Abstract
BACKGROUND
Metabolic syndrome (MetS) adversely affects the vasculature and cerebral white matter (CWM) integrity. Arterial stiffening has been associated with diminished CWM integrity. Physical activity (PA) can ameliorate components of MetS and subsequently affect arterial stiffening and CWM integrity. Our aim was to determine the role of PA on mitigating the adverse influence of MetS on arterial stiffness and CWM integrity.
METHODS
In a cross-sectional study design, sixty-six middle-aged adults (40–62 years) composed of 18 sedentary MetS (Sed MetS), 21 physically active MetS (Active MetS), and 27 healthy individuals absent of MetS risk factors were studied. Carotid artery stiffness was assessed via simultaneous ultrasound and tonometry. CWM integrity was measured using diffusion tensor imaging (DTI) through metrics of fractional anisotropy (FA) and mean diffusivity (MD).
RESULTS
Carotid β-stiffness index in Active MetS was lower than Sed MetS but was not different from Healthy controls (6.6±1.5, 7.7±2.1, and 5.6±1.6 au, p=0.001). CWM integrity was significantly greater in Active MetS subjects compared to Sed MetS subjects but statistically equal to Healthy controls in the anterior limb of the internal capsule, and splenium of the corpus callosum, uncinate fasciculus, and superior corona radiata (all p<0.05).
CONCLUSIONS
Middle-aged individuals with MetS who habitually perform PA demonstrated lower arterial stiffness and more favorable CWM integrity than their sedentary peers, indicating that PA may be effective in mitigating the adverse effects of MetS on the vasculature and brain at midlife.
Keywords: Arterial Stiffness, Brain Structure, Cognition, Midlife
Introduction
Abdominal obesity, dyslipidemia, elevated blood pressure, and insulin resistance are frequently accumulated concurrently throughout the lifespan into adulthood. When three or more of these components exist in a single individual they promote synergistic deleterious effects in a condition known as metabolic syndrome (MetS) (Alberti et al. 2009). The World Health Organization has declared MetS to be a global epidemic (Potenza and Mechanick 2009) and over a third of U.S. adults are afflicted by MetS (Aguilar et al. 2015). This prevalence is troubling, as individuals with MetS not only demonstrate arterial stiffening associated with increased risk for cardiovascular disease and mortality, but also elevate their probability of acquiring vascular dementia by several fold (Raffaitin et al. 2009).
Cerebral white matter (CWM) plays an integral role in conducting neural information between cortical structures allowing the brain to work in synchrony. We have previously observed that midlife visceral adiposity is associated with arterial stiffening and white matter hyperintensities representative of white matter damage foci (Strasser et al. 2015; E. P. Pasha et al. 2017). Taken together, these findings indicate that arterial stiffening and abdominal obesity, two characteristics of MetS, may leave this population particularly vulnerable to early CWM alterations and subsequent cognitive dysfunction (Yates et al. 2012). Considering aortic stiffness has been associated with regional damage to CWM integrity and is elevated in elderly individuals with Alzheimer’s disease and vascular dementia, determining effective strategies to combat arterial stiffening and CWM damage in at risk populations at midlife is valuable for prolonging the cognitive health span (Tarumi et al. 2015; Hanon et al. 2005).
Regular physical activity (PA) can reduce arterial stiffness and attenuate or even abolish arterial stiffening that occurs with advancing age (Boreham et al. 2004). Aerobic training can also reduce visceral adiposity and improve metabolic syndrome components (Thompson et al. 2001). These modifiable cardiovascular risk factors may contribute to carotid artery stiffening and CWM deterioration, making PA an attractive method to mitigate the adverse effects of MetS on arterial stiffening and CWM integrity. However, investigations relating PA to CWM integrity are extremely limited, with investigations surrounding aerobic fitness and the brain of much greater prevalence. Further, the clinically important question of whether PA can simultaneously mitigate damage to the vasculature and CWM due to MetS has never been addressed.
Accordingly, the primary aim of the present investigation was to determine whether individuals with MetS who are physically active demonstrate lower arterial stiffness and more favorable CWM integrity than their sedentary peers. Because healthy cognitive function is diminished by MetS, executive function, processing speed, and memory were also examined (Yates et al. 2012). To accomplish these aims, rather than observing stiffness of the abdominal aorta, we measured arterial stiffness of the carotid artery because of its anatomical relevance to cerebral circulation as the vessel supplying the brain using a robust imaging-based technique (Maillard et al. 2016). Additionally, the carotid artery is vulnerable to arteriosclerotic changes related to aging and exposure to MetS components (Della-Morte et al. 2010).
CWM integrity was determined using diffusion tensor imaging (DTI). DTI is a magnetic resonance imaging (MRI) sequence that characterizes the three-dimensional diffusion of water in tissue by producing scalar metrics capable of describing CWM integrity in vivo (Alexander et al. 2007). Increased fractional anisotropy (FA) or reduced mean diffusivity (MD) are reflective of greater CWM integrity (Alexander et al. 2007). Specifically, FA measures the magnitude of water diffusion in the principle direction of white matter tracts whereas MD measures the average of the rate of water diffusion in all three directions.
In short, arterial stiffness, CWM integrity, and cognitive function were compared in healthy controls and groups of MetS patients who were either physically active or not. Our working hypothesis was that MetS patients who are physically active do not demonstrate arterial stiffening, reduced CWM integrity, or impaired cognitive function. Specifically, we hypothesized that arterial stiffness would be greatest in sedentary MetS participants and similar between active MetS and healthy controls. Similarly, we hypothesized lower FA, higher MD, and poorer cognitive function in MetS participants compared with either active MetS or healthy controls.
