Abstract
Despite contributing significantly to the burden of global disease, the translation of new treatment strategies for diseases of the central nervous system (CNS) from animals to humans remains challenging, with a high attrition rate in the development of CNS drugs. The failure of clinical trials for CNS-therapies can be partially explained by factors related to pharmacokinetics/pharmacodynamics (PKPD) such as lack of efficacy or improper selection of initial dosage. A focused assessment is needed for CNS-acting drugs in first-in-human studies to identify the differences in PKPD from animal models as well as choose the appropriate dose. In this review, we summarize available literature from human studies on the pharmacokinetics and pharmacodynamics in brain tissue, cerebrospinal fluid and interstitial fluid for drugs used in the treatment of psychosis, Alzheimer’s disease and neuro-HIV and address critical questions in the field. We also explore newer methods to characterize pharmacokinetic/pharmacodynamic relationships that may lead to more efficient dose selection in CNS drug development.
1. Introduction
Disorders of the brain contribute significantly to global disease burden. Psychiatric, neurological, developmental and substance abuse disorders affect more than 1 billion people worldwide.(1) As of 2010, these were the leading cause of years lived with disability (YLD) globally, accounting for approximately 30% of all YLDs.(2) However, CNS drug development is extremely challenging. Compared to non-CNS drug development, these programs have a lower clinical approval rate (6% versus 13%) and a longer time to market (12 years versus 6–7 years).(3–6) This has led to several companies withdrawing drug development programs in the neurosciences(7–9) signaling an uncertain future for novel research in CNS disorders.
Difficulty selecting initial drug dosage, untoward toxicities and lack of efficacy are cited as some driving forces behind the high attrition rate of CNS therapies.(10) A robust concentration-effect analysis can provide valuable, reproducible information regarding both the therapeutic as well as adverse effect drug profile over a wide range of doses and greatly aid the development of CNS-acting drugs. However, a report from 2007 indicated that there were very few sets of pharmacodynamic data generated from human studies over a wide range of doses or concentrations.(11) Although concentration-effect relationships are assessed in animals, animal models do not always accurately predict human disease, especially in case of CNS disorders.(12) Difference in blood-brain barrier (BBB) permeability, drug metabolizing enzymes and transporters can lead to differences in drug exposure in the human brain compared to animals(13) and only rarely can drug be sampled from the human brain for pharmacokinetic (PK) measures. Further, animal models may only mimic some mechanisms of human CNS disease or contain targets not seen in humans, challenging the translation of efficacy and/or toxicity of novel therapeutics. Therefore, to address these issues a focused pharmacokinetic/pharmacodynamic (PKPD) assessment is required in humans to identify differences from animal models and adjust dosing. This has been accomplished by employing alternate methodologies such as in-vitro systems, translational studies or in-silico modelling to supplement the understanding of pharmacology within the CNS.
This review is broadly divided into three parts. In section 3, existing methods to measure PK and PD in the brain tissue, cerebrospinal fluid (CSF) and interstitial fluid (ISF) are reviewed. While there is abundance of PKPD information from animal models in the CNS, less complete information is available from human studies. In sections 4–6, we examine clinical PKPD analyses at relevant target sites in the CNS for antipsychotics, anti-Alzheimer’s drugs and antiretrovirals and examine the utility of available information and the need for more research to answer critical questions in the field. In the absence of clinical results, available animal data are presented and cautiously interpreted for clinical relevance. Finally, new methods to improve CNS drug development are examined in section 7.
2. Methodology
An extensive literature search was performed to identify research articles and conference abstracts published in Embase (including articles in the MEDLINE® database) using terms for drugs used to treat disorders of the brain and CNS, combined with terms for PK or PD and terms for the brain and CNS. A full search strategy is provided in the supplementary material. These searches were augmented by targeted searches in PubMed, Google Scholar, and Google Books, which combined terms from the full search strategy, plus additional terms for PK or PD measures or factors affecting these measures. The bibliographies of relevant review articles were also hand searched for additional relevant studies.
3. Pharmacokinetic considerations for drugs acting in the CNS
3.1 Measures of drug pharmacokinetics in the CNS
Drug distribution into the CNS has been characterized by several methods: measuring drug uptake into cultured brain cells (in-vitro), or measuring drug concentration in the brain tissue (ex-vivo) or CSF or ISF (in-vivo).
In-vitro models of the BBB are used as a first line-approach for determining the extent to which investigational agents cross into the brain(14). There are several validated models of the BBB from multiple species(15) and while no ideal cell line exists, the human cell line most widely used and well characterized is the human immortalized endothelial cell line hCMEC/D3. hCMEC/D3 experiments can quantify drug permeability, identify relevant drug-efflux transporter interactions, rapidly screen drug candidates for CNS activity and carry out initial PK studies. However, these models are a static measure of drug PK. For anti-infectives in particular, these models do not account for time-dependent killing and may be less clinically relevant. In-vitro systems also do not fully replicate all in-vivo features of the BBB. For example hCMEC/D3 is more “leaky” than the BBB, and can express lower levels of BBB-specific enzymes and drug transporters(15). Therefore, in-vitro systems may have to undergo modification such as co-culture with other brain cells to replicate tight junctions of BBB.(16) Newer microfluidic technologies such as BBB-on-a-chip or neurovascular-unit-on-a-chip(17) hold promise to mimic the dynamic in-vivo environment.
There are several ex-vivo approaches to measuring drug concentrations in brain tissue either after surgical resection or necropsy. Most PK information comes from brain tissue homogenates using liquid chromatography-mass spectrometry (LC-MS) analysis. These measurements are then used to calculate ISF and intra-cellular fluid (ICF) concentrations(18). Though commonly used, these methods do not provide information about drug localization. Mass spectrometry (MS) imaging has emerged as a method to quantify drug molecules by MS and spatially visualize drug distribution in tissue slices.(19) The advantage of MS imaging is that it can capture drug distribution patterns within different regions of a tissue(20). For example, using Matrix Assisted Laser Desorption Ionization (MALDI) imaging MS, the anti-tubercular drug pretomanid was found to localize predominantly in the corpus callosum of Sprague Dawley rats(21). By using serial sections collected at different time points, it was shown that pretomanid distributed into the corpus callosum 1–2 hours after an intraperitoneal dose of 20mg/kg, and diffused into other parts of the brain at later time points. With advances in imaging technology, this technique may be used to image intracellular drug concentrations and can be coupled with PD targets through immunohistochemistry (IHC) or in-situ hybridization in contiguous slices. While this has not yet been demonstrated for brain cells, Aikawa et al. used hematoxylin and eosin (H&E) along with IHC staining for CD31 and multidrug resistance transporter 1 (MDR1) to show the colocalization of anti-cancer drug alectinib with blood vessels in murine brains(22). A drawback of ex-vivo imaging is that it is a static measurement, and a composite of multiple images from different animals is required to gain information across a dosing interval.
In-vivo imaging techniques, such as Positron Emission Tomography (PET), can provide longitudinal information on drug disposition. PET is a non-invasive imaging technique that relies on the detection of radio-labelled ligands over time. It has been used to measure absolute spatial concentration of drug and determine PK parameters as well as target occupancy of several CNS-acting drugs. While a detailed discussion of PET is beyond the scope of this review, the reader is directed to a 2013 review(23) for a detailed summary on estimating PK parameters using PET studies. Despite the spatial advantages and applicability to human studies, PET scans are expensive, generally limited to fewer patients because of the use of radioactivity, and may not distinguish between parent compound and metabolites.
Other in-vivo drug estimation methods measure drug penetration into fluid compartments of the CNS. Microdialysis involves inserting a dialysis probe into the cerebral region of the brain to measure the protein-unbound concentration in the ISF. This technique is regularly used in animal models for continuous monitoring of drug concentration, but is only applicable during intra-operative procedures in humans.(24) Further, this procedure might not be suitable to measure the concentration of highly lipophilic or protein bound drugs as there can be a high degree of non-specific binding to the microdialysis probe and poor recovery of drug from the fluid.(24,25) Additionally, intracellular active metabolites are not captured using this technique.
The most common approach to generating PK data is drug sampling in CSF. This is done by lumbar puncture for a single sample and spinal catheterization in the subarachnoidal space for continuous sampling. While less invasive than microdialysis, lumbar punctures are painful and not without medical risks, and are not routinely performed. Also, concentrations measured by lumbar puncture can differ based on the location and time of measurement(13). For example, using a mathematical model, phenytoin was predicted to reach 300% greater concentration in cranial CSF than spinal CSF(26). Generally, unbound CSF concentrations are used as surrogates for unbound brain tissue concentrations in animal models(27) based on the free drug hypothesis which stipulates that protein-unbound drug passively moves from the plasma through the BBB and blood-CSF barrier (BCSFB) into the brain and CSF(28). However, this generalization holds true for certain drugs(29,30) with two significant exceptions: i) Drugs that use membrane transporters for influx and efflux (Eg. antidepressants, antiretrovirals [ARVs]) and ii) Drugs with low permeability to cross through the BBB where CSF bulk flow exceeds passive diffusion of the compound into CSF.(31) For substrates of efflux membrane transporters such as P-gp, CSF concentrations tend to overestimate ISF concentrations.(32) While the exact reason for this observation remains unknown, some hypotheses include subapical or apical localization of P-gp on the choroid plexus that results in drug transfer and accumulation into the CSF,(33) or non-functionality of P-gp at the BCSFB.(34) Since the CSF is recycled at a faster rate than ISF, the CSF acts as a “sink” to clear drug.(31) For high permeability compounds, this effect is negligible but for low-permeability compounds, CSF concentrations underestimate the brain or ISF concentrations (Eg. morphine 6-glucuronide). Therefore, the unbound concentration in the brain may differ from the CSF concentration and confound target site assumptions.
