Interactions in the airway ecology of cystic fibrosis may alter organism persistence and clinical outcomes. Better understanding of such interactions could guide clinical decisions.
KEYWORDS: Burkholderia, Pseudomonas aeruginosa, Staphylococcus aureus, airway infections, cystic fibrosis, logistic regression, microbial ecology
ABSTRACT
Interactions in the airway ecology of cystic fibrosis may alter organism persistence and clinical outcomes. Better understanding of such interactions could guide clinical decisions. We used generalized estimating equations to fit logistic regression models to longitudinal 2-year patient cohorts in the Cystic Fibrosis Foundation Patient Registry, 2003 to 2011, in order to study associations between the airway organisms present in each calendar year and their presence in the subsequent year. Models were adjusted for clinical characteristics and multiple observations per patient. Adjusted models were tested for sensitivity to cystic fibrosis-specific treatments. The study included 28,042 patients aged 6 years and older from 257 accredited U.S. care centers and affiliates. These patients had produced sputum specimens for at least two consecutive years that were cultured for methicillin-sensitive Staphylococcus aureus, methicillin-resistant S. aureus, Pseudomonas aeruginosa, Burkholderia cepacia complex, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, and Candida and Aspergillus species. We analyzed 99.8% of 538,458 sputum cultures from the patients during the study period. Methicillin-sensitive S. aureus was negatively associated with subsequent P. aeruginosa. P. aeruginosa was negatively associated with subsequent B. cepacia complex, A. xylosoxidans, and S. maltophilia. B. cepacia complex was negatively associated with the future presence of all bacteria studied, as well as with that of Aspergillus species. P. aeruginosa, B. cepacia complex, and S. maltophilia were each reciprocally and positively associated with Aspergillus species. Independently of patient characteristics, the organisms studied interact and alter the outcomes of treatment decisions, sometimes in unexpected ways. By inhibiting P. aeruginosa, methicillin-sensitive S. aureus may delay lung disease progression. P. aeruginosa and B. cepacia complex may inhibit other organisms by decreasing airway biodiversity, potentially worsening lung disease.
INTRODUCTION
In the United States, cystic fibrosis (CF) affects roughly 30,000 people, reducing life expectancy by >50% (1). Progressive pulmonary disease, marked by recurrent exacerbations, bacterial infection, and declining lung function, drives morbidity and mortality (2–5). Studies of the CF lung reveal a diverse microbiology. Methicillin-sensitive Staphylococcus aureus (MSSA) and Pseudomonas aeruginosa are the two organisms most commonly isolated from the airway (1, 2). Opportunistic organisms, including Burkholderia cepacia complex, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, nontuberculous mycobacteria, and fungal organisms, commonly colonize and infect patients with CF.
The presence of different organisms alters long-term outcomes for patients with CF. MSSA appears to enhance survival, while B. cepacia complex may presage a catastrophic decline in health (6, 7). Acquisition of methicillin-resistant S. aureus (MRSA) or P. aeruginosa is associated with accelerated lung disease (8–12). Published cross-sectional data from the Cystic Fibrosis Foundation Patient Registry (CFFPR) show that dominant airway infections differ with age (1). MSSA most commonly infects pediatric patients, while P. aeruginosa infection increases in frequency with age and commonly dominates the bacterial community in adult patients (13). Without a clear understanding of the underlying microbial interactions, efforts to prevent, treat, or eradicate specific organisms, such as P. aeruginosa, may produce unexpected and undesirable outcomes. A double-blind, randomized controlled study in 2002 showed that prophylactic treatment of MSSA with cephalexin in infants and young children with CF led to earlier colonization with P. aeruginosa (14). Prophylaxis with ciprofloxacin had no effects in preventing infection with P. aeruginosa (15). Similarly, eradication therapy for P. aeruginosa increased the rate of infection with S. maltophilia (15).
While antibiotic therapy may play a role, existing infections themselves appear to alter the rest of the microbiota (7, 16–19) and may thus alter the clinical disease course. In vitro and nonhuman in vivo models show evidence of interspecies interaction between P. aeruginosa and other pathogens, including MSSA and Burkholderia cenocepacia. Mathematical models of disease progression explore potential airway interactions between P. aeruginosa, MSSA, and Burkholderia species; the results from these models are consistent with observational data on CF (1) and illustrate the potential impact of managing these organisms (7).
In this study, we focus on eight common CF airway pathogens and show how the presence of each in a given study year is associated with infections observed in the following year. By improving our understanding of interactions between organisms, we seek to enhance understanding of the underlying mechanisms of changing airway microbial ecology, which may help us anticipate the impacts of changing practice on clinical outcomes.
(Some of the data and results of this study were reported by A. M. Granchelli in preliminary form at the 37th European Cystic Fibrosis Society Meeting, 11 to 14 June 2013, in Gothenburg, Sweden. F. R. Adler and T. G. Liou had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.)
MATERIALS AND METHODS
Study design and data.
We analyzed data for the years 2003 to 2011 from the CFFPR, which contains prospectively collected patient data from 257 Cystic Fibrosis Foundation-accredited care centers and affiliated programs in the United States. Data for the ongoing CFFPR study are gathered according to a defined protocol after obtaining written consent from adult patients or parental consent with assent from minors. The data include patient demographics, clinical measurements of CF disease, treatment information, the number of clinic visits, and culture results from routine quarterly and acute-illness samples. These data were monitored to confirm fidelity to medical charts (20).
We obtained approval from the University of Utah Investigational Review Board (IRB) for the performance of this study, with a waiver of informed consent and approval from the Data Use Committee of the U.S. Cystic Fibrosis Foundation for access to and use of the CFFPR. We continue to participate in data collection for the CFFPR after obtaining written informed consent, with separate approval from the IRB.
Study population and definitions.
Our study cohort included all CFFPR subjects for whom data were available for at least two consecutive years between 2003 and 2011. Since 2003, the CFFPR has recorded each culture result separately for every patient; previously, the CFFPR had reported only a single annualized result per year, thus guiding our study period selection. Patients younger than 6 years were excluded because they cannot reliably perform pulmonary function testing and usually do not produce sputum. We used sputum culture results to determine the presence of infection. The CFFPR records organisms as present or absent for each culture and records the number of cultures obtained in each given year. The presence of infection with a particular organism within a given calendar year was defined as at least one positive culture for that organism within that year. We focused on eight common infections on which the CFFPR contains sufficient data for analysis: MSSA, MRSA, B. cepacia complex, P. aeruginosa, S. maltophilia, A. xylosoxidans, Candida species, and Aspergillus species. We identified, and adjusted for, the following patient characteristics as potential confounders in statistical models: age, age at CF diagnosis, sex, CF-related diabetes, pancreatic sufficiency, weight-for-age Z-score, percentage of predicted forced expiratory volume in 1 s (FEV1%), and acute pulmonary exacerbations (APE). Most of these characteristics have been found previously to predict 5-year survival (6). We defined patients as diabetic in a given year if the condition was present at any time during that year. We defined patients as pancreatic sufficient in a given year if they were noted to be sufficient for all encounters and did not use pancreatic enzymes during that year. For sensitivity analyses to determine the effect of adjustment of associations for different interventions, we used treatment data on oral azithromycin, inhaled aztreonam, tobramycin, recombinant human DNase, and hypertonic saline, days per year of therapy with home intravenous (i.v.) antibiotics, hospitalization days for pulmonary exacerbation treatment, and lung transplantation.
