Abstract
Human neuroimaging studies of natural rewards and drugs of abuse frequently assay the brain’s response to stimuli that, through Pavlovian learning, have come to be associated with a drug’s rewarding properties. This might be characterized as a ‘sensorial’ view of the brain’s reward system, insofar as the paradigms are designed to elicit responses to a reward’s (drug’s) sight, aroma, or flavor. A different field of research nevertheless suggests that the mesolimbic dopamine system may also be critically involved in the motor behaviors provoked by such stimuli. This brief review and commentary surveys some of the preclinical data supporting this more “efferent” (motoric) view of the brain’s reward system, and discusses what such findings might mean for how human brain imaging studies of natural rewards and drugs of abuse are designed.
The mammalian brain’s reward system is critical in the search for food and survival. Distortion of this circuitry is also believed to play a key role in the development of (and perhaps resistance to) drug addiction (Haber & Knutson, 2010; Koob & Volkow, 2016). For this reason, a large body of research in both human and animal behavioral neuroscience has targeted brain reward pathways.
The mesolimbic dopamine system is a central aspect of the brain’s reward circuitry. A considerable body of research presumes what might be termed a “sensorial” view of dopamine, wherein striatal dopamine transmission is a response to an exogenous stimulus— either a drug as the direct result of its pharmacologic actions, or a stimulus that has become associated with the drug’s actions via Pavlovian learning. The capacity of a drug- (or even non-drug reward) associated stimulus— a sight, smell, or taste— to induce dopamine transmission has been interpreted as reflecting drug/reward wanting (the incentive salience model Robinson & Berridge, 1993), or as a teaching signal that enables organisms to calculate reward probabilities and predict when a reinforcer will be available (the reward learning model; Schultz, Dayan, & Montague, 1997). Perhaps more plausibly, a combination of these two phenomena may be operative, as Berridge (2012) notes that most studies of mammalian reward prediction and prediction error are conducted during when animals are in states of deprivation to heighten wanting and assure that they engage the paradigm.
In the context of these two theoretical views (i.e. incentive salience or reward learning and prediction), a large number of human brain imaging studies have examined responses to drug, food, or monetary reward “cues.” Using functional magnetic resonance imaging (fMRI), and less often positron emission tomography (PET), such work has demonstrated that drug and natural reward (food) cues do, indeed, provoke ventral striatal activity (Noori, Cosa Linan, & Spanagel, 2016). Consistent with the incentive salience model, many clinically oriented studies conceptualize these Pavlovian stimuli as tempting individuals into reward consumption through this striatal activation. In experiments of this sort, little is nevertheless required of subjects but to observe and detect the presence of the reward cues. However, another body of findings suggests that this “sensorial” view may not capture the entire picture of striatal activity.
An alternate (or much more likely, complementary) line of thinking derived from largely animal work indicates that mesolimbic dopamine is critical to the execution of motivated behaviors. This preclinical work suggests that it may therefore be important to separate responses to reward-related cues from responses related to the effortful behavior to acquire rewards. Indeed, the midbrain’s dopaminergic input into the telencephalon targets what are commonly understood to be key elements of the motor system: the basal ganglia, of which the ventral striatum is a part. As designed (and perhaps as constrained by the nature of the experimental environment and apparatus), many human brain imaging studies are less well equipped to account for the motoric aspect of reward related behavior.
In this manuscript I will first briefly review the relevant anatomic pathways. I will then review the preclinical data supporting this more “efferent” (motor) view of the brain’s mesolimbic dopamine system in reward, focusing in particular on food and alcohol, although the concepts should, in principle, extend to any addictive drug. I will then discuss how these data might be considered when designing human brain imaging experiments to accommodate this more efferent theory of the brain’s reward system.
Reward system anatomy
Excellent in-depth reviews of the primate and human reward system are available elsewhere (e.g., Haber & Knutson, 2010). For brief context, and in broad overview, midbrain dopamine neurons in the substantia nigra (SN), ventral tegmental area (VTA), and retrorubral area (RRA) project to the striatum (caudate and putamen; Figure 1).
