Rapid screening of urinary tract infection is important to determine antibiotic treatment and reduce unnecessary urine culture. We evaluated the performance of the new flow cytometry-based UF-5000 automated urine analyzer (Sysmex, Kobe, Japan).
KEYWORDS: urinary tract infection, flow cytometry, urine culture, bacterial discrimination
ABSTRACT
Rapid screening of urinary tract infection is important to determine antibiotic treatment and reduce unnecessary urine culture. We evaluated the performance of the new flow cytometry-based UF-5000 automated urine analyzer (Sysmex, Kobe, Japan). A total of 1,430 urine samples from 1,226 patients were analyzed and compared to urine cultures to which a Previ Isola (bioMérieux, Marcy l'Etoile, France) system was applied. In total, 878 of 1,430 urine cultures (61.4%) produced ≥103 CFU/ml bacterial growth (309 with Gram-negative [GN] bacteria, 517 with Gram-positive [GP] bacteria, and 52 mixed cultures), with 336 samples (23.5%) presenting ≥105 CFU/ml bacterial growth. The ≥105 CFU/ml bacterial growth was detected by a ≥71 bacteria/μl UF-5000 bacterial count with 95% sensitivity and 84% specificity. Using a cutoff of <15 bacteria/μl to determine whether or not to culture, 50.9% of samples were below the cutoff, 94.8 and 99.5% of which presented <104 and <105 CFU/ml of bacterial growth, respectively. The bacterial discrimination performance of the UF-5000 for GN bacteria was superior to that for GP bacteria, and in ≥105 CFU/ml monobacterial samples, the sensitivity and specificity for reporting GN bacteria were 91.7 and 90.0%, respectively. In summary, UF-5000 demonstrated potential utility for the rapid screening of negative bacterial cultures. However, this utility is dependent on the patient population; cutoff optimizations must be performed for specific populations. In addition, UF-5000 presented improved performance in characterizing GP and GN bacteria, although the concurrence rates were not high enough to replace routine cultures.
INTRODUCTION
Urinary tract infections (UTIs) are important health care problems for hospitalized and community patients (1). Various bacterial species cause UTIs; approximately 80 to 90% of etiologic bacteria are Gram-negative (GN) bacteria, most commonly Escherichia coli (60 to 80% of cases), in addition to Klebsiella spp., Enterobacter spp., Proteus spp., and Pseudomonas spp. (2). Gram-positive (GP) bacteria, including Enterococcus spp., Staphylococcus spp., and Streptococcus agalactiae, are also important pathogens of UTIs; however, these bacteria might often be contaminants in urine culture.
The gold standard for diagnosing UTIs is urine culture and the microbiological confirmation of etiologic bacteria. However, this method requires substantial time for final reporting, usually 24 to 48 h. In addition, most cultured urine samples yield no or insignificant bacterial growth (3). Therefore, a rapid screening test for UTIs and the determination of samples requiring culture are needed for timely and appropriate clinical decision making. Previously, Gram staining or other manual methods such as urine particle counting were used to predict negative and positive samples. However, these tests provide limited information and have low sensitivity and specificity, and their clinical usefulness is therefore limited.
Automated urine analysis instruments for the rapid and objective analysis of urine microscopy have been developed and are continuously being improved. A flow cytometry based urinalysis analyzer, the Sysmex UF-1000i (Sysmex, Kobe, Japan), can detect and quantify bacterial concentrations in the urine, and its implications for screening for UTI have been investigated (3–9). This automated urine analyzer can also detect cells and particles, including white blood cells (WBCs) and red blood cells (RBCs), in addition to microorganisms in the urine, which increases the sensitivity and specificity for the detection of UTIs. Diagnostic algorithms that reduce the need for urine culture and rapid clinical decision have been suggested based on the results of these studies, with promising results when applied in clinical settings (3–9). However, the discrimination of Gram-positive and negative bacteria was limited, and further improvements would be helpful (8). Recently, a newer version of the previous UF-1000i, the UF-5000 (Sysmex), was developed. This instrument was designed for more accurate bacterial identification and applied a newer flow cytometer data analysis algorithm for more accurate bacterial counting and Gram-negative and Gram-positive bacterial discrimination. In the present study, we evaluated the performance of the Sysmex UF-5000 in screening of UTIs in clinical samples compared to urine culture results and investigated the potential clinical impact.
MATERIALS AND METHODS
Patients and sample preparation.
A total of 1,430 urine samples from 1,226 patients were included in this study. The consecutive urine samples had been routinely submitted to the microbiology laboratory of a tertiary hospital for urine culture over a 3-month period (July 2016 to September 2016), and samples that could be used for UF-5000 analysis within 3 h were included in the study. Urine samples were collected in a sterile urine cup for inoculation of agar plates. The remaining urine samples were used for the UF-5000 analysis and analyzed within 3 h. Among the total patients, 667 (54.4%) were males, and the median age was 59 years (range, 0 to 95 years). In addition, 691 (56.4%) were hospitalized patients, and 535 (43.6%) were outpatients. Among the cohort, 399 adult patients had the following medical comorbidities: advanced cancer (n = 210), hematologic malignancies (n = 45), chronic kidney disease (CKD) with diabetes mellitus (DM) under renal dialysis (n = 51), posttransplantation status (n = 6), and neurologic diseases, such as stroke and dementia, with bedridden status and multiple comorbidities (n = 87). We categorized these patients as an immunocompromised group in this study. Forty-six patients were treated for urinary stones, and 16 patients were treated for urinary obstruction. Pediatric patients accounted for 215 (17.5%) of the total cohort. Among the included patients, 12 patients were treated in the hospital for more than 10 days during the sample collection period (median hospital admission, 43 days; range, 11 to 563 days), and ≥4 urine culture samples were collected for this study. We investigated changes in the results of the sequential samples from these patients. These patients had the following medical conditions: (i) elderly bed-ridden patients with neurologic diseases, paraplegia, and multiple comorbidities (case 1, case 2, case 3, case 8, case 10, and case 11); (ii) patients with malignancies (case 4, case 5, and case 7), including bladder cancer (case 6); and (iii) patients with ureter stone and multiple additional comorbidities, including DM and CKD (case 9 and case 12). This study complied with the Declaration of Helsinki, and the study protocol was reviewed and approved by the Institutional Review Board of Chungnam National University Hospital (CNUH 2016-02-026).
UF-5000 analysis.
The bacterial mode and cell count mode tests of the UF-5000 were performed according to the manufacturer's instructions. The samples were directly aspirated into the instrument without any preparation. Bacterial counts were measured via flow cytometric analysis after nuclei acid staining and analysis of fluorescence and the forward and side scatter. Bacteria were flagged as being Gram-negative, Gram-positive, or mixed infection (“Gram Positive?” [GP], “Gram Negative?” [GN], “Gram Pos/Neg?” [GP/GN]) when the bacterial counts were >100/μl. When classification was not possible, samples were flagged as “unclassifiable.” Other data generated by the UF-5000, including the RBC count, WBC count, epithelial cell count, and yeast-like cell count, were also analyzed.
Urine culture and identification of bacteria.
Urine was inoculated and streaked using an automated urine streaking system, Previ Isola (bioMérieux, Marcy l'Etoile, France). According to the manufacturer's instructions, 10 μl of urine was inoculated and streaked onto 5% sheep blood agar plates (bioMérieux) and MacConkey agar plates (bioMérieux). When streaking failure was noticed by systemic error messages or visual inspection of the agar plates, restreaking was immediately performed by Previ Isola. After the automated application of urine by the Previ Isola system, the agar plates were incubated at 37°C in 5% CO2 for 18 to 24 h. Bacterial colonies were then counted, and the amount of bacterial growth was assessed as no growth, 103 CFU/ml, 103 to 104 CFU/ml, 104 to 105 CFU/ml, or ≥105 CFU/ml. When the urine bacterial count was >103 CFU/ml, the bacteria underwent identification using the Vitek 2 system (bioMérieux), and susceptibility testing was performed using the Vitek 2 Gram-negative and Gram-positive antibiotic susceptibility testing card.
