Abstract
Some evidence suggests that there may be a limited “window of opportunity” for beneficial effects of hormone therapy after menopause in women. We tested whether the timing of cyclic estradiol (E2) treatment impacted its effect on cognitive function in aged, surgically menopausal (ovariectomized) rhesus monkeys. Monkeys were assigned to one of four treatment conditions after ovariectomy: either vehicle or E2 treatment for the duration of the protocol, vehicle for the first 2 years of the protocol followed by E2 for the remainder (delayed treatment), or E2 for the first 11 months of the protocol followed by vehicle for the remainder (withdrawn treatment). Delayed treatment addressed the hypothesis that E2 treatment initiated more than 2 years post-ovariectomy would have a reduced effect on cognitive function. Withdrawn treatment mirrored current clinical advice to women to use hormone therapy in the initial post-menopausal period then discontinue it. Two periods of cognitive testing assessed treatment effects on cognition over time. E2 treatment predominantly affected a prefrontal cortex-dependent test of spatiotemporal working memory (delayed response). Monkeys with delayed E2 treatment modestly improved in delayed response performance over time, whereas vehicle-treated monkeys declined. Monkeys with withdrawn E2 treatment maintained their performance across assessments, as did monkeys treated with E2 across the entire protocol. These findings suggest that a “window of opportunity” for hormone treatment after cessation of ovarian function, if present in nonhuman primates, lasts longer than 2 years. It also supports the notion that beneficial effects of hormone therapy may persist after discontinuation of treatment.
Keywords: estrogen, macaque, aging, prefrontal, working memory
The Women’s Health Initiative (WHI) Memory Study (WHIMS) found that treatment of older women, aged 65 to 79 at study onset, with conjugated equine estrogen (CEE) alone, or CEE plus progestin (medroxyprogesterone acetate, MPA), had no beneficial effects on global cognitive function, was associated with decline in global cognitive function in some women, and increased the risk of mild cognitive impairment (MCI) and Alzheimer’s disease (Espeland et al., 2004; S. R. Rapp et al., 2003b; Shumaker et al., 2004; 2003). These studies contradicted research conducted in animal models, as well as with human female participants, which supported the notion that hormone therapy (HT) after menopause benefits cognitive function (Hao et al., 2007; Markowska & Savonenko, 2002; P. R. Rapp, Morrison, & Roberts, 2003a; Voytko, 2000; 2002).
Considerable discussion and subsequent research has been devoted to understanding the findings from the WHIMS and analyzing its limitations (Craig, Maki, & Murphy, 2005; Maki, 2004; Sherwin, 2006; Sherwin & Henry, 2008). One possibility is that women enrolled in the study may have been too far from the onset of menopause to benefit from HT. This hypothesis is consistent with the idea that there may be a critical period or “window of opportunity” for HT to have beneficial effects on cognitive function, such that benefits are seen when HT is initiated soon after menopause but not when it is delayed (Maki, 2006a; 2006b; Resnick & Henderson, 2002; Sherwin, 2006; Zandi et al., 2002). Alternatively, observational studies that identified beneficial effects of HT may have suffered from a “healthy user” bias, such that women that elect HT are in better health and better educated than women who do not.
Studies in animal models directly address many of the challenges present in studies of humans. Specific advantages include the ability to more precisely match treatment and control groups for chronological age, health status, life history, and other subject characteristics. Furthermore, direct control of formulation of HT, verification of compliance with treatment, exclusion of confounding factors such as diet and other medications that could modulate HT effects, and verification of treatment effectiveness in terms of circulating hormone levels are all possible in animal studies but difficult or impossible to achieve in observational or epidemiological studies with women. Nonhuman primates, specifically rhesus monkeys (Macaca mulatta), are well-suited for modeling the relationship between neuroendocrine and cognitive aging in humans, because many aspects of reproductive physiology are similar in rhesus monkeys and women (Gilardi, Shideler, Valverde, Roberts, & Lasley, 1997), and the profile of cognitive aging in rhesus monkeys parallels that of humans (Baxter, 2001; Herndon, Moss, Rosene, & Killiany, 1997; J. A. Roberts, Gilardi, Lasley, & Rapp, 1997). As observed in humans, prefrontal cortex dysfunction is a key signature, exemplified by impaired performance in the spatial delayed response task, indicating poor spatial working memory (Bartus, Fleming, & Johnson, 1978; Lyons-Warren, Lillie, & Hershey, 2004).
Although the outcome of the WHIMS has made it clear that HT is not suitable for preventing dementia or maintaining cognition in women over 65, many unanswered questions remain. In particular, the “window of opportunity” hypothesis (Resnick & Henderson, 2002; Sherwin, 2007) suggests that HT is only effective in maintaining cognitive function, and delaying the onset of dementia, if it is initiated within a limited time after menopause. On this view, women enrolled in the WHIMS were beyond the critical period, and thus did not benefit from HT. In contrast, in observational studies reporting beneficial neurocognitive effects of HT, women began treatment soon after the onset of menopause and so received the maximal benefit. This hypothesis finds support from some studies in animal models; aging rodents appear to experience maximal benefits from HT at a point in the lifespan that corresponds to late middle-age in humans (Frick, 2009). Spatial memory in rats is improved when HT is initiated immediately or 3 months after ovariectomy (OVX), but not 10 months after OVX (Gibbs, 2000). Elevation of apical spine density in CA1 by estradiol (E2) is less effective 10 weeks post-OVX in rats with no intervening hormone treatment (McLaughlin, Bimonte-Nelson, Neisewander, & Conrad, 2008). Beneficial effects of E2 on hippocampal synaptic physiology in rats that underwent OVX at 2 months of age are lost between 15 and 19 months post-OVX, a consequence of ovarian hormone deprivation rather than chronological age (Smith, Vedder, Nelson, Bredemann, & McMahon, 2010; Vedder, Bredemann, & McMahon, 2014). Rhesus monkeys ovariectomized for 10 years or longer show no benefit from E2 treatment in their delayed response performance (Lacreuse, Wilson, & Herndon, 2002), although performance on a hippocampal-dependent spatial memory task is enhanced. Aside from the one study reporting an insensitivity of very long-term ovariectomized rhesus monkeys to E2, the question of the “window of opportunity” has received little attention in nonhuman primate models. Thus, we investigated in our model whether a substantial delay between surgical menopause and initiation of HT is associated with a reduction in the beneficial cognitive effects of HT.
