Abstract
Anticancer activities of flavonoids derived from Tephroseris kirilowii (Turcz.) Holub. were evaluated in human cancer cells. We isolated and identified, for the first time, eight flavonoids from T. kirilowii and found that three of them (IH: isorhamnetin, GN: genkwanin, and Aca: acacetin) inhibited cell proliferation in a variety of human cancer cell lines. These active flavonoids caused cell cycle arrest at G2/M phase and induced apoptosis and autophagy in human breast cancer cells. Molecular docking revealed that these flavonoids dock in the ATP binding pocket of PI3Kγ. Importantly, treatment with these flavonoids decreased the levels of PI3Kγ-p110, phospho-PI3K, phospho-AKT, phospho-mTOR, phospho-p70S6K, and phospho-ULK. Pretreatment with PI3Kγ specific inhibitor AS605240 potentiated flavonoids-mediated inactivation of AKT, mTOR, p70S6K, ULK, and apoptosis. Taken together, these findings represent a novel mechanism by which downregulation of PI3Kγ-p110 and consequent interruption of PI3K/AKT/mTOR/p70S6K/ULK signaling pathway might play a critical functional role in these flavonoids-induced cell cycle arrest at G2/M phase, apoptosis, and autophagy. Our studies provide novel insights into the anticancer activities of selected flavonoids and their potential uses in anticancer therapy.
Introduction
Traditional Chinese medicines have been recently recognized as a new source of anticancer drugs and neoadjuvant chemotherapy to enhance the efficacy of chemotherapy and to alleviate the side effects of cancer chemotherapy1,2. However, the mechanisms of actions are still largely unknown. Flavonoids are a group of more than 4000 polyphenolic compounds that occur naturally in a variety of plant origin3. A growing number of studies indicate that flavonoids or flavonoid derivatives play critical roles in cancer chemoprevention and chemotherapy. A number of epidemiological studies indicate that high flavonoid intake may be correlated with a decreased risk of cancer, and provide evidence for the protective roles of flavonoids against cancer4,5. In vitro studies indicate that anticancer activities of flavonoids may be related to inhibiting cell proliferation, adhesion, and invasion, inducing cell differentiation, cell cycle arrest, and apoptosis, etc.6,7. In vivo studies demonstrate that flavonoids could inhibit carcinogenesis by affecting the molecular events in the initiation, promotion, and progression stages8. The clinical trials of flavonoids in human have been exploited to achieve cancer preventive or therapeutic effects9. Based on these results, flavonoids could be developed as promising agents for cancer chemoprevention and chemotherapy.
Tephroseris kirilowii Turcz. Holub (Compositae) is a perennial herb widely distributed in China10. The whole plant of T. kirilowii exhibit a wide range of biological activities against many types of diseases such as urethral infection, oedema, eczema, scabies, vaginal trichomoniasis, and leukaemia in Chinese-folk medicine11–13. The main constituents of T. kirilowii are alkaloids and flavonoids. Recently, natural compounds from flavonoids have been found to exhibit anti-cancer effects through multiple molecular mechanisms that involve the modulation of apoptosis, cell cycle arrest and autophagy14–16. However, the types of flavonoids in T. kirilowii have not been characterized, nor have the mechanisms of flavonoids-mediated anticancer activities been elucidated in depth. The purpose of the present study is to isolate and characterize the structures of flavonoids from T. kirilowii, to evaluate the effects of flavonoids on anticancer activities, and to elucidate the molecular mechanisms of flavonoids-mediated anticancer activities. In this study, we isolated and identified, for the first time, eight flavonoids from T. kirilowii: isorhamnetin, isorhamnetin-3- glucoside, kaempferol, apigenin, acacetin, chrysin, 7,8-dihydroxyflavanone, and genkwanin. Using bioactivity-guided screening of selected flavonoid compounds isolated from T. kirilowii, we found that three compounds among these flavonoids exhibited anticancer activities. We provide the evidence that isorhamnetin, genkwanin, and acacetin inhibited cell proliferation in human cancer cells through cell cycle arrest at G2/M phase and induction of apoptotic and autophagic cell death. Molecular docking revealed that these flavonoids dock in the ATP binding pocket of PI3Kγ. Mechanistic studies revealed that interruption of the PI3K/Akt/mTOR/ULK signaling pathway plays a critical role in these flavonoids-mediated cell cycle arrest and induction of apoptotic and autophagic cell death via reducing PI3Kγ-p110 expression. Our studies provide novel insights into the anticancer activities of selected flavonoids and their potential uses in anticancer therapy.
Materials and Methods
Isolation, purification and identification of flavonoids from Tephroseris kirilowii (Turcz.) Holub
Tephroseris kirilowii (Turcz.) Holub. was purchased from a vendor (Guoan Chinese Herbal Medicine Co. Ltd. Zhengzhou, Henan, China). T. kirilowii was extracted with 70% MeOH for 3 days at room temperature to obtain a crude extract. This extract was suspended in 10% aqueous MeOH and partitioned between hexane, CHCl3, EtOAc, and BuOH to obtain the corresponding dried extracts. The EtOAc extract was subjected to silica gel column chromatography using CHCl3-MeOH solvent systems of increasing polarity to afford fractions A to C. Fraction A-C purified respectively by SephadexLH-20 CC (CHCl3/MeOH, 10:90) to yield eight flavonoids. These eight flavonoids were further purified by high-performance liquid chromatography (HPLC).
The structures of flavonoids were identified by spectroscopic analyses including MS and NMR (nuclear magnetic resonance).
Chemicals and antibodies
AS-605240 (S1410) and nocodazole were purchased from Selleck Chemicals (Shanghai, CA). Antibodies against PI3Kγ (5405 T), phospho-Akt (Ser473) (4051), Akt (2920), phospho-mTOR (Ser2448) (2971 L), mTOR (2972), phospho-ULK1 (Ser757) (6888), ULK1 (8054S), phospho-p70S6K1 (Thr389) (9204), cleaved caspase-3 (9661S), pro-caspase-3 (9668S) and GAPDH (5174) were from Cell Signaling Technology (Beverly, MA); XIAP (610716) and Mcl-1 (559027) were from BD Biosciences; PARP was from Epitomics (32561).
Cell culture
MDA-MB-231, MCF-7, A549, SMMC-7721, Eca109, HEB and MCF-10A cells were provided by the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in DMEM, RPMI1640 and MEBM medium contained 10% fetal bovine serum (FBS) and antibiotics at 37 °C in a humidified atmosphere and 5% CO2 in air.
Cell viability (MTT) assay
Cells (5 × 103) were seeded in each well of 96-well plates and treated as indicated experimental conditions for 24 h. 20 μl MTT (5 mg/ml) was added per well and incubated at 37 °C for 4 h. MTT assay was performed according to the manufacturer’s instruction. The cell viabilities were normalized to the control group. The IC50 values were calculated by using linear-regression analysis.
