad1, and Vlcad1 in livers of HFD-fed mice. Mann-Whitney test was used to evaluate the statistical significance. Data represent mean ± SEM. *p < 0.05; **p < 0.01; and ***p < 0.001.
3.5 Nrf1 mediates the inhibitory effect of miR-378-ASO on hepatosteatosis by modulating FAO
Next, we performed loss-of function for miR-378 and determined whether Nrf1 mediates the inhibitory effect of miR-378-ASO on hepatosteatosis. To this end, C57Bl6 mice maintained on HFD were then randomly allocated into three groups and received the different treatments for 6 weeks: Group I received scramble (control); Group II received miR-378-ASO; and Group III received a combination of miR-378-ASO and Nrf1 shRNA used to determine whether knockdown of upregulated Nrf1 can offset the effect of miR-378-ASO on hepatosteatosis. We observed a 68% reduction of hepatic miR-378 expression in mice that had received the miR-378-ASO compared to those receiving the scramble control, and a three-fold increase in mRNA levels of Nrf1 (Supplementary Figure 3C–D). Mice receiving a combination of miR-378-ASO and Nrf1 shRNA exhibited reduced miR-378 and Nrf1 (Supplementary Figure 3C–D).
By 8 weeks after injection, inhibiting miR-378 led to a slight decrease in liver to body weight ratio (Supplementary Figure 3E) and a 51% reduction in hepatic triglycerides (Figure 5A–B). miR-378 knockdown improved the ability of hepatocytes to oxidize fatty acid (Figure 5C), which was consistent with improved degree of hepatosteatosis. Expression of genes controlling FAO including Cpt1α, Pparα and Acad1 was significantly increased in miR-378 deficient mice (Figure 5D). To determine whether Nrf1 was required for improved hepatosteatosis by miR-378-ASO treatment, we further compared mice treated with miR-378-ASO or a combination of miR-378-ASO and Nrf1 shRNA. Additional treatment of Nrf1 shRNA significantly knocked down high levels of Nrf1 that was induced by miR-378-ASO (Supplementary Figure 3C–D), which subsequently impaired FAO of hepatocytes and restored the development of hepatosteatosis (Figure 5A–C). Additional treatment of Nrf1 shRNA further knocked down increased genes controlling FAO (Figure 5D). In addition, both miR-378 deficiency and the additional treatment of Nrf1 did not alter the expression of lipogenic genes including Srebp1c, Fasn, Scd1 and Gpat (Supplementary Figure 3F). These findings indicated that Nrf1, at least in part, mediated the inhibitory effect of miR-378-ASO on hepatosteatosis via modulation of FAO.
Figure 5. Nrf1 was required for miR-378 to promote hepatosteatosis.
(A) H&E and Oil-Red staining of livers from three groups of mice treated with scramble control (n=10), miR-378-ASO (n=10) or a combination of miR-378-ASO and MC-TTR-Nrf1-shRNA (n=10). (B) Hepatic lipid content in livers of the above three groups of mice. Mann-Whitney test was used to evaluate the statistical significance. (C) FAO in hepatocytes from livers of the above three groups of mice. (D) mRNA levels of Acox1, Ppara, Cpt1a, Acad1, and Vlcat1 in livers from the three groups of mice. Student’s t test was used for statistical analysis. Data represent mean ± SEM. Data represent mean ± SEM. **p < 0.01.