In case of in-vivo measurements made at a single time point, the concentration of drug in brain or CSF may be normalized to a simultaneously-collected plasma concentration. While this is a common means of estimating the extent of drug uptake into the CNS, and allows for comparisons of uptake between drugs, the rates of entry and elimination of the drug in plasma, CSF and brain compartments differ.(35) For example, the CSF:plasma concentration ratio for ciprofloxacin increases by as much as 1400% over 24 hours.(35) One approach to avoid this confounding is to use sparse serial sampling in a group of animals or humans to characterize the drug’s full PK profile in the CSF and plasma and calculate the ratio of drug exposure in the 2 compartments by measuring the area under the concentration-time curve. This approach has been performed for several anti-infective drugs(36) during ventricle catheterization when CNS infections need to be monitored(37) or excess CSF fluid needed to be drained.(38,39) Due to difficulties in obtaining multiple CSF samples from patients, population PK modeling has been used with sparse CSF and plasma sampling in order to obtain exposure profiles of various drugs such as abacavir.(40)
3.2 Intracellular versus extracellular drug concentrations
When considering the site of action, it is important to distinguish between extracellular and intracellular CNS drug concentrations. For drugs that act on receptors on neuronal cell membranes such as anti-epileptic drugs (AEDs) and anti-Alzheimer’s drugs, it is preferable to measure drug concentration in the ISF where the PD effect is exerted. Extracellular acting drugs have been measured in brain tissue homogenates, but this approach may be misleading. For AEDs and other basic drugs (pKa >7) where brain volume of distribution is greater than brain water volume (0.8 mL/g), ISF concentrations are over-estimated by brain tissue homogenate due to non-specific binding in brain tissue.(41,42) For anti-infective and anti-cancer drugs which act on intracellular targets, the unbound intracellular drug concentration is the most appropriate PK measure linked with activity. Friden and colleagues demonstrated a method to indirectly estimate unbound intracellular drug concentration. Briefly, in-vitro volume of distribution of unbound drug in brain (Vu,brain) is measured in brain slices from drug-naïve animals incubated in drug containing buffer (brain slice method(43)) and fraction of unbound drug in the brain (fu,brain) is measured by adding drug to brain homogenates from drug-naïve animals.(18) The ratio of intracellular to extracellular unbound drug concentration (Kp,uu,cell) is given by equation 1.
| (1) |
Using this method, intracellular drug concentrations of gabapentin, oxycodone, morphine and codeine were found to be greater than extracellular concentrations.(18)
3.3 Factors affecting pharmacokinetics of drugs in the CNS
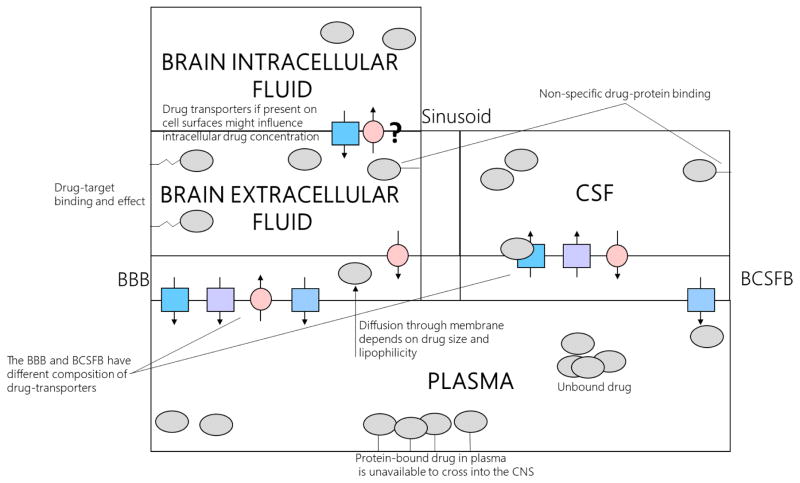
Many factors have been identified to affect drug exposure in the CNS. These have been summarized in figure 1. For an in-depth analysis on specific classes of drugs, the reader is referred to two excellent reviews.(36,44)
Figure 1. Factors affecting the pharmacokinetics and pharmacodynamics of drugs in central nervous system.
This figure demonstrates various factors the influence pharmacokinetic and pharmacodynamic activity in CNS and highlights various compartments of drug action. Effect of physicochemical properties, specific and non-specific protein binding and drug transporters are illustrated. Drug transporters along the membranes may also co-localize which leads to bidirectional movement of drugs. Legend: BBB – blood brain barrier, BCSFB – blood CSF barrier
Protein Binding – Protein binding influences the entry and activity of drug into the CNS. Drugs that are highly protein bound in the plasma concentrate to a lesser extent in the CSF and brain tissue. Conversely, for drugs that accumulate intracellularly in brain tissue such as gabapentin and morphine, the degree of plasma protein binding is low (3% for gabapentin and 20% for morphine). The degree of protein binding varies between plasma, CSF and tissue, based on the concentration of drug-binding proteins. For albumin, concentrations range from 35–50g/L in plasma and are <250mg/L in CSF. For AAG, concentrations are approximately 0.77g/L in plasma and 8.4mg/L in CSF.(31) These proteins can also be synthesized by microglial cells.(45) Therefore, while highly protein-bound drugs (>95% protein binding) such as efavirenz and fluoxetine have lower total drug concentrations in the CSF compared to blood plasma, protein-unbound drug concentrations are similar in both fluids. In general, use of unbound drug concentrations in the CSF leads to mechanistic PKPD relationships(46) and better translatability between species.(47)
Drug Efflux Transporters – Drug efflux transporters such as MDR1 (P-glycoprotein), BCRP and MRP4 are highly expressed at the BBB.(48–50) MDR1 and MRPs have also been identified on the surface of astrocytes.(48) Studies using transporter knockout (KO) mice have shown that MDR1 KO increases brain concentrations of MDR1 substrates by 10–100 fold(51), while the KO of BCRP and MRP4 has minimal effect.(52,53) Therefore, MDR1 inhibition should be a viable option to increase the CNS exposure of drugs in rodent models. Indeed, it has been shown that the co-administration of MDR1 inhibitors (eg. cyclosporin or zosuquidar) increases CNS brain penetration of MDR1 substrates such as nelfinavir or paclitaxel.(54) For indinavir,(55) increased CSF penetration was demonstrated in HIV-infected patients when MDR1 inhibitor ritonavir, was given concomitantly. Although there was increase in plasma exposure, this was driven by a 5-fold increase in trough concentration. Linear regression analysis showed that increase in CSF concentrations (2.67 fold) was not explained by increase in plasma concentrations alone, and inhibition of efflux transporters at the BBB might also contribute to increased CSF exposure of indinavir.
Physicochemical properties – Lipophilic drugs show greater permeability through the lipophilic BBB. In a study of compounds ranging from highly polar (sucrose, logD = −4.49) to highly lipophilic (estradiol, logD = 4.14), the log brain uptake index (BUI) of estradiol in Sprague-Dawley rats was 232 times higher than sucrose.(56) However, a higher lipophilicity also results in a higher degree of non-specific tissue binding.(57) In a study of 7 compounds that ranged in BBB permeability by 160-fold, the highly lipophilic compound fluoxetine showed the greatest permeability through the BBB (evidenced by the highest permeability surface area product of 600 ml/kg*hr) of Sprague-Dawley rats but a free drug fraction (0.23%)(58) that was lower than plasma (6–15%). Similarly efavirenz has a permeability surface area product of 2.4 ml/kg*hr through the BBB and a free fraction of only 0.197%(59) in rat brain tissue, compared to 1% in blood plasma.
3.4. Measures of drug pharmacodynamics in the CNS
Many PD targets are utilized in CNS disorders. In the following section, common clinical PD endpoints are summarized. The pros and cons of these measures are outlined in Table 1.
Table 1.
Commonly used pharmacodynamic measures for CNS drugs and the advantages and disadvantages of each technique
| Class of drugs / pharmacodynamic measure | Advantages | Disadvantages |
|---|---|---|
|
| ||
| I. Drugs used in psychiatric and neurological disorders | ||
| 1. Receptor occupancy/ binding affinity | + Direct measure of efficacy of drug + Can be used for interacting drugs on different receptor sites |
+ Discrepancy between in-vitro and in-vivo values + Species differences may result in difficulties in translation |
| 2. Change in behavioral symptoms and clinical ratings scales | + Are easy to understand clinically + Non-expensive + Can be made longitudinally to track progression of the disorder + Non-invasive |
+ Difficult to translate between animal models and humans + Some disorders (Alzheimer’s) present with several ratings systems which may not always agree. This causes issues with interpretation |
| 3. Neuroimaging markers | + Can provide more detailed information than subjective tests | + PET scans are expensive + With fMRI, there is exposure to high-intensity magnetic fields |
|
| ||
| II. Anti-infectives | ||
| 1. Mitigation of symptoms | + Easy to make PD measurement | + May be subjective + May be difficult to interpret PKPD relationship |
| 2. Bacterial count/ viral load in CSF | + Straight-forward correlation | + Invasive procedure which can be painful + Not enough information on whether CSF measurements approximates brain tissue measurements |
| 3. Neurocognitive scores for HAND | + Non-invasive procedure +Technique accounts for comorbidities |
+ Research tool that is not used clinically |
-
Receptor occupancy/ binding affinity:
Receptor occupancy and binding affinity are related in that receptor binding affinity is an in-vitro measure of the concentration of ligand resulting in a ligand-receptor complex while receptor occupancy is the proportion of receptors that have formed a ligand-receptor complex in-vivo relative to the baseline receptor density. The examples of the N-methyl-D-aspartate (NMDA) receptor agonists amantadine and memantine are illustrative of the binding affinity concept. Amantadine and memantine have weak affinity to the σ site of the NMDA receptor with binding affinities of 20.25 ± 16.48 uM and 19.98 ± 3.08 uM respectively.(60) The drugs showed higher affinity to the PCP binding site on the receptor (10.5 ± 6.1 uM for amantadine and 0.54 ± 0.23 uM for memantine). By taking into account the therapeutic concentrations of these drugs attained in the human brain,(60) it was determined that amantadine acted at both the σ and PCP binding site, while memantine only acted at the PCP binding site. Receptor occupancy studies have been performed for several classes of drugs by means of PET scans and clinical data are available for dopamine D1 and D2 receptors (antipsychotics(61–63)), histamine H1 receptor (antidepressants(64)) and serotonin 5-HT 2 receptor (antipsychotics(63)).
