Abstract
Clip rotation after clipping is a major cause of delayed cerebral ischemia and may occur after any of several intraoperative monitoring techniques. We experienced 3 cases of clip rotation in 3 patients after clipping between March 2011 and December 2013. One of these patients has permanent motor weakness of the left upper extremity because of delayed occlusion of the right M1 lenticulostriate artery. The other two developed delayed occlusion of the frontopolar artery or of the A1 perforating artery, but did not have any neurologic deficits. Clinicians need to exercise great care not to compromise distal blood flow after clipping intracranial aneurysms. We present 3 cases in which clip rotation occurred after aneurysm clipping and progressively compromised blood flow of a nearby branching artery.
Keywords: Clip, Intracranial aneurysm, Ischemia, Rotation
Introduction
Ischemic complication is a major complication associated with poor outcomes following aneurysm clipping. Recent studies have highlighted the frequency of infarct development before the onset of vasospasm and its contribution to neurological morbidity [1, 2, 3]. Such early infarcts have been ascribed either to a brain insult triggered by aneurysmal rupture or to complications associated with aneurysm-securing procedures [4, 5]. To detect blood flow insufficiency of parent arteries and perforators during aneurysm surgery, Doppler ultrasonography, conventional cerebral angiography, endoscopic observation, electrophysiological monitoring, and fluorescence angiography have been used – even awake aneurysm surgery has been attempted [6]. Nonetheless, cerebral ischemia after intracranial aneurysm clipping may occur regardless of the intraoperative monitoring technique employed. Here, we report 3 cases of clip rotation after clipping resulting in kinking of a branching artery or perforator associated with or without neurologic deficit.
Case Reports
We retrospectively reviewed the clinical records of all patients that had undergone surgical treatment for ruptured or unruptured intracranial aneurysms between March 2011 and December 2013. The operative video records of each patient were reviewed. Baseline patient characteristics, that is, demographic, radiographic, intraoperative, and postoperative data, were noted. The following inclusion criteria were applied: (1) treatment by microsurgery to clip an intracranial aneurysm; (2) the development of any neurologic deficit or ischemic lesion by postoperative imaging attributed to compromised blood flow of a branching artery after clipping; (3) confirmation of clip rotation by postoperative imaging. Clinical outcomes were analyzed using the modified Rankin scale (mRS) at 6 months after clipping.
Case 1
A 67-year-old male patient visited our clinic because of an asymptomatic unruptured intracranial aneurysm identified by magnetic resonance angiography. Cerebral catheter angiography revealed an aneurysm at the lenticulostriate artery of the M1 segment of the right middle cerebral artery (MCA) (Fig. 1a). Conventional cerebral angiography showed that the lenticulostriate artery arose from the neck of the aneurysm and the presence of a small daughter sac at the neck of the aneurysm. Atherosclerotic changes in the dome and neck of the aneurysm were identified during microsurgery. The aneurysm was clipped using a slightly downward curved clip of blade length 10.2 mm. The tip of the clip crossed the branching artery. Distal blood flows in the MCA and the lenticulostriate artery were verified by intraoperative micro-Doppler ultrasound. Intraoperative motor-evoked potential and somatosensory-evoked potential monitoring were not used in this case. Immediately after surgery, the patient was drowsy and left hemiparesis was detected. Computed tomographic angiography (CTA) revealed occlusion of the lenticulostriate artery at the clip tip (Fig. 1b). Clip rotation was identified by overlapping the image of postoperative anteroposterior-view skull X-ray and preoperative cerebral catheter angiography (Fig. 1c). Diffusion-weighted imaging revealed acute cerebral infarction in the right putamen, internal capsule, and corona radiate (Fig. 1d). We discussed re-exploration and clip repositioning with the patient and his family but they refused reoperation and an invasive study including cerebral catheter angiography. The left hemiparesis persisted and at 6 months after clipping his mRS was 3.
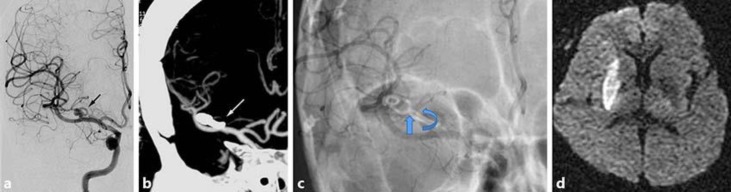
Fig. 1.
