Although chronic respiratory disease and immunosuppression are risk factors for Corynebacterium species respiratory infection, data are scarce regarding this disease in lung transplantation. Our aim was to describe the clinical characteristics and outcomes of lung transplant recipients (LTR) with respiratory isolation of Corynebacterium spp.
KEYWORDS: respiratory tract infections, lung transplantation, stenting, biofilm, bacterial infections
ABSTRACT
Although chronic respiratory disease and immunosuppression are risk factors for Corynebacterium species respiratory infection, data are scarce regarding this disease in lung transplantation. Our aim was to describe the clinical characteristics and outcomes of lung transplant recipients (LTR) with respiratory isolation of Corynebacterium spp. This was a retrospective observational study performed at a referral center in Barcelona, Spain (2014 to 2016). We included all LTR in whom Corynebacterium spp. were isolated in at least one good-quality lower respiratory tract specimen. Overall, 24 of 527 (4.6%) LTR at risk during the study period were included. The main epidemiological, clinical, and microbiological data were analyzed. The most frequently isolated species were C. striatum (11/24), C. pseudodiphtheriticum (3/24), and C. amycolatum (3/24). All 19 (76%) patients who underwent bronchoscopy showed abnormalities, mainly mucosal plaques at the bronchial suture and purulent secretions. Clinical cure was achieved in 8/12 (67%) patients who fulfilled the CDC definition of lower respiratory tract infection (LRTI). To assess the clinical relevance of Corynebacterium spp., only patients with monomicrobial isolation (n = 18) were evaluated. LRTI was diagnosed in 9, and a nonsignificant association was found with a significant number of Corynebacterium sp. CFU/ml (7/9 LRTI versus 2/9 non-LRTI, P = 0.057). Persistent infection was associated with metallic bronchial stent implantation (4/4 versus 2/14, P = 0.005). The isolation of Corynebacterium spp. in respiratory specimens of lung transplant recipients may herald a respiratory tract infection or bronchial suture damage. Bronchial stent implantation is a risk factor for the persistence of Corynebacterium species infection.
INTRODUCTION
Corynebacterium is a genus of aerobically growing Gram-positive bacilli (1, 2) that colonize the skin and mucosa in humans. The clinical relevance of non-diphtheriae Corynebacterium species had been a controversial issue. Historically, these microorganisms were considered contaminants, but multiple studies have described their pathogenicity in certain populations, such as immunocompromised patients, those with chronic respiratory disease or implanted prosthetic material, and patients undergoing prolonged hospitalization (1–12). In most of these reports (4, 6–12), non-diphtheriae Corynebacterium spp. were isolated in respiratory tract specimens, and the studies considered Corynebacterium spp. to be a recognized respiratory pathogen in a variable percentage of patients.
Chronic lung diseases such as chronic obstructive pulmonary disease (COPD) (3, 6, 8, 9) and cystic fibrosis (4) are risk factors for Corynebacterium species infection, and patients with these conditions may require lung transplantation. Nevertheless, data regarding Corynebacterium species infections in lung transplant and other solid organ transplant recipients are scarce and limited to case reports (7, 13–15). The clinical significance of Corynebacterium species isolation in respiratory tract specimens has not been established in this population. Hence, our aim with this study was to describe the clinical characteristics and outcomes associated with the recovery of Corynebacterium species isolates from respiratory specimens of lung transplant recipients.
(This study was presented in part as an oral communication [number 052] at the XXI SEIMC meeting [Malaga, Spain], May 2017.)
MATERIALS AND METHODS
Patients and setting.
A retrospective study (January 2014 to December 2016) was performed at Vall d′Hebron University Hospital, a 1,000-bed tertiary referral hospital in Barcelona (Spain), where 60 to 70 lung transplantation procedures are performed per year. On 1 January 2014, 329 adult lung transplant recipients were being followed in our outpatient clinic, and from that date to 31 December 2016, 198 new lung transplantations had been performed. Therefore, we assume that 527 lung transplant recipients, 21 of whom had received a metallic bronchial stent, were at risk of Corynebacterium species respiratory infection during the study period.
