Abstract
Background
The hazardous effects of alcohol consumption on both hippocampus and memory have been well established. However, the longitudinal effects of ethanol on the developing brain and related consequences on memory are not well explored. Given the above, we investigated the longitudinal effects of college drinking on hippocampal volume in emerging college adults.
Methods
Data were derived from the longitudinal Brain and Alcohol Research in College Students (BARCS). A subset of 146 freshman (mean agebaseline=18.5 yrs) underwent brain MRI scans at baseline and 24 months later. Four drinking related measures derived from a monthly survey were reduced to a single alcohol use index (AUI) using principal component analysis. Gray matter volumetric change (GMV-c) data were derived using a longitudinal pipeline. Voxel-wise hippocampal/para-hippocampal GMV-c associations with the drinking index were derived using a multiple regression framework within SPM12. Supplementary associations were assessed between GMV-c and memory scores computed from the California Verbal Learning Test (CVLT-II; assessed at the end of the study), and between GMV-c and total alcohol induced memory blackouts.
Results
Larger AUI was associated with an accelerated GMV decline in the hippocampus/para-hippocampus. Also larger hippocampal volume decline was associated with poorer memory performance and more memory blackouts.
Conclusion
Our study extends prior cross-sectional literature by showing that heavier drinking burden while in college are associated with greater hippocampal GMV decline that is in turn associated with poorer memory scores, all of which could ultimately have a significant impact on student success.
Keywords: substance use, college, binge, hippocampus, morphometry, cortical
Introduction
Heavy alcohol use is a significant public health concern, especially among emerging adults ages 18-25 who show the highest rates of alcohol use, binge drinking, and alcohol dependence (1). This age range corresponds to the time most commonly spent in college, where heavy drinking negatively impacts academic achievement (2). Further research shows that college students drink more alcohol than non-college individuals of similar age during a period when lifetime years of alcohol consumption peak (3).
The hippocampus and surrounding medial temporal lobe structures play a key role in learning and memory formation (4, 5). Animal models support the hypothesis that alcohol impairs memory formation, in part by disrupting hippocampal activity and neurogenesis (6). Rodent studies have shown that adolescent alcohol exposure could increase the relative proportion of immature, more excitatory synapses in hippocampus, thus contributing to effects such as memory blackouts, excito-toxicity and other alcohol induced memory deficits (7). The hippocampus also exhibits enhanced NMDA-mediated neurotoxicity during alcohol withdrawal, leading to excessive neuroexcitation and ultimately to neuronal damage (8). However, in humans, the impact of alcohol on the hippocampus has yielded conflicting evidence. Smaller hippocampi have been observed in adolescent heavy drinkers (9, 10), with greater volume deficits being associated with earlier alcohol use (10). In contrast, other studies have not found decreased hippocampal volume in adolescent drinkers (11-14).
Prior research consistently identifies memory dysfunction in adolescent and young adult heavy drinkers in addition to older adults with alcohol use disorders (15, 16). Both human and animal studies suggest that adolescents and young adults may be more adversely affected due to the critical neuromaturation that occurs during these years (17, 18). Animal studies indicate that the adolescent brain is especially sensitive to alcohol. Acute exposure disrupts long-term hippocampal potentiation in adolescent rodents at doses that have little/no effect in adults (19). Alcohol impairs spatial memory acquisition in adolescent rats (20-22) and humans (23). Shorter alcohol exposure periods cause longer-term electrophysiological effects in the adolescent versus adult hippocampus (7). Also, alcohol-induced memory blackouts are quite common among college-attending individuals, with nearly half of the drinking students reporting at least one lifetime blackout (24). Blackouts may also be related to the negative effects of alcohol on the hippocampus (6). Research suggests that alcohol use can interfere with memory formation by altering nerve-cell communications or steroid formation especially in the hippocampus (25, 26). However such memory blackouts do not always necessarily correspond precisely with excessive alcohol intake or binge drinking, suggesting differential sensitivity to alcohol effects (27).