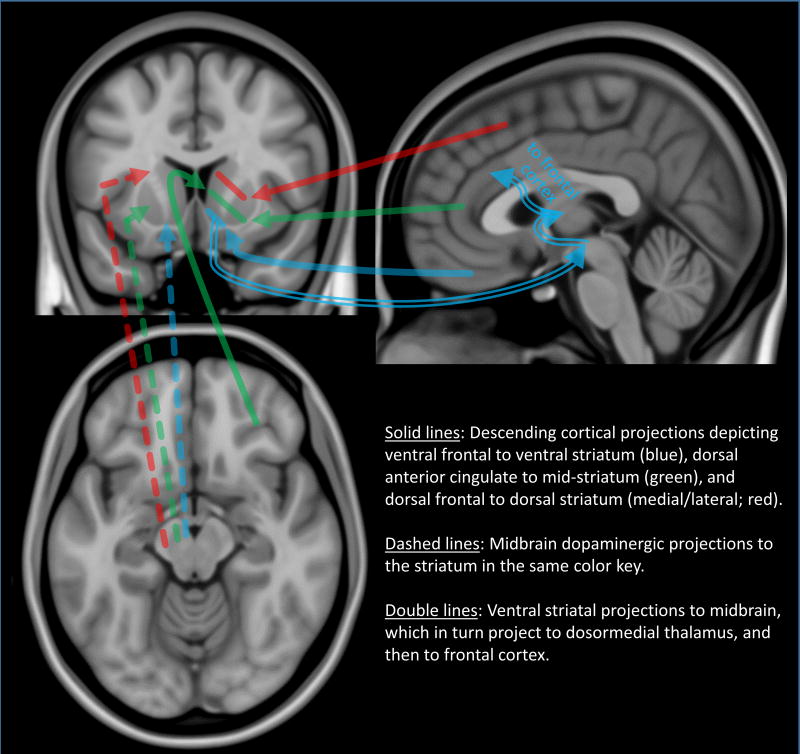
Figure 1.
Schematic of approximate reward pathways between cortex and striatum, as adapted from Haber et al (2000, 2006), Haber & Knutson (2010), and Sesack and Grace (2010). See the respective publications for more detail.
The ventrolateral tier of the SN projects to the lateral sensorimotor striatum (putamen). The SN’s dorsal tier targets the ventral caudate and putamen, while the adjacent VTA sends axons to the nucleus accumbens (Haber, Fudge, & McFarland, 2000). Several regions of frontal cortex also receive dopamine afferents from the dorsolateral SN (dorsolateral/dorsomedial prefrontal) and VTA (medial, ventromedial frontal); RRA regions send afferents to these same cortical areas with a similar topological orientation (Williams & Goldman-Rakic, 1998).
In turn, limbic and frontal association cortices project back to the striatum, with ventromedial orbital (particularly important in the representation of a reward's subjective value; see Hare, Camerer, & Rangel, 2009; Hare, Malmaud, & Rangel, 2011) and lateral orbitofrontal cortices targeting the ventromedial striatum. The dorsal anterior cingulate targets medial caudate and putamen, while dorsolateral prefrontal cortex projects to dorsomedial and dorsolateral (caudate and putamen) striatal areas (Haber, et al. 2006). Along with input from other regions not covered here (e.g., amygdala, insula, hippocampus, hypothalamus), this system is thus poised to assimilate information regarding stimulus salience, reward value, and perceived reward probability— all consistent with the abundance of literature showing that drugs and drug-related stimuli evoke dopaminergic transmission in the ventral striatum (Koob & Volkow, 2016).
The ventral striatum does not, however, rest afferent to frontal cortex in this isolated geography. First, intrastriatal integration is accomplished via a series of spiraling straito-nigro-striatal connections, through which information within limbic, association, and motor striatum can be exchanged (Haber et al., 2000). Second, the ventral striatum sends efferent projections to frontal regions via the ventral pallidum, subthalamic nucleus, dopaminergic midbrain, and dorsomedial thalamus (see Sesack & Grace, 2010 for detail on the pathways). Thus, as a whole, the ventral striatum is poised to function in both sensory and motor domains.
Mesolimbic dopamine and reward-related motor behaviors
In the environment, problems often lie not just in the elevated state of desire evoked by food and drug-related stimuli, but in the relentless behavior engendered by these provocative cues, such as persistent drug seeking pursued to the exclusion of more constructive actions (i.e., criterion 3 for an alcohol use disorder; American Psychiatric Association, 2013). In this vein, pre-clinical studies show that striatal dopamine is linked to more than simply a response to rewards or their related sensory properties. Rather, ventral striatal (i.e., nucleus accumbens) dopamine release also appears to be closely associated with the goal-directed motor behavior needed to procure rewards (Salamone, Correa, Nunes, Randall, & Pardo, 2012).