-
Change in behavioral symptoms and clinical ratings scales:
For Parkinson’s, depression and psychosis, the Unified Parkinson’s Disease Ratings Scale (UPDRS)(65), Hamilton – Depression Rating Scale (HAM-D)(66) and Brief Psychiatric Rating Scale (BPRS)(67) respectively are widely used by clinicians to aid with diagnosis and progression of the disease as well as assess PD. In case of Alzheimer’s, the Alzheimer’s Disease Assessment Scale (ADAS) is commonly administered in almost all clinical trials of symptomatic Alzheimer’s.(68) However, several other ratings scales have been developed for Alzheimer’s. A review published in 2010 identified 68 distinct, relevant scales,(68) though only 5 of these scales met the requirements for a robust multi-domain assessment of the disease.
-
Neuroimaging markers:
Neuroimaging modalities can be used for several different PD measures. For example, in case of anti-depressants,(69) PET scans have been used to derive receptor abundance and occupancy profiles in-vivo while functional magnetic resonance imaging (fMRI) provides information about changes in brain structure and white matter integrity.
-
PD endpoints for anti-infectives
Common PD endpoints include time to mitigation of neurological symptoms such as headache, confusion and muscle weakness and lowering of antimicrobial load (eg. bacterial count, HIV viral RNA) in the CSF. Antibiotics are used to target CNS infections on the basis of in-vitro MIC and IC50 (drug concentration that yields 50% inhibition of microbial growth). Similarly, antiretrovirals may be selected for activity in the CNS on the basis of in-vitro IC50 values.(70) HIV also causes a spectrum of neurocognitive deficits in patients and in such instances, neurocognitive test scores, such as the global deficit score (GDS) have been developed as a PD measure to provide a baseline of neurocognitive impairment and track disease progression.(71)
The following three sections examines currently available target site clinical pharmacology data for three disease states: Psychosis/Schizophrenia, Alzheimer’s disease and neuro-HIV. The reader is referred to table 2 for more detailed information on the studies that are referenced in this manuscript.
Table 2.
Summary of pharmacokinetic and pharmacodynamic measurements made in the CSF and brain tissue for anti-psychotic drugs, anti-Alzheimer’s drugs and antiretrovirals in human studies
| Authors | Study population | Sample size | Drug(s) | Parameters and Results | Reference |
|---|---|---|---|---|---|
|
| |||||
| I. Psychosis | |||||
| Pharmacokinetics | |||||
|
| |||||
| Wode- Helgodt et al. | Humans with a psychotic disorder | 44 | Chlorpromazine | CSF and plasma were analyzed. Clinical improvements in patients with drug concentrations greater than 1ng/ml in CSF and 40ng/ml in plasma | (80) |
|
| |||||
| Kornhuber et al. | Postmortem brain tissue of humans treated with Haloperidol | 11 | Haloperidol | Liquid chromatography was used to measure concentrations in 5 brain regions. Drug concentrations were 10x higher than serum concentrations optimal to treat schizphrenia. Elimination T1/2 from brain tissue was estimated by population PK analysis and was 6.8 days | (72) |
|
| |||||
| Rimón et al. | Humans with chronic schizophrenia | 12 | Haloperidol | At steady-state, CSF and serum concentrations 12 hours post-dose were compared. CSF concentrations were 4.3% of serum | (81) |
|
| |||||
| Sampedro et al. | Postmortem brain tissue | 18 | Amisulpiride, haloperidol, levomepromazine, norclozapine, olanzapine, paliperidone, quetiapine, risperidone, Sulpiride, triapride, ziprasidone | Liquid chromatography tandem mass spectroscopy was used to measure concentration of 17 antipsychotics in the prefrontal cortex. Concentrations below lower limit of quantification noted in ten samples. Some samples had high drug concentrations indicating drug overdose | (73) |
|
| |||||
| Nyberg et al. | Humans with a psychotic disorder | 48 | Thioridazine | Paired CSF and blood samples were obtained. Collection time-point is unclear. Mean total concentration was 19.4nmol/L in CSF Free fraction in CSF = 49.1% |
(75) |
|
| |||||
| Pharmacodynamics | |||||
|
| |||||
| Kim et al. | Healthy volunteers | 18 | Aripiprazole | PET was used to measure dopamine receptor occupancy at 3, 45 and 120 hours post- dose. EC50 = 11.1ng/ml from PD modeling. EC50 = 8.63ng/mL from PK-PD modeling |
(82) |
|
| |||||
| Yokoi et al. | Healthy volunteers | 15 | Aripiprazole | PET was used to measure D2 and D3 occupancy after two weeks of daily dosing. Dose dependent receptor occupancy from 40–95% observed |
(61) |
|
| |||||
| Farde et al. | Humans with a psychiatric disorder | 14 | Chlorpromazine, Clozapine, flupentixol, haloperidol, melperone, perphenazine, pimozide, raclopride, sulpride, thioridazine, thioxanthene, trifluperazine hydrochloride | PET was used to measure dopamine receptor occupancy at steady-state, 6 hours post- dose 65–85% D2 receptor occupancy across the 11 drugs |
(62) |
|
| |||||
| Mamo et al. | Humans with schizophrenia | 16 | Ziprasidone | PET was used to measured dopamine and serotonin occupancy after 3 weeks of administration at trough. Occupancy at 5-HT2 was 76% and at D2 was 56% | (63) |
|
| |||||
| II. Alzheimer’s disease | |||||
| Pharmacokinetics | |||||
|
| |||||
| Mochida et al. | Healthy women | 4 | 11C-Donepezil | Women were orally administered 1mg and 30ug 11C-Donepezil and underwent PET scan 2.5 hours post-dose Mean standardized unit value for mean intensity of pixels imaged was 0.9 in the brain, indicating even distribution of radioactivity in brain as the rest of the body | (85) |
|
| |||||
| Valis et al. | Humans with Alzheimer’s | 16 | Donepezil | Donepezil concentrations in CSF were measured via liquid chromatography. CSF concentration higher at 24h (7.54ng/ml) vs 12hr (5.19ng/ml). No accumulation in plasma | (86) |
|
| |||||
| Darreh-shori et al. | Humans with Alzheimer’s | 104 | Donepezil | CSF and blood AChE measured via Ellman’s colrimetric assay. Collection timepoint is unclear, but all patients were at steady-state. CSF concentrations were 10x lower than plasma. CSF AChE-S inhibition was 30–40% after 5mg dose and 45–55% after 10mg dose. |
(87) |
|
| |||||
| Kornhuber et al. | Humans with mild to moderate dementia | 6 | Memantine | Plasma and CSF measured 2–3 hours after dose in 4 patients CSF concentration was 0.05– 0.3uM and 50% lower than in serum | (88) |
|
| |||||
| Rohrig et al. | Postmortem human brain tissue | 1 | Memantine | Brain tissue concentration was 5.7 mg/kg which was 2.7-times higher than the heart blood concentration (2.1 ug/ml) and 6.9-times higher than the femoral blood concentration (0.83 ug/ml) | (89) |
|
| |||||
| Cutler et al. | Humans with Alzheimer’s | 18 | Rivastigmine | Cmax in CSF was lower than plasma by 2–4-fold and Tmax in CSF (1.4–3.8 hours) was longer than plasma (0.5–1.67 hours) | (91) |
|
| |||||
| Pharmacodynamics | |||||
|
| |||||
| Wattmo et al. | Humans with Alzheimer’s | 84 | Galantamine | Alzheimer’s Disease Assessment Scale - cognitive subscale (ADAS-cog) Mini-Mental State Examination (MMSE) instrumental activities of daily living (IADL) Plasma concentrations did not correlate with any of the PD measures |
(93) |
|
| |||||
| III. Neuro-HIV | |||||
| Pharmacokinetics | |||||
|
| |||||
| Yilmaz et al. | HIV-positive humans | 1 | Efavirenz | Liquid chromatography tandem mass spectoscopy was used to quantify drug concentrations. Median concentration in plasma was 3,718ng/ml and in CSF was 16.3ng/ml. CSF penetration was 0.44% of plasma | (37) |
|
| |||||
| Bumpus et al. | Postmortem human brain tissue | 21 | Atazanavir, Efavirenz, Emtricitabine, Lamivudine, Lopinavir, Tenofovir | Drug concentrations were assessed by liquid chromatography and compared to historical CSF measures. Concentrations varied by brain regions and lower in cortical grey matter than other regions for lopinavir (p=0.01) No difference for other drugs and tenofovir had higher concentration in brain tissue than CSF |
(105) |
|
| |||||
| Curley et al. | Virtual cohort of humans | NA/100 virtual simulati ons | Efavirenz | CNS distribution predicted using permeability-limited PBPK model. Median Cmax was 3,184ng/ml, 49.9ng/ml, and 50,343ng/ml in plasma, CSF, and brain tissue. Brain tissue to plasma ratio was 15.8. | (59) |
|
| |||||
| Pharmacodynamics | |||||
|
| |||||
| Smurzynski et al. | HIV-positive humans | 2,636 | Combination ARV therapy | Neuropsychiatric testing scores (NPZ3) – better scores associated with higher CPE for more than 3 ARV drug regimens. No association for regimens less than 3 ARVs | (101) |
|
| |||||
| Baker et al. | HIV-positive humans | 64 | Combination ARV therapy | Neuropsychiatric testing scores (NPZ4) – no relationship with CPE Brain volumetric changes – no relationship with CPE |
(102) |
|
| |||||
| Caniglia et al. | HIV-positive humans | 61,938 | Combination ARV therapy | “intention-to-treat” hazard ratios of 4 neuro-AIDS conditions – high CPE associated with increased risk of dementia | (104) |
4. Clinical pharmacokinetics and pharmacodynamics of antipsychotics in the CNS
Since chlorpromazine was approved over 60 years ago, there are now 21 FDA-approved first- and second-generation antipsychotics for the treatment of pediatric and adult psychosis. Despite significant advances in the field, a critical area that is yet to be fully addressed with these drugs is the variability in PD response required for efficacy, and the relationship to target site exposure. There is also lack of consensus on the appropriate PK target measure to correlate to anti-psychotic efficacy.