Methods
Participants
A total of 66 community dwelling adults aged 40–62 years from the Austin, Texas area took part in this cross-sectional investigation. Included individuals were without pre-existing overt cardiovascular disease (e.g., coronary artery disease, angina pectoris, transient ischemic attack, myocardial infarction, heart failure, or cardiac surgery), neurological disease (e.g., stroke, Parkinson’s disease, and clinically significant traumatic brain injury), or contraindications to MRI as indicated by a Health Research Questionnaire. Individuals with significant depression (>27 on Beck Depression Inventory-II (BDI-II)) and cognitive impairment (<23 on Mini-Mental State Exam) were excluded. Additionally, all recruited subjects were minimally second generation, proficient in English, and educated in the United States. The current analysis comprised only of subjects with metabolic syndrome (≥3 MetS components) and Healthy controls (0 MetS components). The local institutional review board approved this study, and all participants gave their informed consent.
Metabolic Syndrome Characterization
To be included in a MetS group, participants were required to have ≥3 of the following components: abdominal obesity denoted by waist circumference ≥94 cm for men and ≥80 cm for women; elevated triglycerides (≥150 mg/dL); reduced HDL cholesterol (<40 mg/dL for men and <50 mg/dL for women); elevated blood pressure defined as systolic blood pressure ≥130mm Hg and/or diastolic blood pressure ≥85mm Hg; hyperglycemia of elevated fasting glucose (≥100 mg/dL); pharmacological intervention for any condition above. These criteria were selected according to the NHLBI as previously described (Alberti et al. 2009).
All subjects reported for vascular assessments in the morning after having fasted overnight for at least 8 hours and abstained from physical exercise, alcohol consumption, smoking, and caffeine for at least 24 hours. A stadiometer and digital scale measured height to the nearest centimeter and body weight to the nearest tenth of a kilogram respectively for the calculation of BMI as kg/m2. An elastic measuring tape was placed around the trunk at the top of the iliac crest to measure waist circumference (Croft et al. 1995). A blood sample was drawn from the antecubital vein by a certified phlebotomist via venipuncture. Standard enzymatic techniques were used to quantify blood concentrations of triglycerides, HDL-cholesterol, and glucose. Blood pressure was assessed using the automatic oscillometric methods (VP-2000; Omron Healthcare, Kyoto, Japan) in the supine position after comfortably resting for 15 minutes in a temperature controlled laboratory setting. Following this period, arterial stiffness measurements ensued.
Arterial Stiffness Measurement
A longitudinal B-mode image of the left common carotid artery was acquired using an iE 33 Ultrasound System (Philips, Bothell, WA) equipped with a high-resolution linear-array transducer. The image was captured 1–2 cm proximal to the carotid bulb perpendicularly to the blood vessel such that the near and far wall interfaces presented clearly. Digitized images acquired via ultrasound were analyzed later with computerized image-analysis software (Vascular Research Tool Carotid Analyzer, Medical Imaging Applications, Coralville, IA) by a single investigator blinded to subject cardiovascular health after being saved in DICOM format. Concurrent recordings of pulse pressure waveforms from the contralateral common carotid artery were obtained with arterial applanation tonometry (VP-2000; Omron Healthcare, Kyoto, Japan). Ten consecutive pressure waveforms were acquired from each subject for analysis and averaged. Carotid mean arterial pressure and diastolic pressure were calibrated to brachial mean and diastolic pressure obtained oscillometrically to correct for investigator hold-down pressure as described previously (Armentano et al. 1995). β-stiffness, a measure of carotid stiffness that controls for distending pressure, was calculated as β = In (ΔPressure)/[(ΔDiameter)/Diastolic diameter] (O’Rourke et al. 2002). On a separate visit, subjects underwent MRI and a cognitive function battery.
DTI Acquisition
MRI was performed using a 3T Siemens Skyra system (Siemens Medical Solutions, Malvern, PA) with a 32-channel head coil. A diffusion-weighted, spin-echo, echo planar imaging pulse sequence was used to acquire images in 64 directions at b=700 s/mm. One image with b=0 was collected for a non-diffusion weighted reference image. Contiguous 2mm anterior to posterior slices were used to cover the cerebrum with the following parameters: FOV = 256mm, TR = 8300ms, TE = 84ms. Advanced shimming was performed before diffusion weighted imaging in order to optimize the homogeneity of the magnetic field across the brain and to minimize EPI distortions.
Processing DTI images included motion and eddy current correction using affine transformations in FSL (http://www.fmrib.ox.ac.uk/fsl/). Non-brain signal was removed using FSL’s BET (brain extraction tool). Tensor fitting was performed using FSL’s dtifit function. High-dimensional normalization that employs the full tensor in DTI-TK (http://www.nitrc.org/projects/dtitk/) was used according to the methods of Hui Zhang et al. (Zhang et al. 2007). A study-specific template was created using iterative rigid, affine, and diffeomorphic alignments of the full tensor in DTI-TK (http://www.nitrc.org/projects/dtitk/) and registered to standard space using the IIT Human Brain Atlas (www.nitrc.org/projects/iit2).
Fractional anisotropy (FA) and mean diffusivity (MD) are DTI metrics that together fully characterize the diffusion tensor. FA measures the directionality of diffusion. Myelination is thought to result in more directional diffusion parallel to the axon and therefore higher FA. MD measures the magnitude of diffusion. Higher MD indicates more restricted diffusion and is thought to reflect membrane density. FA and MD are sensitive to early disease related CWM alterations (Acosta-Cabronero et al. 2010).