A compelling preclinical example of this phenomenon comes from Roitman and colleagues (Roitman, Stuber, Phillips, Wightman, & Carelli, 2004). In this study using the high-temporal resolution technique of fast-scanning cyclic voltammetry (FSCV) in behaving rodents, ventral striatal dopamine was released in response to food associated cues. However, the authors also showed that ventral striatal dopamine transients peaked at, and were tightly time-locked to, the animal’s goal-directed behaviors to obtain the food reward. In a separate study using in vivo microdialysis (a recording technique with less temporal resolution than FSCV), Ostlund et al (2011) isolated food seeking behaviors from food receipt/consumption, and similarly showed that accumbens dopamine release was related to lever pressing behaviors to obtain reward. Although dopamine did not track lever-pressing (food seeking) rate, or number of lever presses/rewards earned, there was a significant reduction in lever-press related dopamine release after eating to satiety (Ostlund et al., 2011).
Using FSCV with an addictive drug rather than food, Phillips et al. (2003) also showed that dopamine release in nucleus accumbens peaked when animals lever-pressed for cocaine (also see Owesson-White et al., 2009 for a similar result). Compellingly, electrical stimulation of the VTA, which induced dopamine release in nucleus accumbens, led to spontaneous lever pressing for cocaine. Similarly, Adamantidis and colleagues (2011) found that optogenetic stimulation of the VTA reactivated previously extinguished food-seeking behaviors. Further underscoring the dopaminergic origins of such goal directed behaviors, pharmacologic inactivation of burst firing in the VTA slows goal-directed sucrose seeking and attenuates nucleus accumbens dopamine transients during seeking (Cacciapaglia, Wightman, & Carelli, 2011). Conversely, dopamine D2 receptor over-expression in nucleus accumbens enhances food seeking and acquisition behaviors without affecting satiety (Trifilieff et al., 2013).
Thus, the animal literature makes clear that, in addition to responding to cues of reward’s presence, ventral striatal dopamine transmission is also tightly linked to reward-seeking behaviors, themselves.
Effortful behavior
Although simple instrumental behaviors (e.g. an isolated lever press) to obtain reward are associated with ventral striatal dopamine release, a body of preclinical literature further suggests that accumbal dopamine is critical to surmount more imposing obstacles that interfere with access to food and rewards (Salamone, Correa, Farrar, & Mingote, 2007).
In contrast to the studies that measure dopamine transmission, an alternate technique is to test for effortful behaviors after dopamine depletion. Dopamine depletion in animals does not change food liking behaviors or appetite, but it does change how effort is deployed (Salamone et al., 2012). For example, selective dopamine depletion with 6-hydroxydopamine (6-OHDA) lesions in nucleus accumbens does not alter responding for food reward when a low ratio response (low effort) is required, but dopamine depletion does dampen responding at higher (more difficult) response ratios, and shifts choices to a less preferred, yet more easily obtained, food (Cousins & Salamone, 1994). Similarly, the effects of dopamine antagonism on effort are particularly evident when work-related requirements increase unexpectedly (Ostlund, Kosheleff, & Maidment, 2012). St. Onge and Floresco (2009) found that dopamine receptor (D1, D2) antagonism in rodents decreased choices for larger, riskier food rewards, while amphetamine (which increases synaptic dopamine) augmented preference for the larger/riskier reward. Dopamine antagonism in rat nucleus accumbens also has greater effects on efforts to gain access to alcohol than on alcohol consumption itself (Czachowski, Chappell, & Samson, 2001; Czachowski, Santini, Legg, & Samson, 2002). Similar human phenomena have been observed, with dopamine depletion depressing effort to obtain cigarettes (Venugopalan et al., 2011), and amphetamine increasing effort to work for money (Wardle, Treadway, Mayo, Zald, & de Wit, 2011).