Statistical analysis.
The results of the bacterial counts and Gram-positive and Gram-negative discrimination of the UF-5000 were compared to the results of urine culture. To calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the UF-5000 results, different definitions of negative urine samples were used according to the bacterial colony count levels as follows: (i) a negative growth; (ii) bacterial growth ≤ 103 CFU/ml; and (iii) bacterial growth <105 CFU/ml. Data were compared by using Mann-Whitney U test and Kruskal-Wallis analysis for quantitative variables and the χ2 test for categorical variables. Tukey's correction for multiple testing was used to compare two variables. Receiver operating characteristic (ROC) curve analyses were performed, and the area under the curve (AUC) was used to assess the performance of the UF-5000 according to various cutoffs of negative urine cultures. SPSS (version 20.0; SPSS, Inc., Chicago, IL) was used for these analyses. The level of significance was set at P < 0.05.
RESULTS
Positive urine cultures and UF-5000 bacterial counts.
In total, 878 of 1,430 total urine cultures (61.4%) produced growth of ≥103 CFU/ml bacteria, 383 samples (26.8%) presented growth of ≤103 CFU/ml bacteria, 159 samples (11.1%) presented 103 to 104 CFU/ml, and 336 samples (23.5%) presented ≥105 CFU/ml bacterial growth. Of the 878 cultures with bacterial growth, 309 cultures (35.2%) presented only growth of one or more Gram-negative bacteria (282 samples with one bacterial species, 26 samples with two bacterial species, and 1 sample with three bacterial species), 517 cultures (58.9%) presented only growth of one or more Gram-positive bacteria (488 samples with one bacterial species and 29 species with two bacterial species), 52 cultures (5.9%) presented mixed Gram-negative and Gram-positive bacterial growth, 6 presented predominantly Gram-negative bacterial growth with ≥2-log differences in bacterial counts, 14 presented predominantly Gram-positive bacterial growth, and 32 presented equivalent Gram-negative and Gram-positive bacterial growth. In addition, among the no-bacterial-growth samples, 41 presented growth of Candida species.
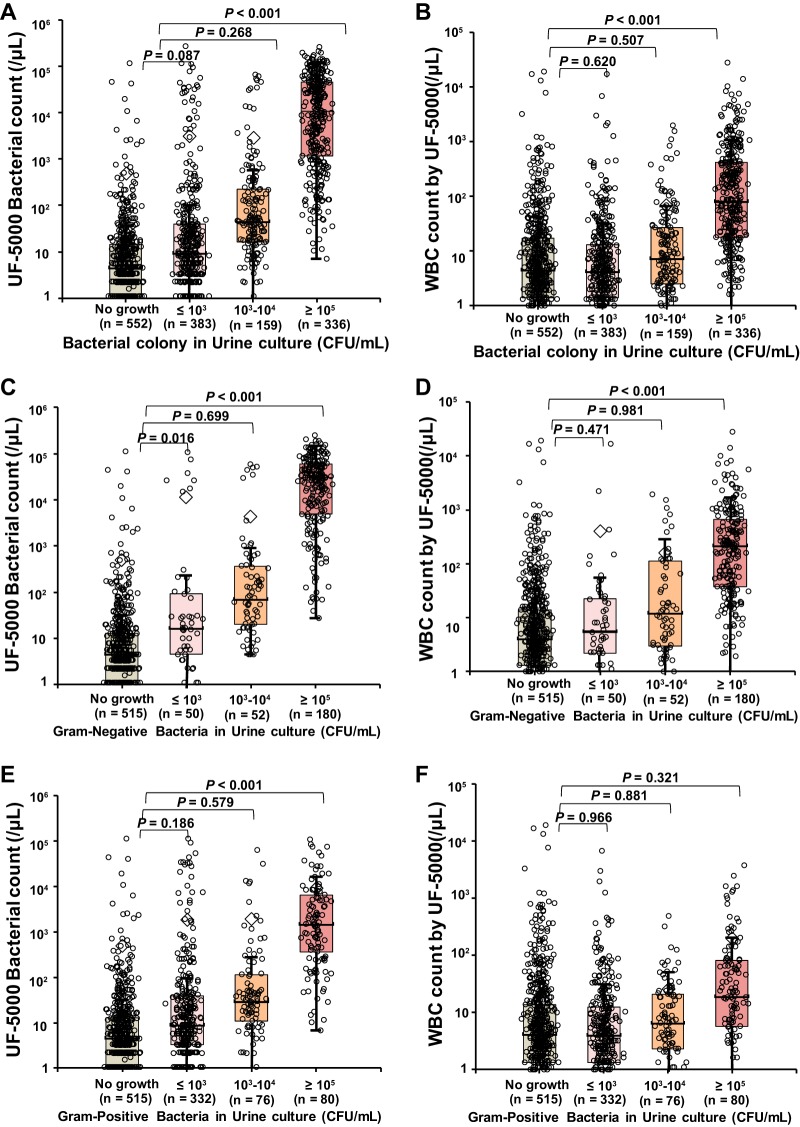
The distribution of bacterial counts and WBC counts by the UF-5000 according to the colony counts is presented in Fig. 1. The median bacterial count detected by the UF-5000 was 17.4 bacteria/μl (range, 0 to 261,967.5 bacteria/μl), distributed as follows when the samples were classified according to bacterial counts in urine culture: median 4.6 bacteria/μl (range, 0 to 115,579.4 bacteria/μl) in the no-growth group; median 9.3 bacteria/μl (range, 0 to 261,967.5 bacteria/μl) in the ≤103 CFU/ml group; median 44.0 bacteria/μl (range, 0 to 65,919.5 bacteria/μl) in the 103 to 104 CFU/ml group; and median 10,779.8 bacteria/μl (range, 0 to 250,978.6 bacteria/μl) in the ≥105 CFU/ml group. The bacterial counts in the samples with bacterial growth ≥105 CFU/ml were significantly higher than the bacterial counts in the samples with no growth (P < 0.001). The median WBC count by UF-5000 was 7.5 WBCs/μl (range, 0 to 27,511.2 WBCs/μl), distributed as follows when the samples were classified according to the bacterial counts in urine culture: median 4.5 WBCs/μl (range, 0 to 18,934.2 WBCs/μl) in the no-growth group; median 4.2 WBCs/μl (range, 0 to 16,765.4 WBCs/μl) in the ≤103 CFU/ml group; median 7.3 WBCs/μl (range, 0 to 1,955.8 WBCs/μl) in the 103 to 104 CFU/ml group; and median 81.0 WBCs/μl (range, 0 to 27,511.2 WBCs/μl) in the ≥105 CFU/ml group. The WBC counts in the samples with bacterial growth ≥105 CFU/ml were significantly higher than the bacterial counts in the samples with no growth (P < 0.001).
FIG 1.
Distribution of bacterial counts and WBC counts measured by the UF-5000 according to the bacterial counts in urine cultures among total samples (A and B), samples with Gram-negative bacteria or no growth (C and D), and samples with Gram-positive bacteria or no growth (E and F).
When the 282 samples with Gram-negative bacterial growth were separately analyzed, excluding polymicrobial growth, and the largest proportion of samples (180 samples, 63.8%) presented high bacterial growth of ≥105 CFU/ml. The bacterial counts and WBC counts by the UF-5000 in samples with Gram-negative bacterial growth ≥105 CFU/ml were significantly higher than those in samples with no growth (median, 29,686.3 bacteria/μl versus 4.5 bacteria/μl, P < 0.001; median, 218.1 WBCs/μl versus 4.1 WBCs/μl, P < 0.001) (Fig. 1C and D). When the 488 samples with Gram-positive bacterial growth were separately analyzed excluding polymicrobial growth, most samples (332 samples, 68.0%) presented low bacterial growth of ≤103 CFU/ml. The bacterial counts by the UF-5000 in samples with Gram-positive bacterial growth ≥105 CFU/ml were significantly higher than those in samples with no growth (median, 1,460.7 bacteria/μl versus 4.5 bacteria/μl, P < 0.001); however, the WBC counts in samples with Gram-positive bacterial growth ≥105 CFU/ml were not significantly higher (median, 19.1 bacteria/μl versus 4.1 bacteria/μl, P = 0.321) (Fig. 1E and F). The lower WBC counts in Gram-positive bacteria suggested that many Gram-positive bacteria were contaminants. There were no significant differences in Gram-negative and Gram-positive bacterial counts between ≤103 CFU/ml and 103 to 104 CFU/ml bacterial growth samples.