In our center, culture and microscopic examination results are systematically recorded in the microbiology department database. We searched this source to retrieve all cases of lung transplant recipients in whom Corynebacterium spp. had been isolated in at least one lower respiratory tract specimen (sputum, tracheal aspirate, bronchoalveolar lavage fluid [BALF], bronchial aspirate, or bronchial biopsy specimen). In patients with sputum and tracheal aspirates, only specimens showing >25 polymorphonuclear leukocytes/low-power field and <10 upper respiratory epithelial cells/low-power field, as assessed by the scoring system of Murray and Washington (16), were considered candidates for inclusion. The study was approved by the clinical research ethics committee of Vall d'Hebron Hospital [PR(AG)404/2016], which also waived the requirement for informed consent.
Data collection.
The clinical data and antibiotic treatment received were recorded in the hospital's electronic medical records. This information and the epidemiological and microbiological data were obtained by chart abstraction and entered into a database specifically created for the study. Two transplant infectious disease physicians (I.L.A. and O.L.) reviewed the charts and recorded the data on a standardized form. Lower respiratory tract samples were taken when the patient had sputum production or bronchoscopy was performed. All patients underwent at least one surveillance bronchoscopy examination within the first year after transplantation. Additional bronchoscopies were performed at the discretion of the attending physician when patients presented with forced ventilatory volume in 1 s (FEV1) deterioration or clinical signs of infection during the follow-up period. All respiratory culture findings before and after lung transplantation were recorded. Lung transplant donor cultures were reviewed to detect any possible donor-to-host transmission. The patients were followed from a minimum period of 6 months to the maximum time available to detect possible relapses or reinfections. The information related to lung transplant recipients followed in the outpatient clinic and data on the number and type of implanted bronchial stents were obtained from the lung transplant unit database.
Definitions.
Lower respiratory tract infections (LRTIs) other than pneumonia, such as bronchitis or tracheobronchitis, were defined according to the Centers for Disease Control and Prevention criteria (17) as follows: no clinical or radiographic evidence of pneumonia, positive culture of material obtained by deep tracheal aspirate or bronchoscopy, and at least 2 clinical signs or symptoms (fever of >38°C, cough, new or increased sputum production, rhonchi, or wheezing) having no other recognized cause.
Clinical cure was defined as the resolution of the pretreatment symptoms without additional antimicrobial therapy and two negative lower respiratory tract cultures during a minimum of 90 days of follow-up after the completion of antibiotic therapy. Clinical failure was established when symptoms persisted or recurred after treatment, additional antimicrobial therapy was required, or Corynebacterium spp. were persistently isolated on follow-up cultures.
The Corynebacterium species bacterial load was considered significant when the CFU/ml of the specimen was >106 CFU/ml in sputum, endotracheal aspirate, or bronchial aspirate and >104 CFU/ml in BALF material (18).
Microbiology data.
Bronchoscopy specimens underwent routine microscopic examination before culture (Gram stain) and were inoculated on chocolate agar, Columbia blood agar, MacConkey agar, and optionally, colistin-nalidixic acid blood agar. For the purposes of the study, a Gram stain was considered positive only when Gram-positive rods were observed as the strongly predominant or unique microorganisms. Species identification was carried out using the Vitek MS matrix-assisted laser desorption ionization–time of flight ([MALDI-TOF] bioMérieux, Marcy-l'Étoile, France) using the direct inoculation method followed by overlaying with 1 μl of α-cyano-4-hydroxycinnamic acid ([CHCA] bioMérieux) according to the manufacturer's instructions. The identification was based on the comparison of the spectra obtained with those included in the Vitek MS v2.0 database. Only results with a 99.9% confidence were considered. Whenever possible, an uncertain strain identity was resolved by 16S sequencing, as previously described (19).