Apoptosis assay
Cells were stained with annexin V-FITC and PI to evaluate apoptosis by flow cytometry according to the manufacturer’s instructions (BD Biosciences PharMingen). In brief, 1 × 106 cells were washed twice with phosphate-buffered saline (PBS) and stained with 2 μl of Annexin V-FITC and 5 μl of PI (50 μg/ml) in 1× binding buffer (10 mM of HEPES, pH 7.4, 140 mM of NaOH, and 2.5 mM of CaCl2) for 15 min at room temperature in the dark. Quantification of apoptotic cells was performed by flow cytometry using a FACScan cytofluorometer (BD Biosciences). Both early (Annexin V-positive, PI-negative) and late (Annexin V-positive and PI-positive) apoptotic cells were included in the cell death determinations.
Cell cycle analysis
Cells were harvested, washed twice with phosphate-buffered saline (PBS) and incubated with nuclei staining buffer (0.1% Triton X-100, 3.8 mM sodium citrate, RNase B (7 kU/ml), and PI solution (50 μg/ml) for 2 h. The cell cycle distribution was measured by using the Becton-Dickinson FACScan cytofluorometer (Mansfield, MA, USA).
Western blots
Cells were processed for western blotting as described previously17. In brief, 20–40 μg of sample proteins were separated using SDS-PAGE and transferred to PVDF membranes (Bio-Rad, 162–0177). The membrane was probed with antibodies as indicated. The membrane was then incubated horseradish peroxidase-conjugated secondary antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD), and the signal was detected using a chemiluminescence reagent kit (Amersham Pharmacia, Piscataway, NJ).
Molecular docking study
The binding modes of three flavonoids (IH, GN, and Aca) with kinases were predicted by Surflex-Dock in SYBYL2.0. The crystal structures of kinases were retrieved from the Protein Data Bank (http://www.pdb.org/pdb/) and prepared by SYBYL-X 2.0 (including residue repair and energy minimization). In the process of molecular docking, we extract the original ligands out of the protein and generate banding pocket. Subsequently, original ligands, Aca, IH, and GN were docked into the pocket respectively.
PI3K kinase assay
ADP-Glo luminescent assay for PI3Kγ was performed according to the standard protocols of Promega. In brief, the kinase reaction was done in 96-well plate. Each well was loaded with test items and reaction buffer containing PI substrate and the PI3K proteins were then added. The reaction was started by the addition of PIP2 and ATP prepared in the reaction buffer and ran for 60 min and subsequently terminated by the addition of ADP-Glo reagent, then read the plate for luminescence detection.
Statistical analysis
Statistical analysis was performed with SPSS 20 software (SPSS, Chicago, Illinois, USA). Data were represented as means ± SD. For comparison between two data sets, a Student’s t test was used. For analysis of three or more sets of data, ANOVA was used. *P < 0.05, **P < 0.01 were considered statistically significant.
Results
Anticancer activity screening of flavonoid compounds from T. kirilowii
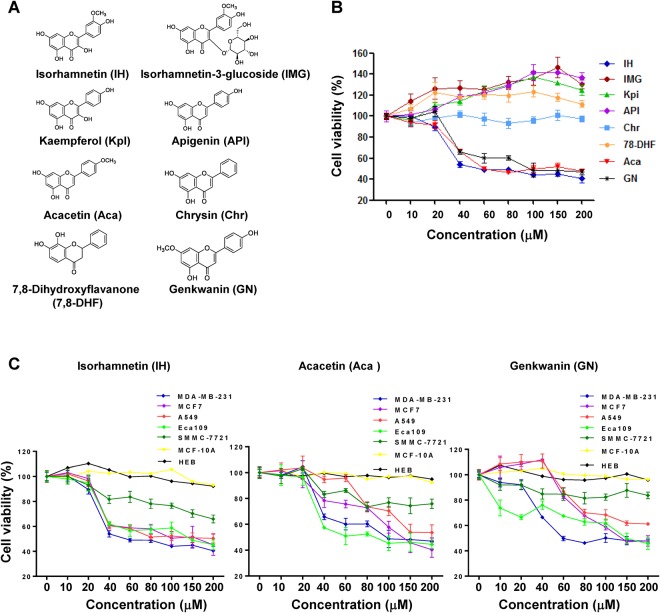
Eight flavonoid compounds were isolated for the first time from T. kirilowii. Their structures were identified by means of spectroscopic analyses as isorhamnetin (IH), isorhamnetin-3-glucoside (IMG), kaempferol (Kpl), apigenin (API), acacetin (Aca), chrysin (Chr), 7,8-dihydroxyflavanone (7,8-DHF), and genkwanin (GN) (Fig. 1A). To evaluate the effects of these flavonoids on the growth of human cancer cells, the growth inhibitory potential of these compounds was determined in human breast cancer MDA-MB-231 cells. We found that the cell viabilities were decreased in a dose-dependent manner in cells treated with IH, GN, and Aca (Fig. 1B). The IC50 values of IH, GN, and Aca for inhibition of cell proliferation in MDA-MB-231 cells were 55.51 μM, 58.54 μM, and 82.75 μM, respectively (Table 1). In contrast, the cell viabilities were slightly increased or not changed in cells treated with other five flavonoids with IC50 values greater than 200 μM (Fig. 1B, Table 1).
Figure 1.
IH, GN and Aca inhibit cell proliferation in multiple cancer cell lines. (A) Structure of IH, IMG, Kpl, API, Aca, Chr, 7,8-DHF and GN. (B) MDA-MB-231 cells were treated with increasing doses of flavonoids for 24 h, and MTT assays were performed to assess cell proliferation. (C) Multiple cancer cell lines were treated with increasing doses of IH, GN and Aca for 24 h, and cell proliferation were measured by MTT assay. IC50 values were presented as mean ± SD for three separate experiments.
Table 1.
IC50 of Flavonoids-inhibited cell proliferation in a variety of cancer.
| Compounds | MDA-MB-231 | MCF-7 | SMMC-7721 | A549 | Eca109 |
|---|---|---|---|---|---|
| IMG | >200 | >200 | >200 | >200 | >200 |
| IH | 55.51 ± 5.22 | 98.76 ± 4.73 | >200 | 148.44 ± 10.34 | 103.71 ± 6.72 |
| Kpl | >200 | >200 | >200 | >200 | >200 |
| API | >200 | >200 | >200 | >200 | >200 |
| Chr | >200 | >200 | >200 | >200 | >200 |
| GN | 58.54 ± 3.67 | 101.40 ± 3.64 | >200 | 120.77 ± 4.24 | 98.2 ± 3.23 |
| 7,8-DHF | >200 | >200 | >200 | >200 | >200 |
| Aca | 82.75 ± 4.35 | 103.91 ± 4.65 | >200 | 157.40 ± 7.54 | 54.7 ± 5.52 |
To determine whether the inhibition of cell proliferation mediated by IH, GN, and Aca was restricted to breast cancer cells, parallel studies were performed in a variety of cancer cells including breast cancer MCF-7 cells, hepatocellular carcinoma SMMC-7721 cells, lung adenocarcinoma A549 cells, and esophageal carcinoma Eca109 cells. The inhibitory effects of cell proliferation mediated by three active flavonoids in these cancer cell lines were similar to that in MDA-MB-231 cells, but had little effect on human normal glial cell line HEB and non-tumorigenic epithelial cell line MCF-10A (Fig. 1C).