Bacterial species and the bacterial counts.
E. coli was the most frequently identified Gram-negative bacterium (231/878, 26.3%). Klebsiella pneumoniae was the second most frequently identified species (58/878, 6.6%), followed by Pseudomonas aeruginosa (36/878, 4.1%). Proteus spp., Citrobacter spp., and Enterobacter spp., which were identified in >1% of cultured bacterial species (Table 1). Among the Gram-positive bacteria, Staphylococcus spp. were most frequently identified (296/878, 33.7%); Staphylococcus epidermidis was the most frequent (243/878, 27.7%), followed by Staphylococcus haemolyticus (28/878, 3.2%) and Staphylococcus aureus (11/878, 1.3%). Staphylococcus saprophyticus was not identified. Enterococcus spp. were identified in 14.1% (124/887) of positive cultures, and Streptococcus spp. were identified in 14.0% of cultures, in which many of the species were unidentified and reported as alpha-hemolytic streptococci (85/878, 9.7%). Most streptococci and staphylococci, other than S. aureus, were suggested to be contaminants, representing approximately 46% of samples with bacterial growth. We compared the distributions of the bacterial, WBC, and RBC counts among different bacterial species in specimens with bacterial growth ≥105 CFU/ml and a predominant growth of one bacterial species (Table 1). When the bacterial, WBC, and RBC counts of each species were compared to the bacterial counts of E. coli, the P. aeruginosa specimens presented significantly lower bacterial counts and significantly higher RBC counts (P = 0.001 and P = 0.007, respectively). Enterococcus and Streptococcus spp. presented significantly lower bacterial counts (P < 0.001 and P = 0.004). These results indicated that a substantial proportion of P. aeruginosa bacteria, as well as many Gram-positive bacteria, were contaminating bacteria. In the samples with Candida spp., the bacterial counts were slightly elevated, and the WBC and RBC counts were as high as those for the samples with bacterial growth.
TABLE 1.
Bacterial, leukocyte, and erythrocyte counts in urine specimens as measured by the UF-5000a
| Organism | No. of samples (%) | UF-5000 results for nonmixed cultures with bacterial growth ≥ 105 CFU/ml |
|||
|---|---|---|---|---|---|
| No. of nonmixed cultures with CFU ≥ 105/ml (%) | Median count/μl (range) |
||||
| Bacteria | WBCs | RBCs | |||
| Escherichia coli | 231 (26.3) | 115 (13.1) | 39,073.2 (19,601.4–71,559.0) | 258.2 (42.3–921.6) | 9.1 (2.5–33.3) |
| Klebsiella spp. | 61 (6.9) | 31 (3.5) | 19,335.4 (2,242.6–61,058.4) | 202.6 (31.0–391.1) | 6.8 (2.9–70.7) |
| Pseudomonas aeruginosa | 36 (4.1) | 15 (1.7) | 1,767.2 (528.8–12,989.5)* | 111.9 (15.1–412.7) | 24.7 (3.2–925.2)* |
| Proteus spp. | 16 (1.8) | 9 (1.0) | 19,788.5 (4,901.5–45,281.0) | 54.1 (10.5–62.0) | 14.1 (5.4–24.6) |
| Citrobacter spp. | 14 (1.6) | 3 (0.3) | 3,059.1 (130.4–3,476.2) | 234.4 (17.7–252.9) | 12.8 (1.9–14.4) |
| Enterobacter spp. | 13 (1.5) | 3 (0.3) | 13,496.3 (648.7–172,850.4) | 1,101.2 (26.1–2,525.5) | 10.2 (1.8–368.1) |
| Acinetobacter spp. | 7 (0.8) | 1 (0.1) | 22,803.6 | 10.7 | 244.9 |
| Morganella morganii | 6 (0.7) | 1 (0.1) | 17,218.5 | 169.5 | 1.8 |
| Serratia marcescens. | 3 (0.3) | 2 (0.2) | 20,399.6 (4,983.8–35,815.4) | 167.0 (125.1–208.9) | 6,108.2 (443.3–11,773.1) |
| Providencia spp. | 3 (0.3) | 2 (0.2) | 3,259.2 (72.4–6,446.0) | 73.5 (59.5–87.5) | 1,927.4 (5.8–3,848.9) |
| Stenotrophomonas maltophilia | 3 (0.3) | 2 (0.2) | 149.9 (141.4–158.4) | 270.6 (250.6–290.6) | 172.0 (30.9–313.0) |
| Raoultella planticola | 1 (0.1) | 1 (0.1) | 37,782.9 | 155.0 | 2,340.1 |
| Enterococcus spp. | 124 (14.1) | 52 (5.9) | 1,677.0 (638.9–6,110.7)* | 17.0 (6.2–80.7) | 13.2 (3.9–51.2) |
| Staphylococcus spp. | 296 (33.7) | 15 (1.7) | 625.5 (250.0–16,561.7) | 26.9 (4.0–146.1) | 20.5 (4.8–115.9) |
| Streptococcus spp. | 123 (14.0) | 11 (1.3) | 50.0 (15.1–474.1)* | 12.9 (3.2–22.6) | 4.5 (1.9–32.6) |
| Diphtheroid rod | 52 (5.9) | 9 (1.0) | 3,741.8 (226.3–5,806.2) | 89.6 (48.3–175.1) | 32.9 (10.8–74.6) |
| Candida spp. | 45 (5.1) | 30 (3.4) | 33.8 (6.9–163.0)* | 85.6 (28.9–321.5) | 183.4 (62.0–2,255.4) |
| Culture negative | 515 (58.7) | NA | 4.5 (1.1–12.8) | 4.1 (1.3–13.7) | 5.7 (1.7–38.9) |
NA, not assessed. Culture-negative samples included cultures with no bacterial growth, and the UF-5000 results for bacterial, WBC, and RBC counts are presented from the samples with no bacterial growth. Percentages were calculated from a total of 878 samples with positive bacterial growth. *, P < 0.05 compared to the results of E. coli after Tukey's correction for multiple testing.
Determining cutoffs for urinary bacterial infection and negative urine culture.
We evaluated the sensitivities and specificities of UF-5000 bacterial counts in detecting bacterial growth in urine cultures at different colony count levels. In the evaluation, samples with polymicrobial growth were excluded. For samples with ≥105 CFU/ml, the UF-5000 bacterial count presented high AUC values (0.952) in the ROC analysis, and the UF-5000 WBC count presented a lower AUC (0.839) (Fig. 2). We evaluated different cutoff bacterial counts according to the different sensitivities for the diagnosis of positive urine cultures with ≥105 CFU/ml (Table 2). We chose bacterial count cutoffs at sensitivities of 90%, 95%, and 99%. With a cutoff bacterial count ≥169 bacteria/μl, the sensitivity and specificity were 90 and 89%, respectively, and with a cutoff ≥71 bacteria/μl, the sensitivity and specificity were 95 and 84%, respectively. With a cutoff ≥15 bacteria/μl, the sensitivity was high at 99%; however, the specificity was 67%. When bacterial count cutoffs with high PPV for predicting ≥105 CFU/ml bacterial growth were investigated, using a cutoff ≥746 bacteria/μl, the PPV was 76.8%. At an even higher bacterial count, with 50,000 bacteria/μl, the PPV of predicting ≥105 CFU/ml bacterial growth was 87.8%.
