Abstract
Background
We have previously demonstrated that the adipose tissue derived hormone leptin controls reproductive function by regulating the hypothalamic-pituitary-gonadal axis in response to energy deficiency. Here, we evaluate the activins-follistatins-inhibins (AFI) axis during acute (short-term fasting in healthy people) and chronic energy deficiency (women with hypothalamic amenorrhea due to strenuous exercise [HA]) and investigate their relation to leptin and reproductive function in healthy subjects and subjects with HA.
Methods
The AFI axis was investigated in: a) A double-blinded study in healthy subjects having three randomly assigned admissions, each time for four days: in the isocaloric fed state, complete fasting with placebo treatment, complete fasting with leptin replacement, b) A case-control study comparing women with HA vs healthy controls, c) An open-label interventional study investigating leptin treatment in women with HA over a period of up to three months, d) A randomized interventional trial investigating leptin treatment vs placebo in women with HA for nine months.
Results
The circulating levels of activin A, activin B, follistatin and follistatin-like 3 change robustly in response to acute and chronic energy deficiency. Leptin replacement in acute energy deprivation does not affect the levels of these hormones suggesting an independent regulation by these two hormonal pathways. In chronic energy deficiency, leptin replacement restores only activin B levels, which are in turn associated with an increase in the number of dominant follicles.
Conclusions
We demonstrate for the first time that the AFI axis is affected both by acute and chronic energy deficiency. Partial restoration of a component of the axis, i.e. activin B only, through leptin replacement is associated with improved reproductive function in women with HA.
Keywords: acute energy deficiency, chronic energy deficiency, energy, fasting, obesity, PCOS
1. Background
Human reproduction is an energy demanding process that requires sufficient energy stores [1]. Conditions related to energy deprivation (i.e. excessive exercise, or reduced nutritional intake) downregulate the hypothalamic-pituitary-gonadal axis (HPG-axis) and often lead to amenorrhea and infertility [Hypothalamic Amenorrhea, (HA) due to strenuous exercise, leading to energy expenditure vs. intake imbalance, or anorexia nervosa, affecting up to 10% of young women] as well as to other clinical manifestations [e.g. osteoporosis, low thyroid hormones, and insulin-like growth factor I (IGF-1), hyperactive adrenal axis] [2]. We have previously demonstrated that leptin orchestrates the processes linking adipose tissue, energy balance, and reproduction [1, 3, 4]. Acute energy deprivation after 72h of fasting reduces circulating leptin levels to ~10% [5]. Similarly, chronic energy deprivation in women with HA is associated with hypoleptinemia. Most importantly, leptin replacement can restore menstruation and fertility and improve other hypothalamic-pituitary-peripheral axes in women with HA [6, 7]. The beneficial effects of leptin on reproductive function are partially achieved by restoration of the pulsatile secretion of gonadotropins [5]; however the exact mechanism and/or the presence of additional mediators participating in this process remains unknown. This is particularly important, given that not all the women with HA respond to leptin treatment [6, 7]. Thus, leptin-independent mechanisms/hormonal systems should contribute to the downregulation of reproductive function observed in energy deprivation states. Identifying such mechanisms may help to develop new treatments for women with HA or even target other conditions related to sub-/infertility due to energy imbalance.
The activin-follistatin-inhibin system (AFI) is considered a key regulator of the HPG-axis [8, 9]. Activin A and activin B as well as inhibin B belong to the transforming growth factor beta (TGF-β) superfamily and exert a variety of functions in different organs, including pituitary and ovarian regulation, gonadal development, bone metabolism, adrenal growth/function, retinal development and hematopoiesis [8]. Follistatins [Follistatin and Follistatin-like 3 (FSTL3)] are glycoproteins that do not share structural similarities with inhibins and activins. Follistatin is primarily secreted by the liver, pituitary, and ovary and antagonizes myostatin-induced inhibition of myogenesis [10]. FSTL3 (previously known as follistatin-related protein) is primarily expressed, at the gene level in placenta, testis, endometrium, adrenal glands, and skeletal muscle [8]. FSTL3 has similar structure and function with follistatin, but is considered less potent. Altogether, activins stimulate the HPG-axis while in contrast, inhibins and follistatins downregulate it by antagonizing activins [8].
Another hormone belonging to the TGF-β superfamily with important structural similarities to inhibins and major role in reproduction is the Anti-Müllerian Hormone (AMH) [11]. In males, AMH is secreted from the testis and is responsible for the regression of the Müllerian ducts during fetal sex differentiation. In females, AMH is secreted by the ovary and is involved in the dominant follicle selection in the follicular phase of menstrual cycle. AMH is considered a reliable marker of the primordial ovarian follicle reserves.
We have previously reported that acute energy deprivation affects hormones of the AFI axis [12–15]. Circulating activin A levels were decreased and follistatin levels were increased both in males and females after 72 hours of fasting [13, 14]. Leptin administration did not affect the levels of activin A and follistatin neither in acute nor in chronic energy deprivation states [12–14]. This demonstrates that the AFI axis may be an independent system regulating the reproductive function according to energy balance.
Given the structural and/or functional similarities/integrations between activin A, activin B, follistatin, FSTL3, inhibin B and AMH, and in order to fully describe the role of AFI as system linking energy sufficiency with reproductive function and related disease states, we investigated in the current study: a) whether circulating activin B, FSTL3, inhibin B, and AMH change in response to acute energy deprivation (short-term fasting), whether these changes are associated with alterations of HPG axis and whether they are leptin dependent (Study 1), b) whether the circulating concentrations of the AFI hormones change during the menstrual cycle in healthy women and whether they differ in women with HA (chronic energy deficient state) (Study 2), c) whether any changes observed in the AFI axis in women with HA (chronic energy deficient state) are mediated by leptin (Study 3 and Study 4) and d) whether any changes observed in AFI axis are related to important improvement of reproductive function and capacity in women with HA (chronic energy deficient state) (Study 3) and can therefore be targeted in the future with novel treatments.
2. Materials and methods
2.1 Study design
To address our aims, the following studies have been utilized (s. Fig.1):
Fig. 1.