A related question of considerable clinical significance is whether the beneficial effects of hormone therapy persist after discontinuation of treatment. In view of evidence from the WHI for increased risk of heart disease, stroke, blood clots, and breast cancer (Anderson et al., 2003; 2004; Wassertheil-Smoller et al., 2003), current clinical advice is for women to take hormone therapy for as short a period as possible after the onset of symptoms of menopause (for example, hot flashes), but to avoid chronic hormone therapy (Santen et al., 2010). Women randomly assigned to receive HT or placebo for 2-3 years immediately after menopause in a trial examining effects of HT on bone loss, showed lowered risk of cognitive impairment when examined 5-15 years later relative to women who had never taken HT, even if they discontinued HT after the end of the trial (Bagger et al., 2005). Testing whether beneficial effects of chronic HT persist in monkeys after discontinuation of treatment complements such studies, given the advantages of the monkey model outlined above, and the ability to determine whether corresponding changes in neurobiological markers also endure. Other therapies that improve cognition in aged animals, such as neurotrophic factors, result in improvements in cognition that persist after discontinuation of treatment (Frick, Price, Koliatsos, & Markowska, 1997). Evidence in favor of potential enduring benefits of HT after it is ended may inform women’s choices about embarking on a short course of HT post-menopause, versus avoiding HT altogether.
To date, our studies in nonhuman primates have focused on a cyclic regimen of E2, which has beneficial effects on cognitive function in aged, surgically menopausal (ovariectomized) rhesus monkeys, as well as positive effects on density of dendritic spines and other indicators of “synaptic health” in prefrontal cortex (Hao et al., 2007; Hara et al., 2014; Morrison & Baxter, 2014; P. R. Rapp et al., 2003a). This contrasts with continuously administered E2 or E2 regimens combined with progesterone, which have sometimes not shown the same beneficial effects (Baxter et al., 2013; Kohama et al., 2016; Ohm et al., 2012; but see Voytko, Murray, & Higgs, 2009). Thus, in these studies, we used a cyclic E2 regimen (dosing E2 by injection every 21 days) similar to previous studies demonstrating positive effects of E2 on brain and cognitive function (Hao et al., 2007; P. R. Rapp et al., 2003a). To that end, monkeys in the different hormone conditions completed two standard tests of cognitive function widely used by our group - the delayed response task and the delayed nonmatching-to-sample task.
Method
Subjects and overview of experimental timeline
The experiments were performed at the California National Primate Research Center (CNPRC) under protocols approved by the University of California, Davis Institutional Animal Care and Use Committee. The initial subject group included 41 behaviorally-naive female rhesus monkeys (Macaca mulatta), aged 18.3-22.5 years at OVX (age mean ± SD, 20.1 ± 1.1 years). Based on an approximate 3:1 ratio of human:rhesus monkey lifespan (Tigges, Gordon, McClure, Hall, & Peters, 1988), this corresponds to an approximate age of 60 human years at OVX. Monkeys at this age reliably show cognitive deficits (Bachevalier et al., 1991; P. R. Rapp & Amaral, 1991). We used a surgical menopause/OVX model which provides a precise time of loss of ovarian function, and because natural menopause in rhesus monkeys occurs around 25 years of age (Walker & Herndon, 2008) which poses a practical challenge for prolonged behavioral testing studies. Thirty-two of the monkeys were pair-housed with other monkeys on this study or colony monkeys, and 9 were singly housed. Pair-housed monkeys were separated at night to facilitate urine collection for hormone assays during the initial phase of the experiment and monitoring of food intake.
Monkeys selected for inclusion in this study received physical examinations by a member of the CNPRC veterinary staff, to ascertain the presence of any health conditions that would confound results. Monkeys that passed the physical examination then received OVX surgery, a post-OVX evaluation of hormone status to verify OVX effectiveness, followed by assignment to treatment condition and beginning of one of four treatment protocols a mean of 109.5 days after OVX (range 63-175 days). This variation in post-OVX washout interval was dictated by scheduling considerations for behavioral testing and hormone treatments, was similar between the four treatment groups, and brief relative to the total protocol duration. As described in the introduction, there were 4 treatment groups. Group Veh received vehicle treatment throughout the experimental protocol, and group E2 received E2 treatment throughout the experimental protocol. Group Withdrawn received E2 treatment for the first ~11 months of the protocol (16 E2 treatments spanning 336 days) followed by vehicle treatment thereafter. Group Delay received vehicle treatment for the first ~24 months of the protocol (35 vehicle treatments spanning 735 days) followed by E2 treatment thereafter until perfusion. The initial number of monkeys in each condition was: Veh: N = 11; E2: N = 11; Withdrawn: N = 10; Delay: N = 9. A timeline for the entire experimental protocol is presented in Figure 1.
Figure 1.
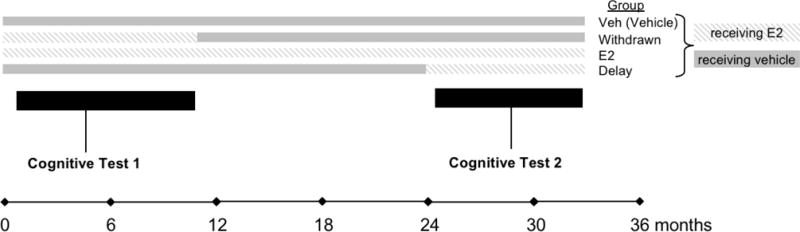
Schematic of the experimental timeline of hormone treatment and experimental groups. Group Veh (vehicle) received vehicle throughout the experiment, group E2 received E2 treatment throughout the experiment, group Withdrawn received E2 treatment for ~11 months and then began vehicle treatment, and group Delay received vehicle treatment for ~24 months and then began E2 treatment. The two periods of cognitive testing are timed to coincide with E2 treatment in group Withdrawn, and then the onset of E2 treatment in group Delay.