Corynebacterium species antibiotic susceptibility was evaluated by disk diffusion and Etest according to the Clinical and Laboratory Standards Institute recommendations (20) using Mueller-Hinton agar supplemented with 5% sheep blood.
Statistical analysis.
Quantitative variables are expressed as the medians and interquartile ranges (IQRs). Associations were tested using the Wilcoxon rank sum test. Data for categorical variables are expressed as the numbers and percentages. Associations were tested using the Fisher exact test. Significance tests were two-sided, and a P value of <0.05 was considered statistically significant. Statistical analyses were performed using STATA version 14.
RESULTS
Patient characteristics.
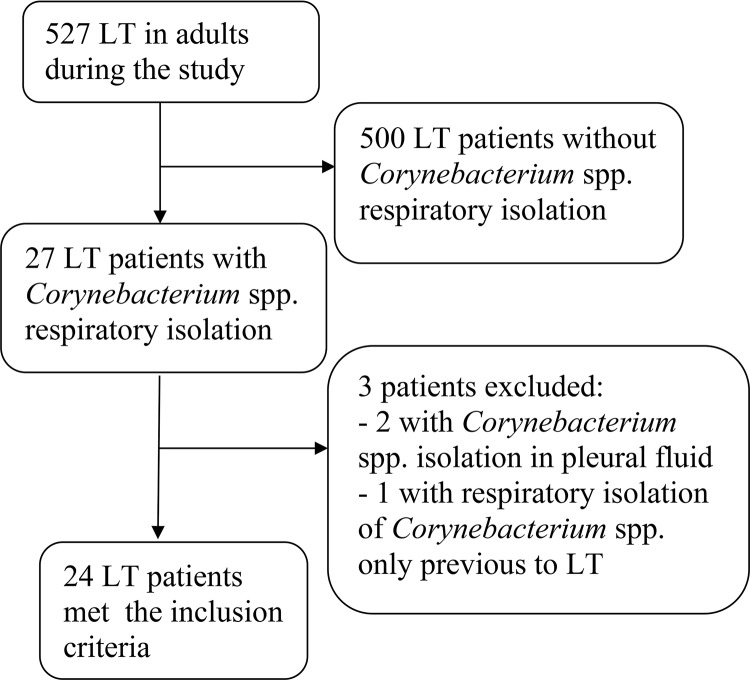
Of 527 lung transplant recipients at risk of infection during the study period, Corynebacterium species isolates were recovered from respiratory specimens of 24 (4.6%) patients meeting the inclusion criteria. A flowchart showing the inclusion of all potential candidates for the study is shown in Fig. 1. Clinical and epidemiological characteristics of the participating patients are summarized in Table 1. The most common underlying diseases were idiopathic pulmonary fibrosis and COPD. The median interval from lung transplantation to Corynebacterium species isolation was 64 days (IQR, 26 to 820 days). Nearly half of the patients (11/24 [46%]) were hospitalized at the diagnosis, and 5 (21%) were hospitalized in the intensive care unit with orotracheal intubation. All patients who underwent bronchoscopy on the basis of clinical criteria (19/24 [79%]) showed some abnormalities, mainly mucosal plaque at the bronchial suture (10/19 [53%]) or purulent secretions (9/19 [47%]). Pathology results were only available for nine patients, five without LRTIs (three with no abnormalities, two suggestive of infection [pneumonitis with the presence of fibrin], and one with acute cellular rejection) and four with LRTIs (three suggestive of infection and one with no abnormalities).
FIG 1.
Flow diagram of patients included in the study. LT, lung transplantation.
TABLE 1.