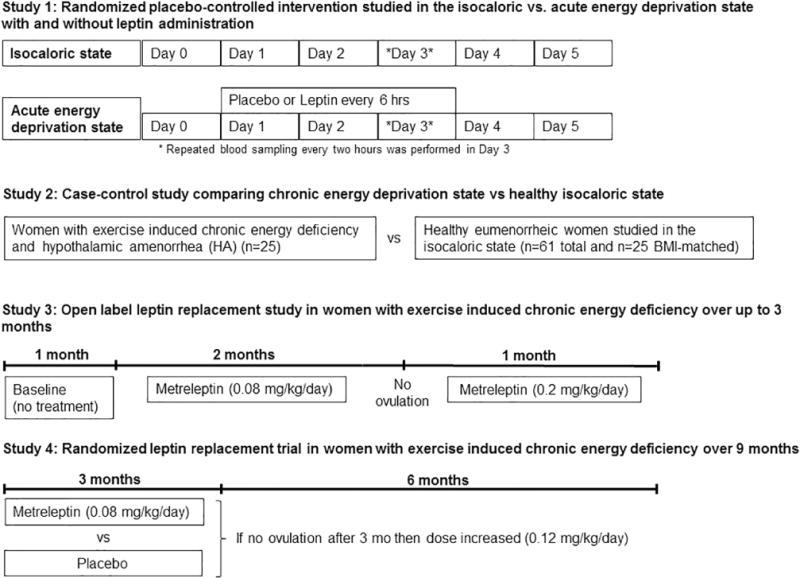
Schematic summary of the four clinical studies that were used in order to investigate the changes of circulating AFI hormones in response to acute and chronic energy deficiency
2.1.1 Study 1: Clinical Research Center (CRC) – based randomized placebo-controlled intervention in the isocaloric and fasting state (acute energy deprivation state)
Following previously described protocols [5, 16], seven healthy lean men [age 23.2±3.7yr; body mass index (BMI) 23.6±1.7 kg/m2] and six healthy lean women (age 22.8±3.4 yr; BMI= 21.7 ±2.2 kg/m2) with regular menstrual cycles (length 26–32d) and no history of oral contraceptive use the last six months were studied under three conditions for 72h: isocaloric fed state, complete fasting state with administration of placebo, or with replacement dose of leptin in a double-blinded randomized fashion. Six men and six women completed all three studies. The isocaloric fed state consisted of four standardized meals per day. During the fasting state, only a standardized volume of calorie-free fluids, electrolytes, and vitamin supplements were allowed. The replacement dose of leptin was for the males 0.04 mg/kg daily on d1 and 0.025 mg/kg four times per day on d2 and 3; for the females 0.08 mg/kg daily on d1 and 0.05 mg/kg four times per day on d2 and 3. Leptin (Amylin, Inc., San Diego, CA; previously known as r-metHuLeptin, provided by Amgen, Inc., Thousand Oaks, CA) was administrated subcutaneously (s.c.). Repeated blood sampling every two hours was performed in day 3 (from 8 am of day 3 till 8 am of day 4).
2.1.2 Study 2: Case-control study comparing chronic energy deficiency vs healthy state
Twenty-five women with HA studied in the context of study 3 and study 4 (s. below) and 61 healthy controls, thereof 25 BMI-matched were included in this study. The women had secondary HA for at least six months coincident with a period of increased exercise or low weight. Their weight had been stable for at least six months and was within 15% of the ideal body weight. They were otherwise healthy (no active eating disorders) and had not been receiving medications for three months or more. The subjects in the control group were apparently healthy young female medical students with regular menstrual cycles and they were either in the follicular phase of their menstrual cycle [n=25, days mean± standard deviation (SD): 6.64±4.20], or in the luteal phase (n=32, days mean ± SD: 20.53±4.87) and four could not report with certainty the day of their menstrual cycle. They had no considerable weight change (>5%) during the previous six months, no change in their physical activity in the last 3 months and no excessive exercise within the last 24 hours. Additionally, they had no history of concomitant medication that could affect muscle or lipid metabolism [protocol described in [17]].
2.1.3 Study 3: Open label leptin replacement study in women with hypothalamic amenorrhea (chronic energy deficiency state) over a period of up to 3 months in a single-arm study
Eight lean women with HA due to strenuous exercise and hypoleptinemia, without active eating disorders and with stable weight (inclusion criterion: within ±15% of ideal body weight for ≥ six months) received metreleptin (0.08 mg.kg−1.day−1, self-injected subcutaneously twice daily: 40% in the morning and 60% in the evening) initially for two months [Protocol published in [7]]. Subjects who had not ovulated in the first two months, continued with a third month of treatment at an increased dose of 0.2 mg (divided as described above). All subjects had visits weekly to undergo pelvic ultrasonography and measure hormone levels, starting one month before initiation of leptin treatment (=baseline month, where measurements were performed at the beginning and end of the month). The pelvic ultrasonography provided information about the number of follicles, the maximum follicle diameter, the ovarian volume, and the endometrial thickness. For one subject no serum was available for the planned measurements and was excluded from the study.
2.1.4 Study 4: Outpatient randomized placebo controlled clinical trial investigating leptin replacement in women with hypothalamic amenorrhea (chronic energy deprivation state) over 9 months
Subjects characteristics, study design, primary and secondary outcomes have been previously published in [6]. This was a randomized, double-blinded, placebo controlled study (parallel design) with a 1:1 allocation ratio to receive either metreleptin or placebo. The study protocol was approved by the institutional review board of Beth Israel Deaconess Medical Center (BIDMC) and was performed in the General Clinical Research Center (GCRC) of BIDMC, where participants were randomized between October 2005 and December 2009. The trial was registered at the US National Institutes of Health (ClinicalTrials.gov) #NCT00130117. Eligible participants were women between 18 and 35 years old with secondary hypothalamic amenorrhea for at least 6 months coincident with strenuous exercise and/or low body weight. Body weight was stable for at least 6 months before screening and within 15% of ideal body weight (inclusion criterion). At the time of screening, fasting morning leptin was <5 ng/ml. Exclusion criteria were: Any significant medical history such as renal or hepatic disease, diabetes mellitus, myocardial ischemia, malignancy, alcoholism, drug abuse, smoking, active eating disorder, depression or other psychiatric disease, anemia, any conditions which oral contraceptive use is contraindicated, other causes of amenorrhea (e.g. hyperprolactinemia, hypothyroidism or hyperthyroidism, Cushing’s syndrome, polycystic ovarian syndrome, congenital adrenal hyperplasia, primary ovarian failure), medications known to affect hormones or bone mineral density (e.g. glucocorticoids, antiseizure medications, thyroid hormones, estrogens), breast feeding, pregnancy or wanting to become pregnant during the next 6 months.
Primary outcome of the study was the difference between the placebo and leptin treated group in change of bone mineral content at anteroposterior spine from baseline to 36 weeks and secondary outcomes included changes in bone markers and in bone mineral density as well as reproductive outcomes. For our study, primary outcomes were differences between the placebo and leptin treated group in change of the circulating levels of activin A, activin B, follistatin, FSTL3 and AMH from baseline to 36 weeks of treatment. Sample size was defined in this study by an interim analysis after seven subjects per arm had completed 24 weeks of study according to the changes observed for the initial primary outcome. No preliminary data were available to perform formal power calculations based on specific prior changes of the molecules of interest. Our study had 88% power to detect differences ≥30% with an SD=25% of the mean, two-tailed and at an α=0.05. Twenty participants were finally assigned randomly to receive either metreleptin or placebo. Randomization tables were produced by the Harvard Catalyst biostatisticians with SAS and delivered directly to the Research Pharmacy for use such that study staff that recruited subjects (medical doctors, care providers) as well as the participants would remain blinded. All participants received calcium (600 mg twice daily) and vitamin D (400 IU daily). Metreleptin or placebo was self-administered s.c. once daily between 7:00 and 11:00 pm for 36 weeks. The dose of metreleptin was 0.08 mg.kg−1.day−1 (self-injected subcutaneously twice daily) for 12 weeks and subjects who had begun menstruating remained in this dose till the completion of the study. The dose in subjects who had not menstruated at week 12 was increased to 0.12 mg/kg. If a subject lost >5% of her baseline weight, dose was reduced by 0.04 mg/kg. After the completion of the treatment, subjects were followed for another 16 weeks. Subjects had visits every four weeks in GCRC for clinical and hormonal assessment. Blood draw was performed in all cases in the morning after a ten-hour overnight fasting. In the arm allocated to receive metreleptin, one participant was discontinued from the study due to injection-site reaction soon after the baseline visit and was not included in the analysis. Additionally, three participants were also discontinued, one at week 24 due to traveling, one at week 24 due to pregnancy and one at week 28 due to persistent weight loss (s. Fig. S1). These three participants were included in the analysis. Three participants in the placebo-treated group at weeks 4, 16 and 24 decided not to continue the study because of traveling. Subject that discontinued the therapy at week 4 was not included in the analysis. Subject characteristics are described in supplementary Table 2.