3.6 Nrf1 inhibits transcription of miR-378
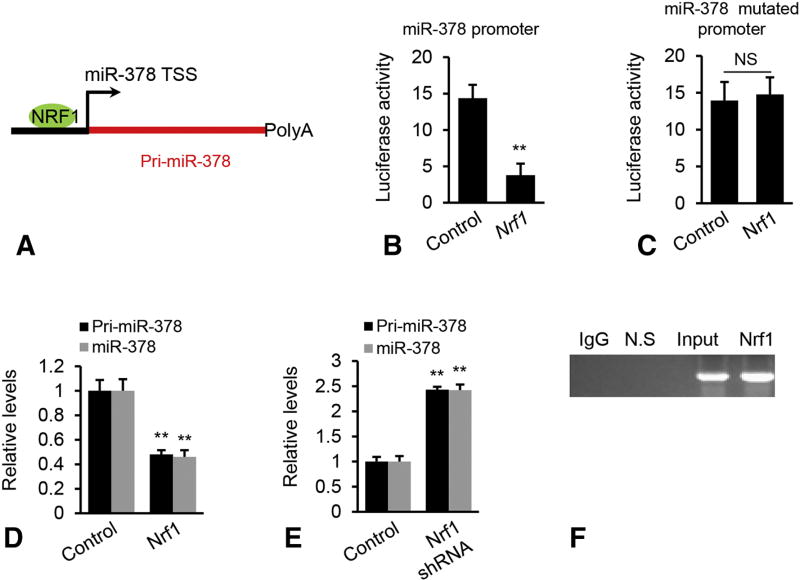
To investigate the potential mechanisms for increased miR-378 in fatty livers, we predicted potential binding sites for transcription factors within the promoter regions of miR-378 using Malinspector [27]. The analysis revealed a highly conserved binding motif for Nrf1 within the promoter of miR-378 (Figure 6A). Nrf1 expression was reduced in fatty livers [28] (Supplementary Figure 4). The action of Nrf1 on the promoter of miR-378 was then examined by luciferase assay. Overexpression of Nrf1 significantly repressed activity of miR-378 promoter (Figure 6B). To test whether the binding motif for Nrf1 within the miR-378 promoter was essential for increased expression of miR-378, we introduced mutations into the Nrf1 binding motifs, and examined the effects of the mutation on the transcriptional activity. As expected, the mutation impaired the inhibitory effect of Nrf1 on the transcription of miR-378 (Figure 6C). Nrf1 overexpression strongly reduced levels of mature miR-378 and miR-378 primary transcript (PrimiR-378), whereas inhibition of endogenous Nrf1 resulted in upregulation of both mature and primary transcript of miR-378 (Figure 6D–E). Together, our findings suggested that miR-378 biogenesis was modulated via transcriptional activity of Nrf1. Chromatin immunoprecipitation (ChIP) revealed that DNA fragments containing Nrf1 binding sites within the Pri-miR-378 promoter were immune-precipitated from genomic DNA of mouse livers by an Nrf1 antibody (Figure 6F), indicating that Nrf1 can physically bind to the promoter region of miR-378. Nrf1 expression was lower in livers of HFD treated mice, indicating that the binding affinity of Nrf1 on the miR-378 promoter should be lower in livers of HFD- than SD-treated mice. Thus, we analyzed the percentage of DNA precipitated by Nrf1 antibody relative to the amount of input DNA (% input). As expected, % input was 4% in livers of HFD-treated mice compared to 9% in livers of SD-treated mice (Supplementary Figure 5).
Figure 6. Nrf1 impaired transcription of miR-378.
(A) Schematic representation of Nrf1 binding site within the promoter of miR-378. (B–C) Luciferase activity of the reporter construct containing either the WT or mutated miR-378 promoter after treatment with miR-378 mimics. In addition to the luciferase reporter vector, Hepa1–6 cells were also transfected with miR-378 mimics or scramble (control). NS: no significance. (D) Levels of mature miR-378 and Pri-miR-378 in Hepa1–6 cells after Nrf1 overexpression. Hepa1–6 cells were transfected with MC-TTR-Nrf1 expression vector or empty vector (Control). (E) Levels of mature miR-378 and Pri-miR-378 in Hepa1–6 cells after Nrf1 knockdown. Hepa1–6 cells were transfected with MC-TTR-Nrf1-shRNA expression vector or empty vector (Control). (F) In vivo ChIP assays were performed using genomic DNA isolated from mouse livers treated with HFD; and the binding of Nrf1 to the endogenous promoter of miR-378 was detected using specific Nrf1 antibodies; N.S.: non-specific control, which is located 10 kb downstream of predicted Nrf1 binding site. Student’s t test was used for statistical analysis. The data shown are representative of an experiment repeated three times and conducted in triplicate. Data represent mean ± SEM. *p < 0.05; **p < 0.01.