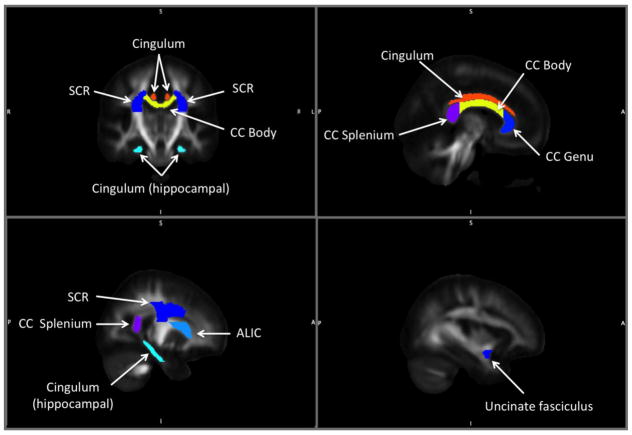
Bilateral regions-of-interest (ROIs) were determined a priori based on existing literature and are displayed in Figure 1. Segments of the corpus callosum (CC) have been investigated previously as CWM regions vulnerable to a multitude of deleterious aging processes, including abnormal cognitive aging (Kennedy and Raz 2009), dementia (Head et al. 2004), and arterial stiffening (Tarumi et al. 2015). The corona radiata and internal capsule have also been identified as CWM tracts particularly susceptible to the effects of arterial stiffening (Tarumi et al. 2015). The cingulum, cingulum (hippocampal), and uncinate fasciculus were selected because of their previously reported relationships with aerobic fitness (Marks et al. 2007). The Johns Hopkins International Consortium for Brain Mapping template was used to define ROIs in standard space. FA maps were calculated from each subject’s tensor map in standard space using DTI-TK. Each participant’s FA map was masked by each ROI and the resulting FA ROIs were thresholded at 0.2 in order to exclude signal from non-white matter voxels (S. M. Smith et al. 2006). The average FA value from each ROI was extracted. MD maps were calculated in subject space. In order to define ROIs in subject space, inverse deformation fields were calculated from standard space to subject space and applied to binarized FA ROIs.
Figure 1.
Cerebral white matter regions-of-interest related to abnormal cognitive aging, dementia, arterial stiffening, and aerobic fitness. ALIC=anterior limb of internal capsule; CC=corpus callosum; SCR=superior corona radiata
Cognitive Assessment
A cognitive performance battery was administered to assess cognitive status and generate study specific executive function and memory domain scores. The Mini Mental State Examination, Wechsler Abbreviated Scale of Intelligence-II Full Scale Intelligence Quotient-2 (WASI-FSIQ) subtest, Beck Depression Inventory-II (BDI-II) were used to determine the general cognitive status of subjects. The Trail Making Test A, Trail Making Test B, WAIS-III Digit Span, and Stroop interference tasks were used to construct an executive function domain score. The processing speed domain was measured by constructing a domain score using the same technique with the Trail Making Test A, Stroop word, and Stroop color tasks. The California Verbal Learning Task (CVLT-II) short delay free recall, long delay free recall, and recognition discriminability comprised the memory domain. Z scores of each task were inverted where appropriate (e.g., time-based tasks) for directional congruity, summated within each domain, and averaged to create the final domain score. Testing was conducted with research assistants trained in administration of these tests. To foster testing standardization, the same assistants performed all scoring.
Physical Activity Behavior
Participants reported days of engaging in low, moderate, and vigorous intensity PA for intervals of at least 15-minute during free time in a 7-day period using the same classifications as the Godin leisure-time physical activity questionnaire (Godin and Shephard 1985). Low PA was defined as “minimal effort” (e.g. yoga, archery, golf, easy walking), moderate PA was defined as “not exhausting” (e.g. fast walking, tennis, easy bicycling, easy swimming), and vigorous PA was described as “heart beats rapidly” (e.g. running, jogging, hockey, vigorous swimming). Frequency of moderate to vigorous PA was calculated by summing the self-reported bouts from the moderate and vigorous categories. This questionnaire has a high two week retest reliability coefficient of 0.94 for vigorous PA with a weaker retest reliability for moderate PA of 0.46 (Godin and Shephard 1985). Summed moderate to vigorous PA was chosen as the PA stratification because exercise at these intensities is recommended by the American College of Sports Medicine (ACSM) for health maintenance (Haskell et al. 2007).
Group stratification
The subject population was first stratified by MetS components. Individuals with ≥3 MetS components created a MetS cohort that was further separated into sedentary (Sed MetS, n=18) and active (Active MetS, n=21) groups based on a median split of self-reported frequency of moderate to vigorous PA. This methodology was selected to establish physically active and inactive cohorts. Although some members of the Sed MetS cohort reported completing some bouts of PA, it was described as sedentary because weekly frequency and duration of PA in this group was nearly absent following the median split. Individuals without MetS components populated the Healthy control group (n=27).
Statistical Analyses
For descriptive characteristics, differences for categorical variables between groups were determined using Chi-squared test while group differences in scalar variables were measured using analysis of variance. Variable homoscedasticity across groups for arterial stiffness and DTI outcomes was assessed with Levene’s test with all passing as p>0.05. To determine differences between regional CWM integrity, analysis of covariance with age, sex, and years of education were included. Data shown are represented as mean ± standard deviation unless stated otherwise. The continuous relationships of β-stiffness and moderate to vigorous PA frequency to DTI metrics was assessed with bivariate correlations and partial correlations controlling for age, sex, and years of education.
To account for inflation of type-1 error due to multiple comparisons, rather than using a strict Bonferroni correction that would be too conservative in light of the intercorrelated outcomes investigated in this study, a modified Bonferroni was employed (Sankoh et al. 1997). Additionally, hypotheses were a priori and small effects were of interest for clinical implications, furthering our rationale for a less conservative α. Based on two degrees of freedom and mean intercorrelations of eight outcomes with r=0.52 and r=0.67 for FA and MD respectively, an α<0.018 of α<0.022 were determined (quantitativeskills.com). Ultimately, the mean of these two α<0.02 was selected for statistical significance. Differences in arterial stiffness and cognitive function domains were deemed significant at the conventional α<0.05. SPSS version 24 (SPSS Inc; IBM, Armonk, NY) was used to perform all statistical analyses.