Our choice of these time intervals for the Withdrawn and Delay treatment groups were based in part on the 3:1 ratio of human:rhesus monkey in terms of lifespan (Tigges et al., 1988). An E2 treatment of about a year in duration in group Withdrawn would correspond to women using HT for about 3 years after the onset of menopause and then discontinuing it, consistent with many current clinical guidelines (Marjoribanks, Farquhar, Roberts, Lethaby, & Lee, 2017; Santen et al., 2010). For group Delay, E2 treatment would begin on average ~27 months post-OVX, allowing for about 3 months post-OVX washout and then 24 months of vehicle treatment; the actual range was 798-879 days (mean, 835.8 days). This corresponds to ~2.3 years post-OVX, or nearly 7 years in human lifespan. Although this does not equal the interval between menopause and start of HT in many of the WHIMS participants, it does represent a substantial period of time in the rhesus monkey lifespan without circulating ovarian hormones before HT is initiated, and one that was practical in terms of logistical constraints with working with aged monkeys.
Behavioral testing consisted of acclimation to the test apparatus, training to criterion and testing across delays on the delayed response (DR) task, and training to criterion and testing on the delayed nonmatching-to-sample task (DNMS). The first testing period began the day after the second E2 or vehicle treatment the monkey received, and the second testing period was timed to begin coincident with the day after the second E2 treatment in monkeys in the Delay group, 777 days after the beginning of the treatment protocol in each monkey. The first test period also incorporated DR and DNMS testing with distraction after completion of delay testing in each task, identical to that described in Baxter et al. (2013), but those results will not be presented here because distraction testing was not included in the second behavioral test period and our focus is on change in performance between the two test periods. Some monkeys also were tested in behavioral tasks in an automated, touchscreen-based apparatus (like that in Baxter, Gaffan, Kyriazis, & Mitchell, 2007) after completion of DNMS in the second behavioral test period but before perfusion, as part of pilot data collection for another planned study. Monkeys continued the hormone treatment they were receiving during the second behavioral test, either E2 or vehicle depending on their group assignment, until perfusion.
Nine of the 41 monkeys failed to complete the entire experimental protocol, with 5 going to perfusion early before the end of the first behavioral test period and another 4 between the end of the first behavioral test period and the end of the second. As a result, the final sample was N = 8 monkeys in each condition who completed the entire experimental protocol, with a time interval between OVX and perfusion averaging 3.35 years (range 2.8-4.0 years). The mean total treatment duration for monkeys that completed the entire protocol was 1055 days for group Veh and 1105 days for group E2. The mean total treatment duration was 1189 days for group Withdrawn, consisting of 16 E2 treatments given over 336 days and vehicle treatments begun 21 days after the last E2 treatment for the remainder of the protocol (mean 832 days). The mean total treatment duration for group Delay was 1110 days, consisting of 35 vehicle treatments given over 735 days and E2 treatments begun 21 days after the last vehicle treatment for the remainder of the protocol (mean 354 days).
OVX, washout, and hormone treatment
For OVX surgery, monkeys were sedated with 10 mg/kg ketamine i.m., given 0.04 mg/kg atropine s.c., then intubated, placed on isoflurane anesthesia to effect, and positioned in dorsal recumbency. A ventral caudal midline abdominal incision visualized the body of the uterus and both ovaries. Ovarian vessels and the Fallopian tubes were isolated, ligated, and severed. The abdominal wall was then closed in three layers with two layers of 2/0 absorbable suture and the final subcuticular layer with 3/0 absorbable suture. The animals were recovered in the CNPRC surgical recovery unit and given three days of post-operative analgesia, 1.5 mg/kg oxymorphone i.m. three times daily.
Urinary metabolites of estrogen (E1C) and progesterone (PdG) were analyzed by enzyme immunoassay in the Primate Assay Laboratory at the CNPRC as previously described by Shideler and colleagues (Shideler, Gee, Chen, & Lasley, 2001) to ensure intact ovarian activity before inclusion in the study and the success of the OVX surgery. For urine collection, cage pans were placed in the late afternoon for overnight sampling, and 3 ml of urine was collected by 0900 the following morning. Samples were collected daily for 6 weeks. Urine was centrifuged and decanted to remove any solid material and then frozen until analysis by enzyme immunoassay. Hormone concentrations were indexed to creatinine (Cr) to adjust for differences in urine concentration. These assays confirmed effectiveness of OVX, similar to the description in Baxter et al. (2013).
Monkeys receiving E2 treatment at any phase of the study received two i.m. injections of 100 μg estradiol cypionate in 1 ml peanut oil vehicle, 9 hours apart, with the first injection at approximately 0600 on the treatment day and the second at 1500. Pilot studies indicated this produced more consistent peak serum E2 levels after dosing compared to single E2 injections (as in P. R. Rapp et al., 2003). Monkeys receiving vehicle treatment received two i.m. injections of 1 ml peanut oil vehicle only on the same schedule. Treatments were given every 21 days.
Behavioral testing
Delayed response (DR) testing
Testing took place in a manual test apparatus, identical to previous descriptions (O’Donnell, Rapp, & Hof, 1999; P. R. Rapp et al., 2003a; P. R. Rapp, Kansky, & Roberts, 1997). A white noise generator was used throughout training to mask extraneous sound. Acclimation and familiarization to the apparatus at the beginning of testing included offering the monkey food rewards in the test tray, as well as the opportunity to displace objects and plaques covering food wells in order to obtain reward. DR training was conducted in phases, with and without delays, as in previous studies (Baxter et al., 2013; P. R. Rapp et al., 2003a). Trials were initiated by raising the opaque barrier of the apparatus, and the monkey watched through a Plexiglas screen while one of the lateral wells of the stimulus tray was baited with a food reward (e.g., raisin or peanut). The lateral wells were subsequently covered with identical plaques and, during the initial phase of training, the clear barrier was raised immediately to permit a response (0 s delay). After the monkey displaced one of the plaques, the opaque barrier was lowered to impose a 20 sec intertrial interval (ITI). Daily test sessions consisted of 30 trials, with the left and right food wells baited equally often according to a pseudorandom sequence. Monkeys were trained until they achieve a criterion of 90% correct (9 errors or less in 9 consecutive blocks of 10 trials). Testing subsequently continued in an identical fashion except that a 1 sec delay was imposed between the baiting and response phase of each trial, and continued until criterion performance was re-achieved. In the next phase the memory demands of the DR task were made progressively more challenging by introducing delays of 5, 10, 15, 30 and 60 sec; testing was otherwise conducted as before (i.e. 30 trials/day, 20 sec ITI). Monkeys were tested for a total of 90 trials (3 days of 30 trials per day) at each retention interval.
