Abstract
We have previously reported clinical data to suggest that colonization factor I (CFA/I) fimbriae of enterotoxigenic Escherichia coli (ETEC) can bind to Lewis a (Lea), a glycan epitope ubiquitous in the small intestinal mucosa of young children (<2 years of age), and individuals with a genetic mutation of FUT2. To further elucidate the physiological binding properties of this interaction, we engineered Chinese Hamster Ovary (CHO-K1) cells to express Lea or Leb determinants on both N- and O-glycans. We used our glyco-engineered CHO-K1 cell lines to demonstrate that CfaB, the major subunit of ETEC CFA/I fimbriae, as well as four related ETEC fimbriae, bind more to our CHO-K1 cell-line expressing Lea, compared to cells carrying Leb or the CHO-K1 wild-type glycan phenotype. Furthermore, using in-silico docking analysis, we predict up to three amino acids (Glu25, Asn27, Thr29) found in the immunoglobulin (Ig)-like groove region of CfaB of CFA/I and related fimbriae, could be important for the preferential and higher affinity binding of CFA/I fimbriae to the potentially structurally flexible Lea glycan. These findings may lead to a better molecular understanding of ETEC pathogenesis, aiding in the development of vaccines and/or anti-infection therapeutics.
Introduction
Enterotoxigenic Escherichia coli (ETEC) is a leading cause of severe diarrhoeal illness in young children (<5 years of age) in low and middle-income countries. It is also a major cause of traveller’s diarrhoea to ETEC endemic areas1,2. The bacterium has evolved to produce one or more of at least 23 distinct fimbrial (known as colonisation factors, CFs) or non-fimbrial adhesins, enabling ETEC to bind to the small intestinal epithelium before producing diarrhoeagenic enterotoxin(s)3. Thus, ETEC adherence factors are prerequisites for the initiation of pathogenesis, representing a critical point at which ETEC infections could be prevented4.
In previous clinical studies, we have demonstrated that Bangladeshi children expressing the histo-blood group antigen (HBGA) Lewis a (Lea, Le(a + b−) phenotype, FUT2 non-secretor status) are more likely to have symptomatic ETEC infection compared to children expressing the HBGA Lewis b (Leb, Le(a−b+) phenotype, FUT2 secretor status)5,6. Interestingly, we have also observed that Bangladeshi children with the Le(a+ b−) phenotype are more likely to be infected by ETEC expressing the colonisation factor antigen I (CFA/I) and the related ETEC CF family fimbriae or pili6. The likely explanation for this being, CFA/I could bind to Lea glycolipid structures present in the small intestinal mucosal layer of very young children (<2 years of age) and individuals with FUT2 non-secretor status7,8.
CFA/I was the first human specific immunogenic ETEC CF to be described. It is a representative member of the antigenically defined ETEC CF class 5 pili, which are also commonly referred to as the α clade fimbrial usher protein (FUP) family4,9. Together, this ETEC CF group (CFA/I, CS1, CS2, CS4, CS14, CS17, CS19 and PCF071) accounts for the largest group of human specific ETEC CF expressing strains causing diarrhoeal disease worldwide2,4. Like other ETEC CF family members, CFA/I is comprised of a four gene operon, encoding for a long rigid homopolymorphic shaft with >1,000 copies of a major subunit (CfaB), with one or a few copies of the tip residing minor subunit (CfaE)4.
With regard to ETEC CFA/I binding to host cells, the minor subunit CfaE binds to the surface of erythrocytes4,10. The major subunit CfaB has been shown to bind to glycosphingolipids and human small intestinal glycolipid structures, such as those expressing Lea or asialo-GM17. It has also been shown that specific monoclonal antibodies raised against CfaB inhibits ETEC CFA/I binding to cultured intestinal epithelial cells11–13. Moreover, an antibody that reacts strongly with the first 25 amino acids of the N-terminal fragment of CfaB has been shown to inhibit ETEC CFA/I bacterial adhesion to human jejunal enterocytes14,15. In contrary, it has been reported by others that CfaE of ETEC CFA/I binds to intestinal tissue and asialo-GM1 glycans that are expressed on erythrocytes and cultured intestinal epithelial cells10,16.
X-ray structural analysis has revealed CfaE and CfaB to have similar barrel like structures, with CfaE containing two and CfaB possessing one exposed hydrophobic immunoglobulin (Ig)-like fold(s), that structurally interact and complement each other9. Interestingly, a 12-amino acid stretch of the CfaB Ig-like fold (V24EKNITVTASVD35) that is located in the N terminal fragment of CfaB, shares structural similarities with all ETEC CF major subunits of the type 5 pili family. This 12 amino acid stretch of CfaB also shared structural similarities with class 1 pili from bacteria that can cause urinary and respiratory infections by binding to host glycolipids containing HBGAs9,17.
The aim of the present study was to create glycan defined Chinese hamster ovary (CHO-K1) cell line models of the human small intestinal mucosa, and to study the binding capabilities of ETEC CFA/I and the related CFs to Lewis Lea and Leb antigens expressed on the cell surface. We also perform computational molecular docking analysis to help understand why CFA/I binds to Lea but not Leb expressing glycans, as well as potentially identify novel CFA/I Lea glycan binding sites.
Results
Glyco-engineered CHO-K1 cells were produced expressing either Lea or Leb
ETEC colonises the epithelial surface of the small intestinal mucosa, where intestinal villi and crypts express abundant Lea and/or Leb glycans4,18,19. To create defined HBGA Lea and Leb glycan models of the human small intestinal mucosa, CHO-K1 cells expressing the P-selectin glycoprotein ligand-1/immunoglobulin fusion protein (PSGL-1/mIgG2b; CHO-CP55 cells), were co-transfected with plasmids encoding: the extended core 1 glycan (GlcNAcβ3Galβ3GalNAcα) enzyme B3GNT3, the type 1 chain glycan (Galβ3GlcNAc) encoding enzyme B3GALT5, and the Lewis gene-encoding enzyme FUT3 alone (generating Lea cells; CHO-PSGL-Lea-a1) or together with the H gene-encoding FUT1 (generating Leb; CHO-PSGL-Leb-b1) (see Supplementary Figure S1 and Table S1 online, and Materials and Methods for further details)20–24.