FIG 2.
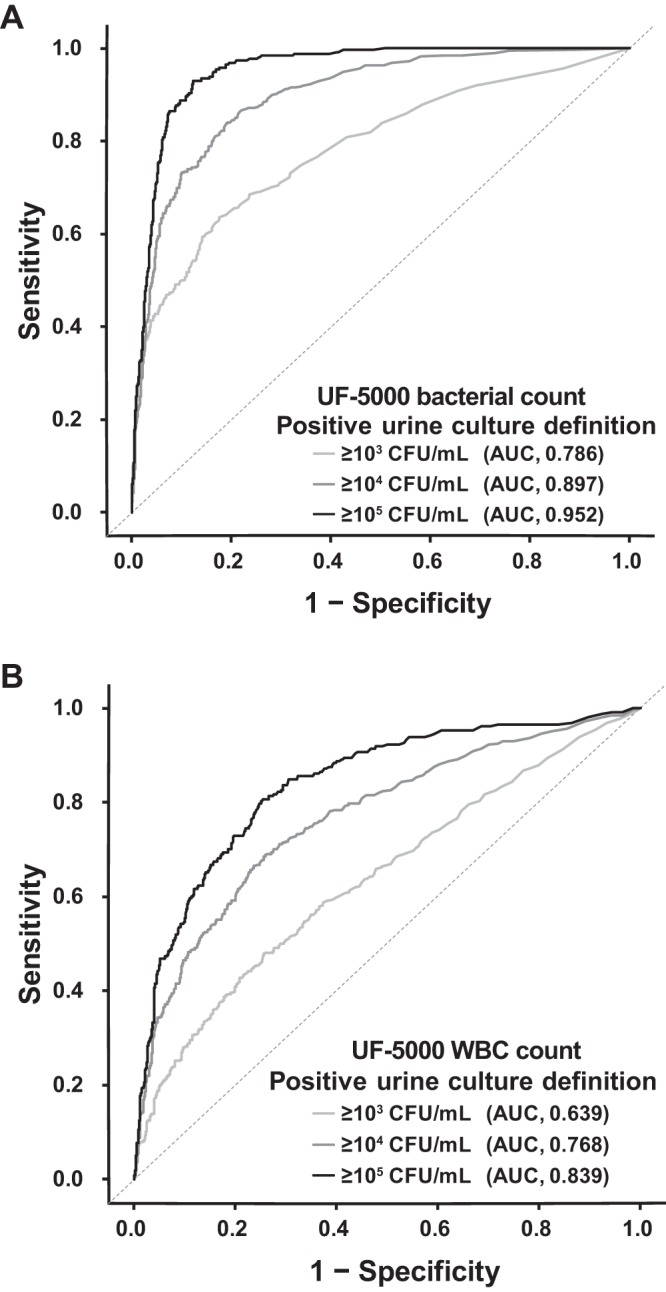
ROC analysis to detect positive urine cultures according to different levels of bacterial colony counts determined by a UF-5000 bacterial count (A) and a WBC count (B).
TABLE 2.
Evaluation of the diagnostic performance of the Sysmex UF-5000 in detecting blood cultures positive for bacterial growth according to different thresholds
| Various criteria for UF-5000 cutoffs and other parametersa | Sensitivities for detection of ≥105 CFU/ml of bacterial culture at various UF-5000 cutoff levels (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 80 | 85 | 88 | 90 | 91 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | |
| UF-5000 bacterial count only | ||||||||||||
| Bacterial count cutoff (no./μl) | 746 | 449 | 255 | 169 | 133 | 122 | 72 | 71 | 50 | 41 | 28 | 15 |
| Specificity (%) | 93.9 | 92.7 | 90.5 | 88.8 | 87.9 | 87.6 | 83.7 | 83.6 | 81.1 | 79.2 | 74.4 | 67.4 |
| PPV for ≥105 CFU/ml (%) | 76.8 | 74.8 | 70.2 | 67.0 | 65.7 | 65.6 | 60.9 | 59.5 | 56.2 | 54.2 | 49.3 | 40.7 |
| Samples below UF-5000 cutoff (%) | 78.8 | 76.8 | 74.6 | 72.8 | 71.9 | 71.3 | 68.0 | 67.7 | 65.5 | 63.8 | 59.8 | 50.9 |
| NPV for no growth (%)* | 49.8 | 50.8 | 51.7 | 52.6 | 52.8 | 53.1 | 54.0 | 54.1 | 54.6 | 55.6 | 57.8 | 61.1 |
| NPV for <104 CFU/ml (%)* | 84.1 | 85.5 | 86.4 | 87.2 | 87.8 | 88.3 | 88.9 | 89.2 | 90.0 | 91.0 | 92.5 | 94.8 |
| NPV for <105 CFU/ml (%)* | 95.0 | 96.3 | 96.8 | 97.2 | 97.5 | 98.0 | 98.2 | 98.5 | 98.7 | 99.0 | 99.4 | 99.5 |
| UF-5000 bacterial count or >15 WBCs/μl | ||||||||||||
| Bacterial count cutoff (no./μl) | 52,895 | 7,678 | 2,989 | 1,659 | 1,176 | 735 | 601 | 422 | 264 | 59 | 45 | 28 |
| Specificity (%) | 74.5 | 72.8 | 72.3 | 71.8 | 71.6 | 71.2 | 70.9 | 70.9 | 70.2 | 66.4 | 65.2 | 62.0 |
| PPV for ≥ 105 CFU/ml (%) | 44.3 | 44.4 | 44.9 | 44.7 | 44.9 | 45.2 | 45.1 | 45.4 | 45.0 | 42.4 | 41.7 | 39.8 |
| Samples below UF-5000 cutoff (%) | 63.5 | 60.9 | 59.9 | 59.3 | 58.9 | 58.1 | 57.7 | 57.5 | 56.8 | 53.5 | 52.4 | 49.6 |
| NPV for no growth (%) | 48.4 | 50.2 | 50.9 | 51.1 | 51.4 | 52.1 | 52.2 | 52.4 | 52.9 | 55.1 | 55.9 | 58.2 |
| NPV for <104 CFU/ml (%)* | 84.0 | 86.0 | 86.8 | 87.1 | 87.6 | 88.5 | 88.8 | 89.2 | 89.6 | 91.0 | 92.1 | 93.6 |
| NPV for <105 CFU/ml (%)* | 93.6 | 95.3 | 96.2 | 96.6 | 97.0 | 97.7 | 98.0 | 98.4 | 98.6 | 99.0 | 99.3 | 99.7 |
| UF-5000 bacterial count or >50 WBCs/μl | ||||||||||||
| Bacterial count cutoff (no./μl) | 4,138 | 1,387 | 880 | 648 | 509 | 400 | 205 | 126 | 59 | 49 | 40 | 15 |
| Specificity (%) | 85.0 | 84.2 | 83.8 | 83.2 | 83.0 | 82.8 | 80.8 | 79.7 | 76.9 | 75.7 | 74.2 | 61.6 |
| PPV for ≥105 CFU/ml (%) | 57.3 | 57.6 | 58.0 | 57.6 | 57.6 | 57.9 | 55.4 | 54.3 | 51.3 | 50.4 | 49.1 | 39.5 |
| Samples below UF-5000 cutoff (%) | 71.9 | 70.3 | 69.3 | 68.4 | 68.1 | 67.5 | 65.6 | 64.6 | 62.1 | 60.9 | 59.6 | 49.3 |
| NPV for no growth (%) | 49.2 | 50.1 | 50.8 | 51.1 | 51.3 | 51.8 | 53.0 | 53.3 | 54.5 | 55.0 | 55.7 | 60.4 |
| NPV for <104 CFU/ml (%)* | 84.1 | 85.4 | 86.5 | 87.0 | 87.3 | 87.9 | 88.7 | 89.2 | 90.2 | 91.2 | 91.8 | 94.8 |
| NPV for <105 CFU/ml (%)* | 94.3 | 95.6 | 96.5 | 97.0 | 97.3 | 97.9 | 98.2 | 98.4 | 98.8 | 99.1 | 99.4 | 99.5 |
*, The percentage of specimens with no bacterial growth, <103 CFU/ml bacterial growth, and <104 CFU/ml bacterial growth among samples below the UF-5000 cutoff for each sensitivity level.