Statistical analysis.
We fitted cross-sectional univariable logistic regression models (21) with the presence of each organism in a given year as the outcome and every other organism studied as an individual explanatory variable. Cross-sectional multivariable models were then fitted with the presence of each organism as the outcome and with all other organisms as explanatory variables with and without additional adjustment for clinical characteristics. These models were fitted separately in each calendar year, from 2003 to 2011. We fitted similar multivariable logistic models relating organisms in each year t to the presence of each organism in year t+1, where t is a particular year from 2003 to 2010. We fitted these models with and without additional adjustment for clinical characteristics. Finally, we fitted a single combined model across all observation years for each patient using generalized estimating equations with an independence working correlation matrix. This model uses multiple observations per individual across the study years. The use of the combined model increases the power of our analysis and reduces the size of confidence intervals (22, 23). It makes the assumption that the associations between organisms from one year to the next are the same across the calendar years. See Text S1 in the supplemental material for detailed methods and the individual steps to fitting the combined model. Sensitivity analyses examined the impact of adjusting for the treatments used. All analyses were performed using the R statistical system (24).
RESULTS
Participants.
We found 28,042 patients aged 6 years or older from 257 care centers and affiliated programs accredited by the U.S. Cystic Fibrosis Foundation who were included in the CFFPR between 1 January 2003 and 31 December 2011 with at least two consecutive years of culture data (Table 1). These patients had a total of 538,458 sets of sputum culture results, of which 537,396 (99.8%) were included in our analysis (see Fig. 1). Culture results were excluded only for lack of same-patient cultures in contiguous years. From 2003 to 2011, the median cross-sectional age of patients increased from 16.6 to 19.1 years (Table 1). The prevalence of CF-related diabetes nearly doubled over this period, from 9.62% to 17.5%. A minority of patients were pancreatic sufficient; this proportion increased between 2003 and 2011 from 6.53% to 11.2%. There were marginal changes in the median FEV1% and the mean number of APE during the study period. The changes in pediatric and adult groups were similar (see Tables S1 and S2 in the supplemental material).
TABLE 1.
Selected characteristics of study patients
| Patient characteristic | Value for the following yr: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |
| No. enrolled | 15,626 | 16,428 | 16,933 | 17,483 | 18,305 | 18,786 | 19,384 | 19,818 | 20,534 |
| No. of culturesa | 42,995 (0.996) | 47,516 (0.999) | 51,826 (1.000) | 55,922 (0.999) | 61,442 (1.000) | 65,319 (1.000) | 68,312 (1.000) | 70,294 (0.999) | 74,409 (1.000) |
| No. of cultures per patientb | 2 (1–4) | 2 (1–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–5) |
| Age (yr)b | 16.6 (11.4–24.0) | 17.0 (11.7–24.5) | 17.2 (11.8–24.8) | 17.6 (12.0–25.3) | 17.8 (12.1–25.9) | 18.1 (12.2–26.4) | 18.4 (12.4–27.0) | 18.7 (12.5–27.4) | 19.1 (12.7–27.9) |
| No. (%) of enrolled patients | |||||||||
| Female | 7,446 (47.7) | 7,811 (47.5) | 8,050 (47.5) | 8,319 (47.6) | 8,764 (47.9) | 9,060 (48.2) | 9,393 (48.5) | 9,618 (48.5) | 9,949 (48.5) |
| With pancreatic sufficiency | 1,018 (6.53) | 1,198 (7.30) | 1,189 (7.03) | 1,365 (7.81) | 1,457 (7.96) | 1,654 (8.80) | 1,830 (9.44) | 2,419 (12.2) | 2,300 (11.2) |
| With diabetes | 1,503 (9.62) | 1,744 (10.6) | 1,919 (11.3) | 2,410 (13.8) | 2,664 (14.6) | 2,845 (15.1) | 3,022 (15.6) | 3,270 (16.5) | 3,601 (17.5) |
| FEV1%b | 81.0 (58.7–97.0) | 81.5 (59.2–97.2) | 82.0 (59.6–97.5) | 82.6 (60.2–97.7) | 83.0 (60.4–98.5) | 83.2 (60.7–98.7) | 83.5 (60.7–99.0) | 83.9 (61.3–99.1) | 83.7 (61.3–99.0) |
| No. of acute pulmonary exacerbationsb | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Wt-for-age Z-scoreb | −0.52 (−1.23 to 0.18) | −0.47 (−1.20 to 0.21) | −0.45 (−1.17 to 0.22) | −0.40 (−1.12 to 0.25) | −0.38 (−1.10 to 0.27) | −0.33 (−1.06 to 0.30) | −0.29 (−1.04 to 0.33) | −0.27 (−1.04 to 0.35) | −0.25 (−1.03 to 0.36) |
| No. (%) of enrolled patients with: | |||||||||
| MSSA | 7,852 (50.2) | 8,292 (50.5) | 8,497 (50.2) | 8,704 (49.8) | 9,140 (49.9) | 9,299 (49.5) | 9,574 (49.4) | 9,759 (49.2) | 10,104 (49.2) |
| P. aeruginosa | 9,969 (63.8) | 10,469 (63.7) | 10,656 (62.9) | 10,729 (61.4) | 11,100 (60.6) | 11,123 (59.2) | 11,323 (58.4) | 11,482 (57.9) | 11,767 (57.3) |
| MRSA | 2,034 (13.0) | 2,642 (16.1) | 3,155 (18.6) | 3,588 (20.5) | 4,150 (22.7) | 4,576 (24.4) | 5,005 (25.8) | 5,514 (27.8) | 5,801 (28.3) |
| Burkholderia complex | 584 (3.74) | 580 (3.53) | 622 (3.67) | 607 (3.47) | 622 (3.40) | 618 (3.29) | 619 (3.19) | 697 (3.52) | 756 (3.68) |
| S. maltophilia | 1,868 (12.0) | 2,064 (12.6) | 2,243 (13.2) | 2,320 (13.3) | 2,440 (13.3) | 2,457 (13.1) | 2,616 (13.5) | 2,881 (14.5) | 3,010 (14.7) |
| A. xylosoxidans | 1,028 (6.58) | 1,095 (6.67) | 1,166 (6.89) | 1,240 (7.09) | 1,236 (6.75) | 1,355 (7.21) | 1,343 (6.93) | 1,468 (7.41) | 1,501 (7.31) |
| Candida species | 1,295 (8.29) | 1,177 (7.16) | 1,365 (8.06) | 1,370 (7.84) | 1,391 (7.60) | 1,609 (8.56) | 1,661 (8.57) | 3,129 (15.8) | 3,559 (17.3) |
| Aspergillus species | 2,458 (15.7) | 2,677 (16.3) | 2,724 (16.1) | 2,913 (16.7) | 2,996 (16.4) | 3,190 (17.0) | 3,141 (16.2) | 3,409 (17.2) | 3,678 (17.9) |
The values in parentheses are the fractions of the numbers of patients enrolled in each year in the study that had usable culture data.