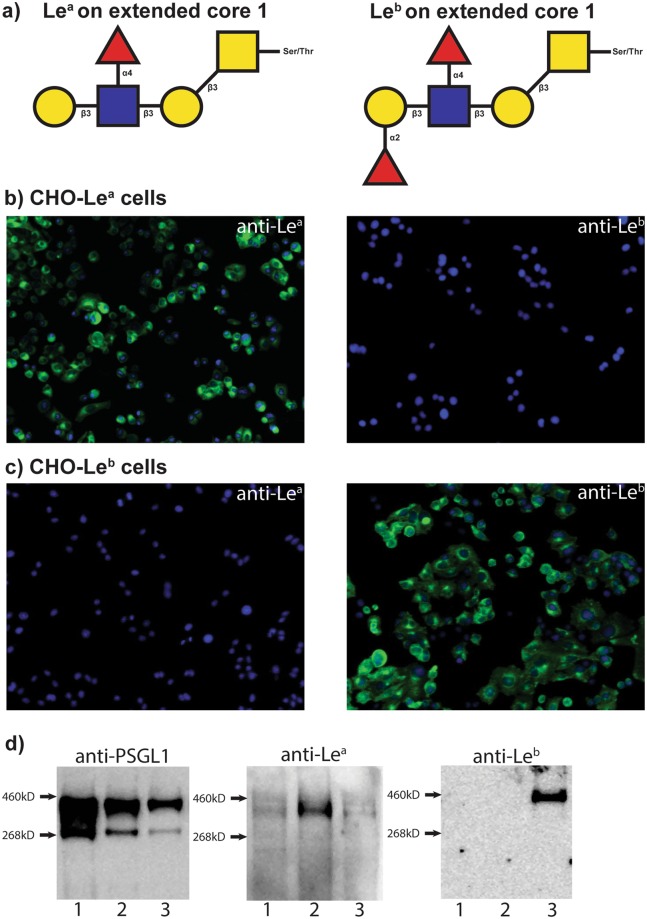
Immunocytochemistry with anti-Lea and anti-Leb antibodies showed that all DAPI stained cells of the selected and expanded clone CHO-PSGL1-Lea-a1 were positively stained (in green) for Lea, but not for Leb (Fig. 1b). Similarly, the DAPI stained CHO-PSGL1-Leb-b4 clone was only positively stained for Leb (Fig. 1c). Subsequent analysis by SDS-PAGE and Western blotting of the purified PSGL-1/mIgG2b produced by these clones, revealed CHO-PSGL1-Lea-a1 expressed only Lea, whereas the CHO-PSGL1-Leb-b4 clone predominantly expressed Leb (Fig. 1d and Supplementary Fig S7 online). Therefore, the CHO-PSGL1-Lea-a1 (CHO-Lea), CHO-PSGL1-Leb-b4 (CHO-Leb) cell lines along with the Lewis antigen negative control cell lines CHO-CP55 and CHO-K1, were used for the ETEC CF-binding characterization experiments.
Figure 1.
Glyco-engineered CHO-Lea and CHO-Leb cell lines express Lewis antigens. (a) SNFG (Symbol Nomenclature for Glycans) diagrams of the Lea and Leb expressing glycan structures that were engineered to CHO-K1 cell lines for this study. For further details please also see Supplementary Fig. S1 and Table S1 online. [(b,c)] Immunocytochemistry staining of the CHO-Lea and CHO-Leb cell lines. (b) CHO-Lea cells stained with anti-Lea and anti-Leb antibodies. (c) CHO-Leb cells stained with anti-Lea and anti-Leb antibodies. Lewis antigens are visualized with Alexa Fluor 488-conjugated antibodies (green) and host cell nuclei with DAPI (blue). Magnification x20. (d) SDS-PAGE and Western blot analysis of PSGL-1/mIgG2b proteins expressed in the CHO-CP55 (lane 1), CHO-Lea (lane 2) and CHO-Leb (lane 3) cell lines. In each lane, 1.5 μg of protein was loaded. Membranes were probed with either anti-PGSL1, anti-Lea or anti Leb antibodies followed by an anti-mouse IgG secondary antibody.
ETEC CFA/I fimbriae binds to CHO-Lea cells
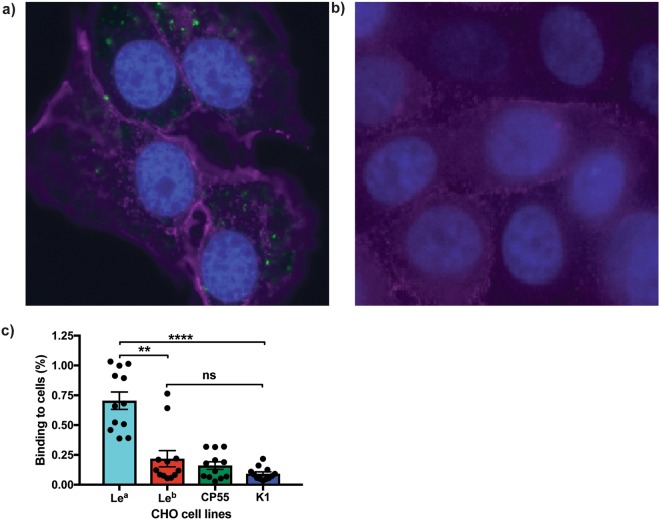
In previous studies, we have demonstrated that ETEC CFA/I fimbriae bind to Lea-5 glycolipids separated onto thin layer chromatograms7. To evaluate the binding specificity of ETEC CFA/I fimbriae to defined Lea or Leb expressing glycans, the glyco-engineered CHO-K1 cells expressing either Lea (CHO-Lea) or Leb (CHO-Leb) determinants on their cell surface, along with the negative glycan phenotype control cell lines (CHO-CP55 and CHO-K1) were infected with a recombinant E. coli Top10-CFA/I bacterial strain. Using immunocytochemistry, we observed the CFA/I strain attached more to CHO-Lea (Fig. 2a) than to CHO-Leb cells (Fig. 2b). Furthermore, when we measured the binding of the recombinant CFA/I strain using a quantitative immunofluorescence assay (Fig. 2c), we observed approximately three-fold, significantly higher, bacterial binding to the CHO-Lea cell line than to the CHO-Leb (P = 0.0065), CHO-CP55 and CHO-K1 cell lines (P = <0.0001).
Figure 2.
ETEC CFA/I fimbriae bind to the CHO-Lea cells. Immunofluorescence staining of (a) CHO-Lea and (b) CHO-Leb cells infected with TOP10-CFA/I. Infected cell lines were stained with anti-CFA/I antibody (Alexa Fluor 488, green), Lea antibody (Alexa Fluor 647, purple), Leb antibody (Texas Red, purple) and host nuclei stain (DAPI, Blue). All images are taken at x40 magnification. (c) TOP10-CFA/I bacteria adhere to the CHO-Lea cell line more than to CHO-Leb, CHO-CP55 and CHO-K1 cells. Graphs represent the percentage of TOP10-CFA/I bacteria binding to each of the cell lines, as measured by quantifiable immunofluorescence analysis. Statistical analysis was performed using ANOVA with Dunn’s multiple comparisons test. **Indicates P = 0.0065, ****Indicates P = <0.0001. Data presented as Mean and SEM of at least three independent experiments.
The ETEC CFA/I major subunit CfaB binds more to CHO-Lea cells than the ETEC CFA/I minor subunit CfaE
It has previously been reported that CFA/I has two distinct binding activities with CfaE binding to receptors of unknown structures on the surface of erythrocytes and intestinal epithelial and cultured cells, whilst the major subunit CfaB binds to various glycolipids present on human small intestinal tissue4,7,16. To assess if either the major CfaB subunit or the minor CfaE subunit are responsible for the binding of CFA/I to Lea, we infected our glyco-engineered cell lines with a CFA/I recombinant strain without the minor subunit CfaE (Top10-CFA/IΔE). Using our semi-quantitative immunofluorescence assay, we found that the CFA/IΔE strain bound at a significantly higher percentage to the CHO-Lea cell line compared to the CHO-Leb (Fig. 3a, P = 0.0376), CHO-CP55 and CHO-K1 cell lines (Fig. 3a, P = <0.0001).
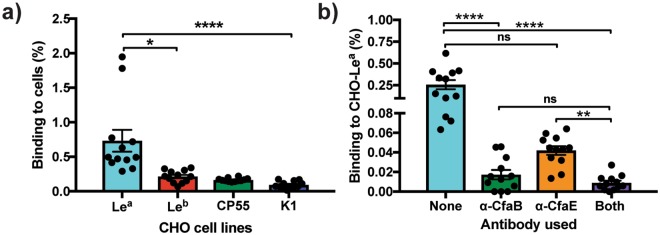
Figure 3.