IH, GN, and Aca cause cell cycle arrest at G2/M phase
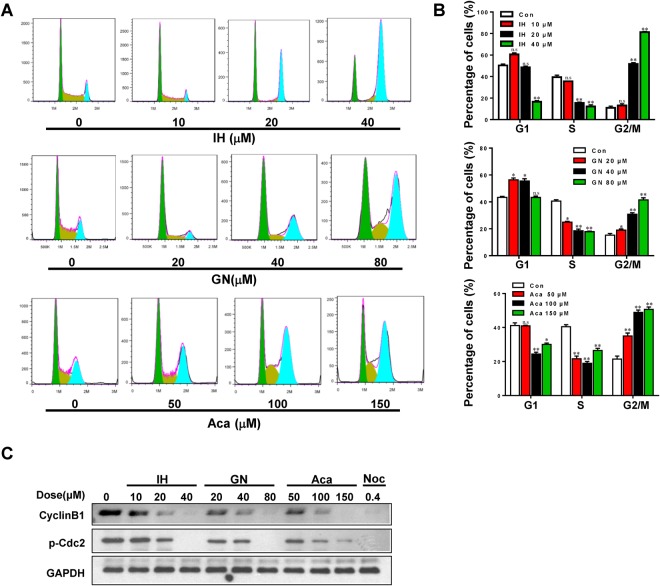
To explore the mechanism by which IH, GN, and Aca inhibit cell proliferation, we next investigated the effects of these active flavonoids on cell cycle progression in human breast cancer MDA-MB-231 cells by using flow cytometry assay. The typical histograms of cell cycle distribution in cells treated without or with IH, GN, and Aca for 24 h were shown in Fig. 2A. Treatment with these flavonoids resulted in increase in percentage of cells at G2/M phase and decrease in percentage of cells at G1 and S phase in a dose-dependent manner (Fig. 2B). These results suggest that flavonoids-mediated inhibitory effects of cell proliferation were accompanied by cell cycle arrest at G2/M phase. G2/M cell cycle arrest was also observed in another breast cancer MCF-7 cells-treated with IH, GN, and Aca (Fig. S1).
Figure 2.
IH, GN and Aca cause cell cycle arrest in MDA-MB-231. MDA-MB-231 cells were treated with IH, GN, Aca at indicated concentrations for 24 h. (A,B) Cells were stained with cell cycle staining solution and analyzed using a flow cytometer. The percentage of cells in each phase is showed as mean ± S.D. for three independent experiments (n.sP > 0.05, *P < 0.05 or **P < 0.01 vs. the control). (C) Cell lysates were prepared and analyzed by western blotting using antibodies against phospho-Cdc2 and cyclin B1.
To explore the mechanism by which these flavonoids induce cell cycle arrest at the G2/M phase, we determined if flavonoids modulate the expression of G2/M cell cycle regulatory molecules using western blotting. Exposure of MDA-MB-231 cells to IH, GN, and Aca resulted in marked decrease in levels of phospho-Cdc2 and cyclin B1 in a dose-dependent manner (Figs 2C, S2). These events were also confirmed in MCF-7 cells-treated with IH, GN, and Aca (Fig. S1). Such findings suggest that repression of Cdc2 and cyclin B1 is likely to be involved in flavonoids-induced G2/M arrest.
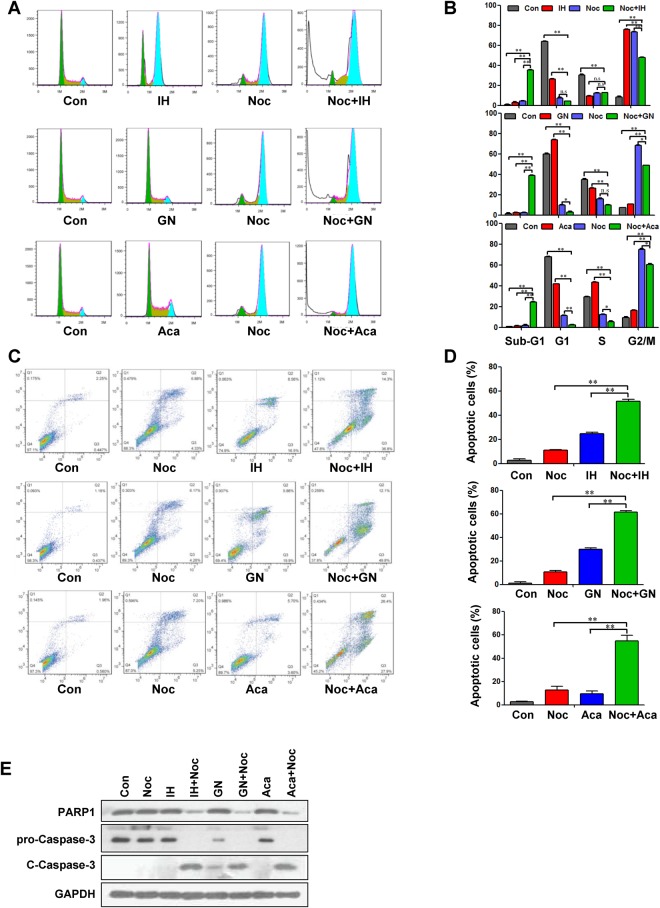
To determine whether G2/M-arrested cells are susceptible to flavonoids-induced cell death, we examined the effect of IH, GN, and Aca in cells synchronized at the G2/M phase by nocodazole treatment. Treatment of G2/M-synchronized cells with low concentrations of IH, GN, and Aca resulted in a pronounced increase in the proportion of sub-G1 cell population (Fig. 3A,B). Furthermore, treatment of G2/M-synchronized cells with flavonoids also resulted in a marked increase in apoptosis (Figs 3C–E, S3). Such findings suggest that G2/M-arrested cells might be sensitive to undergoing induction of apoptosis-mediated by IH, GN, and Aca.
Figure 3.
Pretreatment with nocodazole enhance IH, GN and Aca-induced apoptosis in MDA-MB-231 cells. MDA-MB-231 cells were pretreated with 100 ng/ml nocodazole for 12 h, followed by treatment with or without 30 μM IH, 20 μM GN and 100 μM Aca for 24 h. (A,B) Cells were stained with cell cycle staining solution and analyzed using a flow cytometer. The percentage of cells in each phase is showed as mean ± S.D. for three independent experiments (n.sP > 0.05, *P < 0.05 or **P < 0.01). (C,D) Cells were stained with Annexin V/PI, and the percentage of apoptotic cells were determined using flow cytometry. Data was showed as mean ± S.D (**P < 0.01) for three independent experiments. (E) Cell lysates were prepared and analyzed by western blotting using antibodies against PARP1, pro-caspase-3 and cleaved-caspase 3 (C-Caspase 3).
