Abstract
Pulmonary tumor thrombotic microangiopathy (PTTM) is a rare disease that shows hypoxia with severe pulmonary hypertension related to malignant tumor. Diagnosis is difficult due to rapid clinical progression and the need to demonstrate pathological findings from lung biopsy. A 64-year-old woman visited our hospital with hypoxia and pulmonary hypertension. Diffuse granular shadows in the centrilobular area and ground-glass shadows in both lungs and left ovarian tumor were found on radiological imaging. PTTM was suspected, but pulmonary artery blood aspiration by right cardiac catheter failed to detect cancer cells. We could not obtain lung or ovary biopsies because of hypoxia or pulmonary hypertension. The patient died due to respiratory failure. Signet ring cell carcinoma of unknown primary, PTTM, and Krukenberg tumor were diagnosed on autopsy. Since early diagnosis facilitates adequate treatment, physicians should not miss the opportunity for biopsy in cases of suspected PTTM.
Keywords: Signet ring cell carcinoma, Unknown primary, Pulmonary tumor thrombotic microangiopathy
Introduction
Pulmonary tumor thrombotic microangiopathy (PTTM) is characterized by tumor microemboli associated with fibrocellular and fibromuscular intimal proliferation in small arteries of the lung [1, 2]. Clinical manifestations of PTTM include rapid progression of hypoxia and pulmonary hypertension. Sixty percent of reported PTTM cases were diagnosed from postmortem examination. Krukenberg tumor is known as a metastatic carcinoma of the ovaries and the pathological type is signet ring cell carcinoma with abundant mucus [3]. We herein report a case of rapidly progressing respiratory failure and pulmonary hypertension revealed as signet ring cell carcinoma of unknown primary with PTTM and Krukenberg tumor on postmortem examination.
Case Presentation
A 64-year-old Japanese woman visited our hospital with a history of dry cough and progressive dyspnea for several weeks. She had no fever, sputum or weight loss. She had no history of smoking or dust exposure. Uterine fibroid had been identified 1 year before this admission. Physical examination showed: body temperature, 35.9°C; blood pressure, 98/68 mm Hg; heart rate, 98 beats/min; and respiratory rate, 22 breaths/min. Cardiac II sounds were enhanced without cardiac murmur or crackles on chest auscultation. Other physical examinations yielded normal results, including assessment of consciousness, abdomen, and skin.
Chest radiography showed diffuse small granular shadows in bilateral lung fields (Fig. 1a). Chest computed tomography (CT) showed diffuse centrilobular granular shadows and a tree-in-bud appearance (Fig. 1b, c). Contrast-enhanced abdominal CT revealed a 6.5 × 6.3-cm tumor in the pelvic area (Fig. 1d, e). Laboratory findings were as follows: hemoglobin, 10.3 g/dL; white blood cell count, 6,300/μL; platelet count, 5.0 ×104/μL; prothrombin time/international normalized ratio, 2.0; activated partial thromboplastin time, 30.9 s; fibrinogen, 425.3 mg/dL; fibrin degradation product, 34.4 μg/mL; total protein, 6.4 g/dL; albumin, 3.8 g/dL; aspartate aminotransferase, 36 IU/L; alanine aminotransferase, 17 IU/L; lactate dehydrogenase, 391 IU/L; alkaline phosphatase, 3,011 IU/L; and C-reactive protein, 0.64 mg/dL. Tumor markers were as follows: carcinoembryonic antigen, 4.5 ng/ml; carbohydrate antigen 19-9, 7.0 U/ml; and cancer antigen 125, 49 U/mL. Arterial blood gas analysis under room air showed: pH 7.47; PO2, 52.0 torr; PCO2, 25.6 torr; HCO3−, 18.4 mM/L; and base excess, −3.9 mM/L. Urinalysis and other biochemical tests were within the normal range. Pulmonary function test showed a normal ventilation pattern (%VC, 86.4%; FEV1.0%, 80.4%), mildly decreased in pulmonary diffusing capacity (%DLCO, 71.2%). Echocardiography showed dilatation of the right atrium and right ventricle, with marked elevation of pulmonary artery pressure (pulmonary artery pressure, 65 mm Hg). Upper gastrointestinal endoscopy showed no abnormal lesions in the esophagus, stomach or duodenum.
Fig. 1.
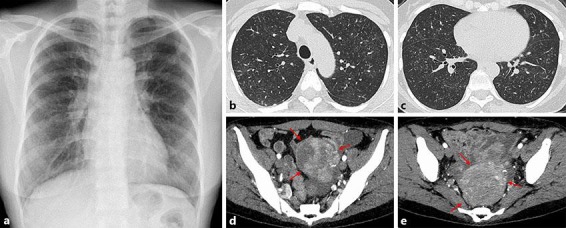
Findings on chest radiography and computed tomography (CT). a Chest radiography shows bilateral micronodular shadows in bilateral lung fields. b, c Chest CT shows diffuse centrilobular nodules and a tree-in-bud appearance. d, e Abdominal CT reveals a 6.5 × 6.3-cm enhanced tumor in the vicinity of the uterus (red arrows).