3.7 Liver-specific inactivation of Nrf1 increases expression of miR-378 and promotes hepatosteatosis
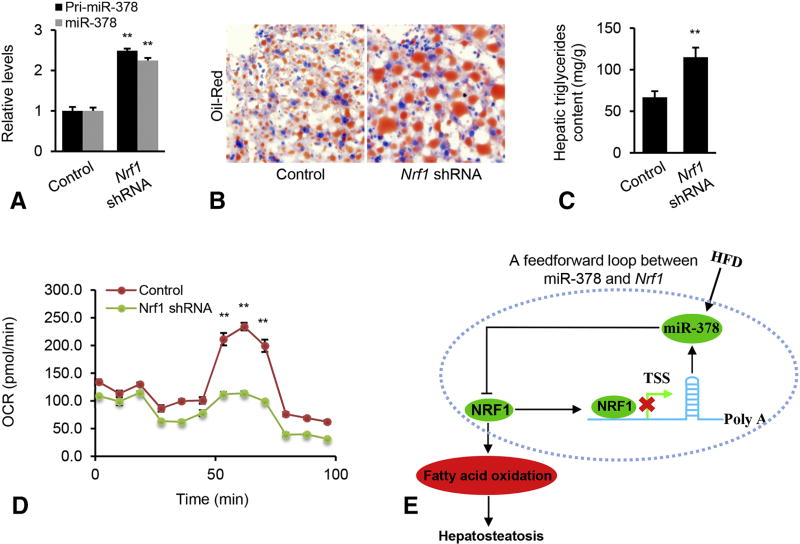
Nrf1 is a transcription repressor of miR-378 and miR-378 directly targets Nrf1 through its 3’UTR. Therefore, we hypothesized that a negative feedback loop between miR-378 and Nrf1 maintains high levels of miR-378 and low levels of Nrf1, which subsequently promotes hepatosteatosis. To test our hypothesis, 8 week-old mice were kept on HFD on for 8 weeks. At 16 weeks of age, the mice were injected with Nrf1 shRNA to antagonize endogenous miR-378 in the liver (Supplementary Figure 6). Upon Nrf1 knockdown, comparable increases of Pri-miR-378 and mature miR-378 were observed (Figure 7A), demonstrating that HFD-induced miR-378 is Nrf1-dependent. To investigate whether Nrf1 knockdown can fully or partially simulate the function of miR-378 in promoting hepatosteatosis, we further analyzed livers from mice treated with Nrf1 shRNA. Similar to the phenotype observed in miR-378-treated mice, Nrf1 inactivation resulted in exacerbated hepatosteatosis (Figure 7B–C). We speculated that the negative feedback loop could maintain high levels of miR-378 after Nrf1 knockdown, which could enhance the inhibition of miR-378 on FAO. As expected, Nrf1 deficiency impaired FAO (Figure 7D). Our findings indicated that miR-378 and Nrf1 formed a negative feedback loop in fatty livers. Once this feedback circuit was activated, miR-378 suppressed expression of Nrf1 and reduced Nrf1 further abrogated its suppression of miR-378 transcription. As a consequence, a reduction in Nrf1 impaired FAO, which subsequently led to aggravated hepatosteatosis (Figure 7E).
Figure 7. Nrf1 knockdown increased miR-378 expression and promoted hepatic lipid accumulation.