We evaluated cutoffs of the UF-5000 bacterial counts for reducing unnecessary urine cultures. With a cutoff <15 bacteria/μl, 50.9% of samples were classified as below the cutoff and, of these, 61.1% of the samples presented no growth, and 94.8 and 99.5% of the samples presented less significant culture results, i.e., <104 CFU/ml and <105 CFU/ml bacterial growth, respectively (Table 2). When the characteristics of the discrepant samples were investigated, three samples with <15 bacteria/μl and ≥105 CFU/ml bacterial growth demonstrated growth of insignificant staphylococci or streptococci; therefore, results for samples from these patients were likely not false-negative results. Among the 31 patients with <15 bacteria/μl and 103 to 104 CFU/ml bacterial growth, 13 patients (41.9%) had insignificant staphylococcal or streptococcal growth, 9 samples (29.0%) demonstrated growth of enterococci, and 9 samples (29.0%) demonstrated Gram-negative bacteria. Among the samples with 103 to 104 CFU/ml Gram-negative bacterial growth, one sample was collected after antibiotic treatment, and five patients had preexisting urological problems. Otherwise, of the samples with 103 to 104 CFU/ml bacterial growth, 97 samples had ≥15 bacteria/μl, and among them, 18/34 (52.9%) samples with ≥169 bacteria/μl demonstrated Gram-negative bacterial growth, and 25/63 (39.7%) samples with 15 to 168 bacteria/μl demonstrated Gram-negative bacterial growth. A substantial number of samples with 103 to 104 CFU/ml bacterial growth were from pediatric patients (40/128, 31.3%).
We also analyzed the sensitivity of bacterial counts analyzed with WBC counts, using arbitrary WBC count cutoffs of 15 and 50 WBCs/μl. As expected, when cutoffs of ≥59 bacteria/μl or >15 WBCs/μl were used, the sensitivity was higher (97%); however, the specificity was lower (66.4%) compared to similar bacterial count level cutoffs without WBC counts (sensitivity, 97%; specificity, 79.2%).
We evaluated the sensitivity and specificity at different cutoffs in samples from a subgroup of 399 immunocompromised patients. The bacterial count cutoffs at sensitivities of 90, 95, and 99% were ≥267 bacteria/μl (sensitivity, 90%; specificity, 93%), ≥71 bacteria/μl, (sensitivity, 95%; specificity, 85%), and ≥27 bacteria/μl (sensitivity, 99%; specificity, 77%), respectively. Using a cutoff of 15 bacteria/μl, 62.9% of the samples showed no growth, and 94.1 and 99.5% of the samples showed bacterial growth with <104 and <105 CFU/ml, respectively. These results were not significantly different compared to the results obtained from the total patient group.
Discriminating Gram-negative and Gram-positive bacteria.
We evaluated the performance of discrimination flagging of Gram-negative and Gram-positive bacteria by the UF-5000 versus urine culture results. Of the total specimens, 71.5% of urine cultures with growth of ≥103 CFU/ml Gram-negative bacteria were flagged as GN or GP/GN, and among samples with ≥105 CFU/ml Gram-negative bacteria, 92.6% of samples were flagged as GN or GP/GN (Table 3). Among the total specimens, 16.8% of urine cultures with growth of ≥103 CFU/ml Gram-positive bacteria were flagged as GP or GP/GN, and among samples with ≥105 CFU/ml Gram-positive bacteria, 81.3% of samples were flagged as GP or GP/GN. Among mixed cultures with ≥105 CFU/ml Gram-negative and Gram-positive bacteria, 41.7% of samples were flagged as GP/GN, and the distribution of flags varied according to the predominant bacteria (Table 3). More than half of samples with ≤104 CFU/ml bacterial growth presented “unclassified” flags (Table 3). We calculated the sensitivity and specificity of the total GN flag, including the GP/GN flag for samples that grew ≥105 CFU/ml Gram-negative bacteria excluding polymicrobial samples (Table 4). The sensitivity of the total GN flag, including the GP/GN flag was 91.7% and the specificity was 90.0%. Among samples with ≥105 CFU/ml Gram-negative bacteria excluding polymicrobial samples, the median UF-5000 bacterial count for true-positive samples was 30,496.8 bacteria/μl (interquartile range [IQR], 8,443.8 to 60,228.8 bacteria/μl), which was significantly higher than that for false-negative samples (median, 333.1 bacteria/μl; IQR, 79.1 to 3,346.8 bacteria/μl; P < 0.001). For the samples that grew ≥105 CFU/ml Gram-positive bacteria excluding polymicrobial samples, the sensitivity and specificity of the GP flag, including the GP/GN flag, were 81.3 and 80.0%, respectively (Table 4). Similarly, the UF-5000 bacterial counts were significantly higher for the true-positive samples (median, 2,373.5 bacteria/μl; IQR, 625.5 to 9,975.8 bacteria/μl) than for the false-negative samples (median, 378.4 bacteria/μl; IQR, 47.7 to 8,709.0 bacteria/μl; P = 0.006). Among the Gram-negative bacterial species, P. aeruginosa, Proteus spp., A. baumannii, Stenotrophomonas maltophilia, and Providencia spp. presented >10% discordant results (Table 4). Among the Gram-positive bacterial species, Streptococcus spp. presented significantly high false-negative results, in addition to diphtheroid rods (Table 4).
TABLE 3.
