Abstract
Primary squamous cell carcinoma (SCC) in the thyroid is extremely rare and has been reported in < 1% of all thyroid cancer cases. Primary SCC in the thyroid was thought to be a transitional form derived from adenocarcinomas; therefore, the majority of reported cases have focused on the conjunction with other histological adenocarcinomas. A 73-year-old male presented to our hospital with bilateral vocal fold palsy and an anterior neck mass. Ultrasound sonography revealed a bulky tumor in the thyroid and bilateral cervical lymphadenopathy. We performed fine-needle aspiration cytology from the thyroid tumor, which revealed SCC. Positron emission tomography/computed tomography showed distant metastases in the lungs, mediastinal lymph nodes, and vertebra. We diagnosed the patient as having stage IVC SCC in the thyroid and administered weekly paclitaxel. Four and a half months after treatment initiation, the tumor progression resulted in aspiration pneumonia, which proved fatal. We performed an autopsy in accordance with the patient's wishes. Pathological findings revealed that all carcinomas in the thyroid, cervical lymph nodes, and lungs were pure SCCs. Immunohistochemical examinations for PAX8, thyroglobulin, and TTF-1 were all negative. Differentiated thyroid carcinomas have 3 major positive markers - PAX8, thyroglobulin, and TTF-1 –, and PAX8 is also sometimes positive for SCC in the thyroid. PAX8 positivity of SCC in the thyroid might, however, be associated with conjunction with other histological adenocarcinomas such as papillary or follicular thyroid carcinoma; therefore, pure SCC in the thyroid might be negative for PAX8.
Keywords: Autopsy, Paired box 8, PAX8, Squamous cell carcinoma, Thyroid
Introduction
Primary squamous cell carcinoma (SCC) in the thyroid is extremely rare, comprising < 1% of all thyroid cancers [1]. Primary SCC in the thyroid has generally been thought to be a transitional form arising from other adenocarcinomas; the majority of such cases, therefore, revealed conjunction with other histologically differentiated adenocarcinomas, such as papillary adenocarcinoma or follicular adenocarcinoma [2], as opposed to the very much rarer pure primary SCC in the thyroid.
SCC in the thyroid proceeds aggressively, frequently invading the surrounding organs, including the larynx, the esophagus, and the trachea. The distinction between primary SCC of the thyroid and an invasion of SCC arising in the adjacent organs is an important one because of the poor prognosis of thyroid SCC [3, 4]. The ability to make this distinction is, however, often confounded by the involvement of organs surrounding the large tumor.
There are 3 major immunohistochemical markers of differentiated thyroid carcinomas: PAX8, thyroglobulin (Tg), and TTF-1 [5]. PAX8 has also been reported as positive in thyroid SCC, and PAX8 staining is useful in distinguishing primary thyroid SCC from invasion or metastasis from extrathyroidal SCC [6]. However, in that report, most thyroid SCC patients did not have pure SCC but instead had SCC in conjunction with papillary or anaplastic thyroid cancer. Therefore, the immunohistochemical features - especially PAX8 relating to pure primary thyroid SCC - were not sufficiently discussed.
Case
A 73-year-old male was referred to our hospital presenting with bilateral vocal fold palsy and a rapidly growing anterior neck mass. We performed ultrasound sonography, revealing a bulky tumor in the thyroid gland and multiple bilateral cervical lymphadenopathies. We performed fine-needle aspiration cytology from both the thyroid tumor and the cervical lymph node, both of which revealed SCC. We found no primary tumor in the pharynx, larynx, or esophagus with laryngoscopy and upper gastrointestinal endoscopy, nor could we discover any primary tumor in the trachea using bronchoscopy. We discovered a bulky thyroid tumor and bilateral cervical lymph nodes involved in the common carotid artery and brachiocephalic artery using computed tomography (CT) with contrast effect (Fig. 1a, b). Using FDG-positron emission tomography/CT, we found FDG uptake in the thyroid, the bilateral cervical lymph nodes, the lungs, the mediastinal lymph nodes, and the vertebra (Fig. 1c). We diagnosed the patient with stage IVC primary SCC in the thyroid and initiated treatment with weekly paclitaxel. He received chemotherapy weekly at 90 mg/m2 according to a 6 weeks on/2 weeks off schedule.
Fig. 1.