In neurophysiological studies, the magnitude of mesolimbic dopamine release predicts the speed with which animals initiate action sequences to obtain sucrose reward (Wassum, Ostlund, & Maidment, 2012)— data consistent with dopamine’s importance to motivational ‘vigor’ (Niv, Daw, Joel, & Dayan, 2007). Midbrain dopamine neuron spiking activity also declines with increasing fatigue and decreasing effort (Pasquereau & Turner, 2013), while striatal dopamine progressively ‘ramps’ as animals navigate to move closer to obtaining a sweet reward (Howe, Tierney, Sandberg, Phillips, & Graybiel, 2013). In humans, the magnitude of amphetamine-provoked dopamine release is correlated with a willingness to exert effort for larger rewards (Treadway et al., 2012).
Not all data in this area are consistent. In a study where cues signaled varying effort, Day et al. (2010) did not observe accumbal dopamine release during lever presses for sucrose pellets. Gan, et al. (2010) also did not find accumbal dopamine release during increased effort demand when animals decided between a reference choice and choices between alternatives differing in reward value or effort. At least in this particular behavioral choice paradigm, accumbal dopamine increased when the alternate choice involved unexpectedly low effort.
Collectively, a number of findings nevertheless strongly suggest that mesolimbic dopamine is important to overcoming response costs in the search for rewards (Phillips, Walton, & Jhou, 2007). In this way, dopamine likely functions not only to facilitate learning the incentive value that reward cues eventually come to possess (Berridge, 2012), but also to translate such information into the motivated effort required to “seal the deal” (Westbrook & Braver, 2016).
Human brain imaging
As previously noted, a substantial proportion of human brain imaging work in the field of alcohol and addiction has been devoted to the ventral striatal response to reward-associated stimuli. A much smaller literature has been devoted to examining the motoric aspects of reward related behaviors, where some findings resemble those in animals. As one example, the monetary incentive delay task (Knutson, Westdorp, Kaiser, & Hommer, 2000) pairs a symbol with the chance to win amounts of money, contingent upon a successfully timed behavior (button press). The brain response often studied (ventral striatal activation) is that to the reward cue, just prior to the motor response. However, at least two studies found that when this motor requirement was omitted, ventral striatal responses to passive (non-instrumental) monetary reward anticipation were either absent or weak (Bjork & Hommer, 2007; Bjork, Smith, Chen, & Hommer, 2012, although two studies do not support this idea; Delgado, Gillis, & Phelps, 2008; Lewis, Porcelli, & Delgado, 2014). Kroemer et al. (2014) also used fMRI to show that higher than average effort was associated with stronger anticipatory cue responses in the ventral striatum.
Our lab’s experiments with PET and the tracer [11C] raclopride to examine dopamine release are also suggestive in this regard. As endogenous dopamine release displaces raclopride, changes in the tracer’s measured binding potential are used to infer dopamine release as a function of behavioral state (Dewey et al., 1993). First, our early work showed no significant striatal dopamine release when alcohol was infused intravenously while healthy subjects were at rest (Yoder et al., 2007), although an effect did occur when alcohol was infused unexpectedly, consistent with the anticipated effects of a prediction error (Yoder et al., 2009). Ramchandani et al (2011) reported significant striatal dopamine release from passive (non-instrumental) intravenous IV alcohol infusion, but only in those who possessed the rare ‘G’ allele of the OPRM1 μ–opioid receptor gene. In a larger, more recent study, we did detect right unilateral dopamine release from passive IV infusion in non-treatment seeking alcoholics, but not healthy controls (Yoder et al., 2016). Subjects were, however, aware that the baseline condition involved no infusion of any sort, and that alcohol infusion was imminent in the subsequently planned challenge condition. Stimulus salience and anticipation could thus affect these results.
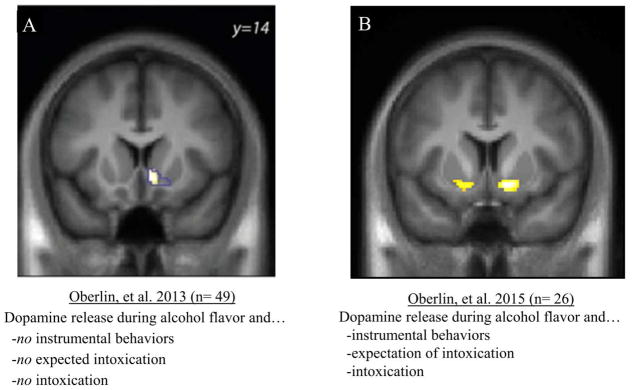
With regard to alcohol-related cues, we recently reported spatially limited (unilateral) alcohol flavor cue-induced ventral striatal dopamine release without (i) instrumental self-administration behaviors (effort), (ii) any expectation of intoxication, and (iii) alcohol intoxication (Figure 2A and Oberlin et al., 2013). However, we showed bilateral ventral striatal dopamine release using an operant self-administration (instrumental) paradigm that delivered alcohol flavor cues in the context of expected and received alcohol intoxication (Figure 2B and Oberlin et al., 2014). This at least suggests that the instrumental behaviors required in this paradigm may be adding to the observed signal.