Although longitudinal studies have been scarce, a study by Squeglia et. al. reported no changes in hippocampal volume after alcohol initiation in adolescence (28). In contrast, a more recent pseudo-longitudinal study (over 30 years) in adults that only captured imaging data at the end of study, reported significant hippocampal atrophy even at moderate levels of alcohol consumption (29). We recently reported that college students with sustained heavy alcohol use demonstrated accelerated volumetric decline in several brain regions encompassing inferior/medial frontal gyrus, parahippocampus, and anterior cingulate (30). This study was performed in a similar BARCS sample (N=129), but only included students who had sustained patterns of light or heavy drinking at baseline/follow-up and used more traditional ways to classify subjects into groups. The above study therefore excluded many subjects that did not classify into one of these two groups. Also, more importantly our previous study dichotomized subjects according to various drinking criteria and thus did not capture direct dose-dependent relationships with alcohol use. In contrast, our current study examined alcohol use as a continuous measure and assessed relationships across all scanned subjects that passed quality control and primarily focused on the hippocampal/para-hippocampal complex.
As mentioned above, the goal of the current longitudinal magnetic resonance imaging (MRI) study was to ascertain alcohol dose-dependent relationships with hippocampal volume change in college students over a span of two years. Our primary prediction was that heavier alcohol intake would be associated with greater hippocampal volumetric decline over the span of two years. We also hypothesized that alcohol related hippocampal changes would be associated with poorer long-term memory performance. Additionally we also predicted that a larger burden of memory blackouts an individual experienced over the two year period would be related to a greater rate of hippocampal volume changes during the same period.
Methods and Materials
Participants
A convenience sample of first-year students (age range 18-23 years) was recruited from two local colleges through email, flyers and classroom visits to solicit participation in the Brain and Alcohol Research in College Students (BARCS) study (2, 30, 31). Recruitment captured greater than 95% of eligible participants. A representative sub-sample of 200 individuals, all free from MRI contraindications, underwent a neuroimaging battery including structural imaging scans at baseline and follow up (24 months apart on average). The final sample (after quality control) included 146 participants. Participants were excluded if: a) s/he was not scanned at one of the two time points, b) scans did not pass quality control at one or both time points (for. excessive motion, bad scan quality, other artifacts), or c) s/he did not provide sufficient information on alcohol use. Additional exclusion criteria included current schizophrenia or bipolar disorder, history of seizures or significant head injury, - alcohol breathalyzer and pregnancy (females). Participants were tested for illicit substance use including marijuana prior to scanning but were not excluded from scanning if they tested positive. Instead this information was recorded and subsequently used to include/exclude subjects from various analyses. For the current analysis we found that only 1 subject had tested positive for marijuana use during scan (consumed 24hrs prior per self-report). Given this information and the nature of our analysis (morphometric as opposed to functional) we decided to include the subject in our analysis. Sample demographics and clinical characteristics are presented in Table 1. All subjects provided written informed consent. The study was conducted in accordance with the declaration of Helsinki and approved by institutional review boards at Central Connecticut State University (CCSU), University of Connecticut, Trinity College, Hartford Hospital and Yale University.
Table 1.
Demographics and clinical characteristics of the subject population.