CfaB, the major subunit of ETEC CFA/I fimbriae, binds to the CHO-Lea cells. (a) TOP10-CFA/IΔE bacteria adhere to the CHO-Lea cell line more than to CHO-Leb, CHO-CP55 and CHO-K1 cell lines. (b) Inhibition of binding of TOP10-CFA/I bacteria to the CHO-Lea cells, using MAbs specific for the CFA/I major subunit CfaB, the minor subunit CfaE, and a mixture of anti-CfaB and anti-CfaE. Graphs represent the percentage of bacteria binding to each of the cell lines, as measured by quantifiable immunofluorescence analysis. Statistical analysis was performed using ANOVA with Dunn’s multiple comparisons test. *Indicates P = 0.0376, **Indicates P = 0.0035, ****Indicates P = <0.0001. Data presented as Mean and SEM of (a) three independent and (b) two independent experiments.
To substantiate these observations, we next performed an inhibition assessment assay using anti-CfaB and anti-CfaE antibodies, the Top10-CFA/I bacterial strain and CHO-Lea cells. We found that pre-incubation of the TOP10-CFA/I strain with the anti-CfaB antibody alone, or in combination with equal amounts of the anti-CfaE antibody, significantly reduced the binding of the Top10-CFA/I strain to the CHO-Lea cells (Fig. 3b, P = <0.0001). There was a slight but no significant decrease (P = <0.08) in the binding to CHO-Lea cells when the Top10-CFA/I strain was pre-incubated with the anti-CfaE antibody alone, compared to no antibody incubation (Fig. 3b).
ETEC CFA/I CF family members also bind to CHO-Lea cells
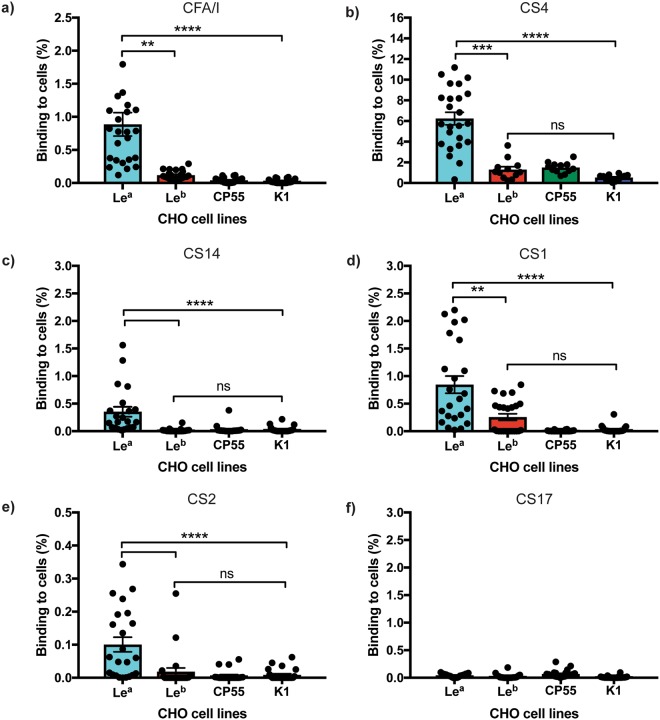
We have previously demonstrated that Bangladeshi children expressing the Lea antigen (Le(a + b−), non-secretor phenotype) are more likely to be suffering with symptomatic ETEC infection if they have been infected by ETEC strains expressing CFA/I, CS1, CS2, CS4, CS14 and CS17 CFs7. To highlight the flexibility of our glycan models, we also infected them with wild-type ETEC reference strains expressing the above CFs (see materials and methods for further details of the ETEC reference stains used). We observed the wild-type ETEC strain expressing CFA/I bound at a significantly higher percentage to CHO-Lea cells, compared to the CHO-Leb (P = 0.0040, Fig. 4), CHO-CP55 and CHO-K1 cells (P = <0.0001, Fig. 4). Similarly, CS4 expressing ETEC bacteria attached strongly to Lea but not the Leb or negative control expressing CHO cells (P = <0.0002, Fig. 4). Moreover, the wild-type ETEC strains expressing CS1, CS2, and CS14 adhered significantly more to the CHO-Lea than to the CHO-Leb, CHO-CP55 and CHO-K1 cells (P = <0.0040, Fig. 4). However, we could see no significant difference in the binding of the ETEC strain expressing CS17 to any of our glycan cell line models (Fig. 4).
Figure 4.
ETEC CFA/I and other type 5 pili family members bind to CHO-Lea cells. (a) ETEC expressing CFA/I+ ST+/LT+ (b) ETEC expressing CS4+, ST−/LT− (c) ETEC expressing CS14+, STh+/LT− (d) ETEC expressing CS1+, ST−/LT− (e) ETEC expressing CS2+, ST−/LT−, and (f) ETEC expressing CS17+, ST−/LT+ and their binding to CHO-Lea, CHO-Leb, CHO-CP55 and CHO-K1 cells. Graphs represent the percentage of bacteria binding to each of the cell lines, as measured by quantifiable immunofluorescence analysis. Statistical analysis was performed using ANOVA with Dunn’s multiple comparisons test. **Indicates P = <0.0040, ***Indicates P = 0.0002, ****Indicates P = <0.0001. Data represent the Mean and SEM of two independent experiments.
Molecular docking predicts the Ig-like groove of CfaB is used by ETEC CFA/I to bind to Lea expressing glycans
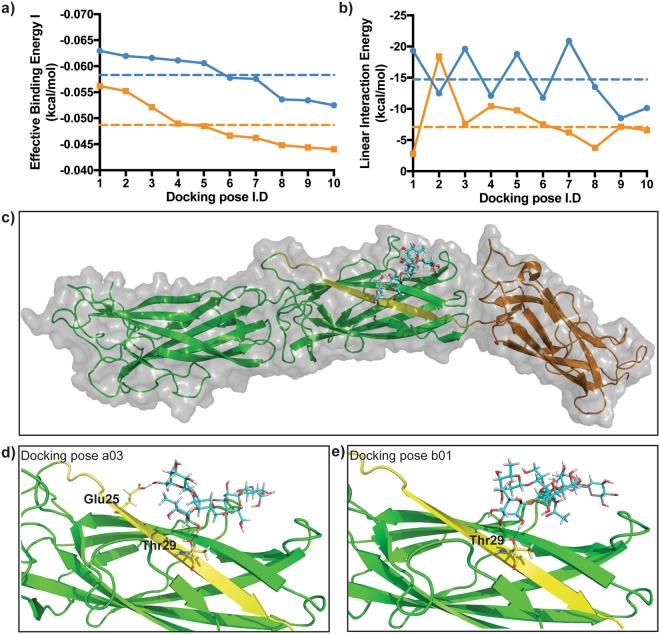
To provide an insight into why CFA/I fimbriae can bind to Lea glycans, we performed molecular docking simulations using pre-existing 3D protein and ligand structures of CFA/I and the Lea-5 and Leb-6 determinants9,25–27. For each glycan, 32 docking simulations were generated around the N terminal Ig-like region of the CfaB major subunit of CFA/I (see Fig. 5c as an example). To predict the most likely of these docking simulations, these poses were ranked based on highest Effective Binding Energy (I and II) (see Supplementary Tables S2 and S3 online, and materials and methods for further details).