A 67-year-old male patient visited our hospital with an asymptomatic unruptured intracranial aneurysm. a Cerebral catheter angiography in the right internal carotid artery demonstrated a saccular aneurysm at the lenticulostriate artery of the middle cerebral artery (black arrow). b Postoperative computed tomographic angiography showed occlusion of the lenticulostriate artery at the clip tip (white arrow). c Clip rotation was identified by overlapping the image of postoperative skull X-ray of anteroposterior view. d Diffusion-weighted image showing infarction of the right lenticulostriate artery territory.
Case 2
A 57-year-old woman presented with a sudden bursting headache. Computed tomography (CT) revealed a subarachnoid hemorrhage in the interhemispheric fissure. Cerebral catheter angiography demonstrated a saccular aneurysm at the frontopolar artery of the right distal anterior cerebral artery of the following dimensions: neck 3.3 mm, height 3.0 mm, width 2.6 mm, and length 3.8 mm (Fig. 2a). On the day of admission, the aneurysm was clipped using a side-curved standard clip of blade length 8.6 mm. The tip of the clip crossed the branching artery. Intraoperative Doppler revealed no velocity change of the branching artery or parent artery after clipping. The clip head and falx cerebri composed a 70-degree angle on intraoperative microscopic view (Fig. 2b). Immediate postoperative lateral-view skull X-ray revealed that the clip head was parallel to the falx cerebri, indicating rotated clip placement (Fig. 2c). However, postoperative CTA well visualized the parent artery and the patient recovered immediately after clipping without any neurologic deficit. Follow-up cerebral catheter angiography performed at 11 days after clipping showed complete obliteration of the aneurysm and slight segmental narrowing of the right callosomarginal artery adjacent to the clip head (Fig. 2d). She was discharged 4 weeks after clipping without any neurologic deficit. Follow-up cerebral catheter angiography performed at 3 months after clipping showed occlusion of the frontopolar artery nearby clip tip, mild luminal narrowing of the distal anterior cerebral artery, and typical string-of-beads appearance with alternating segments of stricture and dilation of the right callosomarginal artery adjacent to the clip head. Dual antiplatelet medication was started with 100 mg aspirin and 75 mg clopidogrel daily to prevent delayed cerebral ischemia. At 6 months, her mRS was 0.
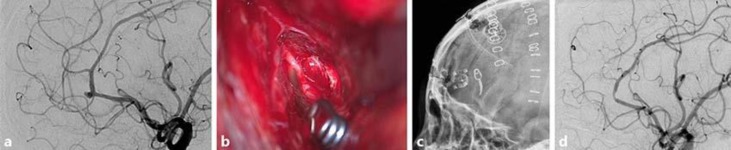
Fig. 2.
A 57-year-old woman presented with a sudden bursting headache. a Cerebral catheter angiography showed a ruptured intracranial aneurysm of a right frontopolar artery. A right frontal interhemispheric approach was adopted with aneurysmal clipping. b The clip head and falx cerebri formed a 70-degree angle and the clip tip crossed the frontopolar artery. c Postoperative lateral skull radiograph demonstrating the plane of the clip head parallel to the falx cerebri due to clip rotation. d Follow-up cerebral catheter angiography demonstrated complete obliteration of the aneurysm and slight segmental narrowing of the callosomarginal artery adjacent to the clip head.
Case 3
A 54-year-old woman was admitted via our emergency room with a sudden bursting headache and vomiting. Brain CT showed subarachnoid hemorrhage of Fisher grade IV. Cerebral catheter angiography showed a left proximal A1 aneurysm directed posteriorly of the following dimensions: neck 2.5 mm, height 1.6 mm, width 2.0 mm, and length 2.7 mm (Fig. 3a). We performed craniotomy using a modified orbitozygomatic approach. The aneurysm was clipped with a 6-mm, 90-degree standard clip. The clip blade was placed crossing the A1 artery at 90 degrees. During the operation, we confirmed that the clip head was parallel to the planum sphenoidale and that the clip tip was parallel to the left internal carotid artery (Fig. 3b). After the operation, CTA showed that the clip blade was parallel to A1, that the clip tip was directed laterally, and that the clip head was vertical to the planum sphenoidale, indicating clip rotation after clipping (Fig. 3c). Five days after the operation, follow-up brain CT showed small lacunar infarction at the left genu of the internal capsule (Fig. 3d). After clipping, the patient fully recovered without any neurologic deficit. At 6 months, her mRS was 0.