Delayed nonmatching-to-sample (DNMS) testing
Testing took place in the same WGTA apparatus as DR. Trials in this task consisted of two phases, initiated when the opaque barrier of the WGTA was raised to reveal an object covering the baited central well of the stimulus tray. After the reward was retrieved, the opaque screen was lowered, and the sample item positioned over one of the lateral wells. The other lateral well was baited and covered with a novel object. During training, a 10 sec delay was imposed and recognition memory was tested by allowing monkeys to choose between the sample and the rewarded novel object. The discriminative stimuli were drawn from a pool of 800 objects according to a pre-determined sequence, ensuring that new pairs of objects were presented on every trial. Twenty trials per day were given using a 30 sec ITI, counterbalancing the left/right position of the novel items. Subjects were tested until they reached a 90% correct criterion by committing no more than 10 errors in 5 consecutive sessions (100 trials). The memory demands of the DNMS task were then made progressively more challenging by introducing successively longer retention intervals of 15, 30, 60, 120 sec (total = 100 trials each, 20/day), and 600 sec (total = 50 trials, 5/day). Monkeys remained in the test apparatus for all delays.
Statistical analysis
Acquisition (behavioral test period 1) or reacquisition (behavioral test period 2) of the DR and DNMS tasks in trials to criterion were compared between groups by either t-tests or one-way ANOVAs, depending on the number of conditions (2 or 4 in the first and second behavioral test periods, respectively). Mixed effects logistic regression was used to model the proportion of trials in which monkeys responded correctly on the DR and DNMS tasks during memory tests in which performance was challenged by progressively longer delays after criterion performance at short delays had been achieved. To account for repeated measures across time in monkeys, random intercepts were included for each monkey and random slopes were included for the time points on which monkeys were tested. Full three-way interactions between treatment group (Veh, E2, Delay, Withdrawn), time point (first or second behavioral test period), and delay interval on task (5-60 sec for DR and 15-600 sec for DNMS) were included in the DR and DNMS models in order to assess the degree to which treatment group modified the change in performance on a given task over time relative to group Veh, conditional on the difficulty of the task (i.e., delay interval between stimulus presentation and choice). Odds ratios (OR) between the odds of responding correctly on a given trial type during the second behavioral test relative to the first represent measures of change in performance over time. The primary parameters of interest are the ratios between these ORs in experimental treatment groups (E2, Delay, Withdrawn) vs. the control group (Veh), which we report as ratios of odds ratios (ROR). A ROR significantly greater than 1 indicates that the change in performance over time either improved more, or declined less, in the experimental group relative to Veh. Hypothesis tests were performed at the 0.05 level of significance and 95% confidence intervals are reported. All tests and confidence intervals were corrected using Dunnett’s method for comparing parameters from three separate experimental groups (E2, Delay, and Withdrawn) to the control group (Veh). All statistical analyses were conducted using R version 3.4.2 (R Core Team, 2014). We adopted this more sensitive approach for these analyses rather than, for example, repeated measures analysis of variance on percent correct scores across delay conditions, because it incorporates into the model random slopes for individual monkey variation, as well as the number of trials carried out at each delay. This increases the amount of information available about the odds of responding correctly on a particular trial, relative to a percent correct measure collapsed across trials.
Results
Delayed response (DR)
For initial acquisition and delay performance during the first behavioral test, the Veh and Delay groups were collapsed into a single “vehicle” group and the E2 and Withdrawn groups into a single hormone treatment group, because at this point in the protocol they were being treated identically. This analysis examined whether E2 treatment initiated within months of OVX had any effect on DR performance. During the first behavioral test, E2 treatment improved rate of acquisition of the DR task at the 0 sec, but not the 1 sec delay: trials to criterion at 0 sec, mean ± SD: vehicle, 267.8 ± 257.5, hormone treatment, 123.8 ± 168.4, t(39) = 2.13, p = 0.040; 1 sec, mean ± SD: vehicle, 344.7 ± 484.4, hormone treatment, 359.1 ± 500.8, t(39) = 0.09, p = 0.93. E2 treatment had no effects on delay performance during the first behavioral test period. There were no significant differences on the delayed response task performance between monkeys on E2 (groups E2 and Withdrawn) compared to monkeys on Veh (groups Veh and Delay) for any of the delay lengths during the first behavioral test (5 sec: OR = 1.21, 95% CI [0.84, 1.75], p = 0.31; 10 sec: OR = 1.38, 95% CI [0.96, 1.98], p = 0.08;15 sec: OR = 1.12, 95% CI [0.79, 1.59], p = 0.54; 30 sec: OR = 0.97, 95% CI [0.69, 1.37], p = 0.87; 60 sec: OR = 0.90, 95% CI [0.64, 1.27], p = 0.56). DR acquisition and performance during the first behavioral test did not correlate with the length of the interval between OVX and treatment initiation (|r|s < 0.28, ps > 0.08). Reacquisition of the DR task at 0 and 1 sec delays at the beginning of the second behavioral test was not influenced by hormone treatment group (Veh, E2, Delay, Withdrawn), Fs(3, 28) < 1, ps > 0.60.