Figure 2.
(A) Unilateral beer flavor-induced ventral striatal dopamine release (compared to control flavor) with no instrumental self-administration behaviors, no expected intoxication, and no alcohol intoxication. (B) Bilateral ventral striatal dopamine release during instrumental self-administration of beer flavor, expected intoxication, and intravenous alcohol (compared to self-administration of a control flavor, no expected intoxication, and saline infusion).
Other studies reporting significant ventral striatal dopamine release from alcohol (Boileau et al., 2003; Setiawan et al., 2014; Urban et al., 2010) also involved traditional instrumental self-administration behaviors through oral ingestion. Clearly our own data leave other possibilities open, such as the expectation of intoxication. Thus, while far from dispositive, the body of findings implies that goal-directed (self-administration) behaviors may well contribute to human ventral striatal dopamine release.
Testable Predictions
In accordance with some approaches to studying animals (e.g., Czachowski & Samson, 2002; Czachowski et al., 2002), reward paradigms for human brain imaging experiments may need to pay much greater attention to dissociating effects that are due to a reward’s Pavlovian associations, and those that might be due to motoric elements involved in either procuring reward or in the behaviors of drug self-administration. In some cases, and as done with animals, this might most cleanly entail separate brain imaging paradigms (imaging data) involving cue exposure and reward seeking/acquisition behaviors so as to minimize any signal overlap between the two. Any act of self-administration would, however, need to be accomplished so as to not measure responses to the drug itself. This latter consideration is not straightforward, as it necessitates avoiding responses related to reward prediction errors (i.e., declines in striatal or midbrain responses related to the unexpected absence of a drug effect; Schultz et al., 1997).
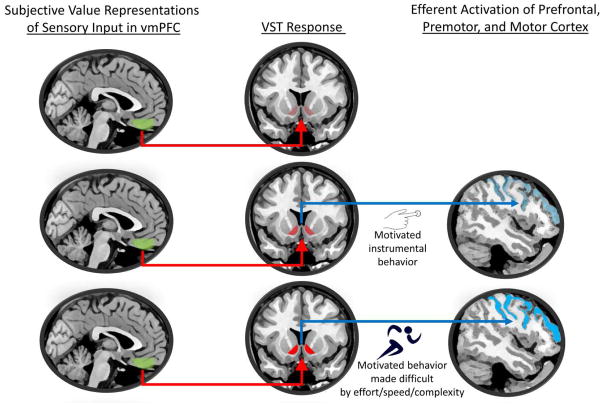
Given the findings reviewed above, and with such a framework in mind, one might then hypothesize a gradient of ventral striatal activity as depicted in Figure 3. With passive exposure to a cue representative of a valued drug or food reward, some degree of ventral striatal activation (a BOLD contrast difference in fMRI or dopamine release measured in PET) should indeed occur, as previously established. This is the “sensory” stage at which most human studies of drug stimuli operate (i.e., Figure 3, top). However, significantly greater ventral striatal activation should become apparent when subjects act to acquire the reward via some instrumental behavior (Figure 3, middle). With rising effort expenditure to overcome obstacles to reward acquisition (such as a greater need for attention, speed, or endurance; Figure 3, bottom), a parametrically greater degree of ventral striatal activation should ensue. Finally, a concomitant prediction would be a corresponding (and correlated) gradient of increasing activity in frontal cortices (motor, premotor, dorsolateral prefrontal) related to the instrumental behaviors, as well their antecedent planning. As suggested by work in animals, however, ventral striatal activation as measured in these circumstances should not predict reward consumption, itself (Czachowski et al., 2001; Czachowski & Samson, 2002; Salamone & Correa, 2002).
Figure 3.