Discrimination of Gram-negative and Gram-positive bacteria by the UF-5000 compared to urine culture resultsa
| Parameters including UF-5000 flags and levels of bacterial colony counts | No. (%) of specimens with Gram-negative, Gram-positive, or mixed bacterial growth in urine cultures |
|||||
|---|---|---|---|---|---|---|
| Gram-negative bacterial growth only | Gram positive bacterial growth only | Positive bacterial cultures with mixed Gram-negative and Gram-positive growth |
||||
| Gram-negative and -positive mixed culture total | Gram-negative predominant | Gram positive predominant | Mixed culture with an equal amt | |||
| Total specimens | ||||||
| Total | 309 | 517 | 52 | 6 | 14 | 32 |
| GP/GN flag | 37 (12.0) | 15 (2.9) | 16 (30.8) | 2 (33.3) | 5 (35.7) | 9 (28.1) |
| GN flag | 184 (59.5) | 24 (4.6) | 16 (30.8) | 4 (66.7) | 1 (7.1) | 11 (34.4) |
| GN flag total including GP/GN flag | 221 (71.5) | 39 (7.5) | 32 (61.5) | 6 (100.0) | 6 (42.9) | 20 (62.5) |
| GP flag | 15 (4.9) | 120 (23.2) | 11 (21.2) | 0 | 7 (50.0) | 4 (12.5) |
| GP flag total including GP/GN flag | 52 (16.8) | 135 (26.1) | 27 (51.9) | 2 (33.3) | 12 (85.7) | 13 (40.6) |
| Unclassified | 73 (23.6) | 358 (69.2) | 9 (17.3) | 0 | 1 (7.1) | 8 (25.0) |
| ≥105 CFU/ml | ||||||
| Total | 204 | 96 | 36 | 5 | 13 | 18 |
| GP/GN flag | 34 (16.7) | 6 (6.3) | 15 (41.7) | 2 (40.0) | 5 (38.5) | 8 (44.4) |
| GN flag | 155 (76.0) | 4 (4.2) | 11 (30.6) | 3 (60.0) | 1 (7.7) | 7 (38.9) |
| GN flag total including GP/GN flag | 189 (92.6) | 10 (10.4) | 26 (72.2) | 5 (100.0) | 6 (46.2) | 15 (83.3) |
| GP flag | 9 (4.4) | 72 (75.0) | 8 (22.2) | 0 | 6 (46.2) | 2 (11.1) |
| GP flag total including GP/GN flag | 43 (21.1) | 78 (81.3) | 23 (63.9) | 2 (40.0) | 11 (84.6) | 10 (55.6) |
| Unclassified | 6 (2.9) | 14 (14.6) | 2 (5.6) | 0 | 1 (7.7) | 1 (5.6) |
| ≥104–105 CFU/ml | ||||||
| Total | 54 | 89 | 16 | 1 | 1 | 14 |
| GP/GN flag | 2 (3.7) | 2 (2.2) | 1 (6.3) | 0 | 0 | 1 (7.1) |
| GN flag | 20 (37.0) | 5 (5.6) | 5 (31.3) | 1 (100) | 0 | 4 (28.6) |
| GN flag total including GP/GN flag | 22 (40.7) | 7 (7.9) | 6 (37.5) | 1 (100) | 0 | 5 (35.7) |
| GP flag | 3 (5.6) | 17 (19.1) | 3 (18.8) | 0 | 1 (100) | 2 (14.3) |
| GP flag total including GP/GN flag | 5 (9.3) | 19 (21.3) | 4 (25.0) | 0 | 1 (100) | 3 (21.4) |
| Unclassified | 29 (53.7) | 65 (73.0) | 7 (43.8) | 0 | 0 | 7 (50.0) |
| ≤103 CFU/ml | ||||||
| Total | 51 | 332 | 0 | 0 | 0 | 0 |
| GP/GN flag | 1 (2.0) | 7 (2.1) | NA | NA | NA | NA |
| GN flag | 9 (17.6) | 15 (4.5) | NA | NA | NA | NA |
| GN flag total including GP/GN flag | 10 (19.6) | 22 (6.6) | NA | NA | NA | NA |
| GP flag | 3 (5.9) | 31 (9.3) | NA | NA | NA | NA |
| GP flag total, including GP/GN flag | 4 (7.8) | 38 (11.4) | NA | NA | NA | NA |
| Unclassified | 38 (74.5) | 279 (84.0) | NA | NA | NA | NA |
GN, “Gram Negative?” flag from UF-5000; GP, “Gram Positive?” flag from UF-5000; GP/GN, “Gram Pos/Neg?” flag from UF-5000; NA, not assessed. GN total included both samples with a GN flag and samples with a GP/GN flag, and GP total included both samples with a GP flag and samples with a GP/GN flag.
TABLE 4.
Sensitivity and specificity of Gram-negative and Gram-positive flagsa
| Performance of UF-5000 flags in different groups of samples | No. of specimens |
% |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | Sen | Spe | PPV | NPV | Con | |
| Total specimens, excluding polymicrobial specimens (n = 1,285) | |||||||||
| GN flag total, including the GP/GN flag | 196 | 949 | 54 | 86 | 69.5 | 94.6 | 78.4 | 91.7 | 89.1 |
| GP flag total, including the GP/GN flag | 118 | 736 | 61 | 370 | 24.2 | 92.3 | 65.9 | 66.5 | 66.5 |
| ≥105 CFU/ml monobacterial samples (n = 260) | |||||||||
| GN flag total, including the GP/GN flag | 165 | 72 | 8 | 15 | 91.7 | 90.0 | 95.4 | 82.8 | 81.2 |
| GP flag total, including the GP/GN flag | 65 | 144 | 36 | 15 | 81.3 | 80.0 | 64.4 | 90.6 | 80.4 |
| ≥105 CFU/ml with monobacterial sample* | |||||||||
| Escherichia coli (n = 115) | |||||||||
| GN flag total, including GP/GN flag | 109 | 0 | 0 | 6 | 94.8 | NA | NA | NA | NA |
| GP flag only | 0 | 106 | 5 | 0 | NA | 95.7 | NA | NA | NA |
| Klebsiella spp. (n = 31) | 1 | ||||||||
| GN flag total, including GP/GN flag | 30 | 0 | 0 | 1 | 96.8 | NA | NA | NA | NA |
| GP flag only | 0 | 31 | 0 | 0 | NA | 100 | NA | NA | NA |
| Pseudomonas aeruginosa (n = 15) | |||||||||
| GN flag total, including GP/GN flag | 13 | 0 | 0 | 2 | 86.7 | NA | NA | NA | NA |
| GP flag only | 0 | 15 | 0 | 0 | NA | 100 | NA | NA | NA |
| Proteus spp. (n = 9) | |||||||||
| GN flag total, including GP/GN flag | 7 | 0 | 0 | 2 | 77.8 | NA | NA | NA | NA |
| GP flag only | 0 | 8 | 1 | 0 | NA | 88.9 | NA | NA | NA |
| Citrobacter spp. (n = 3) | |||||||||
| GN flag total, including GP/GN flag | 3 | 0 | 0 | 0 | 100 | NA | NA | NA | NA |
| GP flag only | 0 | 3 | 0 | 0 | NA | 100 | NA | NA | NA |
| Enterobacter spp. (n = 3) | |||||||||
| GN flag total, including GP/GN flag | 3 | 0 | 0 | 0 | 100 | NA | NA | NA | NA |
| GP flag only | 0 | 3 | 0 | 0 | NA | 100 | NA | NA | NA |
| Other Gram-negative bacteria (n = 9)b | |||||||||
| GN flag total including GP/GN flag | 5 | 0 | 0 | 4 | 55.6 | NA | NA | NA | NA |
| GP flag only | 0 | 6 | 3 | 0 | NA | 66.7 | NA | NA | NA |
| Enterococcus spp. (n = 52) | |||||||||
| GP flag total, including GP/GN flag | 48 | 0 | 0 | 4 | 92.3 | NA | NA | NA | NA |
| GN flag only | 0 | 50 | 2 | 0 | NA | 96.2 | NA | NA | NA |
| Staphylococcus spp. (n = 15) | |||||||||
| GP flag total, including GP/GN flag | 12 | 0 | 0 | 3 | 80.0 | NA | NA | NA | NA |
| GN flag only | 0 | 14 | 1 | 0 | NA | 93.3 | NA | NA | NA |
| Streptococcus spp. (n = 11 | |||||||||
| GP flag total, including GP/GN flag | 4 | 0 | 0 | 7 | 36.4 | NA | NA | NA | NA |
| GN flag only | 0 | 11 | 0 | 0 | NA | 100 | NA | NA | NA |
| Diphtheroid rod (n = 9) | |||||||||
| GP flag total, including GP/GN flag | 6 | 0 | 0 | 3 | 66.7 | NA | NA | NA | NA |
| GN flag only | 0 | 8 | 1 | 0 | NA | 88.9 | NA | NA | NA |
*, Gram-negative bacteria were evaluated for all “Gram Negative?” (GN) flags, including the “Gram Pos/Neg?” (GP/GN) flag, and Gram-positive bacteria were evaluated for all “Gram Positive?” (GP) flags, including the GP/GN flag. Con, concordance rate; FN, false negative; FP, false positive; GN, “Gram Negative?” flag from UF-5000, GP, “Gram Positive?” flag from UF-5000; GP/GN, “Gram Pos/Neg?” flag from UF-5000; NA, not assessed; Sen, sensitivity; Spe, specificity; TN, true negative; TP, true positive.
Other Gram-negative bacteria included Stenotrophomonas maltophilia (n = 2), Providencia spp. (n = 2), Serratia marcescens (n = 2), Acinetobacter baumannii (n = 1), Morganella morganii (n = 1), and Raoultella planticola (n = 1). Of these, a sample with Acinetobacter baumannii, a sample with Stenotrophomonas maltophilia, and a sample with Providencia rettgeri were flagged as GP, and a sample with Providencia stuartii was flagged as unclassified.