IH, GN, and Aca induce apoptosis
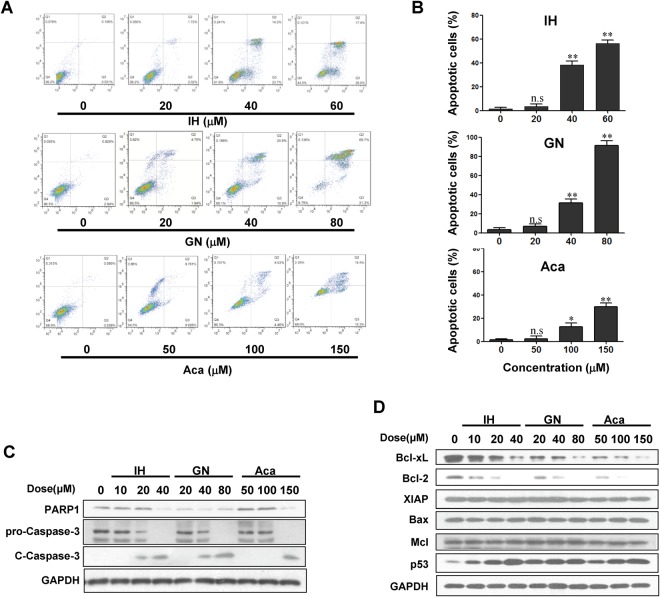
We next investigated whether IH, GN, and Aca inhibited cell proliferation through induction of apoptosis by flow cytometry assay. Treatment of MDA-MB-231 cells with these flavonoids resulted in increase in apoptosis in a dose-dependent manner (Fig. 4A,B). IH and GN exhibited more potent activities in apoptosis induction than Aca. Consistent with these findings, treating cells with same concentrations of flavonoids resulted in decrease in the expression of PARP1 and increase in levels of cleaved caspase-3 (Figs 4C, S4A). These events were also confirmed in MCF-7-treated with IH, GN, and Aca (Fig. S5).
Figure 4.
IH, GN and Aca induce apoptosis in MDA-MB-231. MDA-MB-231 cells were treated with IH, GN, Aca at indicated concentrations for 48 h. (A,B) The percentage of apoptotic cells were determined by flow cytometry using Annexin V/PI staining for three independent experiments. (mean ± SD, n.sP > 0.05, *P < 0.05 or **P < 0.01 vs. the control). (C,D) Cell lysates were prepared and analyzed by western blotting using antibodies against PARP1, pro-caspase-3, cleaved-caspase 3 (C-Caspase 3), Bcl-2, Bcl-xL, p53, Bax, XIAP and Mcl-1.
To gain further insight into the mechanism of flavonoids-induced apoptosis, we examined the expression of Bcl-2 family proteins and the tumor suppressor gene p53 using western blot analysis. Treatment of cells with IH, GN, and Aca resulted in decrease in levels of Bcl-2 and Bcl-xL and increase in levels of p53 (Figs 4D, S4B). In contrast, the expressions of other Bcl-2 family proteins like XIAP, Mcl-1, and Bax remained largely unchanged with treatment of flavonoids (Figs 4D, S4B). Together, these findings suggest that upregulation of p53 and downregulation of Bcl-2 and Bcl-xL could be involved in flavonoids-induced apoptosis in breast cancer cells.
IH, GN, and Aca induce autophagic cell death
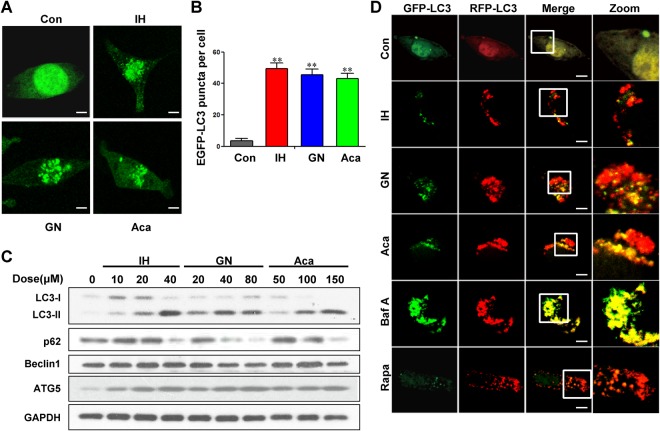
To verify whether the selected flavonoids were capable of inducing autophagy, we first used MDA-MB-231 cells transiently expressing EGFP-LC3. Autophagosome accumulation can be detected with a confocal laser-scanning microscope. Exposure of cells to IH, GN, and Aca resulted in marked increases in EGFP-LC3 puncta formation in MDA-MB-231 cells (Fig. 5A,B). Western blot analysis showed that treatment of cells with IH, GN, and Aca resulted in a dose-dependent accumulation of LC3-II (Figs 5C, S6). Treatment of cells with IH, GN, and Aca also resulted in a dose-dependent decrease in levels of p62 and increase in levels of ATG5 (Figs 5C, S6). These results suggest that these flavonoids may act as autophagy inducers which promoted p62 degradation by autophagy.
Figure 5.
IH, GN and Aca induce autophagy in MDA-MB-231. (A,B) MDA-MB-231 cells transfected with EGFP-LC3 were treated with 40 μM IH, 20 μM GN and 100 μM Aca for 24 h, the EGFP-LC3 puncta were observed under confocal microscopy; scale bars: 10 μm. Quantification of average EGFP puncta per cell for three independent experiments. Data was presented as mean ± SD (**P < 0.01 vs. the control). (C) Cells were exposed to indicated concentrations of IH, GN and Aca for 24 h, the expression of autophagy-related proteins (LC3B-II, p62, Beclin1, ATG5) were detected by western blot analysis, GAPDH was used as a loading control. (D) Cells were transfected with a tandem reporter construct (tfLC3), and were exposed to 30 μM IH, 20 μM GN, 100 μM Aca, 20 nM Baf and 0.25 μM Rapa as indicated. The colocalization of EGFP and mRFP-LC3 puncta was examined by confocal microscopy. Scale bars: 10 μm.
To further examine whether the effects of these flavonoids are due to induced autophagic flux, cells transfected with a tandem reporter construct (tfLC3) were treated with IH, GN, and Aca followed by assessment of EGFP-LC3 and mRFP-LC3 puncta colocalization. EGFP-fluorescence is quenched in acidic environments, whereas mRFP is more stable under acidic conditions18. As shown in Fig. 5D, exposure of cells to IH, GN, and Aca caused pronounced formation of LC3 puncta that displayed only red fluorescence intensity, which was similar to that in cells treated with rapamycin (a typical autophagy inducer). In contrast, treatment with bafilomycin A, a vacuolar-type ATPase inhibitor that blocks autophagic degradation, caused pronounced formation of LC3 puncta that displayed both green and red fluorescence intensity producing a yellow overlay. Induction of autophagy was also observed in MCF-7 cells-treated with IH, GN, and Aca (Fig. S7). Thus, these data clearly demonstrate that these flavonoids act as autophagy inducers, which promote autophagic degradation.