(A) Levels of mature miR-378 and Pri-miR-378 in livers of HFD-fed mice received MC-TTR-Nrf1-shRNA (n=9) or MC-TTR-miR-378-MM (Control, n=9). (B) Oil-Red staining of livers excised from the above two groups of mice. (C) Hepatic lipid content in the above two groups of mice. (D) Reduced FAO rate in hepatocytes of mice treated with MC-TTR-Nrf1-shRNA compared to control mice. (E) A negative feedback loop circuit between miR-378 and Nrf1 maintains the pathogenesis of hepatosteatosis in dietary obese mice. Once this circuit is triggered, it maintains activation of miR-378 biogenesis and suppression of Nrf1, which subsequently impairs fatty acid oxidation and promotes hepatosteatosis. Mann-Whitney test was used to evaluate the statistical significance. Data represent mean ± SEM. *p < 0.05; **p < 0.01.
4. Discussion
Data from CDC in 2017 showed that nearly 70% of adults and 30% of youth are either overweight or obese. Furthermore, the incidence rate for both groups is increasing by 30% annually, suggesting more NAFLD patients and underscoring the importance of identifying new molecular pathways and targets for the design of new therapeutic approaches. We have identified a previously unrecognized negative feedback loop composed of miR-378 and Nrf1 that promotes the development of hepatosteatosis. Specifically, in response to HFD challenge, miR-378 transcription was activated and increased miR-378 further repressed expression of Nrf1 by binding to its 3’UTR. Reduced Nrf1 due to increased miR-378 further released its suppression on miR-378 biogenesis. Activation of this negative feedback loop impaired FAO, which subsequently led to hepatic lipid accumulation. Importantly, increased miR-378 expression is not restricted to murine obesity models of NAFLD, but is also detected in human NASH patients (under review in Hepatology). In addition, we also found that the binding motif of miR-378 within the Nrf1 3′ UTRs is conserved between human, rat, and mouse, further suggesting the potential of miR-378 as a therapeutic target for human NAFLD.
Among these potential target genes, we have functionally validated Nrf1 as a bona fide target of miR-378 both in vitro and in vivo. In other published literatures, Crot1 (Carnitine O-acetyltransferase) and Nrf1 were also identified as direct targets of miR-378 [11, 23]. Crot1 is a mitochondrial enzyme involved in fatty acid metabolism. Loss-of function for Nrf1 causes decreased total content of triglycerides in mouse livers, further indicating the important role of Nrf1 in obesity-regulated fatty liver diseases. However, neither study provided any evidence that Crot1 and/or Nrf1 mediated the inhibitory effect of miR-378 on FAO since miR-378 can simultaneously target many genes. In this study, we treated mice with miR-378-ASO or a combination of miR-378-ASO and Nrf1 shRNA. Such design allowed us to determine whether further knockdown of upregulated Nrf1 due to miR-378-ASO treatment can offset the effect of miR-378-ASO. As a result, we were able to also determine whether Nrf1 rather than other target(s) mediates the inhibitory effect of miR-378 on FAO.
It is well-established that Nrf1 can function as either a transcription activator or repressor [29]; and is able to activate expression of genes involved in FAO [13]. In the normal state, Nrf1 represses transcription of miR-378, which in itself maintains normal levels of Nrf1 mRNA, protein and subsequent FAO since Nrf1 is a direct target of miR-378. As a scenario in NAFLD, one could speculate that increased miR-378 impaired the feedback loop with Nrf1 by reducing protein and mRNA levels of Nrf1, and failing to inhibit transcription of miR-378 and, thereby promoting the development of hepatosteatosis. The balance between miR-378 and Nrf1 is critical to the normal function of the liver; and once that balance is impaired, hepatic lipid accumulation will occur due, in part to impaired FAO.