Based on these findings, the provisional diagnosis was left ovarian cancer and pulmonary hypertension caused by cancer-related lesions. We failed to detect any cancer cells on cytological examination of pulmonary artery blood obtained by right cardiac catheterization. Although we had planned transbronchial lung biopsy or thoracoscopic lung biopsy, or laparotomy for the ovarian tumor, hypoxia and pulmonary hypertension prevented these procedures and the patient died on day 14 after admission, due to respiratory failure.
Pathological findings at autopsy showed fibromuscular intimal proliferation with vessel occlusion in the small arteries of the lung (Fig. 2a). Cancer cells were found in the lymphovascular area of the lung (Fig. 2b). Signet ring cells had proliferated in the enlarged left ovary (Fig. 2c). Signet ring carcinoma cells also showed rich mucus in the ovary (Fig. 2d). Signet ring carcinoma cells were also found in the contralateral ovary, uterus, bone marrow, and thyroid glands (data not shown). Cancer cells were not found in gastric tissue. Finally, signet ring cell carcinoma of unknown primary with Krukenberg tumor and PTTM was diagnosed.
Fig. 2.
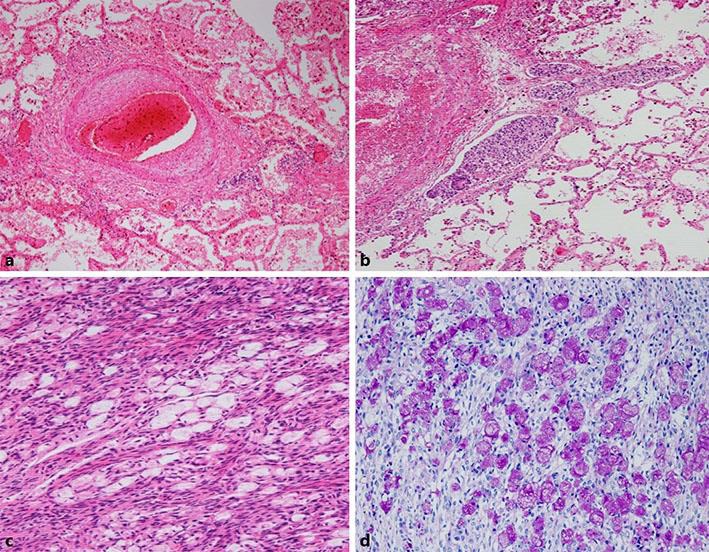
Pathologic findings for the right upper lobectomy specimen. a In the small pulmonary arteries and arterioles, fibrocellular intimal proliferation is apparent, resulting in stenosis (hematoxylin and eosin, ×40). b Extensive lymphovascular invasion of atypical cells in the lung (hematoxylin and eosin, ×40). c Signet ring cells proliferating in the left ovary (hematoxylin and eosin stain, ×100). d Signet ring cells are present against a background of rich mucus in the left ovary (periodic acid-Schiff, ×100).
Discussion
PTTM is difficult to diagnose because of the rapid progression and difficulties of performing biopsy. Previous studies have shown PTTM in 21 patients (3.3%) among 630 consecutive autopsy cases with carcinoma, and 3 of those 21 patients were diagnosed clinically with pulmonary hypertension of unknown cause, with PTTM diagnosed for the first time at autopsy [1]. According to a review of 2,215 consecutive autopsy cases of carcinoma, 30 cases (1.4%) were diagnosed with definitive PTTM. The median survival of patients with PTTM who died was only 9 days from admission or after initiation of oxygen supplementation [4].
The most frequent primary cancer with PTTM was gastric cancer in 58 cases (56.3%), lung cancer in 10 cases (9.7%), breast cancer in 7 cases (6.8%), ovarian cancer in 5 cases (4.9%), unknown primary in 5 cases (4.9%), and bladder cancer in 4 cases (3.9%), according to a literature review of 103 cases of PTTM [5]. The most frequent histological type of cancer related to PTTM was adenocarcinoma [4].
In the present case, PTTM associated with left ovarian cancer was suspected because the patient had a mass lesion in the pelvis. PTTM was diagnosed from autopsy findings. Signet ring cell carcinoma cells had invaded bilateral ovaries, uterus, bone marrow, thyroid glands, and regional lymph nodes around the stomach. Ovarian cancer was difficult to diagnose as the primary tumor, as signet ring carcinoma is rare among ovarian cancers, and cancer cells were not found in the pelvic lymph nodes associated with the ovaries. While signet ring carcinoma cells were also found in regional lymph nodes in the stomach, no cancer cells were evident in the gastric mucosa. However, one limitation was the possible degeneration of gastric mucosa because autopsy was performed 24 h after death. Signet ring cell carcinoma of unknown primary with Krukenberg tumor and PTTM was finally diagnosed.