Values are medians (1st and 3rd quartiles).
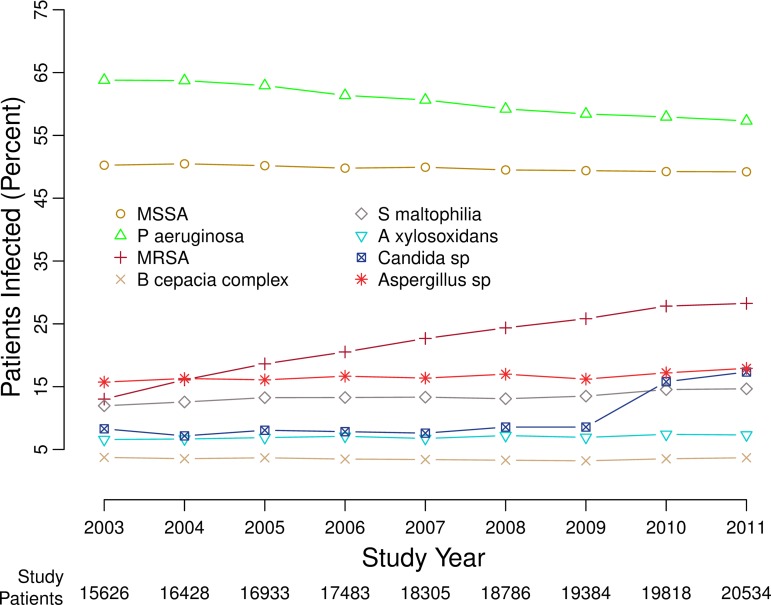
FIG 1.
Percentages of patients with positive cultures for the infections studied, 2003 to 2011. Eight curves show the changing percentages of cultures for the organisms studied, in each of the years from 2003 through 2011, for the patients in the CFFPR who were able to produce sputum samples for microbiologic cultures. The figure is similar to prior figures showing the data in somewhat different ways (59).
Infection prevalence.
There were changes in the percentages of patients with positive cultures for the eight most common infections recorded in the CFFPR between 2003 and 2011 (Table 1 and Fig. 1; also Tables S1 and S2 in the supplemental material). The percentages of patients infected by MRSA and Candida species more than doubled, and the percentages of patients with S. maltophilia, A. xylosoxidans, and Aspergillus species also increased. Between 2003 and 2011, there were small, statistically significant decreases in the percentages of patients infected by MSSA (from 50.2% to 49.2%) and P. aeruginosa (from 63.8% to 57.3%).
Cross-sectional associations between airway infections.
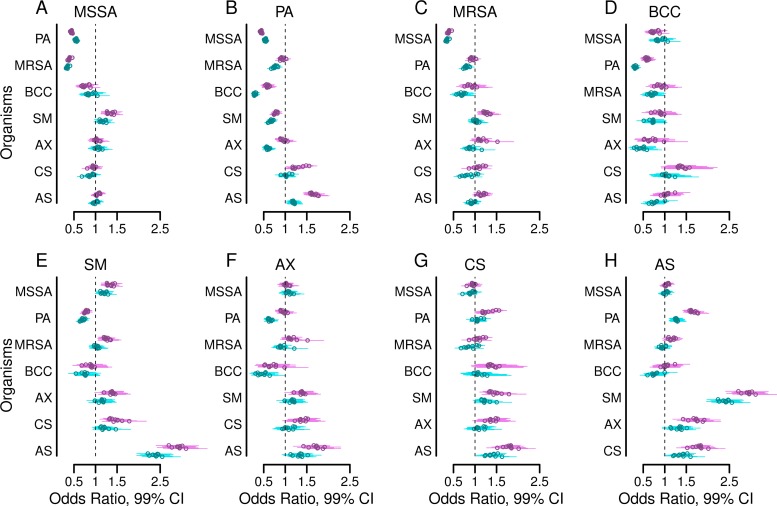
Figure 2 shows estimated odds ratios from multivariable logistic regression models fitted in each year from 2003 to 2011 with each organism as an outcome and the set of all other organisms as explanatory variables, with or without adjustment for clinical variables and the number of cultures. The estimates were remarkably consistent across calendar years. P. aeruginosa infection was negatively associated with MSSA, B. cepacia complex, S. maltophilia, and A. xylosoxidans and was positively associated with Aspergillus species infections for every study year, with or without adjustment for the presence of other organisms (Fig. 2A, D, E, F, and H, respectively). P. aeruginosa and B. cepacia complex were negatively associated in every study year with or without adjustment for other organisms, and additional adjustments for clinical characteristics intensified these associations (Fig. 2B and D). After adjustment for other organisms, with or without adjustment for clinical characteristics, P. aeruginosa infection was negatively associated with MRSA for each year from 2004 through 2011 (Fig. 2C). A. xylosoxidans infections and B. cepacia complex infections showed large negative coefficients in every study year, suggesting that such coinfections are uncommon (Fig. 2D). In contrast, S. maltophilia was associated with Aspergillus species infections every year with large positive coefficients, suggesting that such coinfections are common (Fig. 2H). The odds ratios from logistic regression models examining univariable, unadjusted associations between organisms are similar to the fully adjusted results (see Fig. S1 in the supplemental material).
FIG 2.