Performance across delays, ranging from 5 to 60 sec, for each group on the first and second behavioral tests is illustrated in Figure 2A as proportion correct on each trial type with 95% confidence intervals. Although our primary measure was change in delay performance between first and second behavioral test periods, we also examined whether group differences were present during the first behavioral test period based on assignment to all four treatment groups, even though at this point groups Veh and Delay were being treated identically and groups E2 and Withdrawn were being treated identically. Unexpectedly, performance on the DR task during the first behavioral test differed between at least two treatment groups on the 10 sec delay, X2(3) = 11.26, p = 0.01. Specifically, the odds of responding correctly at a 10 sec delay in the Delay group were half that of the Veh group (OR = 0.55, 95% CI [0.32 – 0.93], p = 0.03). Performance on the 10 sec delay task did not differ significantly between Veh and E2 (OR = 0.97, 95% CI [0.58 – 1.63], p = 0.99) or Veh and Withdrawn (OR = 0.62, 95% CI [0.37 – 1.05], p = 0.084). Because monkeys in the Veh and Delay groups were being treated identically at this point in the protocol, this effect suggests chance variation between these two groups. Furthermore, performance on the DR task did not differ significantly between any two groups for the non-10 sec delays: 5 sec, X2(3) = 2.84, p = 0.42; 15 sec, X2(3) = 6.77, p = 0.08; 30 sec, X2(3) = 1.52, p = 0.68; or 60 sec, X2(3) = 0.537, p = 0.91.
Figure 2. Delayed response (DR) task results.
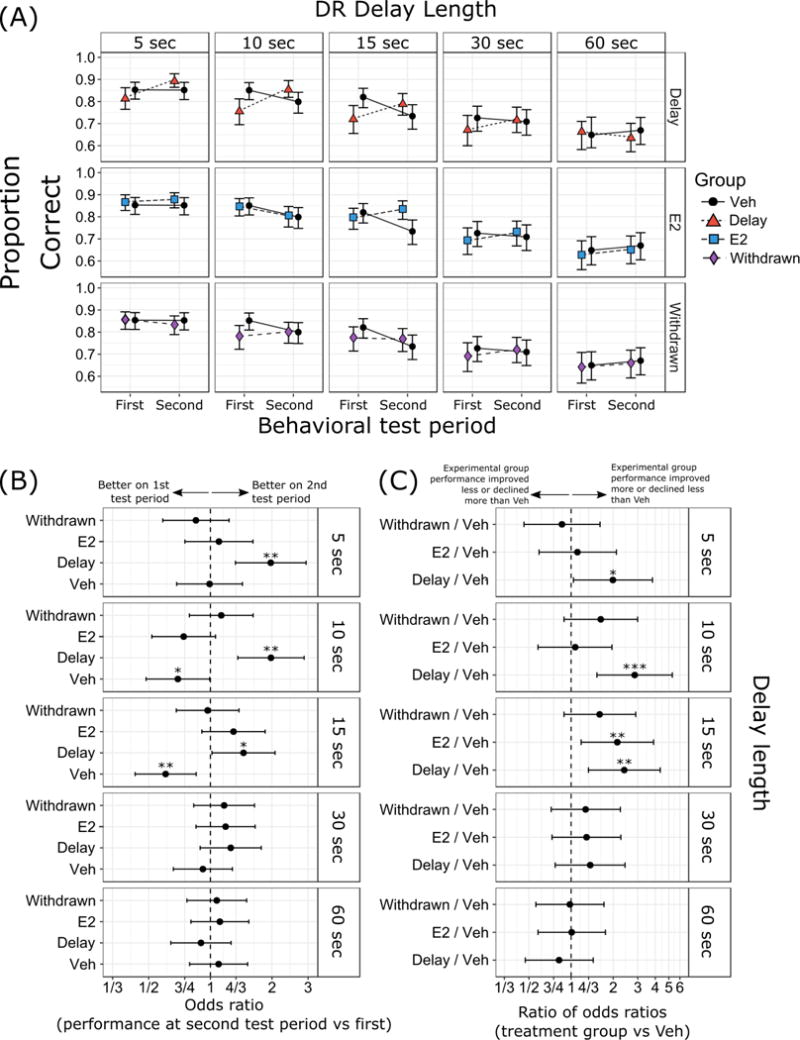
(A) Proportion of correct responses with 95% confidence intervals. (B) Odds ratios (OR) between performance at second test period vs first test period, and (C) ratios of odds ratios (ROR) in performance across time in treatment groups relative to control. Results are separated by delay length (5 sec, 10 sec, 15 sec, 30 sec, and 60 sec delays). Group designations (Veh, E2, Delay, Withdrawn) as in Figure 1. *** p < 0.001, ** p < 0.01, * p < 0.05. p-values in (C) were corrected using Dunnett’s method for comparing parameters from three separate experimental groups (E2, Delay, and Withdrawn) to the control group (Veh).
In our primary analysis of the memory test phase of DR in which performance was challenged with increasing delays, performance improved between the first and second test periods in group Delay compared to group Veh at short and intermediate delays, indicating effectiveness in the delayed E2 treatment begun before the second behavioral test in improving DR performance. With the exception of improved performance at the 15 sec delay in group E2 relative to group Veh between the first and second test periods, performance tended to be stable in the other treatment groups. Changes in performance across time for each group are shown in Figure 2B as odds ratios. These measures show improvement at 5-15 sec delays in group Delay, and declines in performance at 10 and 15 sec delays in group Veh, with each group in terms of their performance in the second behavioral test period relative to the first. Figure 2C shows the differences between treatment groups relative to group Veh in their change in performance over time, as ratios of odds ratios. These measures show significant differences between groups Delay and Veh at 5-15 sec delays, and between group E2 and Veh at the 15 sec delay, all representing greater improvement or less decline of these groups over time relative to group Veh. Thus, overall, monkeys that received E2 post-OVX initially acquired the DR task faster at a 0 sec delay, and monkeys that received delayed E2 treatment tended to show improvement in DR delay performance relative to monkeys that received vehicle throughout the protocol. Monkeys that received constant E2 treatment throughout the study, or monkeys that received E2 initially post-OVX and then had it discontinued, tended to maintain their levels of DR delay performance.