Anti-psychotic drugs are known to penetrate readily into the brain. For example, haloperidol is found in the brain tissue at concentrations that are 10–30 times higher than serum concentrations.(72) Further reports of brain tissue concentration of antipsychotics are available from autopsy tissue: a 2012 analysis in the prefrontal cortex tissue from 18 human autopsy samples noted high concentration of several drugs such as olanzapine (33,378 ng/g) and quetiapine (16,769 ng/g).(73) However, such reports often include no supporting information such as plasma concentrations and post-mortem interval of collection and are therefore difficult to interpret. Given that olanzapine and the other drugs showed a range from undetectable (<2ng/g) to high concentrations, the authors postulated that the exceedingly high concentrations were the result of overdose. Therefore, such studies may not provide accurate information about the therapeutic range of concentrations of antipsychotics. For the newer antipsychotics aripiprazole, lurasidone, and perospirone, clinical brain PK is unknown.(74) However, extensive tissue distribution is evidenced by their large apparent volume of distribution of 400–6000 L.(74) In the absence of brain tissue concentration data, CSF concentration may be predictive of unbound brain tissue PK,(27) although this has not been verified in humans. Antipsychotics extensively enter the CSF(31) and historical estimates of total CSF:plasma protein-unbound concentration ratios for the older agents are indicative of significant binding to CSF proteins. For example, from a study of thioridazine in 48 patients, lumbar puncture followed by venipuncture was performed to obtain ratios of parent drug and metabolite in CSF compared to plasma. The average total CSF:unbound plasma ratio of thioridazine was determined to be 6 and ranged from 1.9 – 16.9,(75) although it is unknown if all the patients in this analysis were under steady state conditions or what the time of sampling of CSF and plasma were relative to the dose.(75) From the same analysis, mean free fraction of thioridazine in the CSF was 49% and the unbound concentration in CSF was twice that in plasma, possibly on account of passive diffusion of thioridazine across BBB. A significant correlation (p=0.002) was shown between the unbound concentration of thioridazine in plasma and CSF,(75) suggesting that unbound concentrations in plasma could potentially be used as a surrogate for CSF concentrations or neuroleptic efficacy. In a later study, the plasma from 53 patients newly started on 200mg/day thioridazine was sampled 12 hours post-dose 6 times over the course of two weeks. However, this analysis did not establish any link between plasma concentrations of thioridazine and anti-psychotic efficacy.(76)
Substrates of drug efflux transporters (eg risperidone and P-gp affinity), may show a lack of correlation between plasma and CSF concentrations. In these cases, other correlates of efficacy such as unbound CSF drug concentrations need to be used. More recent PKPD analyses have explored the relationship between CSF concentration of anti-psychotics and receptor occupancy data.(77,78) In general, while CSF concentrations of antipsychotics correlated with efficacy (eg chlorpromazine),(79,80) this is not always the case due to difficulties in quantifying low CSF drug concentrations (eg. haloperidol).(79,81) Another potential confounder in the relationship between drug concentration and efficacy occurs if there is metabolism to a moiety with anti-psychotic effect. For example, the active metabolite of risperidone, 9-hydroxyrisperidone (paliperidone) is itself a marketed antipsychotic.
The importance of combined PKPD modeling compared to PD alone has been demonstrated for anti-psychotics. Aripiprazole was dosed in 18 subjects from 2mg to 30mg, and PET scans were taken pre-dose and 3, 4, 5, and 120 hours post-dose.(82) Hysteresis was present in the relationship between dopamine receptor occupancy and plasma concentrations due to delayed effect site equilibration. This resulted in the EC50 value changing based on the type of modeling performed. With the combined PKPD analysis of predicted effect site concentration versus receptor occupancy, the EC50 was 8.6ng/ml.(82) However, considering only PD, the EC50 was slightly higher (11.1 ng/mL) due to hysteresis causing a change in the concentration-response slope. Therefore, for drugs where there is discrepancy between the time course of measured plasma concentration and receptor occupancy,(82,83) a combined PKPD analysis results in more reliable estimates of activity and accurate PD endpoints.
5. Clinical pharmacokinetics and pharmacodynamics of drugs used to treat Alzheimer’s disease in the CNS
In the fall of 2017, interpedine and verubecestat were the latest drug failures for Alzheimer’s disease.(84) An examination of the clinical pharmacology of the currently approved drugs for Alzheimer’s identifies several potential sources for failure of clinical trials. Alzheimer’s is a progressive disease where deteriorating brain pathology may lead to altered drug concentrations in the brain. This may be challenging when interpreting PK results from healthy volunteers or animal models. For example, a recent PET scan analysis performed 2.5–3 hour (Tmax) after a single oral dose of 1mg or 30ug 11C-donepezil in four healthy women(85) showed that the mean standardized unit value for mean intensity of pixels imaged (SUVmean) was 0.9 in the brain for both doses which is indicative of an almost even distribution of radioactivity in the brain compared to the rest of the body. However, in a study of donepezil in patients with Alzheimer’s, despite achieving concentrations in the CSF that were ten times lower than plasma, higher concentrations at 24 hours post-dose compared to 12 hours post-dose was observed in CSF but not plasma.(86,87) This is thought to be due to the degradation of P-gp protein in the progressive pathogenesis of Alzheimer’s (donepezil is a substrate of P-gp) that reduces the efflux of drug from CSF.(87) Given the localization of P-gp in BBB and its role in the efflux of drugs from the brain tissue, one might expect similar accumulation of donepezil to occur in brain tissue of Alzheimer’s patients as well, however, this is unknown. Another consideration is the suitability of surrogate PK measurements and their relationship with target site concentrations. For the NMDA receptor antagonist memantine, concentrations in the CSF from 6 patients (0.05–0.3uM) were 50% lower than serum concentrations(88) while the brain tissue concentration of memantine measured from a single autopsy patient (5.7 mg/kg) was 2.7-times higher than the heart blood concentration (2.1 ug/ml) and 6.9-times higher than the femoral blood concentration (0.83 ug/ml).(89) While such data may be too sparse to interpret, memantine is a basic compound (pKa = 10.7),(88) and sequestering within acidic lysosomes via pH partitioning and lysosomal trapping may be responsible for the enhanced brain accumulation of the drug compared to CSF. While clinical brain tissue concentrations are unknown for the acetyl cholinesterase (AChE) inhibitor rivastigmine,(90) continuous CSF sampling in 18 patients at steady state for up to 12 hours post-dose(91) demonstrated that rivastigmine exhibited differential PK in plasma and CSF. The Cmax in CSF was lower than plasma by 2–4-fold and Tmax in CSF was longer than plasma (1.4–3.8 hours compared to 0.5–1.67 hours).
There is limited data on the utility of PD measures in patients with Alzheimer’s disease. For example, an earlier review noted complications of using AChE activity measurements as an outcome measure due to confounding by a number of factors such as diet, concomitant medication or time of lumbar puncture,(92) making the effect size of PKPD analyses more difficult to interpret. Further, while there are some studies that utilize plasma concentrations to correlate with treatment outcomes,(93) plasma concentrations must first be validated as an appropriate surrogate for the target site.
6. Clinical pharmacokinetics and pharmacodynamics of antiretrovirals in the CNS
In 2007, research nosology in the field of HIV was updated(71) to provide guidance on the neurocognitive disorders caused due to HIV – collectively called HIV-associated neurocognitive disorders (HAND). Since this time, the CNS has been implicated as an anatomical reservoir for HIV,(94–97) capable of harboring latent viral infection in macrophage and microglia cells in the brain. To advance our understanding of both the treatment and potential cure for HIV in the CNS, it is imperative to understand the PK of antiretrovirals (ARVs) in the CNS and their relationship with neurocognition and latent reservoirs.