Adjusted cross-sectional associations between airway infections. Forest plots show the adjusted odds ratios (circles) and 99% confidence intervals (bars) of having a positive culture for each of the eight organisms studied within each study year, comparing the presence versus absence of a positive culture for each of the other seven organisms in the same year. The outcomes for methicillin-sensitive Staphylococcus aureus (MSSA) (A), Pseudomonas aeruginosa (B), methicillin-resistant S. aureus (MRSA) (C), Burkholderia cepacia complex (BCC) (D), Stenotrophomonas maltophilia (E), Achromobacter xylosoxidans (F), Candida species (G), and Aspergillus species (H) are shown. Results shown in purple are from models adjusted by the presence of the other six organisms. Results shown in turquoise are from models additionally adjusted for the following clinical characteristics: age, sex, late diagnosis of CF, best FEV1% in each year, annual number of APE, pancreatic sufficiency, diabetes status, and weight-for-age Z-score.
Associations between present and future airway infections.
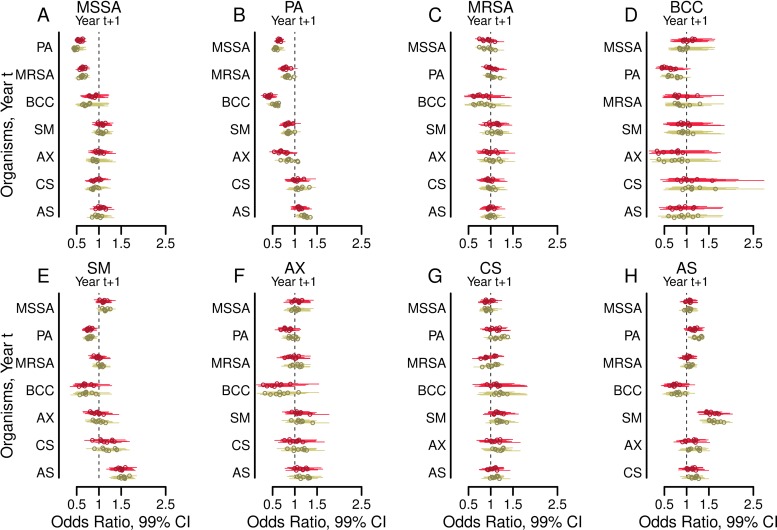
Figure 3 shows the estimated odds ratios from multivariable models for the association of microbial infection status for each of the years 2004 through 2011 (years t+1) with infections in the previous year (where t is a particular year from 2003 to 2010) (21). Associations were similar for every 2-year period examined, with some variation in the degree of statistical significance for individual relationships.
FIG 3.
Adjusted associations between airway infections in the years from 2003 to 2010 (years t) and infections with other organisms in subsequent years 2004 to 2011 (years t + 1). Forest plots show the odds ratios (circles) and 99% confidence intervals (bars) of having a positive culture in year t+1 for each of the eight organisms studied when each of the other organisms was present in the preceding year t (where t is a particular year between 2003 and 2010). Outcomes from years t+1 are shown for methicillin-sensitive Staphylococcus aureus (MSSA) (A), Pseudomonas aeruginosa (B), methicillin-resistant S. aureus (MRSA) (C), Burkholderia cepacia complex (BCC) (D), Stenotrophomonas maltophilia (E), Achromobacter xylosoxidans (F), Candida species (G), and Aspergillus species (H). Results shown in red are from models adjusted by the presence of the remaining six organisms. Results shown in green are from models additionally adjusted for the following clinical characteristics in year t: age, sex, late diagnosis of CF, best FEV1% in each year, annual number of APE, pancreatic sufficiency, diabetes status, and weight-for-age Z-score.
For each organism, the predictor most strongly associated with its presence in year t+1 was the presence of the same organism in year t (see Fig. S2 in the supplemental material). MSSA, B. cepacia complex, S. maltophilia, and A. xylosoxidans in each year t were all negatively associated with P. aeruginosa in the subsequent year t+1 (Fig. 3B). Candida or Aspergillus species were not typically associated with subsequent P. aeruginosa (Fig. 3B). Organisms other than MSSA and B. cepacia complex in each year t were infrequently associated with MRSA in each year t+1. MSSA in 3 years t (2005, 2008, and 2010) and B. cepacia complex infections in 2 years t (2005 and 2007) had potentially significant negative statistical and clinical associations with MRSA (Fig. 3C). Adjustment for clinical variables did not substantially change the estimates or their statistical significance (Fig. 3).
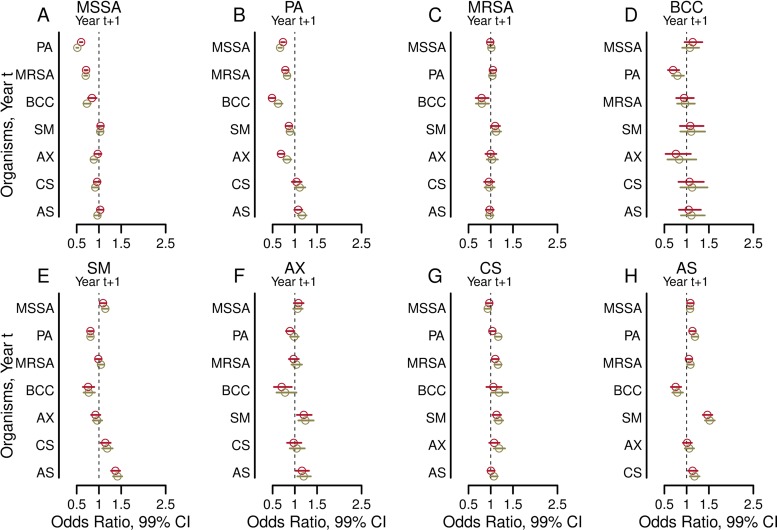
When all observations are used simultaneously in the combined model, the relationships between an organism seen in year t with a different organism in year t+1 (Fig. 4; see also Tables S3 and S4 in the supplemental material) are similar to all relationships seen in multivariable logistic regression models for each of the 2-year models reported above, but with much narrower confidence intervals (Fig. 3). Adjustment for clinical characteristics produced similar results (Fig. 4; Tables S3 and S4). These models again showed that the presence of an organism in year t was most strongly associated with the same organism in year t+1 (see Fig. S3 in the supplemental material). Sensitivity analyses in which we additionally adjusted for clinical treatments (one at a time) given in year t showed that the associations were not substantially altered.
FIG 4.
Adjusted associations from the combined model between an organism seen in year t with a different organism in year t+1. Each forest plot shows the odds ratios (circles) and 99% confidence intervals (bars) from the combined model utilizing the entire 2003–2011 CFFPR data set for each of the eight organisms studied in year t+1 when each of the other organisms was present in the respective year t (where t is a particular year between 2003 and 2010). Outcomes from years t+1 are shown for methicillin-sensitive Staphylococcus aureus (MSSA) (A), Pseudomonas aeruginosa (B), methicillin-resistant S. aureus (MRSA) (C), Burkholderia cepacia complex (BCC) (D), Stenotrophomonas maltophilia (E), Achromobacter xylosoxidans (F), Candida species (G), and Aspergillus species (H). Results shown in red are from models adjusted by the presence of the remaining six organisms. Results shown in green are from models additionally adjusted for the following clinical characteristics in year t: age, sex, late diagnosis of CF, best FEV1% in each year, annual number of APE, pancreatic sufficiency, diabetes status, and weight-for-age Z-score.