| Demographics/Clinical Characteristics | Mean | SD | 25th Percentile | 75th Percentile |
|---|---|---|---|---|
| Age at Baseline (years) | 18.41 | 0.63 | 18 | 19 |
| Age of first drink (years) | 15.73 | 1.76 | 15 | 17 |
| Average time between imaging scans (months) | 24.75 | 4.70 | 21.6 | 28.5 |
| Number of days alcohol was consumed in past 30 days (average across 24 mo) | 3.53 | 3.54 | 0.59 | 5.59 |
| Number of binge episodes during the past 30 days (average across 24 mo) | 2.08 | 2.62 | 0.05 | 3.6 |
| Number of Drinks Consumed during each occasion (average across 24 mo) | 3.05 | 2.83 | 0.47 | 5.23 |
| Maximum number of drinks at a single sitting (average across 24 mo) | 4.37 | 4.02 | 0.86 | 6.83 |
| Average MJ Use (Baseline and Follow up; N=143) | 2.68 | 5.14 | 0 | 3.34 |
| Average memory blackouts during past 6 months (Baseline and Follow up) | 2.25 | 4.56 | 0 | 2 |
| CVLT Total Recall at Follow up (N=140) | 53.71 | 8.92 | 49 | 59 |
| STAI at Baseline (N=134) | 41.72 | 10.58 | 34 | 47.75 |
| BDI at Baseline (N=135) | 4.04 | 5.33 | 1 | 6 |
| N | % | |||
| Sex | ||||
| Male | 61 | 41.80 | ||
| Female | 85 | 58.20 | ||
| Smoking Status | ||||
| Non-Smoker | 130 | 89.00 | ||
| Smoker | 5 | 3.40 | ||
| Missing | 11 | 7.50 | ||
| ADHD Status | ||||
| No | 124 | 84.90 | ||
| Yes | 6 | 4.10 | ||
| Missing | 16 | 11.00 | ||
| Family History of Alcoholism | ||||
| No | 95 | 65.10 | ||
| Yes | 40 | 27.40 | ||
| Missing | 11 | 7.50 |
Longitudinal Variables
Alcohol use survey
Several different measures of alcohol use were recorded for each subject on monthly substance use questionnaires: a) number of days an individual consumed alcohol in the past 30 days (A30); b) number of binge episodes (4 or more drinks for females, 5 or more drinks for males) each individual had during the past 30 days (B30); c) number of drinks he/she had on each drinking occasion (DrinkHave); and d) maximum number of drinks in a 24-hour period at any time in his/her life (DrinkMax). Subjects were required to have responded to 4+ of the monthly surveys to be included in analyses. The above measures were averaged across the two year period. Roughly 77% of the subjects in the study had a minimum of 12 months of self-report data spread out through the two year period. Please see supplementary table 1 for a detailed summary of self-report data available across subjects.
Marijuana use
Subjects reported monthly marijuana (MJ) use on a 1 to 6 scale. 1 was defined as having never used marijuana in the past 30 days, 2 - having used 1-2 times, 3 - having used 3-5 times, 4 – having used 6-9 times, 5 -having used 10-19 times and 6 having used 20+ times in the past month. For the current analysis we computed midpoints of the above data to transform it into a continuous scale (for e.g. if subject reported a scale of 6 then the value was converted to 14.5, which is the midpoint of 10 and 19). The above computed MJ monthly use was averaged across the 2-year period (only subjects with N >= 4 months of available data were included) and used for further analysis (2).
Total Memory blackouts
Subjects were asked at baseline and 2-year follow-up about the number of alcohol induced memory blackouts experienced in the prior 6 months using an in-house interview based on the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA, (32). Data from the two time points were averaged and used for further analysis. Specifically, the subjects were asked the following question: “Have you ever had blackouts when you didn’t pass out while drinking, that is, you drank enough so that the next day you couldn’t remember things you had said or done?”. If the response was ‘yes’ then it was followed by the question: “How many blackouts have you had from drinking in the past 6 months?”
Baseline Variables (Confounders)
Age of first drink
At baseline, individuals were asked the question: how old were you when you had your first alcoholic drink? This response was recorded as their age of first drink.
Lifetime Drinks
At baseline, individuals were asked approximately how many drinks they have had during their lifetime.
Tobacco (Cigarette) Smoking
Individual responses from item 4 from the Fagerstrom (How many cigarettes do you smoke per day) was used to identify cigarette-smokers and non-smokers, converted into a binary yes/no format and employed for further analyses (33).
Family History for Alcoholism (FHA)
FHA was assessed using the Family History Assessment Module (FHAM) (34) based on which subjects were classified as family history positive (FHP) or negative (FHN) for alcoholism.
State Trait Anxiety Index (STAI)
Students completed the trait section of the STAI questionnaire (35). A total STAI sum score was calculated, gender-normed and used for further analysis.
Beck Depression Inventory (BDI)
Students were administered the BDI to assess depressive symptoms at study entry (36). All inventory items were summed to yield a total score.