ARV PK has been extensively studied in the CSF and the reader is referred to two reviews summarizing this topic.(44,98) Using measures of CSF PK of ARVs along with physicochemical properties of the drugs and clinical utility, Letendre and colleagues devised a CNS-penetration effectiveness (CPE) score that accounts for efficacy of ARVs and extent of penetration into the CNS.(99) The scores range from 1–4 with 1 being less effective (having lowest CNS penetration), and 4 being most effective (having highest CNS penetration).(99) ARVs having higher CPE score cause greater reduction in viral load in the CSF in HIV patients.(100) However, the correlation between CPE score and degree of neurocognitive impairment in patients with HAND is variable. For example, while improvement in neurocognitive function was noted by using agents with a higher CPE score in some studies,(101) there are instances where higher CPE was not associated with neurocognitive improvement,(102) or where higher CPE was associated with poorer functioning.(103,104)
Given the contradicting PKPD results, one hypothesis is that brain tissue concentration of ARVs may be a better predictor of neurocognitive impairment in patients with HAND. However, there are sparse clinical data on the agreement between CSF and brain tissue concentrations of ARVs. In a small study by Bumpus and colleagues, sub-compartmental brain tissue concentration of ARVs were evaluated in 9 HIV-positive adults who had AIDS at the time of death.(105) Concentrations in white matter, cortical gray matter and globus pallidus regions of the brain were taken from necropsy samples, and compared to historical CSF concentration data.(105) No difference in brain and CSF concentration was found for efavirenz, emtricitabine, atazanavir and lamivudine. However, for tenofovir, the overall brain concentration of 206 ng/g was 37-fold higher than CSF. For lopinavir, a protease inhibitor, greater accumulation was found in white matter (>400 ng/g) compared to other brain regions (<25 ng/g). Contrary to these data, a recent in-silico model(59) predicted that efavirenz accumulates in brain tissue, with a median tissue-plasma penetration ratio of 15.8. Data recently published in 12 non-human primates(106) showed that tenofovir, emtricitabine, efavirenz, raltegravir, maraviroc and atazanavir all reached higher total concentrations in brain tissue compared to CSF at trough. For efavirenz, the brain tissue to CSF concentration ratio was highest (769-fold) and brain tissue-plasma penetration ratio ranged from 3–5.7, indicating accumulation of efavirenz. Since information on patient adherence was not available for the Bumpus study and comparisons between brain tissue and CSF concentrations were made with historical CSF estimates, low adherence to an ARV regimen before death could explain why efavirenz concentrations were equivalent to the CSF measurements and much lower in these samples than that demonstrated in the nonhuman primates or predicted in the model.
A critical area for future investigation is PKPD correlations as they relate to development of latent reservoirs in target cells of the brain tissue. With advances in mass spectrometry imaging, this work may be able to determine specific distribution patterns of ARVs in the brain(20) that can lead to differential viral growth or establish latency if there is insufficient ARV coverage. Another area of research is to understand the optimal range of intracellular concentration that can prevent HIV cellular infection without CNS toxicity(107).
7. Optimization of Pharmacokinetics/Pharmacodynamics
7.1 Study of biomarkers
In Alzheimer’s disease, abnormal aggregation of protein can manifest as cognitive impairment or dementia.(108) Often, protein accumulation processes begin before clinical manifestations. Therefore, the search for quantifiable proteins or biomarkers in the CSF or blood is important for diagnosis. Biomarkers may also have utility as PD endpoints and a recent review identified amyloid and tau in the CSF as commonly used biomarker outcome measures in ongoing clinical trials for Alzheimer’s disease.(109) As previously demonstrated(110) the utility of these measures comes from the stability of these biomarkers over time and significant differences in concentrations attained between patients with Alzheimer’s and healthy volunteers. Utility of biomarkers to aid in anti-depressant drug development was recently demonstrated by Kielbasas et al. In an indirect response analysis, plasma PK concentrations of the antidepressants atomoxetine, duloxetine and edivoxetine were modeled against the CSF concentration of 3,4-dihydroxyphenylglycol (DHPG),(111) the deaminated form of norepinephrine, as a biomarker. The analysis showed that the antidepressants all had a maximal inhibition of rate of formation of DHPG (Imax) of 33–37% in plasma and that edivoxetine was most potent. However, when in CSF, Imax was much greater for edivoxetine (75%) compared to atomoxetine (53%) and duloxetine (38%). Further investigation of such biomarkers in the clinic can assist in the discovery of novel drug candidates.
Identification of novel biomarkers may also be useful in the field of neuro-HIV as a surrogate measure for neurocognitive impairment(112) to avoid the possibility of confounding with subjective psychiatric tests. In this regard, neurofilament light chain (NFL) has shown promise as a biomarker relating to HAND, although there have been no clinical studies evaluating the correlation of ARV and biomarker concentrations in HIV patients. Similarly, biomarkers should also be explored as a surrogate for establishment of latent HIV reservoir in the brain.(113)
7.2 Modeling and simulation
Several modeling tools have been developed to predict drug disposition within brain. Both a top-down approach (population PK modeling)(78) and a bottom-up approach (PBPK modeling)(26,77,114,115) have been used to predict the brain penetration of various drugs in humans using in-vitro and animal data. Gaohua and colleagues recently developed an extensive PBPK model that incorporated 4 additional compartments of the brain(26): brain blood, brain mass and cranial and spinal CSF. The model was well-suited to describe anatomy and physiology of the brain including passive and active transport mechanisms through the BBB. The model was validated with measured clinical concentrations and in-vitro data for phenytoin and paracetamol and was used to simulate various scenarios that mimicked transporter mediated mechanisms and CSF turnover. A recently developed generic PBPK model that incorporated 5 CSF compartments, including the extravascular drainage from CSF as well as intracellular and extracellular brain compartments was utilized to predict the human brain and CSF PK of nine diverse drugs, including antipsychotics and antidepressants.(116) Such efforts will greatly improve our understanding of CNS target site approximations in humans. Modeling techniques further benefit from incorporating both the PK profile as well as the concentration and effect of endogenous substances. For example(117), a mechanistic monkey PKPD model was developed using plasma and CSF concentrations from the cisterna magna of two novel BACE-1 inhibitors with beta-amyloid and secreted amyloid-precursor protein biomarkers.(117) This model could predict in-vivo inhibition of BACE-1 and effect on amyloid precursor processing by the BACE-1 inhibitors using in-vitro cellular inhibition and enzyme activities as well as drug concentration data.
7.3 PKPD translation from preclinical models
Developing innovative animal models for CNS research could address issues in clinical PKPD such as the relationship between effect site drug concentrations and novel biomarkers, as well as allow for the discovery of novel targets. Zebrafish models have been refined to study several neuro-behavioral disorders such as depression, Parkinson’s disease and attention-deficit hyperactive disorder (ADHD)(118). They offer the advantages of low cost and genetic manipulation over traditional lab species such as rodents, and show a high degree of genetic and physiologic homology to mammals(119). Novel rodent models have also been explored for pediatric epilepsy(120) and CNS involvement in HIV infection(121).
For current animal models, their clinical applicability must be carefully examined. For example, certain animals may lack receptors or drug targets available in humans. Animal models may also differ in expression or activity of drug metabolizing enzymes and transporters. Comprehensive work by Terasaki and colleagues in quantitative targeted absolute proteomics (QTAP) have quantified transporter protein concentrations on the BBB of several species, including humans(50,122) and found interspecies differences in several important transporters. For instance, humans have a greater absolute concentration (fmol/ug of protein) of BCRP compared to mice, while mice have a greater absolute concentration of P-gp, OATP1A2, MRP4 and OAT3. Similarly, absolute transporter concentrations in cynomolgus monkeys track more closely with humans than mice. Since multiple transporters contribute to both the uptake and efflux of drugs at the BBB, the relationship between transporter expression/activity and PK is not straight forward in the CNS. Therefore, while transporter differences at BBB are not currently considered in allometry, models that account for differential transporter-activity between species in CNS are needed to understand if this warrants changes in human dose.
Conclusion
Understanding drug penetration and effect at the various sites of the CNS is essential for neuro-active drug development. Currently, information on brain and CSF drug distribution exists for anti-psychotics, Alzheimer’s drugs and anti-infectives. However, the interaction between drug concentration and effect is still not clearly defined across these areas. Integrating PKPD information for drugs acting in the CNS would allow for better prediction of first in human dose and improve the attrition rate of CNS drug research. In support of this, better utilization of tools such as biomarker identification and modeling can help pave the way for more rigorous explanation of clinical brain PKPD.
Supplementary Material
Key points.
A better understanding of concentration-effect relationships at the target sites in the central nervous system (CNS) is essential for development of neuroactive drugs. However, there is still a paucity of information on these relationships for several drugs acting in the CNS.
The use of newer methods such as biomarker identification and modeling and simulation will allow for better prediction of first in human dosing and may lead to improvements in the success rate of CNS drug development programs.
Acknowledgments
The authors would like to thank Rachael Posey for devising the search strategy for the review article.