All studies described were in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. Written informed consent was obtained from all the participants. Blood draw was performed in all cases in the morning after a ten-hour overnight fasting.
2.2 Hormone measurements
Activin A (Intra-assay Variability:4.25%, Inter-assay Variability: 3.83 %), Activin B (intra-assay CV < 4.1%, inter-assay CV < 3.5%, sensitivity: 4.35 pg/ml), Follistatin-like 3 (intra-assay CV < 3.5%, inter-assay CV < 4.81%, sensitivity: 0.164 ng/ml), Inhibin B (intra-assay CV < 4.03%, inter-assay CV < 6.32%, sensitivity 1.6 pg/ml), AMH (intra-assay CV < 3.69%, inter-assay CV < 4.52%, sensitivity: 1.2 pg/ml) all from Ansh Laboratories (Webster, TX, USA), and Follistatin (R&D Systems, MN; sensitivity 0.03 ng/mL, intra-assay CV 4.9–7.5%, inter-assay CV 5.2–7.3%) were measured using commercially available immunoassays. LH (intra-assay C<10%, inter-assay CV<10%), FSH (intra-assay CV 2.3–3.7, inter-assay CV 5.4–6.7, sensitivity: 0.1 mIU/ml), and Testosterone (intra-assay CV 6.8–13, inter-assay CV 7.7–16.4, sensitivity: 15 ng/dl) levels were measured with an automated immunoassay system (Immulite 1000; Siemens, Deerfield, IL). All samples were measured in duplicates and were repeated if coefficient of variation for any sample was > 15%.
2.3 Statistical analysis
Statistical analysis was performed with SPSS v19.0 (SPSS, Inc., Chicago, IL) for Windows and with Graphpad prism 7 (GraphPad Software Inc, La Jolla, CA). Data for continuous variables are presented as mean ± SDs, unless stated otherwise, and data for categorical variables are presented as numbers and/or percentages, as appropriate. In Study 1, an intention-to-treat analysis with last observation carried forward approach was performed. Two-way analysis of variance (Two-way ANOVA) was performed in continuous variables in Study 1 and post-hoc Tukey’s test was used to compare the means of the three types of treatment (Fed, Fasting+Placebo, Fasting+Leptin). One-way analysis of variance (Friedman test for paired and Kruskal-Wallis test for unpaired measurements) followed by post-hoc Dunn’s test was used to compare the area under the curves (AUCs) and fold-to-baseline AUCs in Study 1 of the three treatment groups. In study 2, normality of distribution of the continuous variables was assessed with Shapiro-Wilks test. Variables that were not normally distributed were logarithmically transformed, as appropriate in this study as well as in study 3 and 4. Independent samples T-test or Mann-Whitney test were used for between group comparisons, in cases of two groups of continuous variables, followed by analysis of covariance (ANCOVA). In Studies 3 and 4, differences between baseline and post-intervention were estimated using ANOVA repeated measures analysis of variance for the single-arm study [Study 3] and mixed model adjusted for baseline for on-treatment analysis for the placebo-controlled prospective study [Study 4], with post-hoc t-tests with Bonferroni correction for both studies. The level of statistical significance was set at 0.05 for all analyses (two tailed or one tailed only where appropriate and noted as such in the results and figures).
3. Results
3.1. Circulating activin B and FSTL3 but not inhibin B and AMH change in a leptin-independent manner whereas circulating LH and testosterone are reduced leptin-dependently in response to acute energy deprivation (Study 1)
As we have previously reported, prolonged fasting for 72h reduces the leptin levels by approximately 80–85% [5, 16]. During the third day of fasting, the daily (24h) luteinizing hormone (LH) secretion is reduced by approximately 30% (Fig. 2A–D); in contrast, the follicle-stimulating hormone (FSH) secretion remains unchanged (Fig. 2E–H). Testosterone in males (not in females) is reduced by approximately 50% (Fig. 3AE–D). Interestingly, leptin replacement during fasting completely prevents the reduction of LH secretion (Fig. 2AE–D). Additionally, leptin attenuates the testosterone reduction in males (Fig. 3B, D).
Fig. 2. LH and FSH circulating levels on day 3 of isocaloric fed state [Fed], 72h complete fasting with placebo [Fa+Pl] or 72h complete fasting with metreleptin replacement [Fa+Lep]) [Study 1].
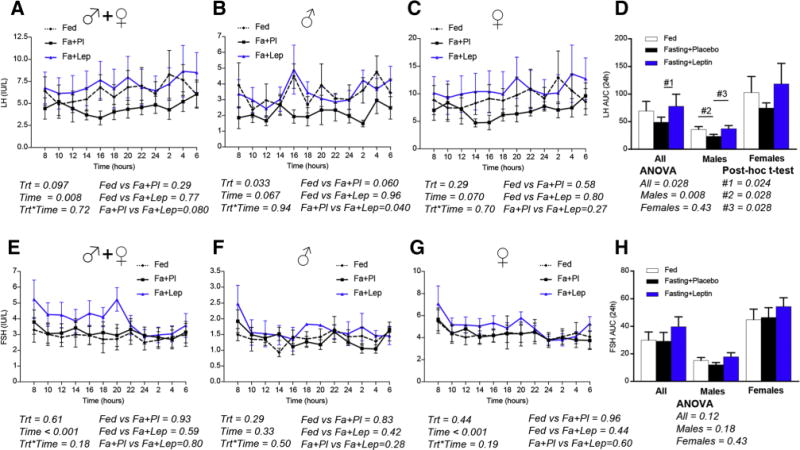
LH is decreased during fasting (A), primarily in males (B) and less (non-significant) in females (C) treated with placebo. Leptin replacement prevents the decrease of LH (A–D). FSH does not change independent of treatment (fed, fasting+placebo or fasting+leptin) (E–H). (Trt [Type of Treatment, i.e. Fed, fasting+placebo or fasting+leptin], Time [hours starting from 8 am on day 3 till 6 am on day 4]). P-values were calculated with two-way ANOVA and post-hoc Tukey’s test was used to compare the three types of treatment (Fed, Fa+Pl, Fa+Lep). One-way analysis of variance (Friedman test for paired and Kruskal-Wallis test for unpaired measurements) followed by post-hoc Dunn’s test was used to compare the area under the curves (AUCs) of the three treatment groups.