Patient characteristics at the time of Corynebacterium species respiratory isolation
| Characteristicsa | No. (%) of patients (n = 24) |
|---|---|
| Male sex | 13 (54) |
| Age (median years [IQR]) | 57.5 [49.4–61.2] |
| Pretransplant diagnosis | |
| IPF | 13 (54) |
| COPD | 10 (42) |
| Hemangiomatosis | 1 (4) |
| Transplant type | |
| Bilateral | 19 (79) |
| Single | 5 (21) |
| Immunosuppressive drugs | |
| TAC+MMF+MPD | 19 (79) |
| TAC+MPA+MPD | 2 (8) |
| TAC+RAP+MPD | 2 (8) |
| TAC+MPD | 1 (4) |
| Days from transplantation to positive culture (median [IQR]) | 64 [26–820] |
| Hospital admission | 11 (46) |
| ICU admission >48 h | 5 (21) |
| Antibiotics in the 3 previous mo | 20 (83) |
| BOS | 3 (13) |
| MPD intravenous pulses in the 3 previous mo | 2 (8) |
| Culture type | |
| BALF or BA | 19 (79) |
| Sputum | 4 (17) |
| Tracheal aspirate | 1 (4) |
| No. of positive cultures at diagnosis | |
| 1 | 10 (42) |
| 2 | 7 (29) |
| 3 | 5 (21) |
| 4 | 2 (8) |
| Culture | |
| Monomicrobial | 18 (79) |
| Polymicrobial | 6 (25) |
| Bronchoscopy performed | 19 (79) |
| Mucosal plaque | 10 (53) |
| Purulent secretions | 9 (47) |
BA, bronchial aspirate; BALF, bronchoalveolar lavage fluid; BOS, bronchiolitis obliterans syndrome; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; MMF, mycophenolate mofetil; MPA, mycophenolic acid; MPD, methylprednisolone; RAP, rapamycin; TAC, tacrolimus.
Donor respiratory tract, blood, and preservation fluid cultures were negative, thereby ruling out donor-derived transmission. Corynebacterium spp. were isolated in sputum before transplantation in only 1 patient, diagnosed with COPD. Perioperative cultures with material from the transplant procedure identified C. striatum, which persisted afterwards. There were no nosocomial outbreaks of Corynebacterium spp. in our center during the study period.
Six of the twenty-four (25%) recipients had received expandable metallic bronchial stents (3 silicone covered and 3 uncovered). In 5 of them, Corynebacterium species respiratory isolation was subsequent to the stent implantation (median, 1,036 days; IQR, 192 to 1,946 days). In the sixth patient, a mucopurulent plaque was found at the bronchial suture 18 days after undergoing single lung transplantation, with BALF and bronchial aspirate positive for C. striatum. This patient did not have respiratory symptoms and no antibiotic treatment was prescribed, but she later developed bronchial suture dehiscence, which required stent implantation. Respiratory isolation of C. striatum persisted. The incidence of Corynebacterium species detection was significantly higher in recipients with a bronchial stent than in those without (6/21 [29%] versus 18/506 [4%], respectively, P < 0.001).
Microbiological data.
C. striatum was the most commonly isolated species (11/24 [48%]). The other species recovered included C. pseudodiphtheriticum (3/24 [13%]), C. amycolatum (3/24 [13%]), and C. tuberculostearicum, C. propinquum, C. argentoratense, and C. accolens (1 each). Identification beyond the genus level was not performed in 3 strains, and specimens were not available at the time of the study. Regarding the overall susceptibility rates, all isolates were susceptible to rifampin, linezolid, and vancomycin, around three-quarters were susceptible to imipenem (14/19 [74%]) and doxycycline (15/19 [79%]), half were susceptible to amoxicillin-clavulanic acid (10/19 [53%]), and only one-quarter (5/19 [26%]) to ciprofloxacin. The susceptibility patterns of the species recovered are summarized in Table 2.
TABLE 2.