FUNDING: This work was funded by the Center for AIDS Research [grant number CFAR P30 AI50410] and the NIH/NIAID [grant number R01AI111891-04]. N Srinivas is supported by the Royster Society of Fellows.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
CONFLICT OF INTEREST: N Srinivas, K Maffuid, and A Kashuba declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
REFERENCE LIST
- 1.Neurological Disorders: Public Health Challenges. 2006. [Google Scholar]
- 2.Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387(10028):1672–85. doi: 10.1016/S0140-6736(15)00390-6. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline : few candidates, frequent failures. Alzheimer’s Res Ther. 2014 Jul;6:1–7. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedetti F, Carlino E, Piedimonte A. Increasing uncertainty in CNS clinical trials : the role of placebo, nocebo, and Hawthorne effects. Lancet Neurol. 2016;15(7):736–47. doi: 10.1016/S1474-4422(16)00066-1. [DOI] [PubMed] [Google Scholar]
- 5.Kesselheim AS, Hwang TJ, Franklin JM. Two decades of new drug development for central nervous system disorders. Nat Rev. 2015;14(12):815–6. doi: 10.1038/nrd4793. [DOI] [PubMed] [Google Scholar]
- 6.Pangalos MN, Schechter LE, Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov. 2007 Jul;6:521–32. doi: 10.1038/nrd2094. [DOI] [PubMed] [Google Scholar]
- 7.Miller G. Is Pharma Running Out of Brainy Ideas? Science (80- ) 2012 Jul;329(2010) doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- 8.Choi DW, Armitage R, Brady LS, Coetzee T, Fisher W, Hyman S, et al. Perspective Medicines for the Mind : Policy-Based “‘ Pull ’” Incentives for Creating Breakthrough CNS Drugs. Neuron. 2013;84(3):554–63. doi: 10.1016/j.neuron.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Goetghebeur PJD, Swartz JE. True alignment of preclinical and clinical research to enhance success in CNS drug development : a review of the current evidence. J Psychopharmacol. 2016 Jul;586(2016):1–9. doi: 10.1177/0269881116645269. [DOI] [PubMed] [Google Scholar]
- 10.de Lange ECM, Hammarlund-Udenaes M. Translational aspects of blood-brain barrier transport and central nervous system effects of drugs: from discovery to patients. Clin Pharmacol Ther. 2015;97(4):380–94. doi: 10.1002/cpt.76. [DOI] [PubMed] [Google Scholar]
- 11.Aronson JK. Concentration-effect and dose-response relations in clinical pharmacology. Br J Clin Pharmacol. 2007;63(3):255–7. doi: 10.1111/j.1365-2125.2007.02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markou A, Chiamulera C, Geyer MA, Tricklebank M. Removing obstacles in neuroscience drug discovery: The future path for animal models. Neuropsychopharmacology. 2009;34(1):74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deo AK, Theil FP, Nicolas JM. Confounding parameters in preclinical assessment of blood-brain barrier permeation: An overview with emphasis on species differences and effect of disease states. Mol Pharm. 2013;10(5):1581–95. doi: 10.1021/mp300570z. [DOI] [PubMed] [Google Scholar]
- 14.Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug Metabolism and Pharmacokinetics, the Blood-Brain Barrier, and Central Nervous System Drug Discovery. 2005 Oct;2:554–71. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud P-O, Deli MA, et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16630991. 0271678X16630991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelt-Menzel A, Cubukova A, Günther K, Edenhofer F, Piontek J, Krause G, et al. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem Cell Reports. 2017;8(4):894–906. doi: 10.1016/j.stemcr.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmiotti CA, Prasad S, Naik P, Abul KMD, Sajja RK, Achyuta AH, et al. In vitro cerebrovascular modeling in the 21st century: Current and prospective technologies. Pharm Res. 2014;31(12):3229–50. doi: 10.1007/s11095-014-1464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridén M, Gupta A, Antonsson M, Bredberg UH-UM. In Vitro Methods for Estimating Unbound Drug Concentrations in the brain interstitial and intracellular fluids. Drug Metab Dispos. 2007;35(9):1711–9. doi: 10.1124/dmd.107.015222. [DOI] [PubMed] [Google Scholar]
- 19.Wiseman JM, Ifa DR, Zhu Y. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc …. 2008:2–7. doi: 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson CG, Bokhart MT, Sykes C, Adamson L, Fedoriw Y, Luciw PA, et al. Mass spectrometry imaging reveals heterogeneous efavirenz distribution within putative HIV reservoirs. Antimicrob Agents Chemother. 2015;59(5):2944–8. doi: 10.1128/AAC.04952-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shobo A, Bratkowska D, Baijnath S, Naiker S, Somboro AM, Bester LA, et al. Tissue distribution of pretomanid in rat brain via mass spectrometry imaging. Xenobiotica. 2016;46(3):247–52. doi: 10.3109/00498254.2015.1067935. [DOI] [PubMed] [Google Scholar]
- 22.Aikawa H, Hayashi M, Ryu S, Yamashita M, Ohtsuka N, Nishidate M, et al. Visualizing spatial distribution of alectinib in murine brain using quantitative mass spectrometry imaging. Sci Rep. 2016 Oct;6(2015):23749. doi: 10.1038/srep23749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varnäs K, Varrone A, Farde L. Modeling of PET data in CNS drug discovery and development. J Pharmacokinet Pharmacodyn. 2013;40(3):267–79. doi: 10.1007/s10928-013-9320-6. [DOI] [PubMed] [Google Scholar]
- 24.Shannon RJ, Carpenter KLH, Guilfoyle MR, Helmy A, Hutchinson PJ. Cerebral microdialysis in clinical studies of drugs: Pharmacokinetic applications. J Pharmacokinet Pharmacodyn. 2013;40(3):343–58. doi: 10.1007/s10928-013-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindberger M, Tomson T, Lars S. Microdialysis sampling of carbamazepine, phenytoin and phenobarbital in subcutaneous extracellular fluid and subdural cerebrospinal fluid in humans: an in vitro and in vivo study of adsorption to the sampling device. J Pharmacol Toxicol. 2002:158–65. doi: 10.1034/j.1600-0773.2002.910402.x. [DOI] [PubMed] [Google Scholar]
- 26.Gaohua L, Neuhoff S, Johnson TN, Rostami-hodjegan A, Jamei M, Centre BE, et al. Development of a permeability-limited model of the human brain and cerebrospinal fluid (CSF) to integrate known physiological and biological knowledge: Estimating time varying CSF drug concentrations and their variability using in vitro data. Drug Metab Pharmacokinet. 2016;31(3):1–49. doi: 10.1016/j.dmpk.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Smith BJ, Chen C, Callegari E, Becker SL, Chen X, et al. Evaluation of cerebrospinal fluid concentration and plasma free concentration as a surrogate measurement for brain free concentration. Drug Metab Dispos. 2006;34(9):1443–7. doi: 10.1124/dmd.105.008201. [DOI] [PubMed] [Google Scholar]
- 28.Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010 Dec;9:929–39. doi: 10.1038/nrd3287. [DOI] [PubMed] [Google Scholar]
- 29.Fridén M, Winiwarter S, Jerndal G, Bengtsson O, Hong W, Bredberg U, et al. Structure-brain exposure relationships in rat and human using a novel data set of unbound drug concentrations in brain interstitial and cerebrospinal fluids. J Med Chem. 2009;52(20):6233–43. doi: 10.1021/jm901036q. [DOI] [PubMed] [Google Scholar]
- 30.Kodaira H, Kusuhara H, Fujita T, Ushiki J, Fuse E, Sugiyama Y. Quantitative evaluation of the impact of active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier on the predictability of the unbound concentrations of drugs in the brain using cerebrospinal fluid concentration as a. J Pharmacol Exp Ther. 2011;339(3):935–44. doi: 10.1124/jpet.111.180398. [DOI] [PubMed] [Google Scholar]
- 31.Shen DD, Artru AA, Adkison KK. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv Drug Deliv Rev. 2004;56(12):1825–57. doi: 10.1016/j.addr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 32.De Lange ECM. Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn. 2013;40(3):315–26. doi: 10.1007/s10928-013-9301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kassem NA, Deane R, Segal MB, Chen R, Preston JE. Thyroxine (T4) transfer from CSF to choroid plexus and ventricular brain regions in rabbit: Contributory role of P-glycoprotein and organic anion transporting polypeptides. Brain Res. 2007;1181(1):44–50. doi: 10.1016/j.brainres.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 34.Westerhout J, Smeets J, Danhof M, De Lange ECM. The impact of P-gp functionality on non-steady state relationships between CSF and brain extracellular fluid. J Pharmacokinet Pharmacodyn. 2013;40(3):327–42. doi: 10.1007/s10928-013-9314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nau R, Zysk G, Thiel A, Prange HW. Pharmacokinetic quantification of the exchange of drugs between blood and cerebrospinal fluid in man. Eur J Clin Pharmacol. 1993;45(5):469–75. doi: 10.1007/BF00315520. [DOI] [PubMed] [Google Scholar]
- 36.Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–83. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yilmaz A, Watson V, Dickinson L, Back D. Efavirenz pharmacokinetics in cerebrospinal fluid and plasma over a 24-hour dosing interval. Antimicrob Agents Chemother. 2012;56(9):4583–5. doi: 10.1128/AAC.06311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chicano-Piá PV, Cercós-Lletí AC, Romá-Sánchez E. Pharmacokinetic model for tobramycin in acinetobacter meningitis. Ann Pharmacother. 2002;36(1):83–6. doi: 10.1345/aph.19114. [DOI] [PubMed] [Google Scholar]
- 39.Kühnen E, Pfeifer G, Frenkel C. Penetration of fosfomycin into cerebrospinal fluid across non-inflamed and inflamed meninges. Infection. 1987;15(6):422–4. doi: 10.1007/BF01647220. [DOI] [PubMed] [Google Scholar]