Figure 5.
Molecular docking predicts CfaB can bind to Lea-5 and Leb-6 glycans. (a) Effective Binding Energy I (kcal/mol) of the ten highest ranked Lea-5 and Leb-6 poses. (b) Linear Interaction Energy (kcal/mol) of the ten highest ranked Lea-5 and Leb-6 poses. Blue lines are Lea-5 poses and orange lines are Leb-6 poses. Dotted lines are respective mean values for the ten highest ranked Lea-5 (blue) and Leb-6 (orange) poses analysed. (c) Overall surface view and cartoon representation of Lea-5 binding to the N-terminal Ig-like groove of the major CFA/I subunit CfaB. (d) Cartoon representation of docking pose a03, showing hydrogen bond interaction of residues Glu25 and Thr29 of the CfaB Ig-like groove with Lea-5. (e) Cartoon representation of docked complex b01, showing hydrogen bond interaction of Thr29 of the CfaB Ig-like groove with Leb-6. For (c) to e) the Lea-5 and Leb-6 ligands are stick representations (cyan) with atoms interacting with CfaB coloured in yellow and hydrogen bonds displayed as black dotted lines. For the CFA/I fimbriae, the minor subunit CfaE is the green ribbon, the major subunit CfaB is the dark yellow ribbon and the CfaB N terminal Ig-like groove is the bright yellow ribbon.
Interestingly, we noticed the ten most likely Lea-5 poses had relatively higher Effective Binding Energy than the ten most likely Leb-6 poses (Fig. 5a and Supplementary Tables S2 and S3 online). Equally, these Lea-5 docking poses had on average, higher Linear Interaction Energy than the Leb-6 docking poses, suggesting Lea-5 interacts more strongly with the CFA/I docking site, compared to Leb-6.
Next, we examined if the Ig-like groove region of CfaB could be binding to the Lewis antigen containing regions of our most likely Lea-5 and Leb-6 docking poses. From this selection, we instantly identified the docking candidate with the highest Effective Binding Energy (I and II) and Linear Interaction Energy to be the Lea-5 docking pose, a03 (Table 1). This selection also predicted Thr29 and up to two other amino acids (Glu25 Asn27) of the CfaB Ig-like groove V24EKNITVTASVD35 region could be binding to the α1,4-FucT (Lea) and/or the neighboring β1,3 Gal and GalNAc moieties of the Lea-5 glycan (Table 1, Fig. 5d, Supplementary Figs S3 and S4 online). In contrast to the most likely Leb-6 docking poses (b01,b05, b07 and b08), we observed that only one CfaB amino acid (Thr29) might bind to just the α1,2-FucT moiety (Leb) of the Leb-6 glycan (Table 1, Fig. 5e, Supplementary Figs S3 and S5 online). Furthermore, the comparatively higher ligand strain energy, the lower ligand strain energy scores and relatively high ligand solvent GB scores of Lea-5 compared to Leb-6 (Table 1), implies less steric hindrance of Lea-5 in the CFA/I docking site suggesting that Lea-5 is a more distorted structure than the Leb-6 glycan.
Table 1.
In-silico docking analysis predicts relatively higher binding affinity of the CFA/I docking site to Lea-5.
| Pose I.D | XP Glide Score | MM-GBSA score | Effective Binding Energy Ia | Effective Binding Energy IIb | Ligand solvent GB | Ligand strain energy | Linear Interaction Energyc |
|---|---|---|---|---|---|---|---|
| Selected Le a -5 docking poses | |||||||
| a03 | −8.898 | −52.583 | −0.061 | −0.907 | −32.956 | 7.368 | −19.627 |
| a04 | −10.036 | −52.168 | −0.061 | −0.899 | −40.076 | 15.161 | −12.092 |
| a06 | −10.182 | −49.319 | −0.058 | −0.850 | −37.542 | 18.196 | −11.777 |
| a08 | −10.310 | −45.768 | −0.054 | −0.789 | −32.258 | 14.674 | −13.509 |
| a09 | −8.005 | −45.628 | −0.053 | −0.787 | −37.112 | 10.394 | −8.517 |
| Selected Leb-6 docking poses | |||||||
| b01 | −8.302 | −56.176 | −0.056 | −0.826 | −53.394 | 0.935 | −2.782 |
| b02 | −8.798 | −55.204 | −0.055 | −0.812 | −36.825 | 0.081 | −18.379 |
| b05 | −8.395 | −48.488 | −0.048 | −0.713 | −38.728 | 11.456 | −9.759 |
| b07 | −8.046 | −46.198 | −0.046 | −0.679 | −39.974 | 5.267 | −6.224 |
| b08 | −9.352 | −44.813 | −0.045 | −0.659 | −41.044 | 5.922 | −3.769 |
Candidate poses selected from the ten highest ranked Lea-5 and Leb-6 docking scores based on lowest Effective Binding Energy I and II, that predict CfaB binding near/on Lewis antigen moieties of either the Lea-5 or Leb-6 glycan. All energy score units are measured in kcal/mol.
aMM-GBSA score/molecular weight of glycan.
bMM-GBSA score/number of heavy atoms in glycan.
cMM-GBSA score - Ligand solvent GB.
Discussion
The surface of the mammalian intestinal tract is covered in a rich diversity of mucosal glycans. Such glycans often express genetically defined HBGAs, with HBGA expression varying in the small and large intestine. HBGA glycan expression evolves from birth, and can contribute to intestinal homeostasis, and microbial composition. However, these genetically defined HBGAs can also act as target binding receptors for the virulence factors of microbial pathogens. Such host-pathogen interactions are thought to contribute to pathogen-host species specify, and pathogen-host tissue tropism17–19,28. For example, FUT2−/− (i.e. HBGA Lea-expressing) individuals are genetically immune to certain norovirus genotypes (GI-1, G-II-3 and G-II-4) compared to FUT2+/+ or FUT2+/− individuals17. Similarly FUT2+/+ pigs are more susceptible to porcine pathogenic ETEC strains expressing F18+ fimbriae29.
We have previously published clinical evidence to suggest that ETEC CFA/I uses the HBGA Lea as a small intestinal host receptor, as Bangladeshi children expressing this Lea antigen (caused by a FUT2 mutation, non-secretor status) are more susceptible to disease caused by ETEC expressing CFA/I and related CF family members, than are Bangladeshi children expressing the Leb antigen (functional FUT2, secretor status)5–7. Expression of HBGAs (Lewis and ABO(H)) on the surface of the human intestinal mucosa is driven by the FUT2 and FUT3 genes, with FUT2 encoding an α1,2-fucosyltransferase (α1,2-FucT) and FUT3 encoding an α1,3/4-fucosyltransferase (α1,3/4-FucT) (Supplementary Fig. S1 online)17. Expression of only FUT3 results in Lea antigen expression, whilst expression of both FUT3 and FUT2 results in the expression of Leb determinants on small intestinal glycans17–19.