IH, GN, and Aca dock in the ATP binding pocket of PI3Kγ
Molecular docking plays an important role in structure-based drug designing, functional site prediction on protein molecular surfaces, protein ligand docking, etc.19,20. A number of evidence revealed that phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK, including ERK, p38, and JNK) signaling pathways play critical roles in the regulation of cell proliferation, apoptosis, and autophagy21,22. To explore the possible interaction between flavonoids and PI3K/Akt or MAPK, molecular docking of three active flavonoids with these signaling pathways was performed. After evaluating the interaction by analyses of the docking scores and binding poses, PI3Kγ was predicted as the most possible target because these flavonoids have similar binding poses as the original ligands in the crystal structure.
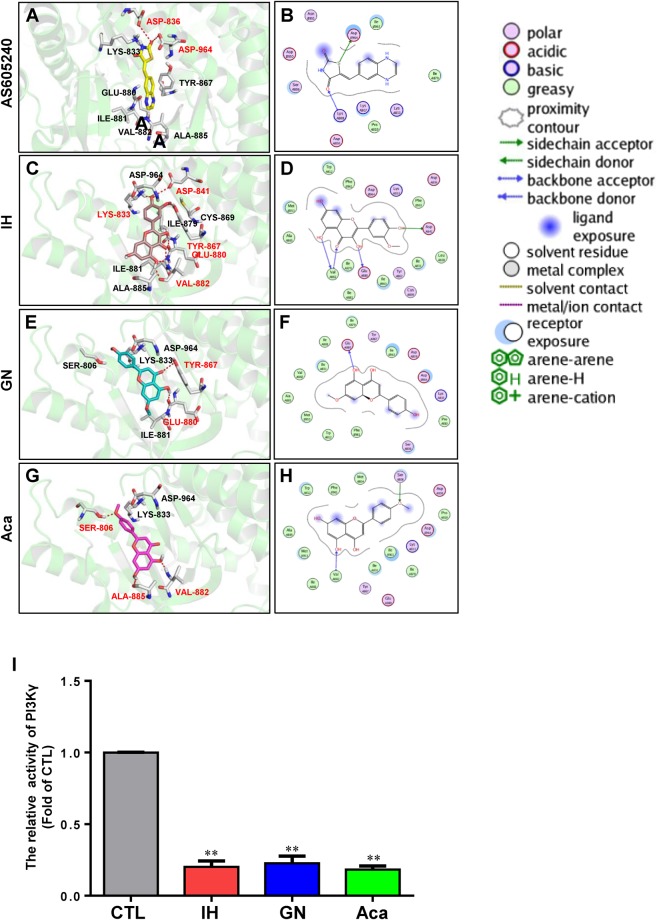
The interaction between PI3Kγ specific inhibitor AS605240 and PI3Kγ in the docking model is almost the same as that in crystal structure (PDBID: 2A5U)23 (Fig. 6A,B), showing that AS605240 formed an interaction with the catalytic lysine (LYS833) within the ATP-binding pocket. As well, other residues, such as ASP-836, ASP-964, TYR-867, GLU-880, ILE-881, ALA-885, and VAL-882 played important roles for their binding interaction.
Figure 6.
Molecular docking of AS605240, IH, GN and Aca in the ATP binding pocket of PI3Kγ (PDB: 2A5U). (A–G) PI3Kγ’s ATP binding domain crystal structure with AS605240, IH, GN and Aca. (B–H) The interaction of AS605240, IH, GN and Aca with protein by 2D for the PI3Kγ’s ATP binding domain crystal structure. (I) The relative catalytic activity of PI3Kγ from three independent experiments. (mean ± SD, **P < 0.01 vs. the control).
Three active flavonoids (IH, GN, and Aca) were also successfully docked into the ATP-binding pocket of PI3Kγ crystal structure. In the PI3Kγ-IH docking model, IH interacted with PI3Kγ by formation of hydrogen bonds with LYS-833, VAL-882, ASP-841, TYR-867, and GLU-880. Moreover, there are hydrophobic interactions of IH with ASP-964, CYS-869, ILE-879, ILE-881, and ALA-885 (Fig. 6C,D). More hydrogen bonds were found in the PI3Kγ-GN docking model with the side chains of TYR-867 and GLU-880. Other residues as LYS-833, ASP-964, SER-806, and ILE-881 showed hydrophobic interaction together with GN (Fig. 6E,F). Similarly, Aca interacted with PI3Kγ by formation of hydrogen bonds with SER-806, ALA-885, and VAL-882. In addition, the hydrophobic interaction of acacetin with LYS-833 and ASP-964 was observed in the PI3Kγ-Aca docking model (Fig. 6G,H). These docking results indicated that these flavonoids are able to bind with PI3Kγ by interaction with the important residues for the catalytic activity, such as LYS-833 and ASP-964, which are similar to that of the known PI3Kγ inhibitor AS605240. By using PI3K kinase assay, we found that treatment of breast cancer MDA-MB-231 cells with IH, GN, and Aca resulted in significant decreases in activities of PI3Kγ (Fig. 6I), further supporting the docking prediction.
IH, GN, and Aca inhibit PI3K/AKT/mTOR/p70S6K/ULK1 signaling pathway via reducing PI3Kγ-p110 expression
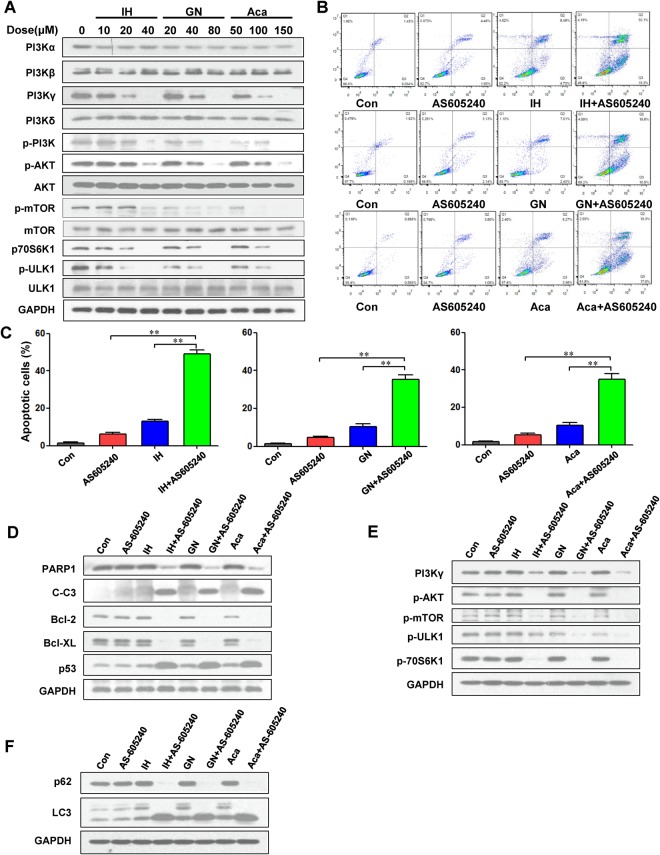
To further confirm whether PI3Kγ is involved in three flavonoids-mediated inhibition of cell proliferation and induction of apoptosis and autophagy, western blot analysis was employed. Exposure of MDA-MB-231 cells to IH, GN, and Aca resulted in dose-dependent decreases in levels of PI3Kγ-p110. In contrast, these flavonoids had little or no effect on expression of PI3Kα, PI3Kβ, and PI3Kδ (Figs 7A, S8A). In addition, these flavonoids had little or no effect on levels of p-ERK, p-p38, and p-JNK (data not shown). It is well known that AKT, mTOR, p70S6K, and ULK are PI3K downstream molecules which regulate cell proliferation, apoptosis and autophagy24. We next examined the effects of three flavonoids on expression of p-AKT, p-mTOR, p-p70S6K, and p-ULK by using western blot analysis. Treatment of MDA-MB-231 cells with IH, GN, and Aca led to decreases in levels of p-AKT, p-mTOR, p-p70S6K, and p-ULK in a dose-dependent manner (Figs 7A, S8A). These events were also confirmed in MCF-7 cells-treated with IH, GN, and Aca (Fig. S9A and S9B). Such findings indicate that inactivation of PI3K/AKT/mTOR/p70S6K/ULK1 signaling pathway could play a crucial role in inhibition of cell proliferation and induction of apoptosis and autophagy mediated by flavonoids via reducing the expression of PI3Kγ.