- 40.Capparelli EV, Letendre SL, Ellis RJ, Patel P, Holland D, Mccutchan JA. Population Pharmacokinetics of Abacavir in Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Abacavir in Plasma and Cerebrospinal Fluid. Antimicrob Agents Chemother. 2005;49(6):2504–6. doi: 10.1128/AAC.49.6.2504-2506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rambeck B, Jürgens UH, May TW, Wolfgang Pannek H, Behne F, Ebner A, et al. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47(4):681–94. doi: 10.1111/j.1528-1167.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 42.Hammarlund-Udenaes M. Active-site concentrations of chemicals - Are they a better predictor of effect than plasma/organ/tissue concentrations? Basic Clin Pharmacol Toxicol. 2010;106(3):215–20. doi: 10.1111/j.1742-7843.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- 43.Kakee A, Terasaki T, Sugiyama Y. Brain efflux index as a novel method of analyzing efflux transport at the blood-brain barrier. J Pharmacol Exp Ther. 1996;277(3):1550–9. [PubMed] [Google Scholar]
- 44.Calcagno A, Di Perri G, Bonora S, AC, GDP, SB Pharmacokinetics and Pharmacodynamics of Antiretrovirals in the Central Nervous System. Clin Pharmacokinet. 2014;53(10):891–906. doi: 10.1007/s40262-014-0171-0. [DOI] [PubMed] [Google Scholar]
- 45.Ahn SM, Byun K, Cho K, Kim JY, Yoo JS, Kim D, et al. Human microglial cells synthesize albumin in brain. PLoS One. 2008;3(7):4–9. doi: 10.1371/journal.pone.0002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Read KD, Braggio S. Assessing brain free fraction in early drug discovery. Expert Opin Drug Metab Toxicol. 2010;6(3):337–44. doi: 10.1517/17425250903559873. [DOI] [PubMed] [Google Scholar]
- 47.Di L, Umland JP, Chang G, Huang Y, Lin Z, Scott DO, et al. Species independence in brain tissue binding using brain homogenates. Drug Metab Dispos. 2011;39(7):1270–7. doi: 10.1124/dmd.111.038778. [DOI] [PubMed] [Google Scholar]
- 48.Lee G, Dallas S, Hong M, Bendayan R. Drug Transporters in the Central Nervous System : Brain Barriers and Brain Parenchyma Considerations. 2001;53(4):569–96. [PubMed] [Google Scholar]
- 49.Hartz AMS, Bauer B. ABC Transporters in the CNS – An Inventory. 2011:656–73. doi: 10.2174/138920111795164020. [DOI] [PubMed] [Google Scholar]
- 50.Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117(2):333–45. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- 51.Löscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRX. 2005;2(1):86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao R, Raub TJ, Sawada GA, Kasper SC, Bacon JA, Bridges AS, et al. Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood-brain barrier. Drug Metab Dispos. 2009;37(6):1251–8. doi: 10.1124/dmd.108.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leggas M, Adachi M, Scheffer G, Sun D, Wielinga P, Du G, et al. MRP4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–21. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalvass JC, Polli JW, Bourdet DL, Feng B, Huang S-M, Liu X, et al. Why clinical modulation of efflux transport at the human blood-brain barrier is unlikely: the ITC evidence-based position. Clin Pharmacol Ther. 2013;94(1):80–94. doi: 10.1038/clpt.2013.34. [DOI] [PubMed] [Google Scholar]
- 55.van Praag RM, Weverling GJ, Portegies P, Jurriaans S, Zhou XJ, Turner-Foisy ML, et al. Enhanced penetration of indinavir in cerebrospinal fluid and semen after the addition of low-dose ritonavir. AIDS. 2000;14(9):1187–94. doi: 10.1097/00002030-200006160-00016. [DOI] [PubMed] [Google Scholar]
- 56.Toda R, Kawazu K, Oyabu M, Miyazaki T, Kiuchi Y. Comparison of drug permeabilities across the blood-retinal barrier, blood-aqueous humor barrier, and blood-brain barrier. J Pharm Sci. 2011;100(9):3904–11. doi: 10.1002/jps.22610. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Chen C. Strategies to optimize brain penetration in drug discovery. Curr Opin Drug Discov Devel. 2005;8(4):505–12. [PubMed] [Google Scholar]
- 58.Liu X, Smith BJ, Chen C, Callegari E, Becker SL, Chen X, et al. Use of a physiologically based pharmacokinetic model to study the time to reach brain equilibrium: an experimental analysis of the role of blood-brain barrier permeability, plasma protein binding, and brain tissue binding. J Pharmacol Exp Ther Donepezil Cerebrospinal Fluid AD Patients Eval Dos Suffic Stand Treat Strateg. 2005;313(3):1254–62. doi: 10.1124/jpet.104.079319. [DOI] [PubMed] [Google Scholar]
- 59.Curley P, Rajoli RKR, Moss DM, Liptrott NJ, Letendre S, Owen A. Efavirenz Is Predicted To Accumulate in Brain Tissue: and In Silico, In Vitro and In Vivo Investigation. Antimicrob Agents Chemother. 2017;61(1):1–10. doi: 10.1128/AAC.01841-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kornhuber J, Schoppmeyer K, Riederer P. Affinity of 1-aminoadamantanes for the sigma binding site in post-mortem human frontal cortex. Neurosci Lett. 1993;163(2):129–31. doi: 10.1016/0304-3940(93)90362-o. [DOI] [PubMed] [Google Scholar]
- 61.Yokoi F, Grunder G, Biziere K, Stephane M, Dogan AS, Dannals RF, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248–59. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 62.Farde L, Wiesel Fa, Halldin C, Sedvall G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry. 1988;45(1):71–6. doi: 10.1001/archpsyc.1988.01800250087012. [DOI] [PubMed] [Google Scholar]
- 63.Mamo D, Sc M, Papatheodorou G, Mann S, Therrien F, Pharm D, et al. A PET Study of Dopamine D 2 and Serotonin 5-HT 2 Receptor Occupancy in Patients With Schizophrenia Treated With Therapeutic Doses of Ziprasidone. 2004 May;:818–25. doi: 10.1176/appi.ajp.161.5.818. [DOI] [PubMed] [Google Scholar]
- 64.Sato H, Ito C, Tashiro M, Hiraoka K, Shibuya K, Funaki Y, et al. Histamine H1 receptor occupancy by the new-generation antidepressants fluvoxamine and mirtazapine: A positron emission tomography study in healthy volunteers. Psychopharmacology (Berl) 2013;230(2):227–34. doi: 10.1007/s00213-013-3146-1. [DOI] [PubMed] [Google Scholar]
- 65.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 66.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799–812. [Google Scholar]
- 68.Robert P, Ferris S, Gauthier S, Ihl R, Winblad B, Tennigkeit F. Review of Alzheimer’s disease scales: is there a need for a new multi-domain scale for therapy evaluation in medical practice? Alzheimers Res Ther. 2010;2(4):24. doi: 10.1186/alzrt48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunlop BW, Mayberg HS. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci. 2014;16(4):479–90. doi: 10.31887/DCNS.2014.16.4/bdunlop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ene L, Duiculescu D, Ruta SM. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life. 2011;4(4):432–9. [PMC free article] [PubMed] [Google Scholar]
- 71.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zöchling R, et al. Persistence of haloperidol in human brain tissue. Am J Psychiatry. 1999;156(6):885–90. doi: 10.1176/ajp.156.6.885. [DOI] [PubMed] [Google Scholar]
- 73.Sampedro MC, Unceta N, Gómez-Caballero A, Callado LF, Morentin B, Goicolea MA, et al. Screening and quantification of antipsychotic drugs in human brain tissue by liquid chromatography-tandem mass spectrometry: Application to postmortem diagnostics of forensic interest. Forensic Sci Int. 2012;219(1–3):172–8. doi: 10.1016/j.forsciint.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Caccia S. Pharmacokinetics and metabolism update for some recent antipsychotics. Expert Opin Drug Metab Toxicol. 2011;7(7):829–46. doi: 10.1517/17425255.2011.575061. [DOI] [PubMed] [Google Scholar]
- 75.Nyberg G, Axelsson R, Mftrtensson E. Cerebrospinal Fluid Concentrations of Thioridazine and Its Main Metabolites in Psychiatric Patients. 1981;148:139–48. doi: 10.1007/BF00568401. [DOI] [PubMed] [Google Scholar]
- 76.Cohen BM, Lipinski JF, Waternaux C. A fixed dose study of the plasma concentration and clinical effects of thioridazine and its major metabolites. Psychopharmacology (Berl) 1989;97(4):481–8. doi: 10.1007/BF00439552. [DOI] [PubMed] [Google Scholar]
- 77.Alqahtani S, Kaddoumi A. Development of a Physiologically Based Pharmacokinetic/Pharmacodynamic Model to Predict the Impact of Genetic Polymorphisms on the Pharmacokinetics and Pharmacodynamics Represented by Receptor/Transporter Occupancy of Central Nervous System Drugs. Clin Pharmacokinet. 2016;55(8):957–69. doi: 10.1007/s40262-016-0367-6. [DOI] [PubMed] [Google Scholar]
- 78.Li CH, Stratford RE, Velez de Mendizabal N, Cremers TI, Pollock BG, Mulsant BH, et al. Prediction of brain clozapine and norclozapine concentrations in humans from a scaled pharmacokinetic model for rat brain and plasma pharmacokinetics. J Transl Med. 2014;12(1):203. doi: 10.1186/1479-5876-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garver DL. Neuroleptic Drug Levels and Antipsychotic Effects: A difficult Correlation; Potential Advantage of Free (or Derivative) versus Total Plasma Levels. J Clin Psychopharmacol. 1989;9(4):277–81. [PubMed] [Google Scholar]
- 80.Wode-Helgodt BB. Clinical effects and drug concentrations in plasma and cerebrospinal fluid in psychotic patients treated with fixed doses of chlorpromazine. Acta Psychiatr Scand. 1978;58(2):149–73. doi: 10.1111/j.1600-0447.1978.tb06929.x. [DOI] [PubMed] [Google Scholar]
- 81.Rimón R, Averbuch I, Rozick P, Fijman-Danilovich L, Kara T, Dasberg H, et al. Serum and CSF levels of haloperidol by radioimmunoassay and radioreceptor assay during high-dose therapy of resistant schizophrenic patients. Psychopharmacology (Berl) 1981;73(2):197–9. doi: 10.1007/BF00429218. [DOI] [PubMed] [Google Scholar]