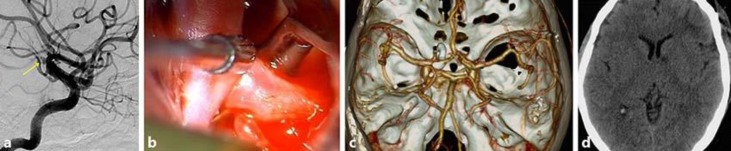
Fig. 3.
A 54-year-old woman presented with subarachnoid hemorrhage, a sudden bursting headache, and vomiting. a Cerebral catheter angiography showed a left proximal A1 aneurysm with bilobulation. Using a modified orbitozygomatic approach, the aneurysm was clipped with a 6-mm, 90-degree-angled standard clip. b The clip blade was parallel to the planum sphenoidale and the clip tip was parallel to the left internal carotid artery. c Postoperative computed tomographic angiography showed that the clip tip was parallel to A1 and the clip head was vertical to planum sphenoidale, indicating clip rotation. d Five days after the operation, computed tomography showed a small lacunar infarction at the genu of the left internal capsule.
Discussion
Case 1: Early Cerebral Ischemia after Clip Rotation
The clip was rotated by progressive brain swelling and repositioning, and kinked the MCA and the lenticulostriate artery, causing the lenticulostriate artery blood flow insufficiency when the retracted brain gradually returned to its original state. Both temporal and frontal lobes played a crucial role in the rotation and tilting of the clip head to an upward inclined orientation. The plane of the clip head had moved parallel to the frontal and temporal surfaces, which was vertical during the operation. The proximal part of the superior temporal gyrus firmly herniated into the lateral frontal-orbital gyrus, thereby compressing the Sylvian cistern and pushing it medially and concealing its deeper portion. We speculated that strangulation of the origin of the lenticulostriate artery may have been caused by clipping, but this could not explain patent blood flow at the lenticulostriate artery evidenced by intraoperative Doppler ultrasound. Intraoperative angiography after removing the brain retraction system and intraoperative electrophysiological monitoring were performed due to nonavailability in this case. Clip rotation may have occurred in the period between clipping and the end of general anesthesia. Urgent readjustment of the clip was needed, but the patient's family denied reoperation because of a lack of confidence regarding neurologic recovery.
Cases 2 and 3: No Neurologic Deficit after Clip Rotation
Intraoperative electrophysiological monitoring was applied in both cases. Intraoperative vascular patency was verified by intraoperative Doppler and fluorescence angiography. In case 2, we detected branching artery occlusion at 11 days by follow-up cerebral catheter angiography. In case 3, we detected a small lacunar infarction at 5 days by follow-up brain CT. In both cases, vasospasm may have occurred, but in both the occlusion of the branching and perforating arteries occurred near the rotation of a clip tip.
In all 3 patients, clip rotation was detected immediately after clipping, by measuring angles between the plane of the clip head and rigid intracranial structures (i.e., parent artery, falx cerebri, and planum sphenoidale). During operation on a relaxed brain, clip rotation cannot be easily predicted. Yasargil [7] described 4 different types of intraoperatively observed anatomical Sylvian fissure variants: category I, a straight wide Sylvian fissure; category II, a straight narrow Sylvian fissure; category III, a herniated frontal lobe into the Sylvian fissure; and category IV, a herniated temporal lobe into the Sylvian fissure. Furthermore, a herniated temporal lobe can push, rotate, or tilt a clip and cause strangulation of a branching artery.
Urgent clip readjustment is needed when an ischemic complication occurs after clipping. Park et al. [8] reported 11 cases of reoperation after clipping due to ischemia. Three of the patients concerned (27.3%) recovered without any neurologic deficits at 6 months after treatment, and the 4 patients with delayed ischemic symptoms achieved relatively (2–4 h after initial clipping) better outcomes than the 7 patients that developed ischemic symptoms immediately after surgery.