Microbiological susceptibility patterns of the Corynebacterium spp. detected. Percentage of susceptible strains
| Organism (n) | % of strains susceptible to:a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEN | AMC | IPM | ERY | RIF | VAN | LZD | GEN | CIP | SXT | DOX | |
| C. striatum (10) | 20 | 50 | 70 | 20 | 100 | 100 | 100 | 10 | 10 | 10 | 60 |
| C. pseudodiphtheriticum (3) | 100 | 100 | 100 | 33 | 100 | 100 | 100 | 100 | 67 | 33 | 100 |
| C. amycolatum (3) | 0 | 0 | 33 | 33 | 100 | 100 | 100 | 0 | 0 | 0 | 100 |
| Corynebacterium spp. (3)b | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 67 | 100 | 100 |
AMC, amoxicillin-clavulanic acid; CIP, ciprofloxacin; DOX, doxycycline; ERY, erythromycin; GEN, gentamicin; IPM, imipenem; LZD, linezolid; PEN, penicillin; RIF, rifampin; SXT, trimethoprim-sulfamethoxazole; VAN, vancomycin.
Data from one isolate each of C. tuberculostearicum, C. argentoratense, and C. accolens.
In patients with polymicrobial cultures, other recovered microorganisms were Pseudomonas aeruginosa (3/6), Escherichia coli, Serratia marcescens, and Haemophilus influenzae (1 each).
Infection, treatment, and evolution.
Twelve (50%) patients fulfilled the criteria for LRTI (tracheobronchitis), and 9 of them had monomicrobial cultures. These patients presented different signs and symptoms: 12 with new onset or worsening of cough, 10 with new onset or change in character of sputum, 6 with worsening of dyspnea, and 4 with fever (>38°C). Chest X-rays did not show pulmonary infiltrates, consolidation, or cavitation at the diagnosis in any patient. All these patients received antibiotic therapy for a median of 15 days (IQR, 14 to 16 days): linezolid was used in 3 patients, amoxicillin-clavulanic acid in 3, ciprofloxacin in 3, teicoplanin in 2, and doxycycline in 1 patient. Clinical cure was achieved in 8/12 (67%) cases. Clinical failure occurred in 4 patients, 1 with polymicrobial and 3 with monomicrobial cultures, 3 of whom had a metallic bronchial stent. Patients with clinical failure were treated with different antibiotics: 2 with ciprofloxacin, 1 with teicoplanin, and 1 with linezolid. In contrast, 3 of 12 (25%) patients without an LRTI showed persistent respiratory isolation of Corynebacterium spp. The median follow-up after the isolation of Corynebacterium spp. in respiratory specimens was 348 days (IQR, 157 to 707 days).
Clinical relevance.
To assess the clinical role of Corynebacterium species detection in lung transplantation, only patients with monomicrobial cultures (18/24 [75%]) were analyzed. Half of these patients (9/18) had LRTIs. The comparisons between the various clinical and microbiological variables in patients with and without LRTIs are shown in Table 3. A nonsignificant association was found between LTRI and a significant number of CFU/ml according to the study definition (7/9 versus 2/9, P = 0.057). Furthermore, the isolation of Corynebacterium spp. occurred at a later time following transplantation in patients with LRTIs than in those without LRTIs (235 versus 28 days, respectively, P = 0.058). Finally, the persistence of infection was significantly associated with the presence of a bronchial stent (4/4 patients versus 2/14, P = 0.005).
TABLE 3.
Comparison of clinical and microbiological characteristics of patients with monomicrobial cultures with and without Corynebacterium species associated infections
| Characteristic | LTRIa (n = 9) | No LTRI (n = 9) | P value |
|---|---|---|---|
| Time to positive culture (days [IQR]) | 235 (55–1,020) | 28 (19–49) | 0.058 |
| Positive Gram stain (n [%]) | 7 (78) | 4 (44) | 0.335 |
| Significant no. of CFU (n [%]) | 7 (78) | 2 (22) | 0.057 |
| More than one culture at diagnosis (n [%]) | 6 (67) | 4 (44) | 0.637 |
LTRI, lower tract respiratory infection.
DISCUSSION
The present analysis of Corynebacterium species isolation in lower respiratory tract specimens from lung transplant recipients has shed some light on the clinical significance of this microorganism in this patient population. Of 527 lung transplant recipients at risk of infection during the study period, Corynebacterium species isolates were recovered from respiratory specimens of 24 patients meeting the inclusion criteria. In our experience, Corynebacterium tended to be the predominant microorganism isolated, and 50% of affected patients had LRTIs, a value similar to the reported rate (56%) of this infection in patients with suspected LRTIs and Corynebacterium species isolation in the general population (12).