Missing data.
Our study population included patients who had two consecutive years of culture data at least once between 2003 and 2011 (Table 1). We analyzed cohorts and patients for missing data. The proportion of patients excluded from any 2-year cohort ranged from 4.6% to 7.5% (see Table S5 in the supplemental material). There were small but statistically significant differences between study patients and patients with missing data. Excluded patients tended to be older, with a higher prevalence of diabetes and slightly decreased lung function; however, they also appeared to be healthier in other ways, with better nutritional status, fewer APE, and higher rates of pancreatic sufficiency (see Tables S6, S7, and S8 in the supplemental material).
DISCUSSION
Our study of microbiology in the human airway reveals microbial interactions that may alter therapeutic responses in individuals with CF. The organisms present currently change the odds of finding other microbes concurrently and in the future. Among the organisms studied, the likelihood of retention is highest for P. aeruginosa, MRSA, and especially B. cepacia complex, the most clinically pathogenic organisms. In contrast, MSSA, the only organism associated with increased 5-year survival in CF (6), is the least likely to persist from one year to the next. P. aeruginosa, MRSA, and B. cepacia complex all reduce the likelihood of culturing MSSA in future years, thus likely reducing patient survival.
Prior studies of small groups of patients using molecular analysis methods have shown that a patient is more likely to retain current infections than to lose them (25, 26). The chronicity of specific infections (27), their interactions with human host defenses, and their clinical outcomes have been studied previously (28). Microbial species-to-species interactions among pathogens similar to those found in the CF airway have been studied in various in vitro and nonhuman in vivo model systems (17, 18, 29–37), and the clinical effects of the combination of P. aeruginosa and MSSA have been explored (38), but interspecies microbial interactions in the CF airway have been minimally explored, and recent calls to expand this knowledge base remain outstanding (16, 39).
Our study answers this call quantitatively by showing precisely that current infections alter the odds of finding other organisms in subsequent years. Our study uses a ∼1,000-fold or larger group of patients than prior studies examining organism persistence alone, thus providing the additional power necessary to explore interspecies interactions with confidence. We demonstrate these associations in extensive univariable and multivariable models (adjusted for other infections and clinical variables), and we demonstrate a lack of sensitivity to various other clinical statuses and all common treatments. The stability of the results despite the variability in clinical states and the prescribing of common treatments underscores the need to understand the potential effects of persistent microbial interactions in order to avoid undesirable clinical outcomes.
The strongest association between organisms was the negative association between MSSA in year t and P. aeruginosa in year t+1, or vice versa (Fig. 3 and 4). All associations were independent of the presence of other organisms (Fig. 3 and 4; also Table S3 in the supplemental material) and the severity of clinical characteristics (Fig. 3 and 4; also Table S4 in the supplemental material) and were insensitive to the use of multiple CF-specific therapies, including chronic and acute antibiotic treatments. MSSA may limit the acquisition and reduce the persistence of P. aeruginosa infection in some patients, and P. aeruginosa may supplant MSSA infection in others (1). These observations are consistent with previous findings that elimination of MSSA leads to more-rapid infection with P. aeruginosa (14).
The two most harmful bacterial pathogens in CF, P. aeruginosa and B. cepacia complex, are also the organisms that were associated with the greatest number of other infections in our study (Fig. 4). The presence of either P. aeruginosa or B. cepacia complex was associated with lower odds of MSSA, S. maltophilia, and A. xylosoxidans in the future. B. cepacia complex was additionally associated with lower odds for concurrent (Fig. 2C, turquoise cluster) and subsequent (Fig. 4C) MRSA infections. By limiting the acquisition or persistence of other infections, P. aeruginosa and B. cepacia complex may decrease microbial diversity. Loss of diversity in CF airway ecology is linked with worsening lung disease, an observation consistent with the high pathogenicity of P. aeruginosa and B. cepacia complex in CF (13, 19).
Our study shows that S. maltophilia and A. xylosoxidans each had less influence on each other and the other six organisms in our study than did P. aeruginosa or B. cepacia complex (Fig. 2, 3, and 4). The decreased impact on microbial diversity may help explain, for example, why S. maltophilia seems less pathogenic than many other organisms in CF (40). Our study supports previous findings that intermittent infection with S. maltophilia does not substantially affect the progression of lung disease or survival (41–43).
Aspergillus and Candida species are the most commonly cultured fungi in the CF airway (44). The extent and nature of interactions between fungal and bacterial infectious agents in the CF airway is unclear. Previous in vitro and nonhuman in vivo model-based research demonstrated inhibition of biofilm formation in both Candida and Aspergillus species by P. aeruginosa (29, 30, 37). Our study, based on clinical observations, suggests that P. aeruginosa and S. maltophilia infections are associated with higher rather than lower odds of concurrent and subsequent Aspergillus infections (Fig. 2H, 3H, and 4H; also Fig. S1 in the supplemental material). MRSA and S. maltophilia were associated with slightly higher odds of subsequent Candida infection (Fig. 4G). Only B. cepacia complex was associated with lower odds of a future fungal infection, and only for Aspergillus species (Fig. 4G). The discrepancies between our observations and those from nonhuman models of P. aeruginosa–Aspergillus interactions (29, 30, 37) may merely reflect differences between model system and human airway conditions but may, alternatively, indicate the presence of important differences in microbial virulence (45). Evidence of interspecies interactions may help explain why approximately one-third of efforts to eradicate pathogens from the CF airway fail (46, 47): perhaps a nontargeted concurrent infection promotes the persistence of a target organism. The clinical impacts of these associations remain uncertain, but their potential for altering disease course and outcomes invites further study.
Our findings suggest that microbial interactions occur in the airways of patients with CF regardless of treatments and events that may modify the presence of microbes. Potential interaction mechanisms may be considered. First, microbes produce antimicrobial agents (31). The strength of associations between MSSA, P. aeruginosa, and B. cepacia complex (Fig. 4A, B, and D) is consistent with prior findings that these organisms produce novel antimicrobials (48–52). Second, organisms may compete for airway resources, such as iron (32, 53). Third, organisms may interact with human host defenses or with each other to modify interactions with yet other organisms (35, 54). Fourth, and finally, our confirmation of the consistency and strength of microbial interactions regardless of the mechanism suggests additional areas for the investigation of clinical impacts. Expanded knowledge of microbial interactions may explain unexpected outcomes of antimicrobial therapy (14, 15), better delineate the pathogenesis of pulmonary exacerbations in CF that punctuate and accelerate the course of disease (55), and improve predictions of long-term outcomes critical to the well-being of patients (6, 7, 56).