Results
Group Characteristics
Descriptive characteristics are presented in Table 1. Per stratification, both MetS groups had greater waist circumference, systolic BP, triglyceride, blood glucose and lower HDL-cholesterol than healthy controls and did not differ from each other except for triglycerides (all p<0.05). Plasma triglyceride concentration in the Active MetS group was significantly greater than the Sed MetS group. Frequency of PA was lower in the Sed MetS group compared with Healthy controls and the Active MetS group (all p<0.05). Physical activity did not differ between the Healthy control and Active MetS groups and seemingly reached the ACSM PA guidelines for frequency (Haskell et al. 2007). Smoking was not significantly different across groups (p>0.05). Ethnicity was also evenly distributed amongst groups (p>0.05). Medication prevalence was greater (p<0.05) in both MetS groups compared with their Healthy peers, but was not different between the MetS groups. No group differences were detected on the MMSE, WASI-FSIQ or BDI-II (all p>0.05).
Table 1.
Selected group characteristics
| Healthy Controls (N=27) | Sedentary MetS (N=18) | Active MetS (N=21) | Test Statistic | P-value | ||
|---|---|---|---|---|---|---|
| Descriptive | Age, y | 50.3 ± 6.5 | 49.3 ± 6.3 | 49.5 ± 7.1 | F=0.126 | 0.882 |
| Sex, M/F | 14/13 | 11/9 | 9/12 | χ2=0.224 | 0.894 | |
| Education, year | 17 ± 2 | 16 ± 3 | 15 ± 2 | F=2.055 | 0.137 | |
| Height, cm | 170 ± 8 | 170 ± 10 | 172 ± 8 | F=0.168 | 0.846 | |
| Body Weight, kg | 68.0 ± 7.7† | 90.7 ± 18.9* | 94.9 ± 13.7* | F=28.055 | <0.001 | |
| BMI, kg/m2 | 23.5 ± 2.3† | 31.3 ± 6.0* | 32.4 ± 5.4* | F=27.136 | <0.001 | |
| Systolic BP, mmHg | 114 ± 7† | 124 ± 11* | 127 ± 15* | F=8.378 | 0.001 | |
| Diastolic BP, mmHg | 70 ± 6 | 71 ± 8 | 73 ± 12 | F=0.132 | 0.919 | |
| Total-C, mg/dL | 200 ± 34 | 193 ± 50 | 217 ± 44 | F=1.620 | 0.206 | |
| HDL-C, mg/dL | 62 ± 14† | 40 ± 13* | 45 ± 19* | F=12.477 | <0.001 | |
| LDL-C, mg/dL | 128 ± 36 | 121 ± 43 | 130 ± 40 | F=0.225 | 0.800 | |
| Triglyceride, mg/dL | 71 ± 27† | 150 ± 62* | 212 ± 109*† | F=23.177 | <0.001 | |
| Glucose, mg/dL | 86 ± 7† | 115 ± 44* | 114 ± 32* | F=7.272 | 0.001 | |
| Waist Circumference, cm | 83 ± 7† | 106 ± 8* | 108 ± 10* | F=65.155 | <0.001 | |
| Post-menopause, n (%) | 4 (15) | 5 (28) | 6 (29) | χ2=3.356 | 0.563 | |
| Health Behavior | MVPA, bouts/week | 4.6 ± 3.1† | 0.1 ± 0.2* | 4.6 ± 2.4† | F=23.246 | <0.001 |
| MVPA, hours/week | 1.7 ± 1.7† | 0.1 ± 0.3* | 1.3 ± 1.1† | F=8.662 | <0.001 | |
| Sit Time, hours/day | 7.5 ± 4.2 | 9.5 ± 4.7 | 7.2 ± 4.3 | F=1.541 | 0.222 | |
| Smoking, n (%) | 6 (9.1) | 2 (3.0) | 3 (4.5) | χ2=1.086 | 0.568 | |
| Medication | Anti-Hypertensive, n (%) | 0 (0.0) † | 6 (9.1)* | 8 (12.1)* | χ2=13.620 | 0.002 |
| Anti-Cholesterol, n (%) | 0 (0.0) | 6 (9.1)* | 7 (10.6)* | χ2=11.208 | 0.004 | |
| Insulin, n (%) | 0 (0.0) | 2 (3.0) | 2 (3.0) | χ2=2.991 | 0.224 | |
| Cognitive Status | MMSE, score | 29 ± 2 | 29 ± 1 | 28 ± 2 | F=0.673 | 0.514 |
| WASI FSIQ-2, score | 114 ± 13 | 116 ± 16 | 111 ± 14 | F=0.664 | 0.518 | |
| BDI-II total, score | 5.7 ± 4.8 | 8.9 ± 5.5 | 7.0 ± 5.2 | F=2.214 | 0.118 | |
| Executive function, z score | −0.6 ± 3.3 | −0.3 ± 1.1 | 0.2 ± 5.3 | F=0.297 | 0.744 | |
| Processing speed, z score | 0.2 ± 0.5† | −0.3 ± 0.5* | 0.1 ± 0.7† | F=4.033 | 0.022 | |
| Memory, z score | 0.3 ± 0.8 | −0.0 ± 0.9 | −0.3 ± 0.7 | F=2.887 | 0.063 |
Data are means ± SD. MetS=metabolic syndrome, BMI=body mass index, BP=blood pressure, C=cholesterol, MVPA=moderate to vigorous physical activity, MMSE=mini mental status exam, WASI FSIQ=Weschler abbreviated scale of intelligence full scale intelligence quotient, BDI=Beck depression inventory
df1=2, df2=63
p<0.05 vs. Healthy Controls
p<0.05 vs. Sedentary MetS
Arterial Stiffness
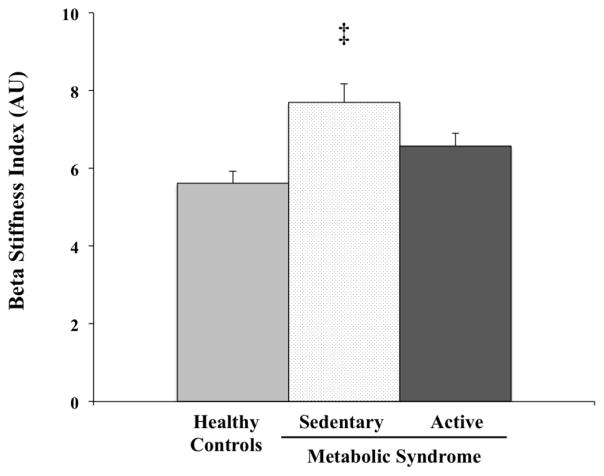
As shown in Figure 2, β-stiffness index was significantly lower in the Active MetS group compared to the Sed MetS group and was not different (p>0.05) from Healthy controls (6.6 au ± 1.5 vs. 7.7 au ± 2.1 vs. 5.6 au ± 1.6, F(2, 63)=8.067, p=0.001). The coefficient of variation of the whole sample for was β-stiffness index was 0.29.