Delayed nonmatching-to-sample (DNMS)
As was the case for DR, for initial acquisition of DNMS during the first behavioral test, we collapsed experimental groups into two and compared “vehicle” and “hormone treatment” groups. E2 treatment did not affect rate of DNMS acquisition, trials to criterion at 10 sec mean ± SD: vehicle, 710.4 ± 620.8; hormone treatment, 812.7 ± 554.0; t(37) = 0.54, p = 0.59. There were no significant differences on DNMS performance between monkeys on E2 (groups E2 and Withdrawn) compared to monkeys on Veh (groups Veh and Delay) for any of the delay lengths during the first behavioral test (15 sec: OR = 1.19, 95% CI [0.90, 1.57], p = 0.23; 30 sec: OR = 1.19, 95% CI [0.85, 1.47], p = 0.42; 60 sec: OR = 0.89, 95% CI [0.69, 1.15], p = 0.36; 120 sec: OR = 1.02, 95% CI [0.80, 1.31], p = 0.86; 600 sec: OR = 1.17, 95% CI [0.88, 1.56], p = 0.27). However, reacquisition of the DNMS task at the beginning of the second behavioral test was better in monkeys that were currently receiving or had previously received E2 (groups E2, Delay, Withdrawn) compared to monkeys in group Veh that had never received E2, means ± SDs: Con, 79.5 ± 117.5; Delay, 7.5 ± 14.9; E2, 0 ± 0; Withdrawn, 12.5 ± 23.75; F(3, 28) = 2.97, p = 0.049.
Performance across delays on the DNMS task, ranging from 15 to 600 sec, for each group on the first and second behavioral tests is illustrated in Figure 3A as proportion correct on each trial type with 95% confidence intervals. DNMS performance during the first behavioral test did not differ significantly between any two groups for any duration of delay (15 sec, X2(3) = 4.14, p = 0.25; 30 sec, X2(3) = 1.66, p = 0.65; 60 sec, X2(3) = 1.60, p = 0.66; 120 sec, X2(3) = 1.40, p = 0.71; or 600 sec, X2(3) = 1.31, p = 0.73).
Figure 3. Delayed nonmatch-to-sample (DNMS) task results.
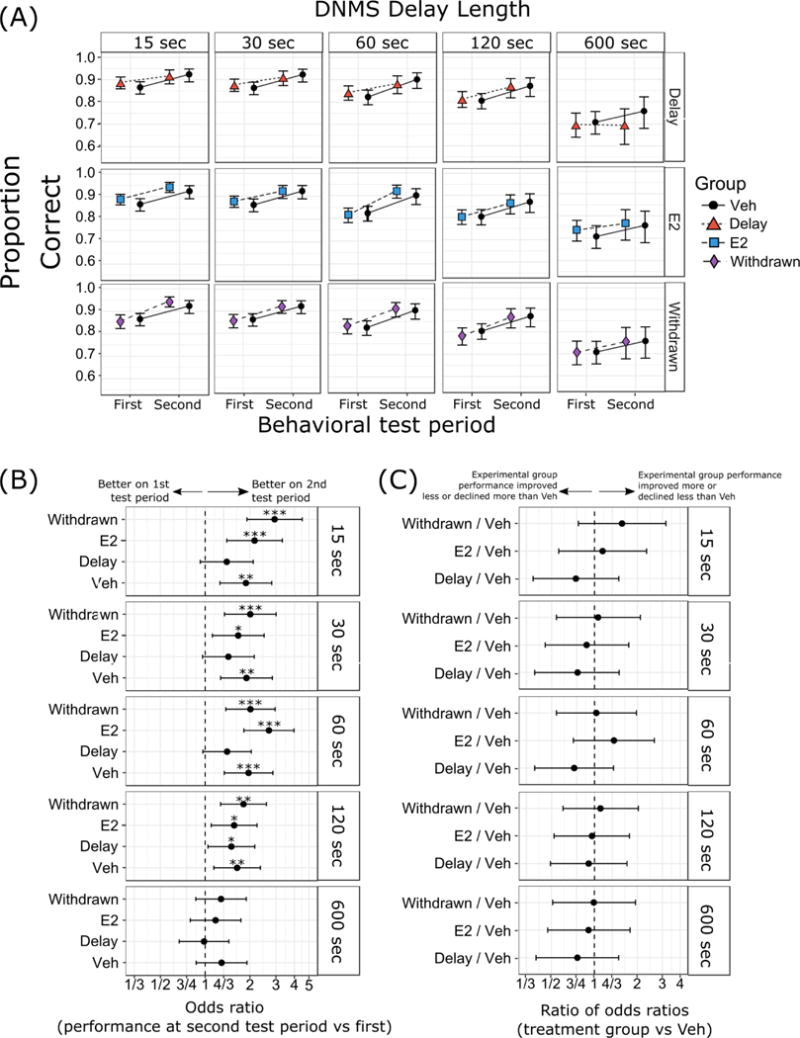
(A) Proportion of correct responses with 95% confidence intervals. (B) Odds ratios (OR) between performance on DNMS at second test period vs first test period, and (C) ratios of odds ratios (ROR) in performance on DNMS across time in treatment groups relative to Veh. Results are separated by delay length (15 sec, 30 sec, 60 sec, 120 sec, and 600 sec delays). Group designations (Veh, E2, Delay, Withdrawn) as in Figure 1. *** p < 0.001, ** p < 0.01, * p < 0.05. p-values in (C) were corrected using Dunnett’s method for comparing parameters from three separate experimental groups (E2, Delay, and Withdrawn) to the control group (Veh).
In our primary analysis, across all delays, change in performance between the first and second behavioral tests in the DNMS task did not differ between the three treatment groups (E2, Delay, Withdrawn) relative to the Veh group. Changes in performance across time are shown as odds ratios in Figure 3B. These measures show marked improvement in most groups between the first and second behavioral tests, except at the longest (600 sec) delay. Figure 3C shows the differences between treatment groups in their performance over time, as ratios of odds ratios. There were no significant differences between any of experimental groups (E2, Delay, Withdrawn) relative to group Veh at any of the delays tested in the DNMS task. Thus, although monkeys that had ever received E2 post-OVX were better at reacquiring DNMS during the second behavioral test, there were no group differences in the change in delay performance over time.