Fig. 3. Testosterone circulating levels on day 3 of three different conditions (isocaloric fed state [Fed], 72h complete fasting with placebo treatment [Fa+Pl], 72h complete fasting with metreleptin replacement [Fa+Lep]) [Study 1].
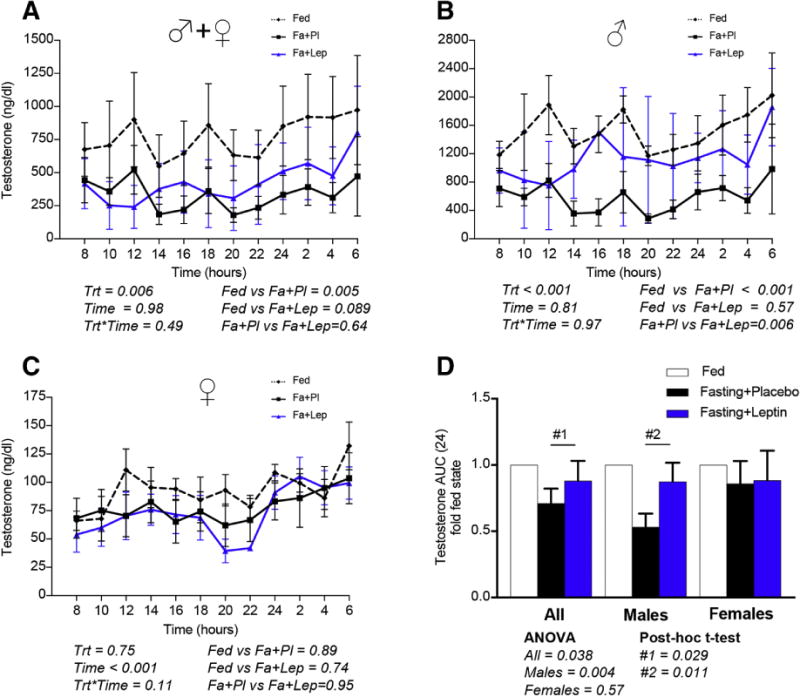
Testosterone is decreased during fasting (A), more robustly in males (B,D) than in females (C,D). Leptin replacement partially prevents the testosterone reduction during fasting (B,D) in males, while it has no effect on females (C,D). (Trt [Type of Treatment, i.e. Fed, fasting+placebo or fasting+leptin], Time [hours starting from 8 am on day 3 till 6 am on day 4]). P-values were calculated with two-way ANOVA and post-hoc Tukey’s test was used to compare the three types of treatment (Fed, Fa+Pl, Fa+Lep). One-way analysis of variance (Friedman test for paired and Kruskal-Wallis test for unpaired measurements) followed by post-hoc Dunn’s test was used to compare the fold-to-baseline AUCs of the three treatment group.
We have previously demonstrated that follistatin is increased and activin A is decreased during acute fasting both in males and females in a leptin-independent manner [13, 14]. Here, we show that activin B, similarly to activin A, is decreased by approximately 25% in fasting conditions both in males and females (Fig. 4AE–C). In contrast to follistatin, FSTL3 is also decreased by approximately 45% in females and 25% in males during fasting (Fig. 4DE–F). Neither AMH nor inhibin B changed in either gender (Fig. S2 AE–F). Leptin replacement during fasting does not affect activin B, FSTL3 and inhibin B (Fig. 4 and Fig. S2), similarly to what we have previously observed for activin A and follistatin. Leptin replacement may only increase circulating AMH levels in females (Fig. S2C).
Fig. 4. (Study 1) Activin B and FSTL3 levels on day 3 of isocaloric fed state [Fed], 72h complete fasting with placebo [Fa+Pl] or 72h complete fasting with metreleptin replacement [Fa+Lep] [Study 1].
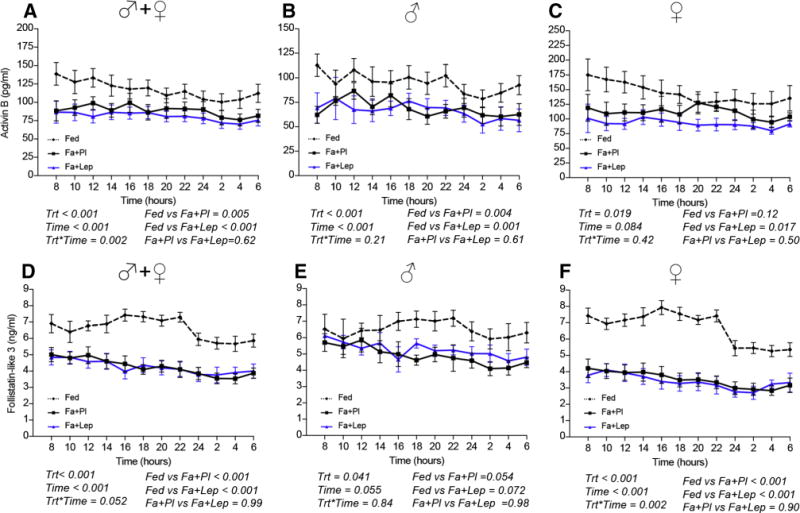
Activin B (A–C) and FSTL3 (D–F) levels are lower on the third day of fasting, independently of treatment with placebo or leptin. (Trt [Type of Treatment, i.e. Fed, fasting+placebo or fasting+leptin], Time [hours starting from 8 am on day 3 till 6 am on day 4]. P-values were calculated with two-way ANOVA and post-hoc Tukey’s test was used to compare the means of the three types of treatment (Fed, Fa+Pl, Fa+Lep).
3.2. The levels of circulating AFI hormones change during the menstrual cycle and are different in healthy women (energy sufficiency state) compared to women with HA (chronic energy deficiency) (Study 2)
A previous study has reported that activin A demonstrates its highest level around midcycle and late luteal/early follicular phase, and its lowest in the midfollicular and midluteal phase [18]. Additionally, several studies have demonstrated that inhibin B decreases in the luteal phase, while AMH does not change through the menstrual cycle [19, 20]. In our population of healthy women (s. table 1 for subject characteristics), inhibin B levels were decreased ~35% in the luteal phase, but activin A, AMH, and FSTL3 remained unchanged (s. Table S1). Both activin B (~13%) and follistatin (~21%) were increased in the luteal compared to the follicular phase (s. Table S1).
Table 1.