Figure 2.
β-stiffness index shown in Healthy Controls and Sedentary and Active individuals with MetS. Data are shown as means ± SEM. ‡Indicates significantly different from Healthy Controls and Active MetS.
White-Matter Integrity
Differences in group mean ROI CWM integrity from the ANCOVA that included age, sex, years of education and a categorical grouping variable are enumerated in Table 2. No group differences in anterior limb of the internal capsule (ALIC), CC genu or body, cingulum (hippocampal), or superior corona radiata (SCR) FA were observed (all p>0.05). CC splenium FA was significantly greater in Healthy controls compared to Sed MetS (0.66 au ± 0.03 vs. 0.64 au ± 0.02 vs. 0.67 au ± 0.03, F(2, 60)=4.952, p=0.010) but not different from Active MetS. Uncinate FA followed the same pattern (0.46 au ± 0.04 vs. 0.44 au ± 0.03 vs. 0.47 au ± 0.03, F(2, 60)=4.889, p=0.011).
Table 2.
Mean DTI white matter integrity coefficients in regions-of-interest with ANCOVA
| Measure | Region | Covariate | Healthy Controls (N=27) | Sedentary MetS (N=18) | Active MetS (N=21) | Test Statistic | df1 | df2 | P-value |
|---|---|---|---|---|---|---|---|---|---|
| FA (AU) | CC Splenium | Corrected Model | 0.66 ± 0.03 | 0.64 ± 0.02‡ | 0.67 ± 0.03 | F=2.663 | 5 | 60 | 0.031 |
| Age | F=3.366 | 1 | 0.072 | ||||||
| Sex | F=0.011 | 1 | 0.916 | ||||||
| Education | F=0.021 | 1 | 0.886 | ||||||
| Group | F=4.952 | 2 | 0.010* | ||||||
| Uncinate | Corrected Model | 0.46 ± 0.04 | 0.44 ± 0.03‡ | 0.47 ± 0.03 | F=4.676 | 5 | 60 | 0.006* | |
| Age | F=3.565 | 1 | 0.064 | ||||||
| Sex | F=4.870 | 1 | 0.031 | ||||||
| Education | F=0.111 | 1 | 0.740 | ||||||
| Group | F=4.889 | 2 | 0.011* | ||||||
| MD (mm2/s) | ALIC | Corrected Model | 0.76 ± 0.05 | 0.80 ± 0.04‡ | 0.76 ± 0.05 | F=5.432 | 5 | 60 | <0.001* |
| Age | F=12.373 | 1 | 0.001 | ||||||
| Sex | F=0.557 | 1 | 0.458 | ||||||
| Education | F=0.204 | 1 | 0.653 | ||||||
| Group | F=7.433 | 2 | 0.001* | ||||||
| SCR | Corrected Model | 0.77 ± 0.03 | 0.80 ± 0.05‡ | 0.76 ± 0.04 | F=2.930 | 5 | 60 | 0.020* | |
| Age | F=4.566 | 1 | 0.370 | ||||||
| Sex | F=2.810 | 1 | 0.598 | ||||||
| Education | F=0.510 | 1 | 0.822 | ||||||
| Group | F=5.039 | 2 | 0.009* | ||||||
| Uncinate | Corrected Model | 0.77 ± 0.04 | 0.81 ± 0.03‡ | 0.77 ± 0.04 | F =3.918 | 5 | 60 | 0.004* | |
| Age | F=3.134 | 1 | 0.082 | ||||||
| Sex | F=1.214 | 1 | 0.275 | ||||||
| Education | F=0.013 | 1 | 0.911 | ||||||
| Group | F=7.795 | 2 | 0.001* |
Data are estimated marginal means ± S.E.
MetS=metabolic syndrome, FA=fractional anisotropy, MD=mean diffusivity, AU=arbitrary unit, ALIC=anterior limb of internal capsule, CC=corpus callosum, SCR=superior corona radiata
Significant F-test, p<0.02
p<0.05 vs. Healthy Controls and Active MetS
In relation to MD, no group differences were detected in the CC genu, body, or splenium, cingulum, and cingulum (hippocampal) (all p>0.05). Remaining ROIs including the ALIC (0.76 mm2/s ± 0.05 vs. 0.80 mm2/s ± 0.04 vs. 0.76 mm2/s u ± 0.05, p=0.011), SCR (0.77 mm2/s ± 0.03 vs. 0.80 mm2/s ± 0.05 vs. 0.76 mm2/s ± 0.04, p=0.009), and uncinate (0.77 mm2/s ± 0.04 vs. 0.81 mm2/s ± 0.03 vs. 0.77 mm2/s ± 0.04, p=0.001) all showed the same pattern of significantly lower MD in both Active MetS and Healthy controls compared to Sed MetS (all p<0.05). Active MetS and Healthy controls were not significantly different in these ROIs (all p>0.05).