Another intriguing finding is that the negative feedback loop between miR-378 and Nrf1 was identified in fatty livers, which maintains the pathogenesis of hepatosteatosis by impairing FAO. Although the physiological role of miR-378 in metabolic diseases has been studied extensively, few studies have addressed the underlying mechanisms of miR-378 dysregulation in human diseases. Our data revealed an important role of Nrf1, a target of miR-378, in repressing transcription of miR-378 both in vitro and in vivo. We demonstrated that knockdown of Nrf1 in the liver led to up-regulated miR-378, which simulated the function of miR-378 in impairing FAO and facilitating hepatic lipid accumulation. These findings are consistent with earlier reports that liver-specific inactivation of Nrf1 led to an substantial lipid accumulation in the liver [22]. It is well known that Nrf1 is a mediator of liver injury and its deficiency in the liver also promoted apoptosis, necrosis, inflammation, and fibrosis [22], suggesting that the negative feedback loop between miR-378 and Nrf1 represents a candidate pathway to cause steatohepatitis, which is beyond the scope of this study.
Conclusions
We have employed detailed molecular approaches and mouse models to demonstrate that the precise regulation of miR-378 expression in livers is essential to control hepatic lipid homeostasis by modulating FAO. The insights obtained from this study further advance our understanding of the physiological roles of miRNA and the multilayer regulation of miRNA biogenesis to address the complexity of lipid homeostasis. The ultimate purpose of our study was to develop miR-378-ASO as a therapeutic agent for NAFLD. Our findings suggested, in fact, that such an approach is feasible. Our data showed that miR-378 knockdown exhibited a therapeutic response for NAFLD in mice. In addition, miR-378 has a conserved binding motif on the 3′ UTR of both human and mouse Nrf1, suggesting that miR-378 as a potential therapeutic target could be applied to human NAFLD patients. Therefore, we will further optimize the dose and duration of miR-378-ASO and investigate its therapeutic potential for NAFLD in future studies.
Supplementary Material
HIGHLIGHTS.
Expression of miR-378 is increased in fatty livers of mice and human hepatocytes with accumulated lipid
Nrf1 is a direct target of miR-378 and Nrf1 is a transcription repressor of miR-378
Liver-specific expression of miR-378 impairs fatty acid oxidation and promotes NAFLD
Ablation of Nrf1 increases expression of miR-378 and promotes NAFLD
A negative feedback loop between miR-378 and Nrf1 promotes the pathogenesis of NAFLD
Acknowledgments
Funding
This work was in part supported by the National Institutes of Health (R01 DK102601, G.S.) and Research Scholar Grant (ISG-16-210-01-RMC, G.S) from the American Cancer Society.
List of abbreviations
- 3’UTRs
3’ untranslated regions
- Acad1
Acyl-coenzyme A dehydrogenase 1
- Acox1
Acylcoenzyme A oxidase 1
- ASO
anti-sense oligonucleotide
- ChIP
chromatin immunoprecipitation
- Cpt1a
Carnitine palmitoyltransferase 1A
- FAO
fatty acid oxidation
- Fasn
fatty acid synthase
- FFA
free fatty acid
- Gpat
glycerol-3-phosphate acyltransferase
- HCC
hepatocellular carcinoma
- H&E
hematoxylin and eosin stain
- HFD
high fat diet
- HITS-CLIP
high-throughput sequencing of RNAs isolated by crosslinking immunoprecipitation from argonaute protein complex
- MC
mini-circle
- NAFLD
non-alcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- Nrf1
nuclear respiratory factory1
- OCR
oxygen consumption rate
- ORF
open reading frame
- Pparα
Peroxisome proliferator-activated receptor alpha
- Ppargc1β
peroxisome proliferator-activated receptor γ coactivator 1 β
- Scd1
stearoyl-CoA desaturase-1
- SREBPs
sterol regulatory element-binding proteins
- Vlcad
Acyl-CoA dehydrogenase, very long chain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors' contributions
G.S., T.Z., and X.Z. conceived and G.S. supervised the project; G.S. and T.Z. designed the experiments; T.Z. performed experiments; X.Z. performed target prediction and database comparison; C.J.S and T.Z. analyzed and interpreted data; and G.S. and G.Y. wrote the manuscript with input from all the authors.