Hypothetical pathways and effects related to (top) passive viewing of reward conditioned stimuli (cues) without opportunity for reward acquisition, (middle) reward cues with consequent instrumental behaviors in service of reward acquisition, and (bottom) reward cues with instrumental reward acquisition behaviors that require effort expenditure, such as to circumvent obstacles. Subjective value of the reward (here presumed constant across rows) is represented in ventromedial prefrontal cortex (vmPFC; green shading), and made available to the ventral striatum (VST; afferent projections as red arrows; see Figure 1 for more detail). Greater activation of the VST (represented as progressively brighter shades of red) should be observed with greater instrumental effort, and thus induce greater activation of prefrontal, premotor, and motor cortex (progressively brighter shades of light blue) via VST efferents (dark blue arrows) to frontal regions (see Figure 1 and Sesack & Grace, 2010 for a hypothetical model of efferent projections).
Conclusions and future directions
The human brain’s response to drug-associated cues is a frequently employed approach in neuroimaging studies of drug and alcohol use disorders, and in the risk for their development. However, the reactive response to a drug-associated cue in the brain’s striatal reward areas may capture only part of the dynamic, and ignore (or depending on the paradigm, blur) striatal aspects of motivated motor behaviors and effort. Separating the effects of reward-related cues from responses related to the effortful behavior to acquire rewards may be important to a broader and more complete understanding of the neurocircuitry changes comprised by addiction and its attendant risk factors for both disease development and treatment relapse.
Acknowledgments
Funding: Writing of this article was supported by NIH Grants R01 AA024588, R01 AA017661, & P60 AA007611.
This paper is based on a lecture given as part of the Yale University - Fulbright - Hadassah Symposium of February 29, 2016. I would like to thank Drs. Evan Morris, Karmen Yoder, and Sarine Janetsian-Fritz for comments on earlier versions of this manuscript.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The author declares that he has no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the studies from the author’s lab.
Reference List
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, … de Lecea L. Optogenetic Interrogation of Dopaminergic Modulation of the Multiple Phases of Reward-Seeking Behavior. The Journal of Neuroscience. 2011;31(30):10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. European Journal of Neuroscience. 2012;35(7):1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: A factorial fMRI investigation. Behavioural Brain Research. 2007;177(1):165–170. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: Effort anticipation, reward anticipation, and reward delivery. Human Brain Mapping. 2012;33(9):2174–2188. doi: 10.1002/hbm.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assad J-M, Pihl RO, Benkelfat C, Leyton M, Diksic M, … Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Cacciapaglia F, Wightman RM, Carelli RM. Rapid Dopamine Signaling Differentially Modulates Distinct Microcircuits within the Nucleus Accumbens during Sucrose-Directed Behavior. The Journal of Neuroscience. 2011;31(39):13860–13869. doi: 10.1523/JNEUROSCI.1340-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacology Biochemistry and Behavior. 1994;49(1):85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of Raclopride in the Nucleus Accumbens on Ethanol Seeking and Consumption. Alcoholism: Clinical and Experimental Research. 2001;25(10):1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcoholism: Clinical and Experimental Research. 2002;26(11):1653–1661. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28(1):39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic Nucleus Accumbens Dopamine Release Encodes Effort- and Delay-Related Costs. Biological Psychiatry. 2010;68(3):306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11(8):880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13(4):350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PEM. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat Neurosci. 2010;13(1):25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal Pathways in Primates Form an Ascending Spiral from the Shell to the Dorsolateral Striatum. Journal of Neuroscience. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-Related Cortical Inputs Define a Large Striatal Region in Primates That Interface with Associative Cortical Connections, Providing a Substrate for Incentive-Based Learning. Journal of Neuroscience. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A. Focusing Attention on the Health Aspects of Foods Changes Value Signals in vmPFC and Improves Dietary Choice. The Journal of Neuroscience. 2011;31(30):11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe MW, Tierney PL, Sandberg SG, Phillips PEM, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013;500(7464):575–579. doi: 10.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. NeuroImage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, Guevara A, Ciocanea Teodorescu I, Wuttig F, Kobiella A, Smolka MN. Balancing reward and work: Anticipatory brain activation in NAcc and VTA predict effort differentially. NeuroImage. 2014;102(Part 2):510–519. doi: 10.1016/j.neuroimage.2014.07.060. [DOI] [PubMed] [Google Scholar]