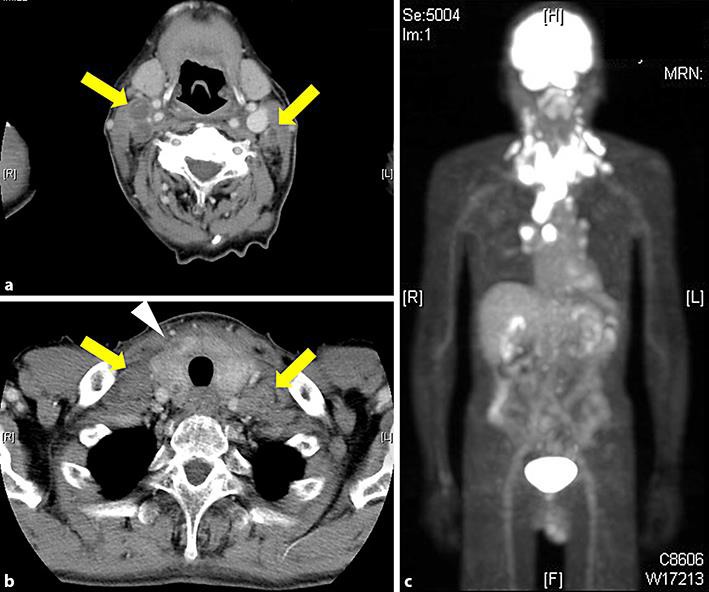
Imaging features at the initial diagnosis. a, b Using computed tomography with contrast effect, we observed that most of the thyroid component was replaced by the tumor and was enlarged. We also observed bilateral cervical lymph node adenopathy. c Positron emission tomography revealed uptakes in the thyroid, bilateral cervical lymph nodes, and mediastinal lymph nodes.
Four months after the initiation of treatment, the objective response obtained from CT presented a stable disease. However, a slightly enlarged tumor resulted in dysphagia and aspiration pneumonia, which led to the patient's death. We performed an autopsy according to the patient's wishes. On a macroscopic level, we found that the greater part of the thyroid was replaced by carcinoma. The tumor and the cervical lymph node metastases involved the common carotid artery and the brachiocephalic artery, but not the trachea or larynx (Fig. 2a, b). The tumor had slightly invaded the external muscle layer of the esophagus; the mucosa of the esophagus, however, was intact. We observed multiple tumor nodules in the bilateral lungs and mediastinal lymphadenopathies. These macroscopic findings indicated primary thyroid cancer with distant metastases.
Fig. 2.
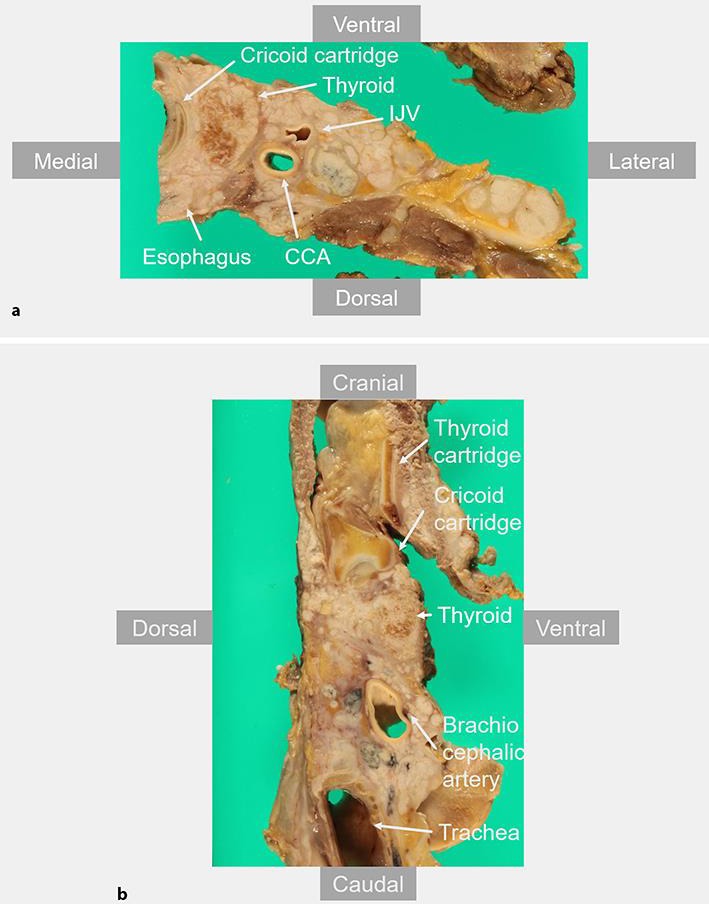
Macro view of the neck from the autopsy specimen. a Axial resection of the neck revealed that the thyroid tumor had invaded toward the cricoid cartridge and the outer muscular layer of the esophagus; however, the mucosa of the larynx and esophagus was intact. The bilateral cervical lymph nodes involved the common carotid artery (CCA) and internal jugular vein (IJV). b Sagittal resection of the neck revealed that the thyroid tumor and lymph nodes were involved with the brachiocephalic artery and had invaded toward the mediastinum. Mediastinal lymph node adenopathy was observed in front of the carina.