Cognitive Function
Group differences in cognitive performance on cognitive function assessments are displayed in Table 1. No differences were observed between groups on executive function or memory performance across groups (all p>0.05). Processing speed was greater in Active MetS compared with Sed MetS group and not different from Healthy controls (0.1 au ± 0.6 vs. −0.3 au ± 0.5, 0.2 au ± 0.5, F(2,63)=4.033, p=0.022).
Associations
Frequency of moderate to vigorous PA was negatively associated with the β-stiffness index, r(65)=−0.255, p=0.041. The β-stiffness index was unrelated to DTI metrics in our ROIs in either bivariate or partial correlations (all p>0.02). Moderate to vigorous PA frequency was associated with DTI metrics of multiple ROIs as shown in Table 3. In partial correlations, moderate to vigorous PA was associated with ALIC, r(60)=−0.359, p=0.004, CC genu, r(60)=−0.344, p=0.006, cingulum, r(60)=−0.321, p=0.011, and uncinate, r(60)=−0.337, p=0.007, MD.
Table 3.
Bivariate and partial correlations of moderate to vigorous physical activity frequency and ROI DTI metrics
| ALIC | CC Genu | CC Body | CC Splenium | Cingulum | Cingulum (hippocampal) | SCR | Uncinate | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | MVPA | FA | 0.214 | 0.248 | 0.212 | 0.287 | 0.206 | 0.224 | 0.013 | 0.288* |
| MD | −0.385* | −0.364* | −0.309* | −0.255 | −0.335* | −0.218 | −0.316* | −0.353* | ||
|
| ||||||||||
| Model 2 | MVPA | FA | 0.195 | 0.219 | 0.188 | 0.265 | 0.186 | 0.221 | 0.002 | 0.272 |
| MD | −0.359* | −0.344* | −0.291 | −0.234 | −0.321* | −0.192 | −0.291 | −0.337* | ||
MVPA=moderate to vigorous physical activity; ALIC=anterior limb of internal capsule; CC=corpus callosum; SCR=superior corona radiata
Model 1: No covariates, df1=1, df2=64
Model 2: Covariates = age, sex, years of education, df1=4, df=60
p<0.02
Discussion
The aim of the present study was to determine the potential role of PA on mitigating the adverse influence of MetS on arterial stiffness, CWM integrity, and cognition in middle-aged adults. The principle findings from the present study are as follows. First, Sed MetS individuals exhibited increased arterial stiffening and diminished CWM integrity compared with healthy controls, indicating MetS-associated increases in arterial stiffness and CWM integrity vulnerability. Second, individuals with MetS who performed greater frequency of moderate to vigorous PA had arterial stiffness and CWM integrity comparable with healthy controls. Third, processing speed was diminished in Sed MetS individuals compared with Active MetS individuals and Healthy Controls.
β-stiffness values within this sample are similar to those reported previously, and fall below that of clinically alarming values (O’Rourke et al. 2002). Interestingly, the difference in β-stiffness between Active MetS group and Sed MetS is similar to the reduction observed in overweight middle-aged adults following a 12 week aerobic exercise intervention (Miyaki et al. 2009). Findings from the current cross-sectional study indicate arterial stiffness and DTI differences between sedentary and active individuals with MetS, with both stiffness and ROI MD being related to frequency of moderate to vigorous PA when analyzed continuously.
It is important to distinguish PA from exercise. PA is a behavior defined by skeletal muscle movement resulting in energy expenditure, while exercise is a subset of PA that is planned with the objective of improving or maintaining physical fitness. Physical fitness, on the other hand, is specified as a set of attributes that people achieve, such as aerobic capacity often described by maximal oxygen uptake or other physical fitness tests (Caspersen et al. 1985). In an epidemiological investigation, performing any duration of PA at midlife was protective of executive function, processing speed, and memory and reduced the odds of acquiring dementia compared with being entirely sedentary (Chang et al. 2010). Longitudinally, higher baseline physical fitness had a protective effect on executive function and verbal memory after a six-year follow-up.
Investigations relating PA to CWM integrity are relatively few with a greater body of existing literature focusing on exercise and cardiorespiratory fitness. A prior investigation reported PA was not related to a reduced rate of white matter lesion progression in a sample of elderly individuals with a spectrum of cognitive impairment (Podewils et al. 2007). In relation to CWM integrity, a comparison of individuals who were physically active demonstrated protection to CWM integrity from carrying the apolipoprotein-E epsilon 4 (APOE4) gene, a genetic marker of significantly heightened Alzheimer’s disease risk, compared to those who engage in low PA with the APOE4 allele (J. C. Smith et al. 2016). In a similar construct, our results are the first to directly relate PA frequency with CWM integrity in individuals with MetS.
In relation to exercise, a small but meticulous study compared CWM integrity between Masters athletes and sedentary but otherwise healthy peers (Tseng et al. 2013). The Masters athletes benefited from regionally greater FA in the right superior corona radiata, bilateral longitudinal fasciculus, bilateral inferior fronto-occipital fasciculus along with lower left posterior thalamic radiation and left cingulum hippocampus MD. Further, physical fitness was positively associated with FA in the Masters athletes and their healthy matched peers. These findings of cardiorespiratory fitness being beneficial for CWM integrity are supported by other investigations showing significant associations between fitness measures and corpus callosum FA in healthy seniors (Johnson et al. 2012).
Intervention studies are necessary to establish dose dependent relationships of PA, exercise, and cardiorespiratory fitness with arterial stiffness, CWM integrity and cognition. Past exercise interventions administered to previously sedentary middle-aged and older populations improved arterial stiffness and CWM integrity. For example, a 3-month aerobic exercise intervention consisting of primarily walking in a sedentary population similarly aged (53 ± 2 years) to the present study restored arterial compliance to similar levels of middle-aged peers and older endurance-trained men (Tanaka et al. 2000). Similarly, a one-year exercise intervention study in older adults compared the effects of walking and stretching on CWM integrity showing regional FA improvements most pronounced in anterior regions specific to the walking intervention (Voss et al. 2013). The present study was able to combine these two observations cross-sectionally in a clinically important subject population of MetS individuals.