- 82.Kim E, Howes OD, Kim B-H, Jeong JM, Lee JS, Jang I-J, et al. Predicting brain occupancy from plasma levels using PET: superiority of combining pharmacokinetics with pharmacodynamics while modeling the relationship. J Cereb Blood Flow Metab. 2012;32(4):759–68. doi: 10.1038/jcbfm.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greenblatt DJ, von Moltke LL, Ehrenberg BL, Harmatz JS, Corbett KE, Wallace DW, et al. Kinetics and dynamics of lorazepam during and after continuous intravenous infusion. Crit Care Med. 2000;28(8):2750–7. doi: 10.1097/00003246-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 84.Carrol J. Another Alzheimer’s drug flops in pivotal clinical trial. Science. 2017 [Google Scholar]
- 85.Mochida I, Shimosegawa E, Kanai Y, Naka S, Isohashi K, Horitsugi G, et al. Whole-Body Distribution of Donepezil as an Acetylcholinesterase Inhibitor after Oral Administration in Normal Human Subjects : A C-donepezil PET Study. Asia Ocean J Nucl Med Biol. 2017;5(1):3–9. doi: 10.22038/aojnmb.2016.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valis M, Masopust J, Vysata O, Hort J, Dolezal R, Tomek J, et al. Concentration of Donepezil in the Cerebrospinal Fluid of AD Patients: Evaluation of Dosage Sufficiency in Standard Treatment Strategy. Neurotox Res. 2016 doi: 10.1007/s12640-016-9672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Darreh-Shori T, Meurling L, Pettersson T, Hugosson K, Hellström-Lindahl E, Andreasen N, et al. Changes in the activity and protein levels of CSF acetylcholinesterases in relation to cognitive function of patients with mild Alzheimer’s disease following chronic donepezil treatment. J Neural Transm. 2006;113(11):1791–801. doi: 10.1007/s00702-006-0526-2. [DOI] [PubMed] [Google Scholar]
- 88.Kornhuber J, Quack G. Cerebrospinal fluid and serum concentrations of the N-methyl-d-aspartate (NMDA) receptor antagonist memantine in man. Neurosci Lett. 1995;195(2):137–9. doi: 10.1016/0304-3940(95)11785-u. [DOI] [PubMed] [Google Scholar]
- 89.Rohrig TP, Hicks CA. Brain tissue: A viable postmortem toxicological specimen. J Anal Toxicol. 2015;39(2):137–9. doi: 10.1093/jat/bku139. [DOI] [PubMed] [Google Scholar]
- 90.Noetzli M, Eap CB. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of alzheimer’s disease. Clin Pharmacokinet. 2013;52(4):225–41. doi: 10.1007/s40262-013-0038-9. [DOI] [PubMed] [Google Scholar]
- 91.Cutler NR, Polinsky RJ, Sramek JJ, Enz A, Jhee SS, Mancione L, et al. Dose-dependent CSF acetylcholinesterase inhibition by SDZ ENA 713 in Alzheimer’s disease. Acta Neurol Scand. 1998;97(4):244–50. doi: 10.1111/j.1600-0404.1998.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 92.Talesa VN. Acetylcholinesterase in Alzheimer’s disease. Mech Ageing Dev. 2001;122(16):1961–9. doi: 10.1016/s0047-6374(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 93.Wattmo C, Jedenius E, Blennow K, Wallin AK. Dose and plasma concentration of galantamine in Alzheimer’s disease - clinical application. Alzheimers Res Ther. 2013;5(1):1–9. doi: 10.1186/alzrt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fois AF, Brew BJ. The Potential of the CNS as a Reservoir for HIV-1 Infection: Implications for HIV Eradication. Curr HIV/AIDS Rep. 2015:299–303. doi: 10.1007/s11904-015-0257-9. [DOI] [PubMed] [Google Scholar]
- 95.Avalos CR, Price SL, Forsyth ER, Pin JN, Shirk EN, Bullock BT, et al. Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques. J Virol. 2016;90(12):5643–56. doi: 10.1128/JVI.00290-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM, et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. Aids. 2017;31(1):5–14. doi: 10.1097/QAD.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80:1415–23. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Decloedt EH, Rosenkranz B, Maartens G, Joska J. Central nervous system penetration of antiretroviral drugs: pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin Pharmacokinet. 2015;54(6):581–98. doi: 10.1007/s40262-015-0257-3. [DOI] [PubMed] [Google Scholar]
- 99.Letendre S. Validation of the CNS Penetration-Effectiveness Rank for Quantifying Antiretroviral Penetration Into the Central Nervous System. Arch Neurol. 2008;65(1):65. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–66. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ. Effects of Central Nervous System Antiretroviral Penetration on Cognitive Functioning in the ALLRT Cohort. AIDS. 2012;25(3):357–65. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baker LM, Paul RH, Heaps-Woodruff JM, Chang JY, Ortega M, Margolin Z, et al. The Effect of Central Nervous System Penetration Effectiveness of Highly Active Antiretroviral Therapy on Neuropsychological Performance and Neuroimaging in HIV Infected Individuals. J Neuroimmune Pharmacol. 2015:487–92. doi: 10.1007/s11481-015-9610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marra CM. HIV-associated neurocognitive disorders and central nervous system drug penetration: what next? Antivir Ther. 2015 doi: 10.3851/IMP2951. [DOI] [PubMed] [Google Scholar]
- 104.Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83(2):134–41. doi: 10.1212/WNL.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bumpus N, Ma Q, Best B, Moore D, Ellis RJ, Crescini M, et al. Antiretroviral Concentrations in Brain Tissue Are Similar to or Exceed Those in CSF. 2015:92103. [Google Scholar]
- 106.Srinivas N, Fallon JK, Sykes C, White N. SHIV INFECTION AND DRUG TRANSPORTERS INFLUENCE BRAIN TISSUE CONCENTRATIONS OF EFAVIRENZ. International AIDS Society; Paris, France: 2017. [Google Scholar]
- 107.Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18(5):388–99. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Llorens F, Schmitz M, Ferrer I, Zerr I. CSF biomarkers in neurodegenerative and vascular dementias. Prog Neurobiol. 2016;138–140:36–53. doi: 10.1016/j.pneurobio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 109.Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s Dement Transl Res Clin Interv. 2017;3(3):367–84. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Bastard N, Aerts L, Sleegers K, Martin J-J, Van Broeckhoven C, De Deyn PP, et al. Longitudinal Stability of Cerebrospinal Fluid Biomarker Levels: Fulfilled Requirement for Pharmacodynamic Markers in Alzheimer’s Disease. J Alzheimer’s Dis. 2013;33(3):807–22. doi: 10.3233/JAD-2012-110029. [DOI] [PubMed] [Google Scholar]
- 111.Kielbasa W, Lobo E. Pharmacodynamics of norepinephrine reuptake inhibition: Modeling the peripheral and central effects of atomoxetine, duloxetine, and edivoxetine on the biomarker 3,4-dihydroxyphenylglycol in humans. J Clin Pharmacol. 2015;55(12):1422–31. doi: 10.1002/jcph.551. [DOI] [PubMed] [Google Scholar]
- 112.McGuire J, Gill A, Douglas S, Kolson D. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neurovirol. 2013;19:S57. doi: 10.1007/s13365-015-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gray LR, Brew BJ, Churchill MJ. Strategies to target HIV-1 in the central nervous system. Curr Opin HIV AIDS. 2016;11(4):371–5. doi: 10.1097/COH.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 114.Ball K, Bouzom F, Scherrmann J-M, Walther B, Declèves X. Physiologically Based Pharmacokinetic Modelling of Drug Penetration Across the Blood-Brain Barrier-Towards a Mechanistic IVIVE-Based Approach. AAPS J. 2013;15(4):913–32. doi: 10.1208/s12248-013-9496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trapa PE, Belova E, Liras JL, Scott DO, Steyn SJ. Insights from an Integrated Physiologically Based Pharmacokinetic Model for Brain Penetration. J Pharm Sci. 2016;105(2):965–71. doi: 10.1016/j.xphs.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 116.Yamamoto Y, Välitalo PA, van den Berg DJ, Hartman R, van den Brink W, Wong YC, et al. A Generic Multi-Compartmental CNS Distribution Model Structure for 9 Drugs Allows Prediction of Human Brain Target Site Concentrations. Pharm Res. 2017;34(2):333–51. doi: 10.1007/s11095-016-2065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu X, Wong H, Scearce-Levie K, Watts RJ, Coraggio M, Shin YG, et al. Mechanistic Pharmacokinetic-Pharmacodynamic Modeling of BACE1 Inhibition in Monkeys: Development of a Predictive Model for Amyloid Precursor Protein Processings. Drug Metab Dispos. 2013;41(7):1319–28. doi: 10.1124/dmd.112.050864. [DOI] [PubMed] [Google Scholar]
- 118.Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci. 2014;35(2):63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nguyen M, Yang E, Neelkantan N, Mikhaylova A, Arnold R, Poudel MK, et al. Developing “integrative” zebrafish models of behavioral and metabolic disorders. Behav Brain Res. 2013;256:172–87. doi: 10.1016/j.bbr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 120.Auvin S, Pineda E, Shin D, Gressens P, Mazarati A. Novel Animal Models of Pediatric Epilepsy. Neurotherapeutics. 2012;9(2):245–61. doi: 10.1007/s13311-012-0119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Honeycutt JB, Sheridan Pa, Matsushima GK, Garcia JV. Humanized mouse models for HIV-1 infection of the CNS. J Neurovirol. 2015;21(3):301–9. doi: 10.1007/s13365-014-0299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ito K, Uchida Y, Ohtsuki S, Aizawa S, Kawakami H, Katsukura Y, et al. Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J Pharm Sci. 2011;100(9):3939–50. doi: 10.1002/jps.22487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.