On examining the histopathology of all of the carcinomas in the thyroid, cervical lymph nodes, and lungs, we observed a palisade arrangement, intercellular bridges, and keratinization with a cancer pearl (Fig. 3a, b, c, d). We interpreted these histopathological features as pure highly differentiated SCC. Consequently, only in the thyroid was primary SCC proven and never in any other organ. We performed immunohistochemical examinations of the primary thyroid cancer, the cervical lymph node metastases, and the lung metastases; all of which were negative for TTF-1, Tg, and PAX8 (Fig. 4a, b, c).
Fig. 3.
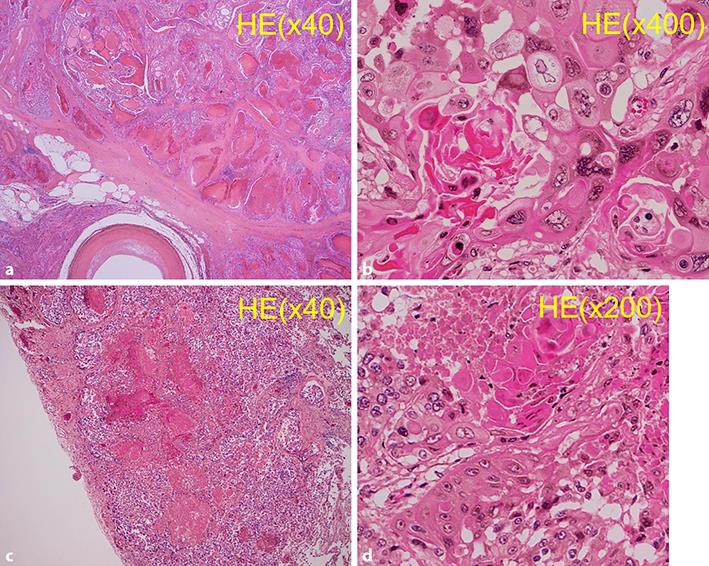
Hematoxylin and eosin staining of the specimen. a Tumor in the thyroid. b Cervical lymph node metastases. Histopathological finding from all carcinomas in the thyroid and the cervical lymph nodes revealed a palisade arrangement, intercellular bridges, and keratinization with a cancer pearl.
Fig. 4.
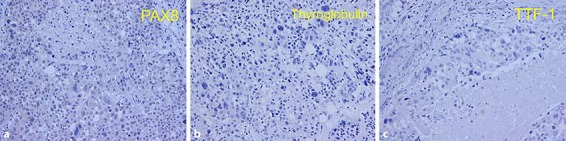
Immunohistochemical features of the thyroid tumor. Immunohistochemical staining for PAX8 (a), thyroglobulin (b), and TTF-1 (c). All were negative for PAX8, thyroglobulin, and TTF-1.
Discussion
Primary SCC in the thyroid behaves aggressively, frequently surrounding great vessels, and even at initial diagnosis has distant metastasis. The most effective treatment for improving the prognosis is definitive surgery; unfortunately, however, extension of the tumor to surrounding organs or frequent distant metastasis makes this very difficult [7]. Although chemotherapy should at least be considered for patients with unresectable SCC in the thyroid to control the disease, effective chemotherapy has never been reported [8]. In this case, we were unsuccessful in reducing the tumor volume or extending the prognosis using chemotherapy with weekly paclitaxel. Clinical trials of molecular-targeted therapy using drugs such as lenvatinib and sorafenib for unresectable thyroid cancer are ongoing; these drugs may show promise to potentially extend survival [9].
Primary SCC in the thyroid has been considered to be a transitional form arising from other adenocarcinomas; therefore, most of the reported cases have been connected with other histologically differentiated adenocarcinomas [2]. The coexistence of well differentiated and poorly differentiated thyroid cancer with SCC could constitute primary thyroid SCC; however, a clear distinction between pure thyroid SCC and invasion or metastasis from extrathyroidal SCC needs to be drawn because of the poor prognosis of the former. Immunohistochemical staining is useful in diagnosing the primary cancer site [10]. The principal immunohistochemical markers of the thyroid - TTF-1 and Tg - are frequently positive in differentiated thyroid cancer, usually positive in anaplastic thyroid cancer, and negative in SCC [5]. The transcription factor PAX8 is known to control the development of the central nervous system, the eyes, the kidneys, and the thyroid gland [11]. Most differentiated thyroid cancer also expresses PAX8, which is used as a positive marker of differentiated thyroid cancer [12]. PAX8 is reportedly also positive in most primary thyroid SCC [6]. Conversely, PAX8 is rarely expressed in SCC of the lungs, the larynx, the thymus, and the skin [11]. PAX8 is therefore useful in distinguishing primary thyroid SCC from extrathyroidal SCC.