Figure 7.
IH, GN and Aca inhibit PI3K/AKT/mTOR/p70S6K/ULK1 signaling pathway via reducing PI3Kγ-p110 expression. (A)MDA-MB-231 cells were treated with IH, GN, Aca at indicated concentrations for 24 h, cell lysates were prepared and subjected to western blot using antibodies against PI3Kα, PI3Kβ, PI3Kγ, PI3Kδ, p-PI3K, p-AKT, AKT, p-mTOR, mTOR, p-p70S6K, p-ULK and ULK1. (B,C) MDA-MB-231 cells were pretreated with 20 μM AS605240 for 2 h, followed by treatment with or without 20 μM IH, 20 μM GN and 50 μM Aca for 48 h. Cells were stained with Annexin V/PI, and the percentage of apoptotic cells were showed as mean ± S.D. for three independent experiments (**P < 0.01). (D–F) Cells were treated as B and C, cell lysates were prepared and subjected to western blot analysis using antibodies against PARP, cleaved-caspase 3 (C-Caspase 3), Bcl-2, Bcl-xL, p53, PI3Kγ, p-Akt, p-mTOR, p-ULK p70S6K, p62 and LC3.
Inhibition of PI3Kγ activity by AS605240 potentiates IH, GN, and Aca-induced apoptosis
The preceding findings implied that inactivation of PI3Kγ might play an important role in flavonoids-mediated lethality. To test this possibility, cells were coexposed to three flavonoids and the PI3Kγ specific inhibitor AS605240, and apoptosis was monitored. Coadministration of a nontoxic concentration of AS605240 (i.e. 20 μM; 6.31%) with a modestly toxic concentration of IH (20 μM; 13.21%), GN (20 μM; 10.39%) and Aca (50 μM; 10.27%) resulted in a pronounced increase in apoptosis (i.e. to 49.22%, 35.43%, and 35.14% respectively) (Fig. 7B,C). Western blot analysis revealed that coadministration of AS605240 and three flavonoids at concentrations that were ineffective or marginally effective by themselves resulted in pronounced increase in activation of caspases-3 and degradation of PARP1 (Figs 7D, S8B). Combined treatment also resulted in potentiation of Bcl-2 and Bcl-xL downregulation, and p53 upregulation (Figs 7D, S8B). In addition, coadministration of AS605240 and three flavonoids resulted in the virtual abrogation of Akt, mTOR, p70S6K, and ULK activations (Fig. 7E,F, Fig. S8C and S8D), Together, these findings suggest that downregulation of PI3Kγ plays a critical role in regulating the lethality of three flavonoids in human breast cancer cells.
Discussion
The ability of flavonoids to inhibit cell proliferation and induce apoptosis or autophagy in human cancer cells has stimulated intense interest in their potential as anti-cancer agents. The present results indicate that three flavonoids (IH, GN, and Aca) isolated from T. kirilowii exhibited anti-cancer activities (i.e. inhibition of cell proliferation by cell cycle arrest at G2/M phase, induction of apoptosis and autophagy). Our results also provide detailed molecular mechanistic information as to how three flavonoids exert their anti-cancer activities on human breast cancer cells (i.e. by inactivation of PI3K, Akt, mTOR, p70S6K, and ULK, upregulation of p53, and downregulation of Bcl-2 and Bcl-xL).
Previous studies indicated that IH suppressed cell proliferation through cell cycle arrest at G2/M phase in human colon cancer cells25. GN inhibited cell proliferation via cell cycle arrest at S phase in mouse melanoma B16F10 cells26. Aca inhibited cell proliferation via cell cycle arrest at G1 phase in human non-small cell lung cancer A549 cells or via cell cycle arrest at G2/M phase in human prostate cancer DU145 cells27,28. Our studies indicated that IH, GN, and Aca inhibited cell proliferation through cell cycle arrest at G2/M phase in human breast cancer MDA-MB-231 cells. Cell cycle progression through G2-M is regulated by activation of a complex consisting of catalytic subunit Cdc2 and regulatory subunit cyclin B1 that controls the entry into mitosis29. Consistent with these reports, treating breast cancer cells with IH, GN, and Aca decreased the levels of phospho-Cdc2 and cyclin B1, suggesting that dephosphorylation of Cdc2 and downregulation of cyclin B1 could be the underlying molecular events contributing to G2/M arrest caused by these flavonoids in breast cancer cells.
Increasing evidence reveal that cell cycle arrests are often followed by or associated with apoptotic death of cancer cells by many cancer therapeutic agents30. Our data show that growth inhibitory effect of these flavonoids was accompanied by an induction of apoptosis, which was accompanied by activation of caspase-3 and degradation of PARP1. We also observed that treatment of G2/M-synchronized cells (by nocodazole) with these flavonoids resulted in a pronounced increase in the proportion of sub-G1 cell population and apoptosis. Such findings suggest that these flavonoids inhibit cell proliferation via cell cycle at G2/M phase, and culminating in apoptosis in human breast cancer cells.
The tumor suppressor gene p53 has been shown to regulate the cell cycle at the G2/M checkpoint in response to DNA damage30. Upregulation of p53 leads to G2/M arrest through the induction of CDK inhibitor p21, which inhibits cdc2 activity by binding directly to Cdc/Cyclin B1 complexes. p53 has also been shown to regulate apoptosis through inhibiting the expression of anti-apoptotic proteins (i.e. Bcl-2, Blc-xL, Mcl-1, etc.)21. Consistent with these reports, our data reveal that p53 plays an important role in these active flavonoids-mediated cell cycle arrest at G2/M phase and apoptosis based on the following results: (i) treatment of breast cancer cells with these flavonoids increased the expression of p53; (ii) these flavonoids induced cell cycle arrest at G2/M phase through inhibiting the expression/activity of p53′s downstream molecules (i.e. activity of Cdc2 and expression of cyclin B1); (iii) these flavonoid compounds induced apoptosis through downregulation of p53′s downstream molecules (i.e. Bcl-2 and Bcl-xL). These findings suggest that upregulation of p53 by these flavonoids not only caused cell cycle arrest at G2/M phase, but also triggered apoptosis in breast cancer cells.