To create a defined glycan model of the human small intestine to study the binding of ETEC CFA/I fimbriae to Lewis antigens, we glyco-engineered the well-defined and naturally HBGA devoid CHO-K1 cell line22. To generate CHO-K1 cells carrying Lea or Leb determinants on their cell surface, we expressed the extended core 1 glycan (GlcNAcβ3 Galβ3GalNAcα) enzyme B3GNT3, the type 1 chain glycan (Galβ 3GlcNAc)-encoding enzyme B3GALT5, and the Lewis gene-encoding enzyme FUT3 alone (generating Lea) or together with the H gene-encoding FUT1 (generating Leb) on a PSGL-1/mIgG2b fusion protein carrying probe (See Supplementary Fig. S1 and Table S1 online for further details)20–24. Expression of the B3GALT5 enzyme facilitates the biosynthesis of type 1 chains (Galβ3GlcNAc) on both N- and O-glycans (on the latter following co-expression of the extended core 1 enzyme B3GNT3), which is the obligate precursor for the Lewis (FUT3) and H-gene (FUT1) enzymes. This enabled us to engineer recombinant CHO-K1 cell lines carrying abundant Lea or Leb antigen substitutions24.
Initially, we evaluated the binding specificity of ETEC CFA/I fimbriae to our stable Lewis antigen-expressing cell lines, by infecting them with recombinant bacteria expressing CFA/I fimbriae. We found the CFA/I expressing strain attached more to the CHO-Lea cell line, than to the CHO-Leb or the Lewis antigen negative control cell lines (CHO-CP55 and CHO-K1). This may suggest that the α1,2-linked fucose of α1,2-FucT which is used to create Leb could be either blocking the CFA/I binding sites on the Lea receptor, or making the binding sites less accessible for the CFA/I fimbriae and thus preventing ETEC CFA/I attachment.
To define which subunit of ETEC CFA/I fimbriae are responsible for the binding of CFA/I fimbriae to our CHO-Lea cell line, we also infected our cell lines with a recombinant E.coli strain expressing CFA/I without the minor subunit (Top10-CFA/IΔE). As expected7, the CfaE deleted strain adhered more to CHO-Lea cells than to CHO-Leb, CHO-CP55 or CHO-K1 cells. Similarly, pre-incubation of the Top10-CFA/I recombinant strain with either anti-CfaB or an equal mix of anti-CfaB and CfaE antibodies, significantly reduced CFA/I binding to CHO-Lea cells. However, the bacterial binding after the pre-incubation of Top10-CFA/I with only anti-CfaE compared to no antibody pre-incubation was reduced, but not statically significant. As mature CFA/I fimbriae contain >1,000 copies of CfaB and one or a few copies of CfaE4, we therefore conclude the major subunit CfaB is naturally the more dominant subunit for CFA/I binding to small intestinal Lea glycans.
To help understand why the CfaB subunit of ETEC CFA/I fimbriae binds to Lea but not Leb glycans, we performed computational molecular docking analysis. Our selected CFA/I docking site encompassed the amino acids V24EKNITVTASVD35 of the highly conserved CfaB Ig-like groove, found in major subunits of ETEC CFA/I related CFs (Supplementary Figs S2 and S5 online) and class 1 pili of bacteria that can cause urinary and respiratory infections by binding to host glycolipids containing HBGAs9,17.
Supporting our binding studies using glycan-defined CHO-K1 cells, as well as previous clinical observations6,7, our in-silico defined CFA/I docking site bound with a relatively higher binding affinity and higher interaction preference to the Lea than to Leb glycan. Moreover, the ligand strain energy as well as the small size of Lea-5, might explain its relative distortion in the CFA/I binding site as compared to that of the Leb-6 glycan. We hypothesise this relative distortion, could be one reason why multiple amino acids (Asn27,Thr29 and Glu25) of the CfaB Ig-like fold region (Supplementary Figs S2 and S5 online), docked to the α1,4-linked fucose and/or several surrounding moieties of the Lea-5 glycan. We now plan to perform more computational simulations as well as further in-vitro analysis to assess these observations further.
To highlight the flexibility of our small intestinal glycan defined like model, we also infected our Lewis expressing CHO-K1 cell lines with wild-type ETEC reference strains expressing CFA/I as well as CFA/I related CFs; the latter having been suggested to also bind to the small intestinal mucosa of children expressing Lea7. As expected, the wild-type ETEC strain expressing CFA/I bound significantly more to CHO-Lea cells than to the Leb or negative cell lines. Similarly, the ETEC strains expressing the related CFs CS1, CS2, CS4, CS14, but not CS17, bound more to CHO-Lea cells.
Interestingly, the amino acids that we hypothesise by molecular docking to be important for CfaB binding to Lea-5, are also highly conserved in the major subunits of the related CFA/I CFs we have studied (see Supplementary Fig. S6 online for more details). However, the binding capacity differences of these wild-type ETEC strains expressing these CFs to CHO-Lea cells may be due to evolved conformational differences amongst family members (Supplementary Fig. S6 online)4. Therefore, one reason why the CS17 ETEC strain does not bind to CHO-Lea cells is that amino acid Arg31, located only a few positions away from the polar Asn27 and Thr29 amino acids (R group, Supplementary Fig. S6 online), is potentially leading to CS17 CF structural and conformational changes near these predicted binding sites.
A critical first step in a microbial pathogenesis is frequently the attachment to host cell glycans. In particular, targets on host tissues include the ubiquitously expressed HBGAs of different mucosae17–19,28. Structural and functional studies are now starting to reveal insights into why individuals expressing different HBGAs are at an increased risk of infections such as those caused by Vibrio cholera, Pseudomonas aeruginosa, norovirus, rotavirus and Helicobacter pylori17,28. By developing Lewis antigen cell-based models of the human small intestine, as well as performing docking studies, we have further defined why HBGA Lea – expressing non-secretor (FUT2−/−) individuals, or young children (<2 years of age) are more susceptible to ETEC expressing CFA/I, or other related CFs family members5,6,8,28. Subsequently, our understanding and characterisation of these host-pathogen binding patterns could represent a critical point at which the adherence of ETEC expressing CFA/I and related CF family members, can be prevented with vaccines and/or anti-infection therapeutics that block this interaction (Fig. 6).
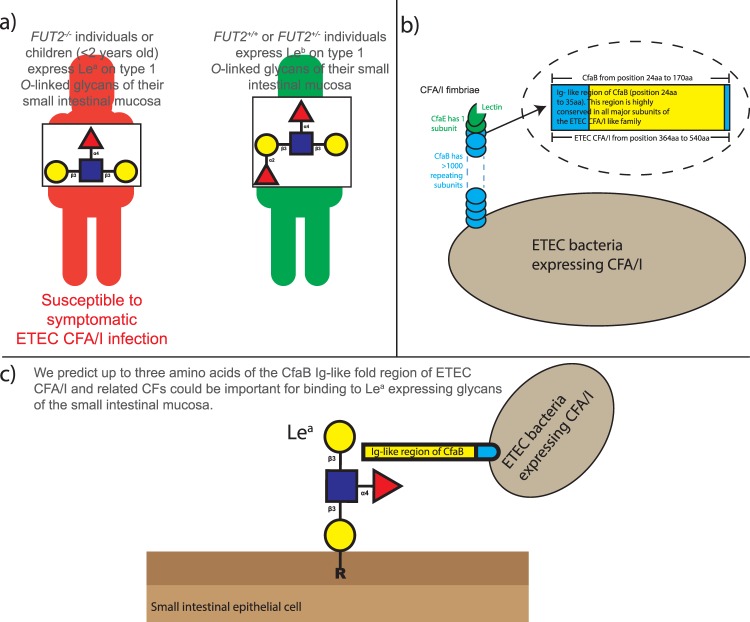
Figure 6.