Competing interests
The authors declare that they have no competing interests
Appendix A
Supplementary Data to this article can be found online
References
- 1.Farrell GC, McCullough AJ, Day CP. Non-Alcoholic Fatty Liver Disease: A Practical Guide. 2013 [Google Scholar]
- 2.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–54. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 4.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Nonslcoholic gatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–60. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao X, Song B-L. SREBP: a novel therapeutic target. Acta Biochim Biophys Sin. 2013;45:2–10. doi: 10.1093/abbs/gms112. [DOI] [PubMed] [Google Scholar]
- 6.Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12:83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 7.Koo S-H. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol. 2013;19:210. doi: 10.3350/cmh.2013.19.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, et al. Nonalcoholic steatohepatitis is associated with altered hepatic microRNA expression. Hepatology. 2008;48:1810–20. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker P, Niesler B, Tschopp O, Berr F, Canbay A, Dandekar T, et al. MicroRNAs as mediators in the pathogenesis of non-alcoholic fatty liver disease and steatohepatitis. Zeitschrift für Gastroenterologie. 2014;52:1–27. [Google Scholar]
- 11.Carrer M, Liu N, Grueter CE, Williams AH, Frisard MI, Hulver MW, et al. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc Natl Acad Sci U S A. 2012;109:15330–5. doi: 10.1073/pnas.1207605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008;283:33554–62. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JY, Kwong M, Lu R, Chang J, Wang B, Yen TB, et al. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 1998;17:1779–87. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickers KC, Shoucri BM, Levin MG, Wu H, Pearson DS, Osei-Hwedieh D, et al. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology. 2013;57:533–42. doi: 10.1002/hep.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayandharan GR, Zhong L, Sack BK, Rivers AE, Li M, Li B, et al. Optimized Adeno-Associated Virus (AAV)-protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses. Hum Gene Ther. 2010;21:271–83. doi: 10.1089/hum.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng R, Wu H, Xiao H, Chen X, Willenbring H, Steer CJ, et al. Inhibition of microRNA-24 expression in liver prevents hepatic lipid accumulation and hyperlipidemia. Hepatology. 2014;60:554–64. doi: 10.1002/hep.27153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Lechon MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O’Connor J-E. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165:106–16. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Friedman R, Farh K, Burge C, Bartel D. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krek A, Grün D, Poy M, Wolf R, Rosenberg L, Epstein E, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 21.Yang J-H, Li J-H, Shao P, Zhou H, Chen Y-Q, Qu L-H. starBase: a database for exploring microRNA–mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Research. 2011;39:D202–D9. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z, Chen L, Leung L, Yen TB, Lee C, Chan JY. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci U S A. 2005;102:4120–5. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon TI, Park JW, Ahn J, Jung CH, Ha TY. Fisetin protects against hepatosteatosis in mice by inhibiting miR-378. Mol Nutr Food Res. 2013;57:1931–7. doi: 10.1002/mnfr.201300071. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Jia Y, Fu T, Viswakarma N, Bai L, Rao MS, et al. Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012;26:628–38. doi: 10.1096/fj.11-194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holness MJ, Bulmer K, Gibbons GF, Sugden MC. Up-regulation of pyruvate dehydrogenase kinase isoform 4 (PDK4) protein expression in oxidative skeletal muscle does not require the obligatory participation of peroxisome-proliferator-activated receptor α (PPARα) Biochem J. 2002;366:839–46. doi: 10.1042/BJ20020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayrhofer P, Schleef M, Jechlinger W. Use of minicircle plasmids for gene therapy. Cancer Gene Therapy: Springer. 2009:87–104. doi: 10.1007/978-1-59745-561-9_4. [DOI] [PubMed] [Google Scholar]
- 27.Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, et al. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–42. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 28.Park EJ, Lee JH, Yu G-Y, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsujita T, Peirce V, Baird L, Matsuyama Y, Takaku M, Walsh SV, et al. Transcription factor Nrf1 negatively regulates the cystine/glutamate transporter and lipid-metabolizing enzymes. Mol Cell Biol. 2014;34:3800–16. doi: 10.1128/MCB.00110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.