Discussion
Our goals in this study were twofold. The first was to test whether E2 treatment, delayed by more than 2 years post-OVX, would still be effective in improving behavior in aged, surgically menopausal rhesus monkeys. The “window of opportunity” hypothesis derived from women in the WHIMS study would predict that E2 treatment after a substantial interval without circulating ovarian hormones would be ineffective. We found that prefrontal cortex-dependent spatial working memory in the DR task improved between the first and second behavioral test periods in monkeys with delayed E2 treatment initiated just before the second behavioral test, suggesting that if a “window of opportunity” exists in rhesus monkeys, it is longer than 2.3 years, the equivalent of ~7 years in humans. Whether comparison of time intervals in terms of lifespan ratio between monkeys and humans is valid in this regard remains an open question. That monkeys eventually become insensitive to effects of hormone therapy is suggested by one study where monkeys more than 10 years post-OVX did not experience any benefit of E2 treatment on the DR task (Lacreuse et al., 2002), but it is unclear whether this relates to the length of the post-OVX interval, the chronological age of the monkeys (Smith et al., 2010), or both. At the very least, our findings suggest that women who do not elect to begin HT immediately when menopausal symptoms are first experienced may still reap some benefit from it. It is notable that there remain very limited data about the impact of HT in women that begin it in their 50s, around the average age of menopause (Marjoribanks et al., 2017). Nevertheless, the behavioral effect of delayed E2 treatment was modest, and limited to the DR task. The more pronounced effect of E2 treatment on DR compared to DNMS is consistent with our previous study (P. R. Rapp et al., 2003a). This may reflect relatively greater sensitivity of prefrontal cortex-dependent mechanisms that support DR to cognitive aging, relative to temporal cortex-dependent processes that support DNMS performance (Morrison & Baxter, 2012; P. R. Rapp & Amaral, 1989; 1991). Monkeys in group Delay also tended to perform worse in the first behavioral assessment than monkeys in group Veh giving them more room for improvement which may have magnified the apparent beneficial effect of E2 in this group.
Our second goal was to determine whether E2 treatment begun soon post-OVX and then withdrawn would produce any lasting beneficial effects on behavior. Memory performance in DR was stable in the second behavioral test relative to the first, indicating that performance did not significantly decline after E2 treatment was withdrawn, and monkeys in this group reacquired the DNMS task more readily than monkeys that had never received E2 post-OVX. This suggests that beneficial effects of E2 on cognition may persist after discontinuation of E2 in monkeys, providing a setting in which to investigate the cellular and molecular mechanisms of this effect.
The effects of hormone treatment were seen in the DR task, in initial acquisition where E2 improved learning of the task, in the pattern of change in delay performance between the first and second tests of DR, and in the reacquisition of DNMS in the second behavioral test. All of these effects suggest a locus of hormone treatment action in the prefrontal cortex, because both DR performance and DNMS acquisition depend on the integrity of the prefrontal cortex (Bachevalier & Mishkin, 1986; Goldman & Rosvold, 1970; Goldman, Rosvold, Vest, & Galkin, 1971; Shamy et al., 2011). Effects of hormone treatment on DR were more pronounced at shorter delays (5-15 sec) and not observed at longer delays (30-60 sec). It is not clear whether this is related to some specific effect on an aspect of shorter term working memory or is related to the monkeys encountering longer delays later in testing when they may have had an opportunity to adapt their behavioral strategies and compensate for any declines in performance. Testing with varying delays within-session could help address this question, although our intention in this study was to maintain continuity of testing sequence with that used in previous experiments from our group (Baxter et al., 2013; P. R. Rapp et al., 2003a).
A limitation of our study is that across both types of testing, there was essentially no effect of E2 during the first behavioral test period, begun shortly post-OVX. This might indicate that post-OVX interval used in this study, approximately 3.5 months, which was shorter than in previous studies in our group (~8 months in P. R. Rapp et al., 2003) may not be sufficiently long for synaptic health (Morrison & Baxter, 2014) and behavior regulated by impacted brain areas to be affected, at least as measured by our task design. This finding may be a feature of our protocol in which monkeys acquire DR post-OVX, given that studies in rodents commonly observe beneficial effects of E2 immediately post-OVX and another study in monkeys behaviorally trained pre-OVX observed DNMS (but not DR) deficits that were ameliorated by hormone treatment 12 weeks post-OVX (Voytko, Higgs, & Murray, 2008). Another possibility is that cohort differences in our population of aged monkeys may have made them more resistent to initial deleterious effects of OVX. For example, the majority of monkeys in the present study were pair-housed whereas all of the monkeys in the 2003 study were singly-housed. The greater enrichment provided by social housing may have insulated monkeys against adverse cognitive effects of OVX. However, this did not compromise our ability to detect changes in behavior over time. This may suggest a period of resilience of behavior following loss of circulating ovarian hormones before synaptic health deteriorates to a point that cognitive impairments are evident. This finding also gives an indication of the importance of timing of HT and is an idea that our future research will continue to investigate.
Acknowledgments
This project was supported by National Institute on Aging (NIA) award P01-AG016765. The California National Primate Research Center is supported by National Institutes of Health (NIH) Office of the Director award P51-OD011107. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank Mary Roberts, Tracy Ojakangas, and Lisa Novik for technical assistance, and Nancy Gee and Bill Lasley for hormone assay data verifying ovariectomy.
Footnotes
Author Note
Mark G. Baxter, Department of Neuroscience and Friedman Brain Institute, Icahn School of Medicine at Mount Sinai; Anthony C. Santistevan and Eliza Bliss-Moreau, Department of Psychology, University of California, Davis, and California National Primate Research Center; John H. Morrison, Department of Neurology, University of California, Davis School of Medicine, and California National Primate Research Center.
A preprint version of this manuscript was posted on BioRxiv at https://www.biorxiv.org/content/early/2018/01/17/248963.
Contributor Information
Mark G. Baxter, Icahn School of Medicine at Mount Sinai
Anthony C. Santistevan, University of California - Davis and California National Primate Research Center
Eliza Bliss-Moreau, University of California - Davis and California National Primate Research Center.