Kobayashi et al. [9] described several techniques to avoid complications after clipping. They described removal of a tiny portion of the temporal lobe pia mater and brain to provide room for the clip head used to treat a ruptured anterior choroidal artery aneurysm. In our 3 cases, subpial resection to provide room for the clip head may have been effective. In case 2, resection of the falx cerebri to provide room for the clip head might also have effectively prevented clip rotation. However, we were unable to predict subtle changes in clip rotation after release of brain retraction. Multiple clipping techniques also help prevent clip rotation. Single clip placement without crossing the branching artery with the clip tip is also important in terms of not strangulating a branching artery. At some point, all surgeons that frequently perform aneurysm operations will encounter a complex aneurysm and feel that available occlusive clip designs are suboptimal. In such cases, the surgeon must rely on his or her creativity and use available clips to secure the aneurysm, and in many cases, to reconstruct the parent vessel [10].
The intracranial operative room required for clipping an intracranial aneurysm by microneurosurgery inevitably disappears because of progressive brain swelling and repositioning after brain retraction is released, and if the available room for proper positioning of the clip head is inadequate, clip rotation might occur and cause strangulation of a branching artery near the aneurysm neck. Close monitoring of the branching artery patency immediately after clipping and proper management are required to avoid ischemic complications after clipping.
Conclusion
Clip rotation is a major cause of cerebral ischemia after clipping. Clinicians should exercise great care not to compromise distal blood flow after clipping intracranial aneurysms.
Statement of Ethics
The authors have no ethical conflicts.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Juvela S, Siironen J. Early cerebral infarction as a risk factor for poor outcome after aneurysmal subarachnoid haemorrhage. Eur J Neurol. 2012 Feb;19((2)):332–9. doi: 10.1111/j.1468-1331.2011.03523.x. [DOI] [PubMed] [Google Scholar]
- 2.Naidech AM, Drescher J, Tamul P, Shaibani A, Batjer HH, Alberts MJ. Acute physiological derangement is associated with early radiographic cerebral infarction after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006 Dec;77((12)):1340–4. doi: 10.1136/jnnp.2006.089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt JM, Rincon F, Fernandez A, Resor C, Kowalski RG, Claassen J, et al. Cerebral infarction associated with acute subarachnoid hemorrhage. Neurocrit Care. 2007;7((1)):10–7. doi: 10.1007/s12028-007-0003-2. [DOI] [PubMed] [Google Scholar]
- 4.Juvela S, Siironen J, Varis J, Poussa K, Porras M. Risk factors for ischemic lesions following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2005 Feb;102((2)):194–201. doi: 10.3171/jns.2005.102.2.0194. [DOI] [PubMed] [Google Scholar]
- 5.Siironen J, Porras M, Varis J, Poussa K, Hernesniemi J, Juvela S. Early ischemic lesion on computed tomography: predictor of poor outcome among survivors of aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007 Dec;107((6)):1074–9. doi: 10.3171/JNS-07/12/1074. [DOI] [PubMed] [Google Scholar]
- 6.Abdulrauf SI, Vuong P, Patel R, Sampath R, Ashour AM, Germany LM, et al. “Awake” clipping of cerebral aneurysms: report of initial series. J Neurosurg. 2017 Aug;127((2)):311–8. doi: 10.3171/2015.12.JNS152140. [DOI] [PubMed] [Google Scholar]
- 7.Yasargil MG. Microneurosurgery. New York: Thieme; 1984. Operative Anatomy; pp. pp. 36–9. [Google Scholar]
- 8.Park W, Ahn JS, Lee SH, Park JC, Kwun BD. Results of re-exploration because of compromised distal blood flow after clipping unruptured intracranial aneurysms. Acta Neurochir (Wien) 2015 Jun;157((6)):1015–24. doi: 10.1007/s00701-015-2408-6. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Tanaka Y. Aneurysm Clip Design, Selection and Application. In: Apuzzo ML, editor. Brain Surgery. New York: Churchill Livingstone; 1993. pp. pp. 825–46. [Google Scholar]
- 10.Giannotta S, Zada G. The fenestrated Yasargil T-bar clip. Neurosurgery. 2012;70((ONS Suppl 2)) onsE339-342; comment onsE342. [Google Scholar]