Limitations.
Our study has several limitations. First, there may be unrecorded treatment decisions that affect airway ecology. However, prior studies show that short-course antibiotic therapy only transiently affects a CF patient's individual microbiota (26, 57). Moreover, our analysis showed that adjustment for various treatments did not materially alter results. Second, we were limited to studying the eight organisms for which sufficient culture data are present in the CFFPR. This excluded direct study of many CF airway organisms that are infrequently present, underreported, not collected during the study period (such as nontuberculous mycobacteria), or identifiable only by nonculture methods (58). The use of culture data is subject to variable rates, by organism, of false-positive or false-negative results; however, similar difficulties affect the recovery of organisms by nonculture methods (58). Furthermore, results from conventional sputum cultures for aerobic organisms are the data that drive clinical decisions in treating patients with CF, are correlated with results from culture-independent methods for identifying the common aerobic infectious agents in CF analyzed in our study, especially P. aeruginosa (26, 58), and provide the basis of prior reports showing associations between organisms and survival outcomes (6, 9). Third, and last, there are potential biases from a lack of data that prevented the inclusion of some patients in the analysis. However, the proportion of patients for whom data were missing was quite low (Tables S6, S7, and S8 in the supplemental material). The patients in the CFFPR during the study period who never had sufficient data for inclusion accounted for 0.2% to 0.9% of all CFFPR patients during each year of the study period (Tables S7 and S8). There may also be data in the CFFPR that were partially available for the adjustment of our models but which we excluded from the analysis for various reasons. For example, we did not use genotype data, because it was unavailable for a large proportion of the patients studied and because it is less successful as a predictor of long-term clinical outcomes than clinical phenotype variables such as those we used previously to predict 5-year survival outcomes (6).
Conclusions.
This study helps clinicians understand how current microbiology may play a role in shaping the overall subsequent microbiology of the CF airway. Mechanistic studies are needed in order to understand specifically how MSSA may limit infection with P. aeruginosa and how P. aeruginosa and B. cepacia complex may limit coinfecting organisms. Such understanding has the potential to influence strategic decisions in CF clinical care. While a bacterial pathogen found in an otherwise healthy host is often met with an attempt at eradication, this strategy in CF may be defeated by interspecies interactions that promote the persistence of multiple species and have unintended consequences even when the treatment seems successful. Eliminating specific infections within the diverse CF airway ecology may disrupt a delicate balance and accelerate the time to infection with more-pathogenic organisms. Determining the potential influence of each organism on the CF microbiome may help clinicians understand the extended impact of modifying a patient's airway ecology and ultimately improve patient survival.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Cystic Fibrosis Foundation (CFF) for the use of Cystic Fibrosis Foundation Patient Registry (CFFPR) data to conduct this study. Additionally, we thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CFFPR. Data are available upon request through the CFF Patient Registry Comparative Effectiveness Research Committee (which may be contacted at datarequests@cff.org). Restrictions on access to data are in place to ensure privacy for all persons in the CFFPR.
A. M. Granchelli, F. R. Adler, and T. G. Liou conceived the initial idea for this paper. All authors participated in the design and execution of the analysis and the interpretation of the data. T. G. Liou obtained funding, acquired the raw data, oversaw the security and integrity of the data, and, with the assistance of Judy Jensen, obtained the necessary permissions from the University of Utah IRB and the CFF Patient Registry Comparative Effectiveness Research Committee to proceed with the study. A. M. Granchelli and T. G. Liou drafted the manuscript, but all authors participated in critical reviews and revisions of the work. Throughout the study, D. R. Cox provided pivotal advice on many statistical aspects. T. G. Liou oversaw clinically oriented aspects of the work.
This project was funded by an award from the Cystic Fibrosis Foundation, Bethesda, MD, USA (LIOU14P0), a Ben B. and Iris M. Margolis Family Foundation of Utah award, continuing funding from the Claudia Ruth Goodrich Stevens Endowment Fund, and National Heart, Lung, and Blood Institute awards T32HL105321 (to A.M.G.) and R01HL125520. R.H.K. was supported by a Medical Research Council Fellowship (MR/M014827/1). F.R.A. received additional support from the Army Research Office (ARO W911NF-15-1-0400), the National Science Foundation Division of Mathematical Sciences (RTG: NSF-DMS 1148230), and the NIH Cancer Systems Biology Consortium (U54 CA209978). The Cystic Fibrosis Foundation, through a separate prospective observational clinical trial, funded the collection of data in the CFFPR that were the basis of the current study, including data from the University of Utah (CC132-16AD). The other funders had no role in data collection. The CFF reviewed the manuscript to ensure that our use of CFFPR data maintains the privacy of protected health information.
The funders of the study had no roles in study design, data analysis, data interpretation, or the writing of this report.
The opinions expressed in this work are solely those of the authors and do not necessarily express the views of the Cystic Fibrosis Foundation, the Margolis Foundation, the Claudia Ruth Goodrich Stevens Family, the National Heart, Lung, and Blood Institute of the National Institutes of Health, the U.S. Army Research Office, the National Science Foundation Division of Mathematical Sciences, the NIH Cancer Systems Biology Consortium, the Medical Research Council in the United Kingdom, the government of the United Kingdom, or the government of the United States.
T. G. Liou reports grant support for clinical trials from Gilead Sciences, Nivalis Therapeutics, Inc., Novartis, Proteostasis Therapeutics, Inc., Savara Pharmaceuticals, and Vertex Pharmaceuticals. In addition, T. G. Liou has a provisional patent on a novel antibiotic; is a member of the Clinical Research Review Committee of the U.S. CFF, for which he reviews grant applications; is a member of the editorial board at Chest; and reviews papers for various journals regarding CF and statistical analyses in medicine. No pharmaceutical companies or other commercial agencies paid any of the authors to write this article.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00354-18.