Autophagy is a catabolic process that enables the sequestration and lysosomal degradation of cytoplasmic organelles and proteins and that is important for the maintenance of genomic stability and cell survival31. Current cancer therapies including chemotherapy and radiation are known to induce autophagy within tumor cells21. Accordingly, small-molecules that induce autophagy may have broad therapeutics applications for cancer treatment. Recently, many flavonoids have been found to exhibit anti-cancer effects through the modulation of autophagy. For example, plant flavonoid wogonin has been shown to induce cancer cell death through inhibition of autophagy32. In contrast, many flavonoids have been shown to induce cell death through induction of autophagy. For example, curcumin is able to induce both autophagy and apoptosis in chronic myeloid leukemia cells via downregulation of the Bcl-2 protein33. Quercetin induces extensive autophagy in epithelial cancer cells, which led to cell cycle arrest and induction of apoptosis34, etc. Our results indicate that IH, GN, and Aca could be autophagic inducers that induce autophagosome accumulation in breast cancer cells based on the following findings: First, treating cells with these flavonoids resulted in a marked increase in EGFP-LC3 puncta formation and accumulation of LC3B-II in breast cancer cells. Second, treatment with these flavonoids decreased the levels of p62, which is a protein that acts as a cargo receptor for the autophagic degradation of substrates and is an indicator of autophagy induction. Third, treatment with these flavonoids increased the levels of ATG5, which is considered to be essential molecule for the induction of autophagy. Fourth, by assessment of EGFP-LC3 and mRFP-LC3 puncta colocalization, we found that cells exposed to these flavonoids led to the production of large amounts of red-only puncta, which is similar to that in cells treated with rapamycin, a typical autophagy inducer. Therefore, our findings suggest that ATG5 participates in these flavonoids-induced autophagosome accumulation.
Phosphoinositide 3-kinase (PI3K) activity is stimulated by diverse oncogenes and growth factor receptors, and elevated PI3K signaling is considered a hallmark of cancer and is a key therapeutic target in the treatment of cancer35. Class I PI3Ks are of particular therapeutic interest, given their importance in the development of human cancers. Class I PI3Ks are heterodimers, comprising a catalytic (p110α, p110β, p110δ, or p110γ) and a regulatory (p85α, p55α, p50α, p85β, p55γ, or p101) subunit. The activated p110 subunit then activates protein kinase B/AKT and downstream effectors including mTOR36. The PI3K/Akt/mTOR/p70S6K pathway is likely the most frequently activated pathway in human cancers, making it an attractive target for anti-cancer drug development. Inhibition of signaling along this pathway can result in both decreased cellular proliferation and promotion of cellular death (including apoptosis and autophagy)37. Increasing evidence indicate that several flavonoids (i.e. procyanidins, luteoloside, and hesperidin) induce apoptosis and/or autophagy through inhibition of PI3K/Akt/mTOR/p70S6K pathway38–40. Based on our results, we speculate that interruption of PI3K/AKT/mTOR/p70S6K signaling pathway may contribute to these flavonoids-induced cell cycle arrest at G2/M phase, apoptosis, and autophagy through reducing PI3Kγ-p110 expression. First, molecular docking indicated that these flavonoids are able to bind with PI3Kγ by interaction with the important resides for the catalytic activity, such as LYS-833 and ASP-964, which are similar to that of the known PI3Kγ inhibitor AS605240. Second, treatment of cells with these flavonoids decreased the expression of PI3Kγ, but did not affect the expression of other PI3K isoforms including PI3Kα, β, and δ. Third, pretreatment of cells with PI3Kγ specific inhibitor AS605240 significantly enhanced these flavonoids-mediated reduction of PI3Kγ and inactivation of AKT, mTOR, and p70S6K. Fourth, pretreatment of cells with PI3Kγ specific inhibitor AS605240 significantly enhanced these flavonoids-mediated apoptosis. Thus, our finding suggest that these flavonoids-mediated cell cycle arrest at G2/M phase, apoptosis, and autophagy might be attributed to PI3Kγ-p110 downregulation, which leads to interruption of PI3K/AKT/mTOR/p70S6K/ULK signaling pathway.
In conclusion, we isolated and identified, for the first time, eight flavonoids from T. kirilowii. Three flavonoids (IH, GN, and Aca) induced cell cycle arrest at G2/M phase, apoptosis, and autophagy in human breast cancer cells, in which downregulation of PI3Kγ-p110 and consequent interruption of PI3K/AKT/mTOR/p70S6K/ULK signaling pathway might be closely involved. Our studies provide novel insights into the anticancer activities of selected active flavonoids and their potential uses in anticancer therapy.
Electronic supplementary material
Acknowledgements
We thank Professor Esteban Dell’Angelica (Department of Human Genetics, David Geffen School of Medicine, University of California) for providing EGFP-LC3 and tfLC3 plasmids. This work was supported by the National Natural Science Foundation of China (81202869; 31571425; 81402013).