John H. Morrison, University of California - Davis and California National Primate Research Center
References
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SAA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women’s Health Initiative randomized trial. JAMA: the Journal of the American Medical Association. 2003;290(13):1739–1748. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SAA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA: the Journal of the American Medical Association. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behavioural Brain Research. 1986;20(3):249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiology of Aging. 1991;12(2):99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C, PERF Study Group Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause (New York, NY) 2005;12(1):12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: debilitating effects on short-term memory. Journal of Gerontology. 1978;33(6):858–871. doi: 10.1093/geronj/33.6.858. [DOI] [PubMed] [Google Scholar]
- Baxter MG. Cognitive aging in nonhuman primates. Functional Neurobiology of Aging; 2001. pp. 407–419. [Google Scholar]
- Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Orbital prefrontal cortex is required for object-in-place scene memory but not performance of a strategy implementation task. Journal of Neuroscience. 2007;27(42):11327–11333. doi: 10.1523/JNEUROSCI.3369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Roberts MT, Gee NA, Lasley BL, Morrison JH, Rapp PR. Multiple clinically relevant hormone therapy regimens fail to improve cognitive function in aged ovariectomized rhesus monkeys. Neurobiology of Aging. 2013;34(7):1882–1890. doi: 10.1016/j.neurobiolaging.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DGM. The Women’s Health Initiative Memory Study: findings and implications for treatment. The Lancet Neurology. 2005;4(3):190–194. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA: the Journal of the American Medical Association. 2004;291(24):2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Hormones and Behavior. 2009;55(1):2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Price DL, Koliatsos VE, Markowska AL. The effects of nerve growth factor on spatial recent memory in aged rats persist after discontinuation of treatment. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 1997;17(7):2543–2550. doi: 10.1523/JNEUROSCI.17-07-02543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21(1):107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biology of Reproduction. 1997;57(2):335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Experimental Neurology. 1970;27(2):291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Vest B, Galkin TW. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. Journal of Comparative and Physiological Psychology. 1971;77(2):212–220. doi: 10.1037/h0031649. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WGM, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proceedings of the National Academy of Sciences. 2014;111(1):486–491. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behavioural Brain Research. 1997;87(1):25–34. doi: 10.1016/s0166-4328(96)02256-5. Retrieved from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9331471&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- Kohama SG, Renner L, Landauer N, Weiss AR, Urbanski HF, Park B, et al. Effect of Ovarian Hormone Therapy on Cognition in the Aged Female Rhesus Macaque. Journal of Neuroscience. 2016;36(40):10416–10424. doi: 10.1523/JNEUROSCI.0909-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiology of Aging. 2002;23(4):589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Lyons-Warren A, Lillie R, Hershey T. Short- and Long-Term Spatial Delayed Response Performance Across the Lifespan. Developmental Neuropsychology. 2004;26(3):661–678. doi: 10.1207/s15326942dn2603_1. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and risk for dementia: where do we go from here? Gynecological Endocrinology: the Official Journal of the International Society of Gynecological Endocrinology. 2004;19(6):354–359. doi: 10.1080/09513590400018207. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006a;138(3):1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Maki PM. Potential importance of early initiation of hormone therapy for cognitive benefit. Menopause: the Journal of the North American Menopause Society. 2006b;13(1):6–7. doi: 10.1097/01.gme.0000194822.76774.30. [DOI] [PubMed] [Google Scholar]
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews (Online) 2017;1:CD004143. doi: 10.1002/14651858.CD004143.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2002;22(24):10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Hormones and Behavior. 2008;54(3):386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nature Reviews Neuroscience. 2012;13(4):240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. Synaptic health. JAMA Psychiatry. 2014;71(7):835–837. doi: 10.1001/jamapsychiatry.2014.380. [DOI] [PubMed] [Google Scholar]
- O’Donnell KA, Rapp PR, Hof PR. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Experimental Neurology. 1999;160(1):300–310. doi: 10.1006/exnr.1999.7192. [DOI] [PubMed] [Google Scholar]
- Ohm DT, Bloss EB, Janssen WG, Dietz KC, Wadsworth S, Lou W, et al. Clinically Relevant Hormone Treatments Fail to Induce Spinogenesis in Prefrontal Cortex of Aged Female Rhesus Monkeys. Journal of Neuroscience. 2012;32(34):11700–11705. doi: 10.1523/JNEUROSCI.1881-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2014. Vienna, Austria. URL http://www.R-project.org. [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 1989;9(10):3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiology of Aging. 1991;12(5):481–486. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8(8):1923–1928. doi: 10.1097/00001756-199705260-00026. Retrieved from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9223078&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2003a;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA: the Journal of the American Medical Association. 2003b;289(20):2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA: the Journal of the American Medical Association. 2002;288(17):2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KV, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport. 1997;8(8):2047–2051. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal Hormone Therapy: An Endocrine Society Scientific Statement. Journal of Clinical Endocrinology & Metabolism. 2010;95(7_Supplement_1):s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH, et al. Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cerebral Cortex. 2011;21(7):1559–1573. doi: 10.1093/cercor/bhq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138(3):1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? Journal of Neuroendocrinology. 2007;19(2):77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: A critical review. Frontiers in Neuroendocrinology. 2008;29(1):88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biology of Reproduction. 2001;65(6):1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA: the Journal of the American Medical Association. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA: the Journal of the American Medical Association. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen’s ability to enhance hippocampal synaptic physiology. Proceedings of the National Academy of Sciences. 2010;107(45):19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. American Journal of Primatology. 1988;15(3):263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Bredemann TM, McMahon LL. Estradiol replacement extends the window of opportunity for hippocampal function. Neurobiology of Aging. 2014;35(10):2183–2192. doi: 10.1016/j.neurobiolaging.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML. The effects of long-term ovariectomy and estrogen replacement therapy on learning and memory in monkeys (Macaca fascicularis) Behavioral Neuroscience. 2000;114(6):1078–1087. [PubMed] [Google Scholar]
- Voytko ML. Estrogen and the cholinergic system modulate visuospatial attention in monkeys (Macaca fascicularis) Behavioral Neuroscience. 2002;116(2):187–197. doi: 10.1037//0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Higgs CJ, Murray R. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience. 2008;154(4):1205–1217. doi: 10.1016/j.neuroscience.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ. Executive Function and Attention Are Preserved in Older Surgically Menopausal Monkeys Receiving Estrogen or Estrogen Plus Progesterone. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;29(33):10362–10370. doi: 10.1523/JNEUROSCI.1591-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML, Herndon JG. Menopause in Nonhuman Primates? Biology of Reproduction. 2008;79(3):398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA: the Journal of the American Medical Association. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA: the Journal of the American Medical Association. 2002;288(17):2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]