Among the 30 urine culture samples showing growth of ≥105 Candida spp., 20 (66.7%) samples had an “unclassified” flag, 8 (26.7%) samples had a GP flag, and 2 (6.6%) samples had GN flags. These samples presented with slightly higher bacterial counts than the normal samples, as shown in Table 1. Samples with Candida spp. showed significantly higher yeast-like cell counts by UF-5000 (median yeast-like cell count for Candida spp., 648.9 cells/μl; for Gram-positive bacteria, 0.7 cell/μl; for Gram-negative bacteria, 0.4 cell/μl; and for no-growth samples, 0.2 cell/μl). Therefore, among the samples with a GP flag, the comparison between the bacterial and yeast-like cell counts will be helpful for the discrimination of samples with Candida and bacterial growth.
Sequential follow-up results of UF-5000 bacterial counts.
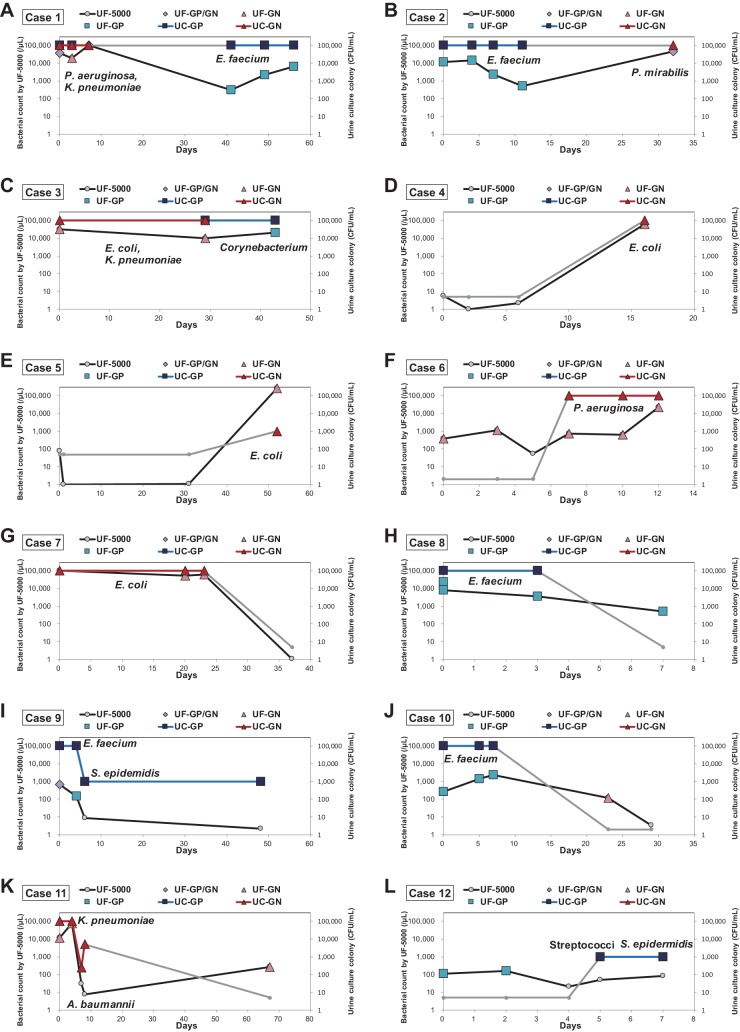
We compared the urine culture results and UF-5000 bacterial counts and flags in patients with sequential samples (Fig. 3). The number of samples followed up ranged from 4 to 6. Patients were followed up after antibiotic treatment (case 1, case 3, case 8, and case 11); they were sequentially monitored for vancomycin-resistant enterococci (VRE) (case 1, case 2, case 8, case 9, and case 10), during chemotherapy treatment (case 4, case 5, case 6, and case 7), and before and after ureterolithotomy (case 9 and case 12). Some patients presented a switch of identified bacteria in the urine culture (Fig. 3A to C), and in these patients, the UF-5000 results presented switched bacterial classification flags and a tendency toward decreased bacterial counts for previously identified species and an increase in newly occurring bacterial species. In patients who underwent VRE surveillance, the UF-5000 results presented matched results and showed decreasing or increasing patterns (Fig. 3A and B). For several patients the emergence of positive urine cultures was noted (Fig. 3D to F), and in these patients, the UF-5000 presented higher bacterial counts, although the extent of the increase varied. In cases where bacterial counts decreased in urine culture (Fig. 3G to J), the UF-5000 presented a decrease in bacterial counts; however, the initial bacterial counts and extent of the decrease did not exactly match with the urine culture colony counts in some patients. In one patient with negative or low bacterial growth in the serial urine culture (Fig. 3L), which is most likely contaminating bacteria, the UF-5000 also presented persistently low bacterial counts.
FIG 3.
Results of urine culture and UF-5000 bacterial counts in several patients (A to L) whose sequential samples were analyzed during follow-up. UF-GP, UF-5000 “Gram Positive?” flag; UF-GN, UF-5000 “Gram Negative?” flag; UF-GP/GN, UF-5000 “Gram Pos/Neg?” flag; UC-GP, urine culture with Gram-positive bacterial growth; UC-GN, urine culture with Gram-negative bacterial growth.
DISCUSSION
The rapid screening of positive urine cultures can enable more prompt decisions regarding patient management and help to reduce unnecessary urine cultures. In the present study, we investigated the Sysmex UF-5000, a new version of an automated flow cytometric urine analyzer, that was developed to improve bacterial counting via enhanced discrimination technologies and gating strategies.
Several previous studies investigated the previous version of an automated flow cytometric urine analyzer, the UF-500i or UF-1000i, for screening urine cultures (3–9). In these studies, bacterial counts were often investigated in combination with WBC counts. Jolkkonen et al. investigated both bacterial counts and WBC counts to screen out samples that were unnecessary for urine culture (3). Based on different age- and gender-specific cutoffs, 64.5% of the samples were classified as not needing culture, and 3.0% of the samples were misclassified compared to conventional culture. In this study, at a total sensitivity of 93%, the specificity was 82.3%, which was slightly lower than our results (specificity 87.6% at a sensitivity 93%, using bacterial counts only as the cutoff criterion). However, a parallel comparison was impossible because the definitions of blood cultures positive for bacterial growth were different, and the study by Jolkkonen et al. excluded samples from urologic diseases or anomalies (3). In another study using the UF-1000i, the best cutoff values were suggested to be 65 bacteria/ml and 100 WBCs/ml, with a sensitivity of 98.2% and a specificity of 62.1% (4). In our results, using a cutoff ≥28 bacteria/μl, the sensitivity and specificity were 98 and 74.4%, respectively, and using a cutoff ≥45 bacteria/μl or >15 WBCs/μl, the sensitivity and specificity were 98 and 65.2%, which was similar to previous results (4). Given the reduced specificity, combining the WBC counts in the cutoff may not substantially improve the diagnostic performance of the UF-5000. In a previous study investigating the UF-1000i cutoff for negative urine cultures and their cost-effectiveness, UF-1000i screening was not warranted due to a high percentage of false-negative results and no evidence of cost saving (10). In that study, only 20% of samples were screened at a cutoff value of 26 bacteria/μl (10). According to our results, 50.9% were screened at a cutoff of 15 bacteria/μl, with 99.5% of NPV for <105 CFU/ml bacterial growth. Therefore, our results are more promising compared to previous results for screening out negative cultures, at least for our population.
In our analysis, we provided data on different sensitivities and specificities for different cutoffs of UF-5000 bacterial count results. Clinical laboratories where negative urine cultures are screened out will consider lower cutoffs to be more appropriate, and we suggest 15 bacteria/μl. In contrast, for clinicians who wish to screen for UTIs through flow cytometric bacterial counts, higher cutoff levels with high PPV for UTI may be needed. In that regard, our results may provide basic information to facilitate the application of this instrument. However, the cutoffs suggested by this study are not representative of various clinical settings or specific patient populations, including immunocompromised patients, pediatric patients, women with acute urethral syndrome, and patients with structural abnormalities in the urinary tract. The utility of the UF-5000 screening method is dependent on the patient population. Therefore, the cutoffs suggested by this study may not be useful in other centers with different patient populations. In our study cohort, we performed a subgroup analysis to evaluate the cutoffs among immunocompromised patients. In this analysis, the diagnostic performances were not significantly different between the immunocompromised patients and total patients. However, the number of patients in the subgroup was small, diseases were heterogeneous, and the immunocompromised group was retrospectively defined. The cutoffs suggested in our patient population were proof of instrumental performance, and further optimization of the cutoff values will be necessary for specific patient populations.