In our case, the histopathological diagnosis was highly differentiated pure SCC, and the thyroid was proven by autopsy to be the primary cancer site. Autopsy is also useful in detecting the primary site or cause of death by unknown primary cancer [13]. This extremely rare case of pure thyroid SCC was proven by autopsy. However, immunohistochemical findings were negative for PAX8. Most previously reported cases of thyroid SCC, positive for PAX8, were not pure SCC but existed in conjunction with papillary or anaplastic thyroid cancer [6]. The presence of preserved immunohistochemical staining for PAX8 in better differentiated areas substantiates the presence of coexisting differentiated or anaplastic carcinoma [5]. An important feature of PAX8 is therefore that pure thyroid SCC may be negative for PAX8, and thyroid SCC with differentiated thyroid cancer may be positive for PAX8.
Conclusions
We encountered highly differentiated pure SCC in the thyroid proven by autopsy. Other coexisting histological adenocarcinoma may influence immunohistochemical features of PAX8. PAX8 may be negative in pure thyroid SCC and positive in thyroid SCC with differentiated thyroid cancer.
Statement of Ethics
This study has been approved by the Institutional Review Board at Tottori University Hospital, Yonago, Japan.
Disclosure Statement
The authors have no conflicts of interest related to this study.
Acknowledgments
We gratefully acknowledge the work of past and present members of our department.
References
- 1.Korovin GS, Kuriloff DB, Cho HT, Sobol SM. Squamous cell carcinoma of the thyroid: a diagnostic dilemma. Ann Otol Rhinol Laryngol. 1989 Jan;98((1 Pt 1)):59–65. doi: 10.1177/000348948909800113. [DOI] [PubMed] [Google Scholar]
- 2.Kleer CG, Giordano TJ, Merino MJ. Squamous cell carcinoma of the thyroid: an aggressive tumor associated with tall cell variant of papillary thyroid carcinoma. Mod Pathol. 2000 Jul;13((7)):742–6. doi: 10.1038/modpathol.3880129. [DOI] [PubMed] [Google Scholar]
- 3.Kebapci N, Efe B, Kabukcuoglu S, Akalin A, Kebapci M. Diffuse sclerosing variant of papillary thyroid carcinoma with primary squamous cell carcinoma. J Endocrinol Invest. 2002 Sep;25((8)):730–4. doi: 10.1007/BF03345109. [DOI] [PubMed] [Google Scholar]
- 4.Kleinhans H, Schmid KW, Verse T. [Primary squamous cell carcinoma of the thyroid gland] HNO. 2013 Jul;61((7)):661–3. doi: 10.1007/s00106-012-2622-y. [DOI] [PubMed] [Google Scholar]
- 5.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012 Nov;22((11)):1104–39. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki A, Hirokawa M, Takada N, Higuchi M, Yamao N, Kuma S, et al. Diagnostic significance of PAX8 in thyroid squamous cell carcinoma. Endocr J. 2015;62((11)):991–5. doi: 10.1507/endocrj.EJ15-0226. [DOI] [PubMed] [Google Scholar]
- 7.Cook AM, Vini L, Harmer C. Squamous cell carcinoma of the thyroid: outcome of treatment in 16 patients. Eur J Surg Oncol. 1999 Dec;25((6)):606–9. doi: 10.1053/ejso.1999.0715. [DOI] [PubMed] [Google Scholar]
- 8.Syed MI, Stewart M, Syed S, Dahill S, Adams C, McLellan DR, et al. Squamous cell carcinoma of the thyroid gland: primary or secondary disease? J Laryngol Otol. 2011 Jan;125((1)):3–9. doi: 10.1017/S0022215110002070. [DOI] [PubMed] [Google Scholar]
- 9.Yasumatsu R, Sato M, Nakagawa T, et al. The treatment and outcome analysis of primary squamous cell carcinoma of the thyroid. Auris Nasus Larynx. 2017 doi: 10.1016/j.anl.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Booya F, Sebo TJ, Kasperbauer JL, Fatourechi V. Primary squamous cell carcinoma of the thyroid: report of ten cases. Thyroid. 2006 Jan;16((1)):89–93. doi: 10.1089/thy.2006.16.89. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan A, Shen SS, Hamilton C, Anjana K, Coffey D, Krishnan B, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011 Jun;24((6)):751–64. doi: 10.1038/modpathol.2011.3. [DOI] [PubMed] [Google Scholar]
- 12.Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol. 2008 Feb;21((2)):192–200. doi: 10.1038/modpathol.3801002. [DOI] [PubMed] [Google Scholar]
- 13.Riihimäki M, Hemminki A, Sundquist K, Hemminki K. Causes of death in patients with extranodal cancer of unknown primary: searching for the primary site. BMC Cancer. 2014 Jun;14((1)):439. doi: 10.1186/1471-2407-14-439. [DOI] [PMC free article] [PubMed] [Google Scholar]