ADHD
Students were administered a select section of the Barkley Adult ADHD Rating Scale – IV (BAARS-IV) to assess ADHD symptoms (37). Students were asked to self-report whether they had ever been formally diagnosed with ADHD and whether they were currently receiving treatment for the same. We coded individuals as having a history of ADHD if they responded ‘yes’ to both questions.
End of study Variable(s)
Memory
Memory status was determined by listing a total recall over the five learning trials of the California Verbal Learning Test Edition 2 (CVLT-II, (38) administered at the two-year follow-up. Total recall is a robust and discriminating measure of overall memory capacity (4)
Principal Component Analysis (PCA) of Alcohol Use Data
The four alcohol use indicators were highly inter-correlated (see supplementary table 2). As a means of data reduction we computed a synthetic drinking variable termed alcohol use index (AUI), using principal component analysis (PCA) as implemented in SPSS v24 (https://www.ibm.com/analytics/us/en/technology/spss/). The above also circumvents the need for further multiple comparison correction that would be necessary if the alcohol use variables were kept separate. PCA was explicitly chosen as opposed to doing a factor analysis given that the underlying latent model was unknown. Based on the Kaiser Criterion components yielding an eigenvalue >1 were retained. Individual component coefficient scores were estimated using the Anderson-Rubin method (39), which ensured orthogonality and normality of the estimated component scores. The newly formed AUI was then used to predict voxel-wise associations with gray matter volume changes as explained further in the statistical analysis section.
Image Acquisition and Processing
Magnetic resonance structural brain images were collected on a Siemens Allegra 3T system (Siemens AG, Erlangen, Germany) located at the Olin Neuropsychiatric Research Center in Hartford, CT. Images were collected using a sagittal T1 MPRAGE sequence with the following parameters TR/TE/TI=2300/2.74/900msec, flip angle=8°, slab thickness=176mm, FOV=176×256mm, matrix=176×256×176, voxel size=1mm3, pixel band-width=190Hz, scan time=10:09.
Computation of Longitudinal Gray Matter Volume (GMV) Change
To compute gray matter volume change maps, structural images from each time point were subjected to symmetric diffeomorphic modeling of longitudinal data, implemented in SPM12 (40). The following steps were implemented to derive the volumetric rate of change maps for each subject: (a) an average image was estimated by optimally registering each time point image by means of a groupwise-consistent 3D non-linear image registration (diffeomorphic) technique with correction for intensity inhomogeneity. This was followed by producing two Jacobian determinant maps that encode for the relative difference in volume between the first/second scan and the average image. A Jacobian difference image was then computed by subtracting the above images to produce a relative volume change image between the two time points. This Jacobian difference map was further divided by the time elapsed between the two scans to derive a rate of change map. (b) the mid-point image was then segmented into its respective GM, WM and CSF constituents (c) The Jacobian rate of difference images from step (a) were multiplied by the GM probabilistic tissue segmented image from step (b) to derive a GMV rate of change map (GMV-c). (d) The images from step (c) were normalized to MNI space using the DARTEL method, respective normalization parameters were then applied to the GMV-c maps to bring them into DARTEL-MNI space. (e) these images were then smoothed with a 6mm FWHM Gaussian kernel and subjected to further statistical analyses as described below. All settings were left at default for the above registration methods.
Creation of Hippocampus and Para-hippocampus Masks
Bilateral binary masks for the hippocampus and the para-hippocampus were derived using the WFU Pickatlas toolbox (41). Masks were then modified in SPM12 to conform to the same dimensions of the above processed GMV-c maps.
Statistical Analyses
We performed a multiple linear regression within the GLM framework in SPM12 wherein the AUI was entered as a predictor variable to derive GMV-c associations on a voxel-by-voxel basis within the hippocampus/para-hippocampus mask. The analyses controlled for effects of age and sex and used a permutation based approach with N=1000 iterations to compute voxel-wise statistics. This was coupled with corrections for multiple comparisons using the threshold free cluster enhancement technique (TFCE) (42). The above permutation analyses and the multiple comparison correction were conducted using the TFCE toolbox (http://dbm.neuro.uni-jena.de/tfce/) implemented in Matlab R7.7. Resulting maps were thresholded at the p<0.05 family wise error (FWE) and displayed using the NeuroElf toolbox (http://neuroelf.net/).