Case-Control study. Anthropometric and hormonal parameters in women with HA vs healthy controls [Study 2]
| Age | Weight (kg) |
Height (cm) |
BMI (kg/m2) |
Leptin (ng/ml) |
Activin A (pg/ml) |
Activin B (pg/ml) |
Follistatin (ng/ml) |
FSTL3 (ng/ml) |
AMH (pg/ml) |
Inhibin B (pg/ml) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| HA (n=25) | 25.8±4.6 | 55.0±4.9 | 164.6±6.2 | 20.7±2.0 | 3.9±1.8 | 244±111 | 76.1±40.2 | 1.3±0.6 | 8.04±1.51 | 6008±3094 | 52.2±40.1 |
| Healthy [total] (n=61) | 19.7±0.8 | 62.8±7.5 | 167.2±4.7 | 22.5±2.5 | 12.3±10.1 | 378±99 | 153±46 | 0.7±0.2 | 9.08±2.07 | 5500±3318 | 48.5±32.7 |
| Healthy [BMI-matched] (n=25) | 19.7±0.9 | 58.9±6.9 | 168.0±5.7 | 20.8±1.8 | 11.4±7.2 | 367±132 | 157±51 | 0.6±0.2 | 8.77±1.70 | 6709±3821 | 60.0±38.7 |
| pa | < 0.0001 | < 0.0001 | 0.061 | 0.001 | < 0.0001 | 0.006 | < 0.0001 | 0.007 | 0.008 | 0.42 | 0.65 |
| pb | < 0.0001 | 0.031 | 0.047 | 0.73 | < 0.0001 | 0.026 | < 0.0001 | 0.002 | 0.018 | 0.99 | 0.62 |
Data are presented as mean ± standard deviation. Healthy [total] refers to all the healthy controls and Healthy [BMI-matched] to a subgroup of healthy controls with BMIs matched to the BMI of the women with HA.
pa = p for comparison between HA and Healthy [total] after adjustment for BMI and age; p-values calculated by ANCOVA
pb= p for comparison between HA and Healthy [BMI-matched] after adjustment for age; p-values calculated by ANCOVA
In order to investigate whether the AFI axis is also affected in the state of chronic energy deficiency, we have compared the circulating levels of the AFI hormones in women with HA with healthy controls. Women with HA demonstrate ~50% lower circulating levels of activin B, 35% of activin A, 12% of FSTL3, and ~85% higher levels of follistatin compared to healthy women (s. Table 1). Finally, inhibin B and AMH levels were similar between both groups.
3.3. Changes in the AFI axis due to chronic energy deprivation are leptin-independent, with the exception of circulating activin B that increases in women with HA treated with leptin (Study 3 and study 4)
We investigated whether the regulation of AFI axis in chronic energy deficiency is controlled by leptin in two studies (Study 3 and Study 4). In the single-arm open-label study (Study 3) (s. table S2 for subject characteristics), treatment with leptin in women with HA increased the activin B levels by ~34% in the first month of treatment, 52% in the second, and 62% in the third (Table 2). In the double-blinded placebo controlled study (Study 4) (s. table S2 for subject characteristics and Fig. S1 for study flow diagram), the increase of activin B levels was ~35% and was observed after twenty weeks of treatment (Table 3). The difference of activin B levels (higher in leptin treated group compared to placebo) remained significant even after adjusting for estradiol and progesterone (p value time*treatment adjusted only for baseline= 0.010, time*treatment- adjusted for estradiol, progesterone and baseline=0.023). Of note, in study 4 the women treated with HA started from higher baseline levels of activin B compared to the subjects in study 3 and followed a different treatment protocol (i.e. decrease of leptin dose by >5% body weight reduction from baseline, maximum dose 0.12 mg. kg−1. day−1, instead of 0.2 mg. kg−1. day−1 in the third month of Study 3) which may explain the delayed and more modest increase of activin B. Activin A, follistatin, FSTL3, and AMH did not change during treatment neither in Study 3 (p for activin A = 0.017 in the analysis of variance (ANOVA), no significant differences in post-hoc tests) nor in Study 4 (Tables 2–3), similar to what we have previously reported for inhibin B.
Table 2.
Single-arm open label study. Circulating hormones in women with HA treated with leptin for max. 3 months [Study 3]
| Activin A (pg/ml) |
Activin B (pg/ml) |
Follistatin (ng/ml) |
FSTL3 (ng/ml) |
AMH (pg/ml) | |
|---|---|---|---|---|---|
|
Baseline (N=7) |
244.0±50.9 | 59.8±19.2 | 1.69±0.19 | 8.3±1.7 | 4726±2671 |
|
Month 1 (N=7) |
225.7±61.2 | 79.9±31.8 | 1.74±0.21 | 7.7±1.2 | 3987±2818 |
|
Month 2 (N=7) |
255.4±59.7 | 91.1±25.2‡ | 1.68±0.21 | 7.7±2.3 | 4012±2963 |
|
Month 3 (N=5) |
298.3±73.8 | 96.9±24.4‡ | 1.84±0.34 | 8.2±1.2 | 3823±3187 |
| Overall P value† | 0.017 | 0.002 | 0.542 | 0.419 | 0.482 |
All data are presented as mean ± Standard deviation. During months 1 and 2 the dose of r-metHuLeptin was 0.08 mg per kilogram per day, and during month 3 the dose was 0.2 mg per kilogram per day. Values are the average over the specified period.
Overall P values were derived by means of repeated-measures analysis of variance. Multiple comparisons were performed with t-test with Bonferroni correction.
p-values<0.05 for the comparison with baseline values. Specifically, Activin B Month 3 vs Baseline p= 0.014, Activin B Month 2 vs Baseline p= 0.034.
Table 3.
Randomized placebo controlled clinical trial. Circulating hormones in women with HA treated with leptin for 36 weeks [Study 4]
| Activin A (pg/ml) | Activin B (pg/ml) | Follistatin (ng/ml) | FSTL3 (ng/ml) | AMH (pg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L | P | L | P | L | P | L | P | L | P | |
|
Week 0 (N=18) |
277±159 | 201±63 | 88.3±54.9 | 75.0±29.3 | 1.36±0.72 | 0.90±0.37 | 8.17±1.47 | 7.63±1.53 | 5490±3606 | 7777±2092 |
|
Week 4 (N=18) |
288±127 | 235±57 | 73.8±24.8 | 58.8±16.1 | 1.46±0.62 | 1.35±0.85 | 8.09±1.68 | 8.57±1.72 | 5553±3244 | 7530±2456 |
|
Week 12 (N=18) |
335±171 | 181±57 | 92.2±53.2 | 67.6±31.9 | 1.13±0.33 | 0.99±0.20 | 6.99±1.15 | 7.04±1.76 | 5598±4177 | 8167±5358 |
|
Week 20 (N=18) |
356±187 | 232±91 | 113.1±46.4 | 52.8±12.9 | 1.36±0.69 | 1.30±0.81 | 7.86±1.96 | 7.53±2.68 | 5730±4104 | 7932±3212 |
|
Week 36 (N=18) |
296±174 | 203±78 | 117.5±45.1 | 53.8±24.1 | 1.01±0.38 | 1.09±0.15 | 6.98±2.39 | 7.26±1.49 | 5768±4335 | 6891±2081 |
| Time | 0.316 | 0.546 | 0.261 | 0.043 | 0.673 | |||||
| Treatment | 0.048 | < 0.0001 | 0.910 | 0.432 | 0.977 | |||||
| Time*Treatment | 0.408 | 0.010 | 0.719 | 0.723 | 0.934 | |||||
All data are presented as mean ± standard deviation (SD). P-values were measured with Mixed Models in SPSS Statistics and adjusted for baseline. L: leptin administration; P: placebo administration.