Working hypothesis. (a) FUT2−/− individuals or children (<2 years old) express Lea on type 1 O-linked glycans of the small intestinal mucosa. We have evidence to suggest they are susceptible to symptomatic ETEC CFA/I (and related CFA/I CF family members) infection. FUT2+/+ or FUT2+/− individuals (>2 years old) express Leb on type 1 O-linked glycans of the small intestinal mucosa. We have not found these individuals to not be susceptible to ETEC CFA/I (and related CFA/I CF family members) infection. (b) ETEC CFA/I fimbriae contains >1,000 copies of a major subunit (CfaB), with one or a few copies of the tip residing minor subunit (CfaE). A 12-aa stretch V24EKNITVTASVD35 of the Ig-like binding groove region of CfaB served as a CFA/I docking site our in-silico docking analysis. This 12 amino acid stretch also shares structural similarities with all ETEC CF major subunits of the CFA/I CF like family, and with class 1 pili from bacteria that can cause urinary and respiratory infections by binding to host glycolipids containing HBGAs. (c) Using our small intestinal like glycan defined CHO-K1 cell lines, we have demonstrated that CfaB of ETEC CFA/I fimbriae, as well as four related CFs, bind more to our CHO-K1 cell-line expressing Lea, compared to cells carrying Leb or the CHO-K1 wild-type glycan phenotype. Using in-silico docking analysis, we predict up to three amino acids (Glu25, Asn27, Thr29) found in the immunoglobulin (Ig)-like groove region of CfaB of CFA/I and related CF fimbriae, could be important for the preferential and higher affinity binding of CFA/I fimbriae to Lea glycans. These findings may lead to a better molecular understanding of ETEC pathogenesis, aiding in the development of vaccines and/or anti-infection therapeutics, which block such host-pathogen interactions.
Materials and Methods
Plasmids used to construct glyco-engineered cell lines
Plasmids encoding the P-selectin glycoprotein-1/mouse immunoglobulin IgG2b Fc fragment (PSGL-1/mIgG2b) fusion protein, the human extended core 1 (GlcNAcβ3Galβ3GalNAcα) enzyme (B3GNT3), the type 1 chain (Galβ3GlcNAc) enzyme (B3GALT5), the Lewis gene α3/4-fucosyltransferase (FUT3) and the blood group H gene-encoded α2-fucosyltransferase (FUT1) were constructed as described previously (See Supplementary Table S1 online for details of the constructed plasmids)20–22,24. The pCMV/FUT1/Zeo rather than a FUT2 expression plasmid was selected to be used in this study, as it has been shown to better support Leb expression in CHO cells24.
Construction of glyco-engineered cell lines
Adherent CHO-K1 cells (ATCC®, Manassas, VA, USA) were seeded in six-well cell culture plates containing Dulbecco’s Modified Eagle’s medium (DMEM, Lonza), supplemented with 10% fetal bovine serum (FBS, Invitrogen, Waltham, MA, USA). All transfection experiments were performed 24 hours after seeding (70–80% cellular confluency). Cellular transfection was performed in accordance with manufacturer’s instructions using Lipofectamine 2000 (Invitrogen). CHO-K1 cells were transfected with the pEF1α/PSGL-1/mIgG2b/PAC plasmid to create the CHO-CP55 cell line. CHO-CP55 cells were then co-transfected with a cocktail of plasmids; pCMV/C1-β1,3GlcNAcT/Neo (human extended core 1 enzyme), pCMV/GalT5/Gpt (type 1 chain enzyme), in combination with of pCMV/FUT3/Hyg for Lea expression (CHO- Lea cell line), and combined with pCMV/FUT1/Zeo for Leb expression (CHO-Leb cell line) (Supplementary Fig. S1 and Table S1 online). To generate stable transfectants, all the five expression vectors were linearized with Avr II (New England BioLabs, Ipswich, MA, USA), and equal concentrations of plasmids encoding glycosyltransferases were used.
Following transfection (48 hours), all the transfected cell lines were incubated in selection medium as stated in the Supplementary Materials and Methods online. Two weeks after transfection, drug resistant clones were picked and transferred to 96-well plates containing their corresponding selection medium and propagated. Lea and Leb expression was assessed by immunocytochemistry and Western blot using monoclonal antibodies specific for the Lea and Leb determinants (see Supplementary Materials and Methods online for further details). Two clones- CHO-PSGL1-lea-a1 and CHO-PSGL1-leb-b4 (Fig. 1) were selected, expanded and used for ETEC cell binding experiments.
Bacterial strains used in infection experiments
The previously constructed recombinant Top10-CFA/I (AmpR) and Top10-CFA/I/E− (CmR) strains were cultured as previously described7. The following wild-type ETEC strains and natural mutants were also used: E3006 (258909-3; CFA/I+ ST+/LT+), E120 (60R936; CS1+, ST−/LT−), E3017 (58R957; CS2+, ST−/LT−), E3037 (62R486; CS4+, ST−/LT−), E3013 (CS14+, STh+/LT−), and E3014 (CS17+, ST−/LT+)4,30. All ETEC strains were cultured as previously described7,30. Expression of CFs was tested by agglutination assays using monoclonal CF antibodies4,30.
In preparation for infection experiments, bacterial cultures were centrifuged at 10,000 × g for 10 minutes. The bacterial pellets were then washed and re-suspended in PBS before being re-centrifuged. Recombinant strains were re-suspended in PBS and ETEC strains were re-suspended in 0.5% D-mannose/PBS solution at an OD 0.8/mL (680 nm) to block possible binding by Type I fimbriae.
Infection of CHO cell lines
Each cell line was seeded at a concentration of 0.5 × 105/mL into microscope well slides (open µ-Slide, Ibidi). Three days after seeding, the well culture media was removed, washed with warm PBS and then replaced with 275 µL of DMEM with 1% FBS and 2nM L-glutamine in preparation for infection experiments. Cell lines were infected in duplicate with 25 µL of the corresponding bacterial suspensions (∼25 MOI) for three hours in a humidified incubator at 37 °C and 5% CO2. Following three washes with PBS, cells were fixed in paraformaldehyde for 10 minutes before being washed a further two times with PBS. Fixed cells were stained by immunofluorescence (see below) to determine the number of adherent bacteria to the cell lines.
Inhibition of binding of the Top10-CFA/I ETEC strain to engineered CHO cells using monoclonal antibodies
The monoclonal anti-CfaB and CfaE antibodies were a kind gift from Professor Weiping Zhang of Kansas State University, USA. The CHO-Lea cell line and the recombinant Top10-CFA/I strain were prepared as described above. A suspension (0.8OD/mL) of the Top10-CFA/I strain was mixed with either a 1:50 dilution of (i) anti-CfaB, (ii) anti-CfaE, (iii) a mixture of anti CfaB and anti-CfaE or (iv) no antibody mix. The bacterial/antibody mixtures were then mixed thoroughly and incubated for 30 minutes at room temperature. Cell lines were then infected with the bacterial mixtures as described above.
Immunoflorescence staining of infected cell lines and quantifiable immunofluorescence analysis
All microscopy was performed using the inverted LSM700 confocal microscope with Zeiss Zen Blue software. Infected cells were stained for immunofluorescence microscopy using the protocol stated in the Supplementary Materials and Methods online.