A unique aspect of the current investigation is the use of a middle-aged MetS population that is particularly vulnerable to arterial stiffening, diminished CWM health, and cognition. We demonstrated that individuals with MetS even at midlife could perform relatively short bouts (at least 15 minutes) of moderate to vigorous PA to protect against arterial stiffening and negative CWM changes. The modesty of the mean frequency and duration of PA performed by Active MetS group is encouraging. PA that improves aerobic exercise is an attractive intervention because it is easily accessible, cost friendly, and generally safe under the guidance of a medical practitioner. Aerobic exercise can be received either through a structured program or the adoption of a lifestyle modification as being physically active as in the present study. Although we did not intervene in our subject’s lifestyle, individuals in the Active MetS group appeared to buffer the effects of MetS related arterial stiffening and CWM health. Further, there are no existing pharmacological interventions specifically tailored for arterial stiffness or CWM integrity and anti-hypertensives have little effect.
The exact mechanisms by which PA enriches CWM integrity remain elusive and are likely complex. As mentioned previously, PA can improve aerobic fitness, which positively correlates with increased CWM integrity (Voss et al. 2013; Johnson et al. 2012). Another probable factor is improving arterial stiffness associated with aging and cardiovascular disease. A fundamental function of large vessels including the aorta and carotid artery is to buffer hemodynamic pulsatility and transfer smooth continuous blood flow from the heart to the peripheral vasculature and ultimately end organs (Nichols et al. 2011). Dysregulation of the Windkessel effect results in exposure of small vessels to pulsatility that enhances arterial stiffening through the loss of elastin and inflict damage to end organs such as the brain (Mitchell 2008). If this pulsatility is transferred to cerebral microvessels incapable of accommodating mechanical stress, atherogenic and inflammatory responses that impair microvascular reactivity may result (Hughes et al. 2015). Such changes can reduce cerebral blood flow to white and gray matter in the brain thereby limiting essential nutrients and prompt intermittent ischemic-like conditions (Tarumi et al. 2011). This phenomenon of reduced cerebral perfusion related to impaired memory has been observed in individuals with MetS (Birdsill et al. 2013). Further, we recently demonstrated that arterial stiffening mediates lower cerebrovascular conductance related to MetS (E.P. Pasha et al. 2017).
Contrary to our hypothesis, despite observing differences in arterial stiffness and CWM integrity, corresponding differences in cognition were not observed in either executive function or memory performance. However, processing speed was poorest in the Sedentary MetS group who also presented with significantly great arterial stiffness. One recent investigation showed the carotid β-stiffness index to be associated with reduced performance on a processing speed task but not memory or CWM integrity (DuBose et al. 2017). Determinants of cognition are multifactorial and are not solely reliant on vascular function and CWM integrity. Thus, other contributors not measured in this investigation (e.g. cortical thickness or social influences) may have enabled the Sedentary MetS group to preserve executive function and memory to a level similar to that of their age-matched peers. Alternatively, considering our middle-aged cohorts, the differences identified in arterial stiffness and CWM integrity may disrupt processing speed earlier than executive function or memory.
The present study benefited from the distinctive and well-characterized subject population. Middle-aged adults without cardiovascular risk factors are increasingly sparse. Additionally, the MetS groups were statistically similar in physiological characteristics outside of the independent variable of PA. Methodologically, the assessment of arterial stiffness via ultrasound and CWM integrity via DTI are reliable and robust (Laurent et al. 2006; Alexander et al. 2007). DTI is particularly suitable for the present study because of its increased sensitivity to CWM changes compared with other conventional structural MRI techniques (O’Sullivan et al. 2001). This characteristic is critical as changes to the CWM at midlife are likely small but could be indicative of early neuropathology.
There are several study limitations that must be addressed. Inherent to any cross-sectional investigation is the inability to make causal inferences. Here, we observed similar arterial stiffness and CWM integrity in active individuals with MetS and healthy controls. While conceivable, we cannot specifically assert that PA was solely responsible for these findings, as external factors such as genetics and others, may have influenced the differences of arterial stiffness and CWM integrity (Persson et al. 2006), such as age, sex, education, smoking, sedentary behavior, and ethnicity. Nonetheless, the homogeneity of these characteristics within our sample likely limits potential biases on cognitive function tests. Second, characterization of PA from a self-reported questionnaire is vulnerable to reporting bias and retest variability. Ideally, activity monitors would be given to participants to more accurately distinguish PA behavior. However, dichotomizing moderate to vigorous PA frequency within our sample resulted in largely disparate PA between MetS groups. An additional confound is duration of MetS. Duration of exposure to MetS, which was not recorded, may have differed between Active and Sed MetS groups. Lastly, type-1 error is possible in this investigation due to multiple comparisons. To combat this, we employed the modified Bonferronni post-hoc test for intercorrelated outcomes reducing the alpha to α <0.02 from the conventional α <0.05. Additionally, it was deemed of clinical value to identify CWM regions potentially vulnerable to the effects of MetS.
Conclusions
Our findings provide novel evidence that moderate to vigorous PA is associated with more favorable vascular and CWM integrity outcomes in middle-aged adults with MetS. These results reinforce the implication that arterial stiffening could be a mechanistic contributor to reduced cerebral CWM integrity and cognitive dysfunction in middle-aged individuals with cardiovascular risk factors and MetS vulnerable for future cognitive decline. Future studies should implement longitudinal PA interventions to better characterize the mechanisms behind the therapeutic benefits of PA on arterial stiffness, CWM integrity, and cognition in MetS individuals.