REFERENCES
- 1.Cystic Fibrosis Foundation. 2016. Cystic Fibrosis Foundation Patient Registry 2015. Annual data report Cystic Fibrosis Foundation, Bethesda, MD. [Google Scholar]
- 2.Lipuma JJ. 2010. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amadori A, Antonelli A, Balteri I, Schreiber A, Bugiani M, De Rose V. 2009. Recurrent exacerbations affect FEV1 decline in adult patients with cystic fibrosis. Respir Med 103:407–413. doi: 10.1016/j.rmed.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Sanders DB, Bittner RCL, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. 2010. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. 2012. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J 40:61–66. doi: 10.1183/09031936.00159111. [DOI] [PubMed] [Google Scholar]
- 6.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. 2001. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler FR, Liou TG. 2016. The dynamics of disease progression in cystic fibrosis. PLoS One 11:e0156752. doi: 10.1371/journal.pone.0156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. 2008. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med 178:814–821. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 9.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. 2010. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 10.Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, Grimwood K. 2001. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 138:699–704. doi: 10.1067/mpd.2001.112897. [DOI] [PubMed] [Google Scholar]
- 11.Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, Green CG, Collins J, Farrell PM. 2001. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol 32:277–287. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Kosorok MR, Farrell PM, Laxova A, West SEH, Green CG, Collins J, Rock MJ, Splaingard ML. 2005. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 13.Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, Zhang Y, Surendra A, Gong Y, Tullis DE, Yau YCW, Waters VJ, Hwang DM, Guttman DS. 2015. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 5:10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stutman HR, Lieberman JM, Nussbaum E, Marks MI, the Antibiotic Prophylaxis in Cystic Fibrosis Study Group. 2002. Antibiotic prophylaxis in infants and young children with cystic fibrosis: a randomized controlled trial. J Pediatr 140:299–305. doi: 10.1067/mpd.2002.121930. [DOI] [PubMed] [Google Scholar]
- 15.Onakpoya IJ, Hayward G, Heneghan CJ. 2015. Antibiotics for preventing lower respiratory tract infections in high-risk children aged 12 years and under. Cochrane Database Syst Rev 2015(9):CD011530. doi: 10.1002/14651858.CD011530.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkins MD, Floto RA. 2015. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J Cyst Fibros 14:293–304. doi: 10.1016/j.jcf.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Beaume M, Köhler T, Fontana T, Tognon M, Renzoni A, van Delden C. 2015. Metabolic pathways of Pseudomonas aeruginosa involved in competition with respiratory bacterial pathogens. Front Microbiol 6:321. doi: 10.3389/fmicb.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bragonzi A, Farulla I, Paroni M, Twomey KB, Pirone L, Lorè NI, Bianconi I, Dalmastri C, Ryan RP, Bevivino A. 2012. Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS One 7:e52330. doi: 10.1371/journal.pone.0052330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldan R, Cigana C, Testa F, Bianconi I, De Simone M, Pellin D, Di Serio C, Bragonzi A, Cirillo DM. 2014. Adaptation of Pseudomonas aeruginosa in cystic fibrosis airways influences virulence of Staphylococcus aureus in vitro and murine models of co-infection. PLoS One 9:e89614. doi: 10.1371/journal.pone.0089614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, Elbert A, Petren KM, Marshall BC. 2016. The Cystic Fibrosis Foundation Patient Registry. Design and methods of a national observational disease registry. Ann Am Thorac Soc 13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW Jr, Lemeshow S, Sturdivant RX. 2013. Applied logistic regression, 3rd ed John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- 22.Liang K-Y, Zeger SL. 1986. Longitudinal data analysis using generalized linear models. Biometrika 73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 23.Prentice RL, Zhao LP. 1991. Estimating equations for parameters in means and covariances of multivariate discrete and continuous responses. Biometrics 47:825–839. doi: 10.2307/2532642. [DOI] [PubMed] [Google Scholar]
- 24.R Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 25.Carmody LA, Zhao J, Kalikin LM, LeBar W, Simon RH, Venkataraman A, Schmidt TM, Abdo Z, Schloss PD, LiPuma JJ. 2015. The daily dynamics of cystic fibrosis airway microbiota during clinical stability and at exacerbation. Microbiome 3:12. doi: 10.1186/s40168-015-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tunney MM, Klem ER, Fodor AA, Gilpin DF, Moriarty TF, McGrath SJ, Muhlebach MS, Boucher RC, Cardwell C, Doering G, Elborn JS, Wolfgang MC. 2011. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax 66:579–584. doi: 10.1136/thx.2010.137281. [DOI] [PubMed] [Google Scholar]
- 27.Lee TWR, Brownlee KG, Conway SP, Denton M, Littlewood JM. 2003. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros 2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 28.Callaghan M, McClean S. 2012. Bacterial host interactions in cystic fibrosis. Curr Opin Microbiol 15:71–77. doi: 10.1016/j.mib.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira JAG, Penner JC, Moss RB, Haagensen JAJ, Clemons KV, Spormann AM, Nazik H, Cohen K, Banaei N, Carolino E, Stevens DA. 2015. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS One 10:e0134692. doi: 10.1371/journal.pone.0134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mowat E, Rajendran R, Williams C, McCulloch E, Jones B, Lang S, Ramage G. 2010. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol Lett 313:96–102. doi: 10.1111/j.1574-6968.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 31.Bakkal S, Robinson SM, Ordonez CL, Waltz DA, Riley MA. 2010. Role of bacteriocins in mediating interactions of bacterial isolates taken from cystic fibrosis patients. Microbiology (Reading, Engl) 156:2058–2067. doi: 10.1099/mic.0.036848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver VB, Kolter R. 2004. Burkholderia spp. alter Pseudomonas aeruginosa physiology through iron sequestration. J Bacteriol 186:2376–2384. doi: 10.1128/JB.186.8.2376-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machan ZA, Pitt TL, White W, Watson D, Taylor GW, Cole PJ, Wilson R. 1991. Interaction between Pseudomonas aeruginosa and Staphylococcus aureus: description of an anti-staphylococcal substance. J Med Microbiol 34:213–217. doi: 10.1099/00222615-34-4-213. [DOI] [PubMed] [Google Scholar]