3.4. The increase of circulating activin B in women with HA (chronic energy deprivation state) treated with leptin correlates with follicular parameters (Study 3)
We hypothesized that activin B could be related with beneficial effects on follicular parameters. In an exploratory analysis, the difference between the highest (achieved during treatment) and baseline levels of activin B (last measurement at baseline month “End of Baseline”, before initiating leptin treatment) correlated positively with the difference between the total and baseline number of dominant follicles (r=0.677, one-tailed p=0.048) (Fig. 5), while no correlation was observed for AMH, activin A, follistatin and FSTL3 (Fig. S3). Finally, activin B levels were not associated with follicle, ovarian, or endometrium size.
Fig. 5. Correlation of activin B levels with number of dominant follicles during leptin treatment [Study 3].
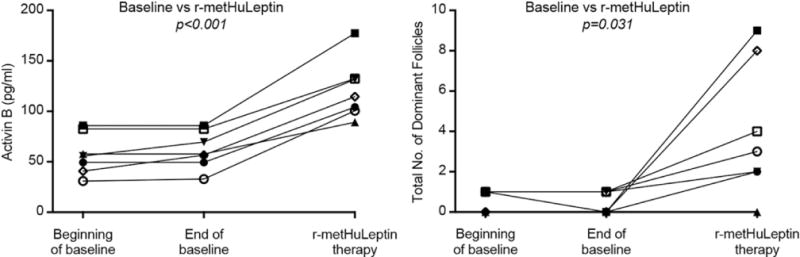
The difference between the highest (achieved during leptin treatment) and baseline levels (“End of Baseline”, which is the last measurement in the baseline month) of activin B correlates positively with the difference between the total and baseline number of dominant follicles (r=0.667, one-tailed p= 0.048) in leptin-treated women with HA (white filled symbols = subjects who ovulated, black filled symbols = subjects who did not ovulate. Bivariate correlations were calculated with Pearson’s coefficient. P-values for the difference between the highest and baseline levels were calculated with paired t-test.
4. Discussion
We demonstrate here, for the first time, that activin B and FSTL3 levels decrease during short-term energy deprivation in healthy individuals. Additionally, we demonstrate that activin A, activin B, follistatin, and FSTL3 levels are altered in disease states, i.e. women with HA, due to chronic energy deficiency, in a similar manner and even more robustly as compared to short-term energy deprivation. Furthermore, we show that short-term leptin treatment does not affect the secretion profile of any of the investigated hormones of the AFI axis in acute energy deprivation in healthy males and females indicating a leptin independent pathway for their regulation. In chronic energy deficiency, long-term treatment with leptin in females with HA restores the circulating levels of activin B, in an estradiol and progesterone-independent manner. The increase in activin B levels during leptin treatment is positively associated with the total number of dominant follicles measured by ultrasound, a finding that supports the role of activin B as regulator of reproductive function in disease state, i.e. in women with HA due to chronic energy deficiency. In contrast to activins and follistatins, the secretion profile of inhibin B and AMH is neither affected by short- nor by long-term energy deficiency.
Combining the results of our previous and the current study, we suggest that two main hormonal systems (leptin-system and AFI-system) control the reproductive function in acute and in chronic energy deficient states (Fig. 6).
Fig. 6. Main hormonal pathways regulating reproductive function in women with hypothalamic amenorrhea (chronic energy deficiency).
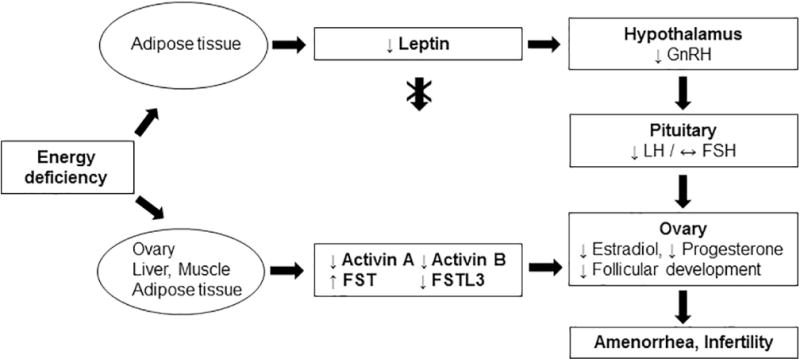
Chronic energy deficiency results in low adipose tissue mass and low circulating leptin. The profound hypoleptinemia downregulates the GnRH secretion from hypothalamus which in turn reduces LH pulsatility in the pituitary gland. Consequently, estradiol and progesterone levels are significantly decreased, follicular development is impaired and amenorrhea with infertility occur. Chronic energy deficiency also leads to a reduction in circulating activins and FSTL3 and to an increase in FST. These hormones are secreted mainly by the gonads and secondarily by the liver (activin B and FST), adipose tissue and muscle. The changes in circulating activins and follistatins are mainly leptin independent (with the exception of activin B) and are not affecting FSH secretion. Restoration of the activin B levels through leptin treatment is associated with an increase in the number of dominant follicles in women with HA, indicating that the alterations of activins and follistatins in chronic energy deficiency may contribute to the impairment of follicular development and consequently to amenorrhea and infertility.
4.1. Leptin is regulating the reproductive function in acute and chronic energy deficiency
Leptin regulates primarily LH secretion in response to energy deprivation [1, 3–5, 16]. The leptin-dependent system demonstrates high-sensitivity even in short-term energy deprivation (i.e. 72h in our studies), since a profound reduction of circulating leptin is observed, leading to a downregulation of LH secretion in both genders and in a decrease of testosterone levels in males. As we have previously demonstrated [16], circulating estradiol levels are not affected in this condition, indicating that despite the leptin-mediated downregulation of LH secretion, ovary function is probably still preserved in healthy females during short term fasting. This is also supported by previous studies demonstrating normal follicle development and/or estradiol production despite the reduction of LH in healthy women who were fasting for three to five days in follicular phase [21, 22].
In contrast, chronic energy deficiency state, often observed in females due to strenuous exercise or reduced caloric intake, is characterized by hypoleptinemia and a robust downregulation of HPG axis leading to amenorrhea and infertility [6, 7, 23]. These changes in reproductive function may have teleological significance given that in periods of significant energy deprivation survival of the organism has precedence over procreation, thus available calories should preferentially be reserved for survival vs. bringing a pregnancy to term. However, recovery of menstruation and fertility in these women remains often challenging even after improvement of energy balance. As we have previously demonstrated, leptin replacement in these conditions leads to ovulation and restoration of menstrual cycles by restoration of LH pulsatility and increase in estradiol and progesterone in many but not all of the women with HA[6, 7]. This indicates that other hormonal mechanisms are additionally involved in the regulation of reproductive function in this state.
4.2. Regulation of activin-follistatin system in response to acute energy deficiency
We report herein a second hormonal system that is affected by short and long term changes in energy balance and may have an impact in reproductive function especially in chronic energy deprivation state. This system consists primarily of activins (activin A and activin B) and follistatins (follistatin and FSTL3), while inhibin B and AMH according to our findings are not affected by energy balance. In acute energy deprivation state, the stimulatory reproductive hormones activin A and activin B are decreased both in males and females. The secretion profiles of the antagonists of activins differ, with follistatin-levels being increased and FSTL3 decreased. This may be due to their differences in the secretion patterns and/or function. Follistatin is secreted by organs that are susceptible to changes during energy deprivation in females, i.e. pituitary and ovary, while FSTL3 is secreted primarily by the endometrium, which has minor involvement in the regulation of HPG axis during energy deprivation [8]. Additionally, follistatin is much more potent than FSTL3 at inhibiting activins [8], indicating that the net outcome from the increase of follistatin and decrease of FSTL3 levels points to a further inhibition of the already downregulated AFI axis due to the reduced circulating levels of activins.