For our supplementary analyses we extracted summary data (average) from any significant GMV-c clusters found in the above primary analysis using the REX toolbox (https://www.nitrc.org/projects/rex/) and then correlated (spearman rho) these values against CVLT-II total recall, average memory blackouts and average MJ use. To explore the effect of possible confounding variables we also performed a follow-up multiple regression analysis with AUI, age of initiation, lifetime drinks, FHA, smoking status, BDI, STAI and ADHD as predictors to look for associations with the above GMV-c clusters. All the above supplementary analyses were conducted in SPSS v24 and also adjusted for age and sex.
Results
Detailed sample descriptors are provided in Table 1.
Alcohol Use Index
All drinking measures were highly correlated across subjects as seen from supplementary table 2. A relatively high Kaiser-Meyer-Olkin measure of sampling adequacy (KMO=0.77) confirmed the validity of using a PCA for structure detection. Principal component analysis revealed one primary factor (eigenvalue = 3.57) accounting for approximately 94% of the total variance in the data. As mentioned previously, we termed this primary factor as the Alcohol Use Index (AUI). Loading indices for the individual drinking metrics on this primary AUI component were as follows A30=0.834, B30=0.91, DrinkHave=0.9, DrinkMax=0.92. The AUI variable also correlated significantly with the number of monthly drinks reported as part of the baseline (rho=0.82; p<0.001) and follow-up (rho=0.74; p<0.001) interview data.
GMV-c Associations
Two clusters within the hippocampal region were significantly correlated (negatively) with AUI after correction for multiple comparisons (p<0.05, FWE corrected; see figure 1 for a visual depiction of these clusters), thus suggesting an increase in alcohol use/exposure was associated with a greater rate of volumetric decline. Cluster 1 was centered on the L hippocampus/para-hippocampus and consisted of 227 voxels. Cluster 2 was centered on the R hippocampus and consisted of 157 voxels.
Figure 1.
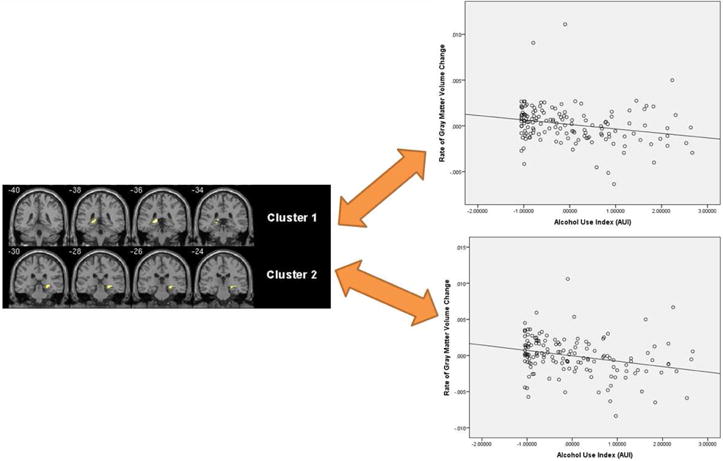
Regions where heavier AUI was associated with a greater rate of volumetric decline. Data is displayed at a p<0.05 TFCE corrected FWE threshold.
GMV-c in the R-Hippocampus correlated with CVLT-TR scores (spearman rho=0.31; p=0.001) and GMV-c from the L-Hippocampus correlated at trend level with CVLT-TR scores (spearman rho =0.166; p=0.07). See supplementary figure 1. GMV-c was also significantly correlated with memory blackouts (L Hippo: rho = −0.22; p = 0.008, R Hippo: rho = −0.27; p = 0.001), suggesting that more memory blackouts were associated with a larger rate of GMV decline in these regions. See supplementary figure 2. GMV-c in the above clusters did not however correlate with MJ use in a large sub-sample (N=121) of our subjects with available substance use data. Relationships between CVLT-TR and blackouts with baseline GMV (adjusted for TIV) were also non-significant.