C. striatum, C. pseudodiphtheriticum, and C. amycolatum were the species most frequently isolated in our study. C. pseudodiphtheriticum has been traditionally associated with respiratory tract infections (2, 4, 10–12, 21), and there are increasing reports of C. striatum-related respiratory infections (3, 7–9, 12). In contrast, although C. amycolatum has been linked to bloodstream infections and endocarditis (1, 22–24) and C. tuberculostearicum to blood, skin, and peritoneal infections (1, 5), there is no previous reported evidence linking these species to respiratory infections (1).
We found that a significant bacterial load of Corynebacterium spp. in the specimens cultured, defined according to the study criteria, was associated with respiratory symptoms. The detection of Corynebacterium as the predominant microorganism in properly collected clinical material is a recommended criterion to consider it a clinically relevant pathogen (1). The detection of a significant number of CFU/ml in cultures indicates a high bacterial load; therefore, the association with respiratory symptoms is biologically plausible. Microbiologists should be aware that the isolation of Corynebacterium spp., particularly when they are the predominant microorganisms in cultures, may be related to an active infectious process and should not be dismissed as nonsignificant overgrowth. Nevertheless, cases must be evaluated individually to place each isolate in the proper clinical context.
Emerging microbiome data support that Corynebacterium spp. may cause respiratory tract infections in the context of a loss of microbially diverse populations and dysregulated host responses. In a cohort of lung transplant recipients with respiratory tract infections, pneumonia (8 patients) and tracheobronchitis (12 patients), pneumonia was characterized by a loss of microbially diverse populations and dominated by the genera Pseudomonas, Staphylococcus, Streptococcus, and in one case, Corynebacterium (25). The authors suggested that Corynebacterium may cause pneumonia as the surrounding microbiome is depleted and corresponding host responses are altered. These data have also been reported in other diseases such as chronic rhinosinusitis (26). Unfortunately, we could not analyze microbiome or host-response data due to the retrospective nature of our study. More studies are needed to validate this hypothesis.
Previous reports have shown that C. striatum, C. amycolatum (1, 6, 7, 14), and C. pseudodiphtheriticum (1) can exhibit reduced susceptibility to antimicrobials. However, C. pseudodiphtheriticum showed a much more favorable susceptibility profile in our study. This factor has potential implications regarding the antibiotic therapy for these patients. Our lung transplant recipients with LRTIs were treated with various antibiotic regimens, and clinical cure was achieved in 67%. Clinical failures were probably due to the resistance to multiple drugs and the difficulty of eliminating the infections at the bronchial sutures. We found that the presence of covered or uncovered metallic bronchial stents was significantly associated with new Corynebacterium species respiratory isolation and persistently positive cultures. The association of Corynebacterium species infection with metallic stents may be related to the ability of these species to form biofilms (27), microbial communities known to be less susceptible to conventional antimicrobial therapies. In a large retrospective study, up to 40% of lung transplant recipients showed new bacterial colonization after metallic stent implantation, mainly of Pseudomonas aeruginosa and Staphylococcus aureus (28), but there were no data regarding Corynebacterium spp.
Of note, although only half of the patients had LRTIs, all those who underwent bronchoscopy had some abnormalities. The most common were mucosal plaques at the bronchial sutures and purulent secretions. Furthermore, one patient with Corynebacterium species purulent plaque in the bronchial suture developed suture dehiscence. Therefore, in lung transplant recipients, respiratory isolation of Corynebacterium spp. in the absence of clinical symptoms but with bronchial suture changes should be interpreted with caution until further data are available.
This study has some limitations. The first are those inherent to its observational and retrospective design. However, the patients were uniformly managed by our lung transplant unit during a lengthy follow-up period. Second, it is a single-center study, and the data obtained cannot be generalized to other lung transplant settings. Third, the absence of pathology data for most patients and the fact that clinical failure could not be properly evaluated in all patients, mainly in those with polymicrobial cultures, are also limitations of the study.