4.3. Regulation of activin-follistatin system in response to chronic energy deficiency
In chronic energy deprivation states, women with hypothalamic amenorrhea due to strenuous exercise (a prototype condition of chronic energy deficiency) demonstrate even more robust changes in activins and follistatin, with a reduction in circulating levels of activin A (~35%), activin B (~50%), and FSTL3 (~12%), and higher levels of follistatin (~85%) compared to healthy controls. To our knowledge this is the first study comparing levels of activin A, activin B, follistatin, and FSTL3 between women with HA and healthy controls. An older study had focused on activin A and reported higher levels in HA [24]. In that study, a two-site enzyme immunoassay was used for the measurement of activin A, which is currently not available and thus its accuracy and precision cannot be independently evaluated. The authors of the study admitted that the increase of activin A is difficult to explain. In contrast, our findings here support the teleological hypothesis of reduced reproductive ability in energy deficient states, when energy stores are primarily used for functions essential for survival.
Activins and follistatins act both on the pituitary gland to regulate FSH secretion, as well as on the gonads to regulate follicle growth, ovary hormonal secretion, germ cell development and Sertoli cell proliferation [8, 9]. Despite the robust changes of the activins and follistatins, we did not observe any reduction of FSH in acute energy deprivation state. This is also supported by previous findings showing that FSH does not change during acute fasting [21]. Whether gonadal function is affected directly by the changes in activins-follistatins during acute fasting remains unclear and should be addressed in future studies. Similarly, in chronic energy deprivation, women with HA usually demonstrate a loss of pulsatility in LH secretion, while the changes in FSH secretion are rather modest. In our studies, baseline FSH measurements were also in the normal range despite the profound differences in baseline levels of activins and follistatins between women with HA and healthy controls. In addition, the levels of FSH did not increase during long-term leptin treatment in women with HA, even in those that responded to therapy i.e. developed menstruation and increased their follicle numbers [6, 7].
4.4. Interaction of the activin-follistatin hormonal system with leptin in acute or chronic energy deficiency
Since changes in activins-follistatins are not associated with alterations in circulating FSH, we investigated whether they are related to parameters of ovary function and whether they are regulated by leptin in conditions of energy deficiency. Our findings demonstrate that with the exception of activin B, neither in acute nor in chronic energy deficiency state leptin replacement affects the circulating levels of the AFI hormones. This shows that most of the changes observed in the AFI system in energy deficiency are leptin-independent. Regarding activin B, we observed a restoration of the circulating levels of the hormone in women with HA during leptin treatment in both prospective clinical studies. The increase in activin B remained significant even after adjusting for estradiol and progesterone, both of which increase during leptin treatment. Since the rise in activin B levels is estradiol- and progesterone-independent, it is possible that leptin directly stimulates activin B synthesis-secretion. Activin B is a homodimer of two βB-subunits while activin A of two βA-subunits. Although the distribution of βA and βB-subunit transcripts is similar in most of the tissues in humans, in anterior pituitary gland and in gonadotropes only the βB-subunit has been detected so far [8]. Interestingly in rats, where both subunits are expressed in the pituitary gland, selective deletion of the leptin receptor in gonadotropes significantly reduces the βA and βB transcripts [25]. Thus, it is possible that the higher activin B levels during leptin treatment in humans derive by an increased secretion of the hormone by the gonadotropes and this needs to be addressed by experimental studies in the future.
4.5 Relation of the leptin-mediated changes in the activin-follistatin hormonal system with reproductive function in chronic energy deficiency
To evaluate the functional relevance of the restoration of the activin B levels during leptin treatment, we performed an exploration analysis and identified a positive association of activin B with the number of dominant follicles in women with HA after treatment with leptin. Animal studies have focused so far on activin A, where they showed that treatment of follicles isolated from goat, rodent, or sheep with activin A stimulates growth and survival of the follicles.[26–28] Activin A-deficient mice die within 24h of birth. On the other hand, activin-B deficient female mice are fertile, but they demonstrate perinatal lethality of their offsprings [29]. Activin B is produced in the early phase of antral follicular development and decreases androstenedione production from primary theca cells in vitro [30]. In our study, both responders and non-responders to therapy demonstrate an increase of activin B levels. This indicates that activin B may have a contributing role in follicular development. Thus, an important question that has to be answered in the future, is whether restoration of the other profoundly affected AFI hormones (i.e. activin A, follistatin and FSTL3) to the levels observed in healthy population will contribute further to the improvement of the reproductive function in women with HA and what the therapeutic potential of such changes would be.
Limitation of the open-label as well as of the randomized clinical trial is the small sample size. Additionally, both studies were designed to address other primary and secondary outcomes than the ones reported here. Finally, in the randomized clinical trial no measurements of follicular parameters were performed, which would have been used to validate our findings from the open-label study.
4.6. Clinical implications of the current findings
The clinical implications of the above findings can be extended to several directions. First of all interventional studies administering activins or inhibiting follistatins (when these molecules become available for clinical evaluation) alone or in combination with leptin replacement, will help to first advance our knowledge of underlying mechanisms and then determine whether restoration of these two main hormonal systems can lead to complete recovery of reproductive ability in women with HA. Current treatment for HA is focusing on increasing caloric intake, decreasing exercise and replacing estrogens and it is only modestly achieving to improve fertility and protect from bone loss. Secondly, interventional studies with activins or follistatin-inhibitors may prove to be useful for evaluating treatments in other conditions related to sub- or infertility, such as the polycystic ovary syndrome (PCOS), where also increased follistatin levels and normal or reduced activin A and B levels are observed independently of obesity [31–33]. Finally, several drugs modulating the functions of activins/follistatins/myostatins are currently in the pipeline. For example, bimagrumab, an antibody against activin receptor type II, is currently evaluated as potential treatment in several conditions, ranging from muscle wasting after hip fracture, to treatment of sarcopenia or even treatment in overweight and obese patients with type 2 diabetes [34]. Similarly, Sotatercept (ACE-011), that prevents the binding of Activin A to its type IIA receptor (ActRIIA) has been associated with increased bone formation and decreased resorption in healthy postmenopausal women [35]. The regulation of the AFI axis by energy status shown herein provides physiological support to the efforts to manipulate the axis by creating agonists or antagonists for therapeutic benefit in disease states that are intimately linked with energy status changes (from lipodystrophy and HA to obesity and diabetes). Since such treatments are practically blocking, among other, the function of activins, should be carefully assessed for possible effects on the reproductive system.
Supplementary Material
Highlights.