Author Contributions
N.G., H.W.Z. and J.J.H. conceived and designed the research study. H.W.Z. isolated and characterized the structures of flavonoids, Q.O.Y. performed the homology modeling and molecular docking. N.G., H.W.Z. and J.J.H. wrote and revised the manuscript. R.Q.F., X.L., Y.H.Z., J.L., L.L., Y.N.L., Q.D. and Q.S.L. participated in the experiments. N.G., H.W.Z. and J.J.H. performed the statistical analysis. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Hong-Wei Zhang and Jin-Jiao Hu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29308-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu H, et al. Antitumor effects of traditional Chinese medicine targeting the cellular apoptotic pathway. Drug design, development and therapy. 2015;9:2735–2744. doi: 10.2147/DDDT.S80902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, et al. Traditional Chinese medicine syndrome-related herbal prescriptions in treatment of malignant tumors. Journal of traditional Chinese medicine = Chung i tsa chih ying wen pan. 2013;33:19–26. doi: 10.1016/s0254-6272(13)60095-3. [DOI] [PubMed] [Google Scholar]
- 3.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. Journal of nutritional science. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo X, et al. The role of targeting kinase activity by natural products in cancer chemoprevention and chemotherapy (Review) Oncology reports. 2015;34:547–554. doi: 10.3892/or.2015.4029. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, DePinho RA, Ernst M, Vousden K. Cancer research: past, present and future. Nature reviews. Cancer. 2011;11:749–754. doi: 10.1038/nrc3138. [DOI] [PubMed] [Google Scholar]
- 6.Ding Y, et al. Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chemico-biological interactions. 2017;275:210–217. doi: 10.1016/j.cbi.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Dai J, et al. Downregulation of NEDD9 by apigenin suppresses migration, invasion, and metastasis of colorectal cancer cells. Toxicology and applied pharmacology. 2016;311:106–112. doi: 10.1016/j.taap.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao, K. et al. Oroxyloside inhibits angiogenesis through suppressing internalization of VEGFR2/Flk-1 in endothelial cells. Journal of cellular physiology, 10.1002/jcp.26198 (2017). [DOI] [PubMed]
- 9.Awan FT, et al. A phase 1 clinical trial of flavopiridol consolidation in chronic lymphocytic leukemia patients following chemoimmunotherapy. Annals of hematology. 2016;95:1137–1143. doi: 10.1007/s00277-016-2683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Li J. [Growth strategies of different age classes of ramets in Kalimeris integrifolia population at the Songnen Plains of China] Ying yong sheng tai xue bao = The journal of applied ecology. 2003;14:2171–2175. [PubMed] [Google Scholar]
- 11.Wang YJ, Liao CC, Chen HJ, Hsieh CL, Li TC. The Effectiveness of Traditional Chinese Medicine in Treating Patients with Leukemia. Evidence-based complementary and alternative medicine: eCAM. 2016;2016:8394850. doi: 10.1155/2016/8394850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, et al. Traditional Chinese medicine in cancer care: a review of case reports published in Chinese literature. Forschende Komplementarmedizin. 2011;18:257–263. doi: 10.1159/000333065. [DOI] [PubMed] [Google Scholar]
- 13.Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends in molecular medicine. 2007;13:353–361. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Wang JT, Wu ZN, Hu MY, Gao HW. [Effect of total flavonoids in Scutellaria barbata in mediating autophagy in tumor cells via PI3K/AKT/mTOR pathway] Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2017;42:1358–1364. doi: 10.19540/j.cnki.cjcmm.20170222.003. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, et al. A natural chalcone induces apoptosis in lung cancer cells: 3D-QSAR, docking and an in vivo/vitro assay. Scientific reports. 2017;7:10729. doi: 10.1038/s41598-017-11369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prieto-Dominguez, N., Garcia-Mediavilla, M. V., Sanchez-Campos, S., Mauriz, J. L. & Gonzalez-Gallego, J. Autophagy as a Molecular Target of Flavonoids Underlying their Protective Effects in Human Disease. Current medicinal chemistry, 10.2174/0929867324666170918125155 (2017). [DOI] [PubMed]
- 17.Gao N, Rahmani M, Dent P, Grant S. 2-Methoxyestradiol-induced apoptosis in human leukemia cells proceeds through a reactive oxygen species and Akt-dependent process. Oncogene. 2005;24:3797–3809. doi: 10.1038/sj.onc.1208530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bizzarri R, Serresi M, Luin S, Beltram F. Green fluorescent protein based pH indicators for in vivo use: a review. Analytical and bioanalytical chemistry. 2009;393:1107–1122. doi: 10.1007/s00216-008-2515-9. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira LG, Dos Santos RN, Oliva G, Andricopulo AD. Molecular docking and structure-based drug design strategies. Molecules. 2015;20:13384–13421. doi: 10.3390/molecules200713384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Ruyck J, Brysbaert G, Blossey R, Lensink MF. Molecular docking as a popular tool in drug design, an in silico travel. Advances and applications in bioinformatics and chemistry: AABC. 2016;9:1–11. doi: 10.2147/AABC.S105289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sui X, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer letters. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Azzi J, et al. The novel therapeutic effect of phosphoinositide 3-kinase-gamma inhibitor AS605240 in autoimmune diabetes. Diabetes. 2012;61:1509–1518. doi: 10.2337/db11-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebrahimi S, et al. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Current medicinal chemistry. 2017;24:1321–1331. doi: 10.2174/0929867324666170206142658. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Yang X, Chen C, Cai S, Hu J. Isorhamnetin suppresses colon cancer cell growth through the PI3KAktmTOR pathway. Molecular medicine reports. 2014;9:935–940. doi: 10.3892/mmr.2014.1886. [DOI] [PubMed] [Google Scholar]
- 26.Nasr Bouzaiene N, Chaabane F, Sassi A, Chekir-Ghedira L, Ghedira K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life sciences. 2016;144:80–85. doi: 10.1016/j.lfs.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Hsu YL, Kuo PL, Liu CF, Lin CC. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer letters. 2004;212:53–60. doi: 10.1016/j.canlet.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R. Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure-activity relationship with linarin and linarin acetate. Carcinogenesis. 2005;26:845–854. doi: 10.1093/carcin/bgi014. [DOI] [PubMed] [Google Scholar]
- 29.Kishimoto T. Entry into mitosis: a solution to the decades-long enigma of MPF. Chromosoma. 2015;124:417–428. doi: 10.1007/s00412-015-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryu, H. et al. The Small Molecule AU14022 Promotes Colorectal Cancer Cell Death via p53-mediated G2/M-phase Arrest and Mitochondria-mediated Apoptosis. Journal of cellular physiology, 10.1002/jcp.26234 (2017). [DOI] [PubMed]
- 31.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annual review of cell and developmental biology. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 32.Wang SF, Wu MY, Cai CZ, Li M, Lu JH. Autophagy modulators from traditional Chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. Journal of ethnopharmacology. 2016;194:861–876. doi: 10.1016/j.jep.2016.10.069. [DOI] [PubMed] [Google Scholar]
- 33.Wu LX, et al. Curcumin derivative C817 inhibits proliferation of imatinib-resistant chronic myeloid leukemia cells with wild-type or mutant Bcr-Abl in vitro. Acta pharmacologica Sinica. 2014;35:401–409. doi: 10.1038/aps.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue Y, Du M, Zhu MJ. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by Escherichia coli O157:H7. Free radical biology & medicine. 2017;108:760–769. doi: 10.1016/j.freeradbiomed.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Li, Z. & Zhang, X. Kinases Involved in Both Autophagy and Mitosis. International journal of molecular sciences 18, 10.3390/ijms18091884 (2017). [DOI] [PMC free article] [PubMed]
- 36.Vogt PK, et al. Phosphatidylinositol 3-kinase: the oncoprotein. Current topics in microbiology and immunology. 2010;347:79–104. doi: 10.1007/82_2010_80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouyang ZH, Wang WJ, Yan YG, Wang B, Lv GH. The PI3K/Akt pathway: a critical player in intervertebral disc degeneration. Oncotarget. 2017;8:57870–57881. doi: 10.18632/oncotarget.18628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, et al. Cytotoxic effects of procyanidins from Castanea mollissima Bl. shell on human hepatoma G2 cells in vitro. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2014;64:166–176. doi: 10.1016/j.fct.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 39.Zhou, M., Shen, S., Zhao, X. & Gong, X. Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines. Biochemical and biophysical research communications, 10.1016/j.bbrc.2017.10.042 (2017). [DOI] [PubMed]
- 40.Saiprasad G, Chitra P, Manikandan R, Sudhandiran G. Hesperidin induces apoptosis and triggers autophagic markers through inhibition of Aurora-A mediated phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and glycogen synthase kinase-3 beta signalling cascades in experimental colon carcinogenesis. European journal of cancer. 2014;50:2489–2507. doi: 10.1016/j.ejca.2014.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.