Our secondary analyses also revealed that rate of GMV decline of the R-Hippocampus cluster was marginally greater in family history positive compared to negative individuals (F=5.67; p=0.02). We did not find any significant associations between GMV-c cluster data and other confounding variables that measured psychiatric status, mood, cigarette use and family history. See supplementary table 3 for complete details.
Discussion
The primary objective of the study was to derive associations between alcohol use (represented by a single metric AUI) and change in hippocampal/para-hippocampal volume in college students over a two-year period. Individuals with heavier drinking patterns had a larger rate hippo-parahippocampal volume decline. This higher rate of GMV decline in the hippocampus was associated with poorer memory, and with greater number of memory blackouts. To our knowledge this is the first report to study the effects of alcohol use on longitudinal hippocampal volume change in college students.
Neuromaturation and effects of alcohol
Adolescence and emerging adulthood are periods of heightened neuroplasticity during which the human brain is undergoing a multitude of changes, including extensive synaptic pruning, myelination and cortical/subcortical reorganization, some of which extend well into the third decade of life (43-45). The limbic system has been identified as more vulnerable to alcohol effects, in part because it undergoes substantial adolescent neurodevelopment (46). Extensive structural remodeling of the limbic system including hippocampus during this age range is part of normal neuromaturation (45). Our results suggest that alcohol consumption has a strong dose-related association with hippocampal volume loss over an extended period of time that might possibly be impacting this important maturation process. These results are consistent with a recent prospective longitudinal study that found accelerated cortical volume pruning in several brain structures among youths who initiated heavy drinking versus those who did not (47).
There could be several reasons for the observed hippocampal volume loss. One mechanism might be neuronal cell death mediated via NMDA receptors. Alcohol is known to inhibit NMDA receptors, which may result in a complex disinhibition of several excitatory pathways leading to a neuronal cell death in cortico-limbic structures including hippocampus (8, 48). Rodent studies demonstrate that intermittent alcohol exposure during adolescence causes long lasting functional and structural hippocampal changes that persist into adulthood (49). The fact that these changes are often accompanied by microglial proliferation (a primary response mechanism to injury) suggests that the adolescent brain might respond to alcohol exposure as injury (50). Our results add to findings from earlier studies that show smaller hippocampi associated with earlier age of initiation, and longer duration of alcohol use disorder, (48, 51). Although we are unable to determine whether there were pre-existing hippocampal differences related to the drinking behavior of our subjects, our results provide longitudinal evidence of a dose-dependent relationship between alcohol use and hippocampal volume decline in the late adolescent/early adult developing brain.
Alcohol, hippocampus and memory
Adolescents respond to alcohol differently from adults, as they are more sensitive to alcohol’s rewarding effects and to its effects on cognitive functions including memory (52). Our study provides converging evidence in that hippocampal/para-hippocampal regions exhibiting accelerated volume loss due to heavy alcohol exposure were additionally correlated both with the average number of memory blackouts individuals had experienced over the two-year period and a measure of memory recall (CVLT-TR) assessed at the end of study. Although we found that AUI and blackouts were strongly correlated, we did not find a significant relationship between alcohol exposure and the above ascertained memory function, likely due to the fact that our assessed metric of alcohol exposure was more chronic than acute, and that memory was not assessed longitudinally.
Effects of co-morbid MJ use and other confounding variables
Alcohol use is often co-morbid with other drug use on college campuses, especially marijuana (2), consistent with this we found alcohol and MJ use were significantly correlated in our sample. However, GMV change in the hippocampus was not associated with self-reported MJ use, a finding consistent with a recent report that suggests daily MJ use was not associated with hippocampal morphometry (among other brain regions) in adolescent and adult users (53). In contrast to the above however, cannabis use has been associated (albeit inconsistently) with other brain regions including the core reward network in young adult users (54). Also, age of first drink, number of lifetime drinks, tobacco use, anxiety, and depression were unrelated to changes in hippocampal volume in our study.