To quantify bacterial adhesion, bacteria were visualised using the Alexa Fluor 488 channel and the CHO cells with the DAPI channel. All immunofluorescence settings remained the same for each experiment performed. Using the Multiple Single Positions (Tiles) position array tool available in the Zen Blue microscope software, six random tiles of each microscopic slide chamber were taken at x20 magnification. For each experiment performed, a total of two chambers per CHO cell line and infection were imaged (i.e. 12 random tile scans per cell line and bacterial infection). Confocal images in Zen format were analysed using the Velocity 3D image software (PerkinElmer, California, USA). Bacterial adhesion was quantified by measuring the mean surface area of bacteria (Alexa Flour 488 channel fluorescence, µM2) and the mean surface area of CHO cells (DAPI channel fluorescence, µM2). Binding of bacteria to CHO cell lines was expressed as a percentage of the total mean surface area of bacteria divided by the total mean surface area of CHO cells multiplied by 100.
Molecular docking
In-silico docking studies were performed using the Glide module31 version 6.2, in Schrödinger, LLC, New York, NY, 2017. The X-ray crystal structure of the fused complex containing the CfaE and CfaB subunits of ETEC CFA/I fimbriae at 2.3 Å was obtained from the RSCB Protein Data Bank (PDB ID: 3F83)9,25,32, and prepared for molecular docking using the Protein Preparation Wizard33 of the Maestro, version 10.5 of the Schrödinger, LLC, New York, NY, 2017 software. Lea-5 (CID:11051152) and Leb-6 (CID:91852492) structures were downloaded from PubChem26,27, and converted to 3D before being processed for in-silico experiments using the Ligprep module34 of the Schrödinger, Maestro v10.5 software. This module generated a number of conformers (32 conformers) for each structure based on various tautomers, stereochemistries, ionization states and checking various ring conformations at a pH range set between 7 ± 2, followed by energy minimization with OPLS-2005 force field31.
Molecular Docking was performed using the Receptor Grid Generation panel module of the Glide, Schrödinger, LLC, New York, NY, 2017 software. A 12-aa stretch V24EKNITVTASVD35 of the Ig-like binding groove region of CfaB was used to a build grid and served as our CFA/I docking site9. The extra precision (XP) method of Glide dock was used for the docking experiments of Lea-5 and Leb-6 conformers to the CFA/I docking site with the sampling of the ligand being kept flexible during docking35.
The relative binding affinity score of our CFA/I docking complex to Lea-5 and Leb-6 was calculated (based on the XP docked complexes) using the MM-GBSA method (Molecular mechanics with generalized born surface area), available in the Schrödinger’s tool Prime software (Schrödinger, LLC, New York, NY, 2017)36,37. Apart from the MM-GBSA (ΔG) energy, a few other parameters were studied to further elucidate the docking results. These include: Effective Binding Energy (see calculations (1) and (2) below) which rescales the binding affinity of the Ligand strain energy to represent the extent of ligand distortion, ligand solvation energy (Lig solv GB) to represent the binding energy of ligand in solvent, and Linear Interaction Energy to represent the extent of binding affinity of ligand towards protein over solvent (see calculation (3) below).
-
Effective Binding Energy I = MM-GBSA/molecular weight of ligand
Molecular weight of Lea-5 ligand = 853.774
Molecular weight of Leb-6 ligand = 999.916
-
Effective Binding Energy II = MM-GBSA/number of heavy atoms in ligand
Number of heavy atoms in Lea-5 ligand = 58
Number of heavy atoms in Lea-5 ligand = 68
Linear Interaction Energy = MM-GBSA − Ligand solvent GB.
Interesting docking sites were selected based on Effective Binding Energy (I and II), the number of interactions from the conserved region of CfaB, and docked complexes interacting with the Lewis antigen or neighbouring moieties of either Lea-5 or Leb-6.
Statistical analysis
Binding of bacteria to the different CHO cell lines were expressed as mean percentages of added bacteria binding to the cells in 12 confocal tile scans. Inhibition results were obtained from two independent experiments performed in duplicate. All other binding experiments were obtained from at least three independent experiments performed in duplicate. Statistical analysis was performed using Graph Pad Prism 6. ANOVA using Dunn’s multi comparisons test was used to compare the mean percentage of bacteria binding to the CHO-Lea, CHO-Leb, CHO-CP55 and CHO-K1 cell lines. For additional verification, the Wilcoxon matched pairs sign rank test was used to compare the mean percentage of bacteria binding to the CHO-Lea cell line compared to CHO-Leb cell line. Significance was set at a P value of <0.05.
Data availability
The raw datasets generated during and/or analysed during the current study are available in the Figshare repository, (https://figshare.com/s/41a7b658f9474b6dfd53).
Electronic supplementary material
Acknowledgements
We gratefully thank Professor Weiping Zhang of Kansas State University, USA for the kind gift of monoclonal anti CfaB and anti CfaE antibodies. We acknowledge the Centre for Cellular Imaging at the Sahlgrenska Academy, University of Gothenburg, Sweden for the use of their imaging equipment, and for the support of their staff. LM, JT and AMS work is supported by the Swedish Research Council (grants 2013-6615 and 2011-3435) and the Swedish Strategic Foundation (grant SB12-0072). JH work is supported by the County Council of Västra Götaland (ALF). SC gratefully acknowledges Professor Leif Eriksson of the Department of Chemistry and Molecular biology, University of Gothenburg, Sweden for his valuable guidance and suggestions in computational analysis of this work. SC acknowledges the Swedish Research Council (grant 2014-3914), the University of Gothenburg Center for Antibiotics Resistance Research, and the Faculty of Science for financial support. The Swedish National Infrastructure for Computing (Grant number 2016/34-30) is gratefully acknowledged for grants of computing time at the Chalmers supercomputing center (C3SE).
Author Contributions
L.M. performed the infection experiments, analysed all data and wrote the manuscript. J.L. glyco-engineered the CHO-cell lines to express Lea and Leb determinants. S.C. preformed the molecular docking experiments. J.T. constructed the recombinant bacterial strains. A.M.S. and J.H. oversaw the project. All authors reviewed the manuscript before submission.
Competing Interests
The authors declare no competing interests.