- Lewis AH, Porcelli AJ, Delgado MR. The effects of acute stress exposure on neural correlates of Pavlovian conditioning with monetary gains and losses. Frontiers in Behavioral Neuroscience. 2014:8. doi: 10.3389/fnbeh.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Daw N, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007;191(3):507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Noori HR, Cosa Linan A, Spanagel R. Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta-analysis. European Neuropsychopharmacology. 2016;26(9):1419–1430. doi: 10.1016/j.euroneuro.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran S, Soeurt C, O'Connor S, Yoder K, Kareken D. Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology. 2014:1–10. doi: 10.1007/s00213-014-3720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: Mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Kosheleff AR, Maidment NT. Relative Response Cost Determines the Sensitivity of Instrumental Reward Seeking to Dopamine Receptor Blockade. Neuropsychopharmacology. 2012;37(12):2653–2660. doi: 10.1038/npp.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular Dopamine Levels in Striatal Subregions Track Shifts in Motivation and Response Cost during Instrumental Conditioning. The Journal of Neuroscience. 2011;31(1):200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Mark Wightman R, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. European Journal of Neuroscience. 2009;30(6):1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. Limited Encoding of Effort by Dopamine Neurons in a Cost-Benefit Trade-off Task. The Journal of Neuroscience. 2013;33(19):8288–8300. doi: 10.1523/JNEUROSCI.4619-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien MLAV, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology. 2007;191(3):483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, … Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving - An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine Operates as a Subsecond Modulator of Food Seeking. The Journal of Neuroscience. 2004;24(6):1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behavioural Brain Research. 2002;137(1–2):3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M. The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine and beyond. Journal of the Experimental Analysis of Behavior. 2012;97(1):125–146. doi: 10.1901/jeab.2012.97-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia Reward Network: Microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Dagher A, Schlagintweit H, Casey KF, Benkelfat C, Leyton M. Differential Striatal Dopamine Responses Following Oral Alcohol in Individuals at Varying Risk for Dependence. Alcoholism: Clinical and Experimental Research. 2014;38(1):126–134. doi: 10.1111/acer.12218. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic Modulation of Risk-Based Decision Making. Neuropsychopharmacology. 2009;34(3):681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, … Zald DH. Dopaminergic Mechanisms of Individual Differences in Human Effort-Based Decision-Making. The Journal of Neuroscience. 2012;32(18):6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, … Javitch JA. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry. 2013;18(9):1025–1033. doi: 10.1038/mp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NBL, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, … Abi-Dargham A. Sex Differences in Striatal Dopamine Release in Young Adults After Oral Alcohol Challenge: A Positron Emission Tomography Imaging Study With [11C]Raclopride. Biological Psychiatry. 2010;68(8):689–696. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan VV, Casey KF, O'Hara C, O'Loughlin J, Benkelfat C, Fellows LK, Leyton M. Acute Phenylalanine/Tyrosine Depletion Reduces Motivation to Smoke Cigarettes Across Stages of Addiction. Neuropsychopharmacology. 2011;36(12):2469–2476. doi: 10.1038/npp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping Up Effort: Effects of d-Amphetamine on Human Effort-Based Decision-Making. The Journal of Neuroscience. 2011;31(46):16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT. Phasic Mesolimbic Dopamine Signaling Precedes and Predicts Performance of a Self-Initiated Action Sequence Task. Biological Psychiatry. 2012;71(10):846–854. doi: 10.1016/j.biopsych.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Braver TS. Dopamine Does Double Duty in Motivating Cognitive Effort. Neuron. 2016;89(4):695–710. doi: 10.1016/j.neuron.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex. 1998;8(4):321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, … Kareken DA. Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug and Alcohol Dependence. 2016;160:163–169. doi: 10.1016/j.drugalcdep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng T, O'Connor S, Morris ED. Heterogeneous Effects of Alcohol on Dopamine Release in the Striatum: A PET Study. Alcoholism: Clinical and Experimental Research. 2007;31(6):965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O'Connor SJ, Kareken DA. When What You See Isn't What You Get: Alcohol Cues, Alcohol Administration, Prediction Error, and Human Striatal Dopamine. Alcoholism: Clinical and Experimental Research. 2009;33(1):139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]