In conclusion, clinical isolates of Corynebacterium spp. recovered from lower respiratory tract specimens in lung transplant recipients may have clinical significance, particularly if a large number of CFU/ml are identified in cultures. In our experience, half of our patients presented LRTIs and resulted in bronchial suture damage. In addition, we found that metallic bronchial stent implantation is a risk factor for the presence and persistence of Corynebacterium species lower respiratory tract infection.
ACKNOWLEDGMENTS
Ibai Los-Arcos has a Rio Hortega contract in the 2016 call for Strategic Action Health from Instituto de Salud Carlos III of the Spanish Health Ministry for the years 2017 to 2018. We thank Celine Cavallo for English language support.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
All authors declare that they have no conflicts of interest related to this study.
REFERENCES
- 1.Bernard K. 2012. The genus Corynebacterium and other medically relevant coryneform-like bacteria. J Clin Microbiol 50:3152–3158. doi: 10.1128/JCM.00796-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funke G, von Graevenitz A, Clarridge JE, Bernard KA. 1997. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev 10:125–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez-Martínez L, Suárez AI, Rodríguez-Baño J, Bernard K, Muniáin MA. 1997. Clinical significance of Corynebacterium striatum isolated from human samples. Clin Microbiol Infect 3:634–639. doi: 10.1111/j.1469-0691.1997.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 4.Bittar F, Cassagne C, Bosdure E, Stremler N, Dubus JC, Sarles J, Reynaud-Gaubert M, Raoult D, Rolain JM. 2010. Outbreak of Corynebacterium pseudodiphtheriticum infection in cystic fibrosis patients, France. Emerg Infect Dis 16:1231–1236. doi: 10.3201/eid1608.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinić V, Lang C, Weisser M, Straub C, Frei R, Goldenberger D. 2012. Corynebacterium tuberculostearicum: a potentially misidentified and multiresistant Corynebacterium species isolated from clinical specimens. J Clin Microbiol 50:2561–2567. doi: 10.1128/JCM.00386-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Díez-Aguilar M, Ruiz-Garbajosa P, Fernández-Olmos A, Guisado P, Campo R, Quereda C, Cantón R, Meseguer MA. 2013. Non-diphtheriae Corynebacterium species: an emerging respiratory pathogen. Eur J Clin Microbiol Infect Dis 32:769–772. doi: 10.1007/s10096-012-1805-5. [DOI] [PubMed] [Google Scholar]
- 7.Verroken A, Bauraing C, Deplano A, Bogaerts P, Huang D, Wauters G, Glupczynski Y. 2014. Epidemiological investigation of a nosocomial outbreak of multidrug-resistant Corynebacterium striatum at one Belgian university hospital. Clin Microbiol Infect 20:44–50. doi: 10.1111/1469-0691.12197. [DOI] [PubMed] [Google Scholar]
- 8.Renom F, Garau M, Rubi M, Ramis F, Galmes A, Soriano JB. 2007. Nosocomial outbreak of Corynebacterium striatum infection in patients with chronic obstructive pulmonary disease. J Clin Microbiol 45:2064–2067. doi: 10.1128/JCM.00152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renom F, Gomila M, Garau M, Gallegos MDC, Guerrero D, Lalucat J, Soriano JB. 2014. Respiratory infection by Corynebacterium striatum: epidemiological and clinical determinants. New Microbes New Infect 2:106–114. doi: 10.1002/nmi2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed K, Kawakami K, Watanabe K, Mitsushima H, Nagatake T, Matsumoto K. 1995. Corynebacterium pseudodiphtheriticum: a respiratory tract pathogen. Clin Infect Dis 20:41–46. doi: 10.1093/clinids/20.1.41. [DOI] [PubMed] [Google Scholar]