In addition, several limitations of this study should be considered. This study was conducted at a single center, and considering that a high proportion of samples tested positive for streptococci and staphylococci, a considerable proportion of samples were of poor quality and inadequate for a diagnosis of UTI. A substantial number of samples showed 103 to 104 CFU/ml bacterial growth, and the proportion of samples with 103 to 104 CFU/ml growth was high for pediatric patients. This study was conducted at a tertiary hospital, and many pediatric patients had a history of antibiotic treatment before their hospital visit. These factors, along with the high rate of contamination, make it difficult to interpret the urine culture results of these patients. The bacterial count results are also difficult to interpret. Therefore, thorough patient history and clinical presentation assessments are particularly important. A urine culture should be performed to diagnose UTI in clinically suspected patients, while a bacterial count may provide supplementary information.
The UF-5000 has been developed for improved performance for the classification of Gram-negative and Gram-positive bacteria. We therefore evaluated the performance of the UF-5000 flagging system in discriminating Gram-negative and Gram-positive bacteria. Previously, the UF-1000i classified bacteria as either “rods” or “cocci/mixed,” not as Gram positive or Gram negative (8). Previous studies using the UF-1000i suggested that bacterial-classification performance was not satisfactory and not sufficient to be of clinical use (8). In another study, a novel combinatorial analysis using UF-1000i bacterial counts, WBC counts, and RBC counts was investigated for the discrimination of bacterial species (9). Therefore, the need for methods to rapidly classify urine bacteria via flow cytometry is very high; however, the classification results provided by previous automated analyzers were not satisfactory and the analyzers remain underdeveloped. Our results showed that in UTIs with ≥105 CFU/ml bacterial growth, the sensitivity and specificity were both approximately 90% for Gram-negative bacteria, with a high PPV of 95.4% using the UF-5000 GN and GP/GN flags. The sensitivity and specificity were lower (both approximately 80%) for Gram-positive bacteria. These results may reflect the differences in the characteristics of the included bacteria, as a greater proportion of Gram-negative bacteria are true pathogens. However, the proportion of Gram-positive bacteria that were contaminating or nonpathological bacteria was high. We suggest that bacteria that are pathogenic for UTI were more accurately classified with a higher bacterial count by UF-5000. When the specific bacterial species were considered, the diagnostic performance was high for the most frequently isolated E. coli and Klebsiella spp. and low for P. aeruginosa, Proteus spp., and other less frequently isolated Gram-negative bacteria. P. aeruginosa is often a colonizing bacteria (11). For Gram-positive bacteria, Enterococcus spp. showed high sensitivity and specificity; however, the sensitivity for Streptococcus spp. was much lower. Most streptococci were suggested to be contaminating or nonpathogenic bacteria. When the UF-5000 bacterial count was compared, false-negative samples for bacterial flags presented significantly lower bacterial counts compared to true positive samples. In other words, lower-count samples tended not to produce accurate bacterial classification results, which were reported as “unclassified.”
Analyses of the sequential follow-up results of patients showed generally matched bacterial count changes and bacterial classification results between the UF-5000 and bacterial culture, although the number of patients was small, and conclusive results could not be obtained. We suggest that because conventional urine culture can provide only semiquantitative results, the more rapid and quantitative results provided by UF-5000 may be useful for patient follow-up and to detect changes in bacterial species and bacterial burden.
The present study is the first to compare the results of an automated urine flow cytometry system and urine cultures using an automated bacterial inoculation system (i.e., Previ Isola). Using the automated bacterial inoculation system, the analytical variability of the procedure in the application of the exact amount of urine for semiquantitative urine culture was reduced, yielding more consistent comparisons.
In summary, UF-5000 bacterial counts demonstrated superior results for the screening of significant bacterial growth in urine cultures and for screening out negative urine cultures compared to those of previous versions, although performance might vary depending on the study population. Improved Gram-negative and Gram-positive bacterial identification was possible with UF-5000, although the concurrence rates were not high enough to replace routine culture. We suggest that in patients without any clinical symptoms of UTI and no urological problems, a bacterial count cutoff of 15 bacteria/μl might be used to screen out negative cultures. In patients with other problems and clinical suspicion for UTI, the bacterial counts and bacterial classification results may be provided as supplementary information. For more effective applications of UF-5000 bacterial counts in clinical practice, further investigations should be performed, including the optimization of cutoff values and the development of an appropriate algorithm to apply this rapid screening machine to diagnostic and treatment decision processes, possibly in combination with other testing modalities for bacterial identification and susceptibility testing, such as matrix-assisted laser desorption ionization–time of flight and molecular testing.
ACKNOWLEDGMENTS
G.C.K. designed the study, performed data management and statistical analysis, and helped write the manuscript. S.Y.K. and H.K. shared the responsibility for data management, statistical analysis, and manuscript writing. Y.P., J.K., and S.H.K. shared the responsibility for study design, data interpretation, and manuscript revision for important intellectual content. All authors read and approved the final manuscript.
REFERENCES
- 1.Foxman B. 2014. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. 2015. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolkkonen S, Paattiniemi EL, Karpanoja P, Sarkkinen H. 2010. Screening of urine samples by flow cytometry reduces the need for culture. J Clin Microbiol 48:3117–3121. doi: 10.1128/JCM.00617-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pieretti B, Brunati P, Pini B, Colzani C, Congedo P, Rocchi M, Terramocci R. 2010. Diagnosis of bacteriuria and leukocyturia by automated flow cytometry compared with urine culture. J Clin Microbiol 48:3990–3996. doi: 10.1128/JCM.00975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Zwet WC, Hessels J, Canbolat F, Deckers MM. 2010. Evaluation of the Sysmex UF-1000i® urine flow cytometer in the diagnostic work-up of suspected urinary tract infection in a Dutch general hospital. Clin Chem Lab Med 48:1765–1771. doi: 10.1515/CCLM.2010.342. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Zhang Y, Xu D, Shao W, Lu Y. 2010. Evaluation of the Sysmex UF-1000i for the diagnosis of urinary tract infection. Am J Clin Pathol 133:577–582. doi: 10.1309/AJCP1GT2JXOCQBCZ. [DOI] [PubMed] [Google Scholar]
- 7.Lee K-M, Kim YK, Lee W-K. 2012. Evaluation of an automated urine flow cytometer for screening of bacterial contamination in platelet concentrates. Laboratory Medicine Online 2:209. doi: 10.3343/lmo.2012.2.4.209. [DOI] [Google Scholar]
- 8.Geerts N, Jansz AR, Boonen KJ, Wijn RP, Koldewijn EL, Boer AK, Scharnhorst V. 2015. Urine flow cytometry can rule out urinary tract infection, but cannot identify bacterial morphologies correctly. Clin Chim Acta 448:86–90. doi: 10.1016/j.cca.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Monsen T, Ryden P. 2015. Flow cytometry analysis using Sysmex UF-1000i classifies uropathogens based on bacterial, leukocyte, and erythrocyte counts in urine specimens among patients with urinary tract infections. J Clin Microbiol 53:539–545. doi: 10.1128/JCM.01974-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broeren MA, Bahceci S, Vader HL, Arents NL. 2011. Screening for urinary tract infection with the Sysmex UF-1000i urine flow cytometer. J Clin Microbiol 49:1025–1029. doi: 10.1128/JCM.01669-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. 2009. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health 2:101–111. doi: 10.1016/j.jiph.2009.08.003. [DOI] [PubMed] [Google Scholar]