Circulating activins and follistatins change in response to acute energy deprivation leptin-independently
Women with hypothalamic amenorrhea due to strenuous exercise (state of chronic energy deficiency) demonstrate an altered circulating profile of activins and follistatins compared to healthy eumenorrheic women
Leptin treatment in women with hypothalamic amenorrhea increases the circulating levels of activin B
The increase in circulating activin B correlates positively with the number of dominant follicles measured during leptin treatment
Acknowledgments
Funding: The current study was funded by NIH K24DK081913. NP was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) –389891681 (PE 2431/2-1).
Abbreviations
- AFI
Activin-follistatin-inhibin system
- AMH
Anti-Müllerian Hormone
- BMI
Body mass index
- FSH
Follicle-stimulating hormone
- FSTL3
Follistatin-like 3
- GCRC
General Clinical Research Center
- HA
Hypothalamic Amenorrhea
- HPG-axis
Hypothalamic-pituitary-gonadal axis
- IGF-1
insulin-like growth factor I
- LH
Luteinizing hormone
- SD
Standard deviation
- TGF-β
Transforming growth factor beta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contribution: C.S.M designed the experiment. All the authors contributed to the conduction of the experiments and acquisition of the data. N.P., J.U. and C.P analyzed the data. N.P. wrote the manuscript with input from all the other authors. N.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Competing interests: CSM is advisor of Ansh Labs and was advisor of Amgen and Aegerion
References
- 1.Chou SH, Mantzoros C. 20 years of leptin: role of leptin in human reproductive disorders. J Endocrinol. 2014;223(1):T49–62. doi: 10.1530/JOE-14-0245. [DOI] [PubMed] [Google Scholar]
- 2.Fourman LT, Fazeli PK. Neuroendocrine causes of amenorrhea–an update. J Clin Endocrinol Metab. 2015;100(3):812–24. doi: 10.1210/jc.2014-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes. 2007;14(6):458–64. doi: 10.1097/MED.0b013e3282f1cfdc. [DOI] [PubMed] [Google Scholar]
- 4.Mathew H, V, Castracane D, Mantzoros C. Adipose Tissue and Reproductive Health. Metabolism. 2017 doi: 10.1016/j.metabol.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Chan JL, et al. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111(9):1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou SH, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108(16):6585–90. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welt CK, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 8.Makanji Y, et al. Inhibin at 90: from discovery to clinical application, a historical review. Endocr Rev. 2014;35(5):747–94. doi: 10.1210/er.2014-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijayarathna R, de Kretser DM. Activins in reproductive biology and beyond. Hum Reprod Update. 2016;22(3) doi: 10.1093/humupd/dmv058. [DOI] [PubMed] [Google Scholar]
- 10.Amthor H, et al. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270(1):19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 11.Dewailly D, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–85. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 12.Brinkoetter M, et al. Leptin treatment reduces body fat but does not affect lean body mass or the myostatin-follistatin-activin axis in lean hypoleptinemic women. Am J Physiol Endocrinol Metab. 2011;301(1):E99–E104. doi: 10.1152/ajpendo.00146.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moragianni VA, et al. Short-term energy deprivation alters activin a and follistatin but not inhibin B levels of lean healthy women in a leptin-independent manner. J Clin Endocrinol Metab. 2011;96(12):3750–8. doi: 10.1210/jc.2011-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vamvini MT, et al. Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin A and follistatin but not myostatin in healthy males. J Clin Endocrinol Metab. 2011;96(11):3416–23. doi: 10.1210/jc.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anastasilakis AD, et al. Circulating follistatin displays a day-night rhythm and is associated with muscle mass and circulating leptin levels in healthy, young humans. Metabolism. 2016;65(10):1459–65. doi: 10.1016/j.metabol.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JL, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103(22):8481–6. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anastasilakis AD, et al. Circulating irisin in healthy, young individuals: day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J Clin Endocrinol Metab. 2014;99(9):3247–55. doi: 10.1210/jc.2014-1367. [DOI] [PubMed] [Google Scholar]
- 18.Muttukrishna S, et al. Changes in peripheral serum levels of total activin A during the human menstrual cycle and pregnancy. J Clin Endocrinol Metab. 1996;81(9):3328–34. doi: 10.1210/jcem.81.9.8784092. [DOI] [PubMed] [Google Scholar]
- 19.Groome NP, et al. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81(4):1401–5. doi: 10.1210/jcem.81.4.8636341. [DOI] [PubMed] [Google Scholar]
- 20.Hehenkamp WJ, et al. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 21.Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88(1):297–311. doi: 10.1210/jc.2002-020369. [DOI] [PubMed] [Google Scholar]
- 22.Olson BR, et al. Short-term fasting affects luteinizing hormone secretory dynamics but not reproductive function in normal-weight sedentary women. J Clin Endocrinol Metab. 1995;80(4):1187–93. doi: 10.1210/jcem.80.4.7714088. [DOI] [PubMed] [Google Scholar]
- 23.Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med. 2010;363(4):365–71. doi: 10.1056/NEJMcp0912024. [DOI] [PubMed] [Google Scholar]
- 24.Petraglia F, et al. Low levels of serum inhibin A and inhibin B in women with hypergonadotropic amenorrhea and evidence of high levels of activin A in women with hypothalamic amenorrhea. Fertil Steril. 1998;70(5):907–12. doi: 10.1016/s0015-0282(98)00283-0. [DOI] [PubMed] [Google Scholar]
- 25.Akhter N, et al. Selective deletion of leptin receptors in gonadotropes reveals activin and GnRH-binding sites as leptin targets in support of fertility. Endocrinology. 2014;155(10):4027–42. doi: 10.1210/en.2014-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva CM, et al. Activin-A promotes the development of goat isolated secondary follicles in vitro. Zygote. 2015;23(1):41–52. doi: 10.1017/S0967199413000294. [DOI] [PubMed] [Google Scholar]
- 27.Guzel Y, et al. Recombinant activin A enhances the growth and survival of isolated preantral follicles cultured three-dimensionally in extracellular basement matrix protein (matrigel) under serum-free conditions. Gynecol Endocrinol. 2014;30(5):388–91. doi: 10.3109/09513590.2014.888411. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Phillips DM, Mather JP. Activin promotes ovarian follicle development in vitro. Endocrinology. 1995;136(3):849–56. doi: 10.1210/endo.136.3.7867593. [DOI] [PubMed] [Google Scholar]
- 29.Vassalli A, et al. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994;8(4):414–27. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- 30.Young JM, et al. Activin B is produced early in antral follicular development and suppresses thecal androgen production. Reproduction. 2012;143(5):637–50. doi: 10.1530/REP-11-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eldar-Geva T, et al. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16(12):2552–6. doi: 10.1093/humrep/16.12.2552. [DOI] [PubMed] [Google Scholar]
- 32.Norman RJ, et al. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16(4):668–72. doi: 10.1093/humrep/16.4.668. [DOI] [PubMed] [Google Scholar]
- 33.Teede H, et al. Follistatin and activins in polycystic ovary syndrome: relationship to metabolic and hormonal markers. Metabolism. 2013;62(10):1394–400. doi: 10.1016/j.metabol.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 34.https://clinicaltrials.gov/ct2/results?cond=&term=Bimagrumab&cntry1=&state1=&Search=Search#tableTop, October 2017
- 35.Ruckle J, et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24(4):744–52. doi: 10.1359/jbmr.081208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