- 34.Pernet E, Brunet J, Guillemot L, Chignard M, Touqui L, Wu Y. 2015. Staphylococcus aureus adenosine inhibits sPLA2-IIA-mediated host killing in the airways. J Immunol 194:5312–5319. doi: 10.4049/jimmunol.1402665. [DOI] [PubMed] [Google Scholar]
- 35.Bernier SP, Workentine ML, Li X, Magarvey NA, O'Toole GA, Surette MG. 2016. Cyanide toxicity to Burkholderia cenocepacia is modulated by polymicrobial communities and environmental factors. Front Microbiol 7:725. doi: 10.3389/fmicb.2016.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAlester G, O'Gara F, Morrissey JP. 2008. Signal-mediated interactions between Pseudomonas aeruginosa and Candida albicans. J Med Microbiol 57:563–569. doi: 10.1099/jmm.0.47705-0. [DOI] [PubMed] [Google Scholar]
- 37.Sass G, Nazik H, Penner J, Shah H, Ansari SR, Clemons KV, Groleau M-C, Dietl A-M, Visca P, Haas H, Déziel E, Stevens DA. 2018. Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J Bacteriol 200:e00345-. doi: 10.1128/JB.00345-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubert D, Réglier-Poupet H, Sermet-Gaudelus I, Ferroni A, Le Bourgeois M, Burgel P-R, Serreau R, Dusser D, Poyart C, Coste J. 2013. Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J Cyst Fibros 12:497–503. doi: 10.1016/j.jcf.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Harrison F. 2007. Microbial ecology of the cystic fibrosis lung. Microbiology (Reading, Engl) 153:917–923. doi: 10.1099/mic.0.2006/004077-0. [DOI] [PubMed] [Google Scholar]
- 40.Waters V, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. 2013. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros 12:482–486. doi: 10.1016/j.jcf.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Waters V, Atenafu EG, Salazar JG, Lu A, Yau Y, Matukas L, Tullis E, Ratjen F. 2012. Chronic Stenotrophomonas maltophilia infection and exacerbation outcomes in cystic fibrosis. J Cyst Fibros 11:8–13. doi: 10.1016/j.jcf.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Waters V, Yau Y, Prasad S, Lu A, Atenafu E, Crandall I, Tom S, Tullis E, Ratjen F. 2011. Stenotrophomonas maltophilia in cystic fibrosis: serologic response and effect on lung disease. Am J Respir Crit Care Med 183:635–640. doi: 10.1164/rccm.201009-1392OC. [DOI] [PubMed] [Google Scholar]
- 43.Goss CH, Otto K, Aitken ML, Rubenfeld GD. 2002. Detecting Stenotrophomonas maltophilia does not reduce survival of patients with cystic fibrosis. Am J Respir Crit Care Med 166:356–361. doi: 10.1164/rccm.2109078. [DOI] [PubMed] [Google Scholar]
- 44.Bakare N, Rickerts V, Bargon J, Just-Nübling G. 2003. Prevalence of Aspergillus fumigatus and other fungal species in the sputum of adult patients with cystic fibrosis. Mycoses 46:19–23. doi: 10.1046/j.1439-0507.2003.00830.x. [DOI] [PubMed] [Google Scholar]
- 45.Inglis RF, Gardner A, Cornelis P, Buckling A. 2009. Spite and virulence in the bacterium Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 106:5703–5707. doi: 10.1073/pnas.0810850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taccetti G, Bianchini E, Cariani L, Buzzetti R, Costantini D, Trevisan F, Zavataro L, Campana S, Italian Group for P aeruginosa Eradication in Cystic Fibrosis. 2012. Early antibiotic treatment for Pseudomonas aeruginosa eradication in patients with cystic fibrosis: a randomised multicentre study comparing two different protocols. Thorax 67:853–859. doi: 10.1136/thoraxjnl-2011-200832. [DOI] [PubMed] [Google Scholar]
- 47.Vallières E, Rendall JC, Moore JE, McCaughan J, Hoeritzauer AI, Tunney MM, Elborn JS, Downey DG. 2016. MRSA eradication of newly acquired lower respiratory tract infection in cystic fibrosis. ERJ Open Res 2:00064–2015. doi: 10.1183/23120541.00064-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gross H, Loper JE. 2009. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep 26:1408–1446. doi: 10.1039/b817075b. [DOI] [PubMed] [Google Scholar]
- 49.Ghequire MGK, De Mot R. 2014. Ribosomally encoded antibacterial proteins and peptides from Pseudomonas. FEMS Microbiol Rev 38:523–568. doi: 10.1111/1574-6976.12079. [DOI] [PubMed] [Google Scholar]
- 50.Janek D, Zipperer A, Kulik A, Krismer B, Peschel A. 2016. High frequency and diversity of antimicrobial activities produced by nasal Staphylococcus strains against bacterial competitors. PLoS Pathog 12:e1005812. doi: 10.1371/journal.ppat.1005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song L, Jenner M, Masschelein J, Jones C, Bull MJ, Harris SR, Hartkoorn RC, Vocat A, Romero-Canelon I, Coupland P, Webster G, Dunn M, Weiser R, Paisey C, Cole ST, Parkhill J, Mahenthiralingam E, Challis GL. 2017. Discovery and biosynthesis of gladiolin: a Burkholderia gladioli antibiotic with promising activity against Mycobacterium tuberculosis. J Am Chem Soc 139:7974–7981. doi: 10.1021/jacs.7b03382. [DOI] [PubMed] [Google Scholar]
- 52.Mahenthiralingam E, Song L, Sass A, White J, Wilmot C, Marchbank A, Boaisha O, Paine J, Knight D, Challis GL. 2011. Enacyloxins are products of an unusual hybrid modular polyketide synthase encoded by a cryptic Burkholderia ambifaria genomic island. Chem Biol 18:665–677. doi: 10.1016/j.chembiol.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, Malfroot A, Cornelis P, Newman DK. 2013. Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. mBio 4(4):e00557-13. doi: 10.1128/mBio.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pernet E, Guillemot L, Burgel P-R, Martin C, Lambeau G, Sermet-Gaudelus I, Sands D, Leduc D, Morand PC, Jeammet L, Chignard M, Wu Y, Touqui L. 2014. Pseudomonas aeruginosa eradicates Staphylococcus aureus by manipulating the host immunity. Nat Commun 5:5105. doi: 10.1038/ncomms6105. [DOI] [PubMed] [Google Scholar]
- 55.Whelan FJ, Surette MG. 2015. Clinical insights into pulmonary exacerbations in cystic fibrosis from the microbiome. What are we missing? Ann Am Thorac Soc 12(Suppl 2):S207–S211. [DOI] [PubMed] [Google Scholar]
- 56.Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, Packer K, Clark T, Carveth H, Chen J, Rogers SL, Lane C, Moore J, Sturrock A, Paine R III, Cox DR, Hoidal JR. 2012. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS One 7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fodor AA, Klem ER, Gilpin DF, Elborn JS, Boucher RC, Tunney MM, Wolfgang MC. 2012. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One 7:e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahboubi MA, Carmody LA, Foster BK, Kalikin LM, VanDevanter DR, LiPuma JJ. 2016. Culture-based and culture-independent bacteriologic analysis of cystic fibrosis respiratory specimens. J Clin Microbiol 54:613–619. doi: 10.1128/JCM.02299-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salsgiver EL, Fink AK, Knapp EA, LiPuma JJ, Olivier KN, Marshall BC, Saiman L. 2016. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest 149:390–400. doi: 10.1378/chest.15-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.