Implications
With alcohol abuse common on college campuses, our finding that heavy drinking affects the hippocampus could have significant implications for students. Declines in hippocampal volume could potentially influence college success and quality of life well into adulthood. This study, along with other reports from the BARCS sample revealing poorer academic performance, behavioral and neurobiological outcomes for individuals with sustained heavy use alcohol and/or MJ use (2, 30, 31, 55, 56), strongly suggest that moderation of alcohol use (and possibly other drugs) while in college is a key factor towards student success.
Strengths and Limitations
The longitudinal design of this study in which both alcohol use and hippocampal volumes in a developmentally vulnerable sample were tracked over time constitutes a unique strength. Our study nevertheless has limitations. We did not capture longitudinal behavioral data that might have helped us better understand the relationships between GMV-c and change in memory functioning. Although the alcohol measures were highly collinear in nature, the AUI might not be capturing the unique variance associated with each. There may be other important interacting variables, such as stress levels, peer pressure, personality factors, or other substance use, which were not examined here but would be critical areas for future inquiries. We did not scan participants before the onset of drinking, making it difficult to determine whether gray matter volume differences were pre-existing; future studies such as the ongoing large scale ABCD project will help delineate patterns of gray matter development in relation to varying patterns of substance use initiation and escalation.
Conclusion
Heavy drinking in college is associated with accelerated hippocampal/para-hippocampal volume decline. Such risky drinking patterns also seem to be associated with more memory blackouts (episodes of alcohol induced memory loss) and worse memory functioning likely mediated via hippocampal brain volume loss. Our study could therefore carry important implications for attempts to regulate college alcohol use behavior, which could have a profound impact on long- and short-term student success.
Supplementary Material
Acknowledgments
This research was made possible by grant support from the National Institute on Alcohol Abuse and Alcoholism (AA016599 and AA19036, Pearlson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.SAMHSA. NSDUH Series H-51. Rockville, MD: Center for Behavioral Health Statistics and Quality; 2016. Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. [Google Scholar]
- 2.Meda SA, Gueorguieva RV, Pittman B, Rosen RR, Aslanzadeh F, Tennen H, et al. Longitudinal influence of alcohol and marijuana use on academic performance in college students. PLoS One. 2017;12:e0172213. doi: 10.1371/journal.pone.0172213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slutske WS, Hunt-Carter EE, Nabors-Oberg RE, Sher KJ, Bucholz KK, Madden PA, et al. Do college students drink more than their non-college-attending peers? Evidence from a population-based longitudinal female twin study. J Abnorm Psychol. 2004;113:530–540. doi: 10.1037/0021-843X.113.4.530. [DOI] [PubMed] [Google Scholar]
- 4.Lezak MD, Howieson DB, Loring DW, Hannay J, Fisher JS. Neuropsychological Assessment Edition 4. 3rd. Oxford University Press; 2004. [Google Scholar]
- 5.Welsh MC, Pennington BF. Assessing frontal lobe functioning in children: views from developmental psychology. Dev Neuropsychology. 1988;4:199–230. [Google Scholar]
- 6.White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus. 2000;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Crews FT, Steck JC, Chandler LJ, Yu CJ, Day A. Ethanol, stroke, brain damage, and excitotoxicity. Pharmacol Biochem Behav. 1998;59:981–991. doi: 10.1016/s0091-3057(97)00538-8. [DOI] [PubMed] [Google Scholar]
- 8.Olney JW, Farber NB. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology. 1995;13:335–345. doi: 10.1016/0893-133X(95)00079-S. [DOI] [PubMed] [Google Scholar]