Footnotes
Lynda Mottram and Jining Liu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29258-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bourgeois AL, Wierzba TF, Walker RI. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016;34:2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 2.Isidean SD, Riddle MS, Savarino SJ, Porter CK. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine. 2011;29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 3.Torres AG, Zhou X, Kaper JB. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect Immun. 2005;73:18–29. doi: 10.1128/IAI.73.1.18-29.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madhavan TP, Sakellaris H. Colonization factors of enterotoxigenic Escherichia coli. Adv Appl Microbiol. 2015;90:155–197. doi: 10.1016/bs.aambs.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Mottram L, Wiklund G, Larson G, Qadri F, Svennerholm AM. FUT2 non-secretor status is associated with altered susceptibility to symptomatic enterotoxigenic Escherichia coli infection in Bangladeshis. Sci Rep. 2017;7:10649. doi: 10.1038/s41598-017-10854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed T, et al. Children with the Le(a+ b−) blood group have increased susceptibility to diarrhea caused by enterotoxigenic Escherichia coli expressing colonization factor I group fimbriae. Infect Immun. 2009;77:2059–2064. doi: 10.1128/IAI.01571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansson L, Tobias J, Lebens M, Svennerholm AM, Teneberg S. The major subunit, CfaB, of colonization factor antigen i from enterotoxigenic Escherichia coli is a glycosphingolipid binding protein. Infect Immun. 2006;74:3488–3497. doi: 10.1128/IAI.02006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kononova SV. How Fucose of Blood Group Glycotypes Programs Human Gut Microbiota. Biochemistry (Mosc) 2017;82:973–989. doi: 10.1134/S0006297917090012. [DOI] [PubMed] [Google Scholar]
- 9.Li YF, et al. Structure of CFA/I fimbriae from enterotoxigenic Escherichia coli. Proc Natl Acad Sci USA. 2009;106:10793–10798. doi: 10.1073/pnas.0812843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhaven TP, Riches JD, Scanlon MJ, Ulett GC, Sakellaris HA. Binding of CFA/I Pili of Enterotoxigenic Escherichia coli to Asialo-GM1 is Mediated by the Minor Pilin, CfaE. Infect. Immun. 2016;84(5):1642–1649. doi: 10.1128/IAI.01562-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudin A, McConnell MM, Svennerholm AM. Monoclonal antibodies against enterotoxigenic Escherichia coli colonization factor antigen I (CFA/I) that cross-react immunologically with heterologous CFAs. Infect Immun. 1994;62:4339–4346. doi: 10.1128/iai.62.10.4339-4346.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lásaro MO, et al. Combined Vaccine Regimen Based on Parenteral Priming with a DNA Vaccine and Administration of an Oral Booster Consisting of a Recombinant Salmonella enterica Serovar Typhimurium Vaccine Strain for Immunization against Infection with Human-Derived Enterotoxigenic Escherichia coli Strains. Infect. Immun. 2004;72(11):6480–6491. doi: 10.1128/IAI.72.11.6480-6491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranallo RT, Fonseka CP, Cassels F, Srinivasan J, Venkatesan MM. Construction and Characterization of Bivalent Shigella flexneri 2a Vaccine Strains SC608(pCFAI) and SC608(pCFAI/LTB) That Express Antigens from Enterotoxigenic Escherichia coli. Infect. Immun. 2005;73(1):258–267. doi: 10.1128/IAI.73.1.258-267.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudin A, Olbe L, Svennerholm AM. Monoclonal antibodies against fimbrial subunits of colonisation factor antigen I (CFA/I) inhibit binding to human enterocytes and protect against enterotoxigenic Escherichia coli expressing heterologous colonization factors. Microbial Pathogenesis. 1996;20:35–45. doi: 10.1006/mpat.1996.0040. [DOI] [PubMed] [Google Scholar]
- 15.Rudin A, Svennerholm AM. Identification of a cross-reactive continuous B-cell epitope in enterotoxigenic Escherichia coli colonisation factor antigen I. Infect. Immun. 1996;64(11):4508–4513. doi: 10.1128/iai.64.11.4508-4513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker KK, Levine MM, Morison J, Phillips A, Barry EM. CfaE tip mutations in enterotoxigenic Escherichia coli CFA/I fimbriae define critical human intestinal binding sites. Cell Microbiol. 2009;11:742–754. doi: 10.1111/j.1462-5822.2009.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooling L. Blood Groups in Infection and Host Susceptibility. Clin Microbiol Rev. 2015;28:801–870. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjork S, Breimer ME, Hansson GC, Karlsson KA, Leffler H. Structures of blood group glycosphingolipids of human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J Biol Chem. 1987;262:6758–6765. [PubMed] [Google Scholar]
- 19.Finne J, et al. Novel polyfucosylated N-linked glycopeptides with blood group A, H, X, and Y determinants from human small intestinal epithelial cells. J Biol Chem. 1989;264:5720–5735. [PubMed] [Google Scholar]
- 20.Liu J, Gustafsson A, Breimer ME, Kussak A, Holgersson J. Anti-pig antibody adsorption efficacy of {alpha}-Gal carrying recombinant P-selectin glycoprotein ligand-1/immunoglobulin chimeras increases with core 2 {beta}1, 6-N-acetylglucosaminyltransferase expression. Glycobiology. 2005;15:571–583. doi: 10.1093/glycob/cwi037. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Holgersson J. Recombinant Galalpha1,3Gal-substituted mucin/immunoglobulin chimeras: a superior absorber of anti-pig antibodies. Transplant Proc. 2000;32:859. doi: 10.1016/S0041-1345(00)01010-1. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Jin C, Cherian RM, Karlsson NG, Holgersson J. O-glycan repertoires on a mucin-type reporter protein expressed in CHO cell pools transiently transfected with O-glycan core enzyme cDNAs. J Biotechnol. 2015;199:77–89. doi: 10.1016/j.jbiotec.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Qian Y, Holgersson J. Removal of xenoreactive human anti-pig antibodies by absorption on recombinant mucin-containing glycoproteins carrying the Galalpha1,3Gal epitope. Transplantation. 1997;63:1673–1682. doi: 10.1097/00007890-199706150-00023. [DOI] [PubMed] [Google Scholar]
- 24.Holgersson J, Lofling J. Glycosyltransferases involved in type 1 chain and Lewis antigen biosynthesis exhibit glycan and core chain specificity. Glycobiology. 2006;16:584–593. doi: 10.1093/glycob/cwj090. [DOI] [PubMed] [Google Scholar]
- 25.The Protein Databank. Structure of fusion complex of the minor pilin CfaE and major pilin CfaB of CFA/I pili from ETEC E. coli (PDB ID number: 3F83) http://www.rcsb.org/pdb/explore.do?structureId=3f83 (2018).
- 26.National Center for Biotechnology Information. PubChem Compound Database; CID = 91852492 https://pubchem.ncbi.nlm.nih.gov/compound/91852492#section=Top (2018).
- 27.National Center for Biotechnology Information. PubChem Compound Database; CID = 11051152. https://pubchem.ncbi.nlm.nih.gov/compound/11051152#section=Top (2018).
- 28.Heggelund JE, Varrot A, Imberty A, Krengel U. Histo-blood group antigens as mediators of infections. Curr Opin Struct Biol. 2017;44:190–200. doi: 10.1016/j.sbi.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Coddens A, et al. Recognition of Blood Group ABH Type 1 Determinants by the FedF Adhesin of F18-fimbriated Escherichia coli. J. Biol. Chem. 2009;284(15):9713–9726. doi: 10.1074/jbc.M807866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjöling Å, Wiklund G, Savarino SJ, Cohen DI, Svennerholm AM. Comparative Analyses of Phenotypic and Genotypic Methods for Detection of Enterotoxigenic Escherichia coli Toxins and Colonisation Factors. J. Clin. Microbiol. 2007;45(10):3295–3301. doi: 10.1128/JCM.00471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friesner RA, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 32.Berman HM, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen WL, Maxwell DS, Tirado-Rives J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. Journal of the American Chemical Society. 1996;118:11225–11236. doi: 10.1021/ja9621760. [DOI] [Google Scholar]
- 35.Friesner RA, et al. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. Journal of Medicinal Chemistry. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 36.Bell, J. A. et al. PrimeX and the Schrödinger computational chemistry suite of programs. International Tables for Crystallography. 534–538 (2012).
- 37.Genheden S, Ryde U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov. 2015;10:449–461. doi: 10.1517/17460441.2015.1032936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw datasets generated during and/or analysed during the current study are available in the Figshare repository, (https://figshare.com/s/41a7b658f9474b6dfd53).