- 11.Manzella JP, Kellogg JA, Parsey KS. 1995. Corynebacterium pseudodiphtheriticum: a respiratory tract pathogen in adults. Clin Infect Dis 20:37–40. doi: 10.1093/clinids/20.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Nhan T-X, Parienti J-J, Badiou G, Leclercq R, Cattoir V. 2012. Microbiological investigation and clinical significance of Corynebacterium spp. in respiratory specimens. Diagn Microbiol Infect Dis 74:236–241. doi: 10.1016/j.diagmicrobio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Burke GJ, Malouf MA, Glanville AR. 1997. Opportunistic lung infection with Corynebacterium pseudodiphtheriticum after lung and heart transplantation. Med J Aust 166:362–364. [DOI] [PubMed] [Google Scholar]
- 14.Tarr PE, Stock F, Cooke RH, Fedorko DP, Lucey DR. 2003. Multidrug-resistant Corynebacterium striatum pneumonia in a heart transplant recipient. Transpl Infect Dis 5:53–58. doi: 10.1034/j.1399-3062.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 15.Roy M, Ahmad S. 2016. Rare case of Corynebacterium striatum septic arthritis. BMJ Case Rep 2016:bcr2016216914. doi: 10.1136/bcr-2016-216914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray PR, Washington JA. 1975. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc 50:339–344. [PubMed] [Google Scholar]
- 17.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.García LS. (ed). 2010. Respiratory tract cultures, p 321–409. Clinical microbiology procedures handbook, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 19.Tang YW, Von Graevenitz A, Waddington MG, Hopkins MK, Smith DH, Li H, Kolbert CP, Montgomery SO, Persing DH. 2000. Identification of coryneform bacterial isolates by ribosomal DNA sequence analysis. J Clin Microbiol 38:1676–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2010. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, 2nd ed CLSI guideline M45-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed] [Google Scholar]
- 21.Van Roeden SE, Thijsen SF, Sankatsing SUC, Limonard GJM. 2015. Clinical relevance of Corynebacterium pseudodiphtheriticum in lower respiratory tract specimens. Infect Dis (Lond) 47:862–868. doi: 10.3109/23744235.2015.1070962. [DOI] [PubMed] [Google Scholar]
- 22.Knox KL, Holmes AH. 2002. Nosocomial endocarditis caused by Corynebacterium amycolatum and other nondiphtheriae corynebacteria. Emerg Infect Dis 8:97–99. doi: 10.3201/eid0801.010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalal A, Urban C, Segal-Maurer S. 2008. Endocarditis due to Corynebacterium amycolatum. J Med Microbiol 57:1299–1302. doi: 10.1099/jmm.0.2008/003343-0. [DOI] [PubMed] [Google Scholar]
- 24.Belmares J, Detterline S, Pak JB, Parada JP. 2007. Corynebacterium endocarditis species-specific risk factors and outcomes. BMC Infect Dis 7:4. doi: 10.1186/1471-2334-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankar J, Nguyen MH, Crespo MM, Kwak EJ, Lucas SK, McHugh KJ, Mounaud S, Alcorn JF, Pilewski JM, Shigemura N, Kolls JK, Nierman WC, Clancy CJ. 2016. Looking beyond respiratory cultures: microbiome-cytokine signatures of bacterial pneumonia and tracheobronchitis in lung transplant recipients. Am J Transplant 16:1766–1778. doi: 10.1111/ajt.13676. [DOI] [PubMed] [Google Scholar]
- 26.Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, Goldberg AN, Lynch SV. 2012. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med 4:151ra124. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandran R, Puthukkichal DR, Suman E, Mangalore SK. 2016. Diphtheroids-important nosocomial pathogens. J Clin Diagn Res 10:DC28–DC31. doi: 10.7860/JCDR/2016/19098.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb J, Fuehner T, Dierich M, Wiesner O, Simon AR, Welte T. 2009. Are metallic stents really safe? A long-term analysis in lung transplant recipients. Eur Respir J 34:1417–1422. doi: 10.1183/09031936.00041909. [DOI] [PubMed] [Google Scholar]