Acknowledgments
Funding: This work was made possible by funding provided by the National Institute of Neurological Disorders and Stroke (R01 NS075565; to A.P.H.) and the National Science Foundation (GRFP; to A.B.).
Footnotes
Compliance with ethical standards: All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest: The authors declare no conflict of interest.
References
- Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain. 2010;133(Pt 2):529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension. 1995;26(1):48–54. doi: 10.1161/01.hyp.26.1.48. [DOI] [PubMed] [Google Scholar]
- Birdsill AC, Carlsson CM, Willette AA, Okonkwo OC, Johnson SC, Xu G, et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Obesity (Silver Spring) 2013;21(7):1313–1320. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boreham CA, Ferreira I, Twisk JW, Gallagher AM, Savage MJ, Murray LJ. Cardiorespiratory fitness, physical activity, and arterial stiffness: the Northern Ireland Young Hearts Project. [Research Support, Non-U.S. Gov’t] Hypertension. 2004;44(5):721–726. doi: 10.1161/01.HYP.0000144293.40699.9a. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, et al. The effect of midlife physical activity on cognitive function among older adults: AGES--Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2010;65(12):1369–1374. doi: 10.1093/gerona/glq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft JB, Keenan NL, Sheridan DP, Wheeler FC, Speers MA. Waist-to-hip ratio in a biracial population: measurement, implications, and cautions for using guidelines to define high risk for cardiovascular disease. J Am Diet Assoc. 1995;95(1):60–64. doi: 10.1016/S0002-8223(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Gardener H, Denaro F, Boden-Albala B, Elkind MS, Paik MC, et al. Metabolic syndrome increases carotid artery stiffness: the Northern Manhattan Study. Int J Stroke. 2010;5(3):138–144. doi: 10.1111/j.1747-4949.2010.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose LE, Voss MW, Weng TB, Kent JD, Dubishar KM, Lane-Cordova AD, et al. Carotid beta-stiffness Index is Associated with Slower Processing Speed but not Working Memory or White Matter Integrity in Healthy Middle-Aged/Older Adults. J Appl Physiol (1985) 2017 doi: 10.1152/japplphysiol.00769.2016. jap 00769 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. [Research Support, Non-U.S. Gov’t] Stroke. 2005;36(10):2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. [Congresses] Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14(4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hughes TM, Craft S, Lopez OL. Review of ‘the potential role of arterial stiffness in the pathogenesis of Alzheimer’s disease’. Neurodegener Dis Manag. 2015;5(2):121–135. doi: 10.2217/nmt.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, et al. Effects of Arterial Stiffness on Brain Integrity in Young Adults From the Framingham Heart Study. Stroke. 2016;47(4):1030–1036. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks BL, Madden DJ, Bucur B, Provenzale JM, White LE, Cabeza R, et al. Role of aerobic fitness and aging on cerebral white matter integrity. [Comparative Study. Ann N Y Acad Sci. 2007;1097:171–174. doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 2008;105(5):1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, et al. Effect of habitual aerobic exercise on body weight and arterial function in overweight and obese men. Am J Cardiol. 2009;104(6):823–828. doi: 10.1016/j.amjcard.2009.04.057. [DOI] [PubMed] [Google Scholar]
- Nichols WW, Nichols WW, McDonald DA. McDonald’s blood flow in arteries: theoretic, experimental, and clinical principles. 6. London: Hodder Arnold; 2011. [Google Scholar]
- O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15(5):426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57(12):2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
- Pasha EP, Birdsill A, Parker P, Elmenshawy A, Tanaka H, Haley AP. Visceral adiposity predicts subclinical white matter hyperintensities in middle-aged adults. Obes Res Clin Pract. 2017;11(2):177–187. doi: 10.1016/j.orcp.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Pasha EP, Birdsill AC, Oleson S, Haley AP, Tanaka H. Impacts of Metabolic Syndrome Scores on Cerebrovascular Conductance Are Mediated by Arterial Stiffening. Am J Hypertens. 2017:hpx132. doi: 10.1093/ajh/hpx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, et al. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66(7):1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Beauchamp N, Lyketsos CG, Kuller LH, Scheltens P. Physical activity and white matter lesion progression: assessment using MRI. [Research Support, N.I.H., Extramural] Neurology. 2007;68(15):1223–1226. doi: 10.1212/01.wnl.0000259063.50219.3e. [DOI] [PubMed] [Google Scholar]
- Potenza MV, Mechanick JI. The metabolic syndrome: definition, global impact, and pathophysiology. [Review] Nutr Clin Pract. 2009;24(5):560–577. doi: 10.1177/0884533609342436. [DOI] [PubMed] [Google Scholar]
- Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, Tzourio C, et al. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City Study. [Research Support, Non-U.S. Gov’t] Diabetes Care. 2009;32(1):169–174. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16(22):2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Smith JC, Lancaster MA, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, et al. Interactive effects of physical activity and APOE-epsilon4 on white matter tract diffusivity in healthy elders. Neuroimage. 2016;131:102–112. doi: 10.1016/j.neuroimage.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, Tanaka H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr Metab Cardiovasc Dis. 2015;25(5):495–502. doi: 10.1016/j.numecd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102(11):1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Tarumi T, de Jong DL, Zhu DC, Tseng BY, Liu J, Hill C, et al. Central artery stiffness, baroreflex sensitivity, and brain white matter neuronal fiber integrity in older adults. Neuroimage. 2015;110:162–170. doi: 10.1016/j.neuroimage.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens. 2011;24(10):1108–1113. doi: 10.1038/ajh.2011.101. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. [Review] Med Sci Sports Exerc. 2001;33(6 Suppl):S438–445. doi: 10.1097/00005768-200106001-00012. discussion S452-433. [DOI] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, et al. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. [Research Support, N.I.H., Extramural] Hum Brain Mapp. 2013;34(11):2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32(9):2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]