Krukenberg tumor generally refers to metastatic carcinoma of the ovary and is characterized by the presence of mucin-filled signet ring cells accounting for at least 10% of the tumor [6]. The primary sites of Krukenberg tumor are reportedly the stomach (67%), colon (9%), breast (7%), biliary duct (2%), and others [3]. Even early gastric cancer with signet ring cell carcinoma within the intramucosal layer could metastasize as Krukenberg tumor [7, 8].
Pulmonary artery blood aspiration from a right cardiac catheter is useful for the diagnosis of PTTM [9]. In fact, we performed pulmonary artery blood aspiration in this case, but could not detect cancer cells on cytological examination. The volume of blood samples and appropriate wedge techniques should be standardized in the future. Although the molecular mechanisms underlying PTTM formation in patients with cancer have yet to be clarified, some reports have suggested the involvement of vascular endothelial growth factor and platelet-derived growth factor [4, 10]. Several cases of early diagnosis and successful treatment of PTTM have also been reported [11, 12]. Basically, cancer treatment should be administered as soon as possible after diagnosis, and evidence is lacking regarding the efficacy of molecularly targeted therapies aimed at vascular endothelial growth factor or platelet-derived growth factor [13].
In conclusion, since early diagnosis leads to adequate treatment, physicians should suspect PTTM and act to rapidly diagnose cancer as an etiology of respiratory failure in cases with hypoxia or pulmonary hypertension of unknown cause.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
All authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.von Herbay A, Illes A, Waldherr R, Otto HF. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990 Aug;66((3)):587–92. doi: 10.1002/1097-0142(19900801)66:3<587::aid-cncr2820660330>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Chinen K, Tokuda Y, Fujiwara M, Fujioka Y. Pulmonary tumor thrombotic microangiopathy in patients with gastric carcinoma: an analysis of 6 autopsy cases and review of the literature. Pathol Res Pract. 2010 Oct;206((10)):682–9. doi: 10.1016/j.prp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006 Mar;30((3)):277–99. doi: 10.1097/01.pas.0000190787.85024.cb. [DOI] [PubMed] [Google Scholar]
- 4.Uruga H, Fujii T, Kurosaki A, Hanada S, Takaya H, Miyamoto A, et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med. 2013;52((12)):1317–23. doi: 10.2169/internalmedicine.52.9472. [DOI] [PubMed] [Google Scholar]
- 5.Fujishiro T, Shuto K, Shiratori T, Kono T, Akutsu Y, Uesato M, et al. A case report of pulmonary tumor thrombotic microangiopathy (PTTM) caused by esophageal squamous cell carcinoma. Esophagus. 2013;10((4)):247–51. doi: 10.1007/s10388-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prat J. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Mod Pathol. 2005 Feb;18(Suppl 2):S99–111. doi: 10.1038/modpathol.3800312. [DOI] [PubMed] [Google Scholar]
- 7.Kakushima N, Kamoshida T, Hirai S, Hotta S, Hirayama T, Yamada J, et al. Early gastric cancer with Krukenberg tumor and review of cases of intramucosal gastric cancers with Krukenberg tumor. J Gastroenterol. 2003;38((12)):1176–80. doi: 10.1007/s00535-003-1227-3. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Hiramatsu A, Koyama T, Oyama Y, Tanaka A, Honma K. A Krukenberg Tumor from an Occult Intramucosal Gastric Carcinoma Identified during an Autopsy. Case Rep Oncol Med. 2014;2014:797429. doi: 10.1155/2014/797429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchigami S, Tsunoda R, Shimizu H, Takae M, Usuku H, Yoshimura H, et al. Pulmonary Tumor Thrombotic Microangiopathy - Antemortem Diagnosis With Pulmonary Artery Wedge Blood Cell Sampling in a Recurrent Breast Cancer Patient. Circ J. 2017 Nov;81((12)):1959–60. doi: 10.1253/circj.CJ-17-0228. [DOI] [PubMed] [Google Scholar]
- 10.Okubo Y, Wakayama M, Kitahara K, Nemoto T, Yokose T, Abe F, et al. Pulmonary tumor thrombotic microangiopathy induced by gastric carcinoma: morphometric and immunohistochemical analysis of six autopsy cases. Diagn Pathol. 2011 Mar;6((1)):27. doi: 10.1186/1746-1596-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa A, Yamadori I, Matsubara O, Matsubara H. Pulmonary tumor thrombotic microangiopathy with circulatory failure treated with imatinib. Intern Med. 2013;52((17)):1927–30. doi: 10.2169/internalmedicine.52.0718. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Uruga H, Fujii T, Mochizuki S, Hanada S, Takaya H, et al. Antemortem diagnosis of pulmonary tumor thrombotic microangiopathy in a patient with recurrent breast cancer: a case report. BMC Cancer. 2016 Aug;16((1)):666. doi: 10.1186/s12885-016-2721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. 2010 Nov;182((9)):1171–7. doi: 10.1164/rccm.201001-0123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