- 9.Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 11.Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche JP, Ferrett H, et al. Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Res. 2013;214:1–8. doi: 10.1016/j.pscychresns.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson S, Malone SM, Thomas KM, Iacono WG. Adolescent drinking and brain morphometry: A co-twin control analysis. Developmental cognitive neuroscience. 2015;16:130–138. doi: 10.1016/j.dcn.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malone SM, Luciana M, Wilson S, Sparks JC, Hunt RH, Thomas KM, et al. Adolescent drinking and motivated decision-making: a cotwin-control investigation with monozygotic twins. Behavior genetics. 2014;44:407–418. doi: 10.1007/s10519-014-9651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neurosci Biobehav Rev. 2016;70:244–259. doi: 10.1016/j.neubiorev.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology (Berl) 1994;115:340–349. doi: 10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- 17.Spear LP. Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neuroscience and biobehavioral reviews. 2016;70:228–243. doi: 10.1016/j.neubiorev.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- 20.Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- 21.Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- 22.Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- 23.Charness ME. Brain lesions in alcoholics. Alcohol Clin Exp Res. 1993;17:2–11. doi: 10.1111/j.1530-0277.1993.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 24.White AM, Jamieson-Drake DW, Swartzwelder HS. Prevalence and correlates of alcohol-induced blackouts among college students: results of an e-mail survey. J Am Coll Health. 2002;51:117–119. 122–131. doi: 10.1080/07448480209596339. [DOI] [PubMed] [Google Scholar]
- 25.Givens B, Williams JM, Gill TM. Septohippocampal pathway as a site for the memory-impairing effects of ethanol. Hippocampus. 2000;10:111–121. doi: 10.1002/(SICI)1098-1063(2000)10:1<111::AID-HIPO12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wechsler H, Wuethrich B. Dying to drink: confronting binge drinking on college campuses. Emmaus, PA: Rodale Press; 2002. [Google Scholar]
- 28.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, et al. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357:j2353. doi: 10.1136/bmj.j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meda SA, Dager AD, Hawkins K, Tennen H, Raskin S, Wood RM, et al. Heavy drinking in college students is associated with accelerated gray matter volumetric decline over a 2 year period. Frontiers in Behavioral Neuroscience. 2017 doi: 10.3389/fnbeh.2017.00176. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, et al. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol Clin Exp Res. 2013;37(Suppl 1):E161–171. doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 33.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 34.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 35.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc 1983 [Google Scholar]
- 36.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 37.Barkley RA, Knouse LE, Murphy KR. Correspondence and disparity in the self-and other ratings of current and childhood ADHD symptoms and impairment in adults with ADHD. Psychol Assess. 2011;23:437–446. doi: 10.1037/a0022172. [DOI] [PubMed] [Google Scholar]
- 38.Delis DC, Wetter SR, Jacobson MW, Peavy G, Hamilton J, Gongvatana A, et al. Recall discriminability: utility of a new CVLT-II measure in the differential diagnosis of dementia. J Int Neuropsychol Soc. 2005;11:708–715. doi: 10.1017/S1355617705050812. [DOI] [PubMed] [Google Scholar]
- 39.Anderson TW, Rubin H. Statistical inference in factor analysis. Proceedings of the the Third Berkeley Symposium on Mathematical Statistics and probability. 1956;5:111–150. [Google Scholar]
- 40.Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci. 2012;6:197. doi: 10.3389/fnins.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 42.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 43.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- 46.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, et al. Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17040469. appiajp201717040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 49.Risher ML, Fleming RL, Risher WC, Miller KM, Klein RC, Wills T, et al. Adolescent intermittent alcohol exposure: persistence of structural and functional hippocampal abnormalities into adulthood. Alcohol Clin Exp Res. 2015;39:989–997. doi: 10.1111/acer.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcohol Clin Exp Res. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- 53.Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci. 2015;35:1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34:5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, et al. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction. 2014;109:585–595. doi: 10.1111/add.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Worhunsky PD, Dager AD, Meda SA, Khadka S, Stevens MC, Austad CS, et al. A Preliminary Prospective Study of an Escalation in ‘Maximum Daily Drinks’, Fronto-Parietal Circuitry and Impulsivity-Related Domains in Young Adult Drinkers. Neuropsychopharmacology. 2016;41:1637–1647. doi: 10.1038/npp.2015.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


