Routine use of whole-genome analysis for infectious diseases can be used to enlighten various scenarios pertaining to public health, including identification of microbial pathogens, relating individual cases to an outbreak of infectious disease, establishing an association between an outbreak of food poisoning and a specific food vehicle, inferring drug susceptibility, source tracing of contaminants, and study of variations in the genome that affect pathogenicity/virulence. We describe the setup, validation, and ongoing verification of a centralized whole-genome-sequencing (WGS) laboratory to carry out sequencing for these public health functions for the National Infection Services, Public Health England, in the United Kingdom.
KEYWORDS: WGS, infectious disease, public health, quality control, validation
ABSTRACT
Routine use of whole-genome analysis for infectious diseases can be used to enlighten various scenarios pertaining to public health, including identification of microbial pathogens, relating individual cases to an outbreak of infectious disease, establishing an association between an outbreak of food poisoning and a specific food vehicle, inferring drug susceptibility, source tracing of contaminants, and study of variations in the genome that affect pathogenicity/virulence. We describe the setup, validation, and ongoing verification of a centralized whole-genome-sequencing (WGS) laboratory to carry out sequencing for these public health functions for the National Infection Services, Public Health England, in the United Kingdom. The performance characteristics and quality control metrics measured during validation and verification of the entire end-to-end process (accuracy, precision, reproducibility, and repeatability) are described and include information regarding the automated pass and release of data to service users without intervention.
TEXT
Routine use of whole-genome analysis for infectious diseases has become possible through the rapid development of whole-genome-sequencing (WGS) technologies together with significant reductions in DNA sequencing costs. Whole-genome analysis can be used to enlighten various scenarios pertaining to public health, including identification of microbial pathogens, relating individual cases to an outbreak of infectious disease, establishing an association between an outbreak of food poisoning and a specific food vehicle, tracing the source of contaminants within a manufacturing process, inferring drug resistance genotypically, and studying how variations in the genome affect pathogenicity/virulence.
The need to provide a WGS service for Public Health England (PHE) was acknowledged following submission of a detailed business case. For the sequencing provision, a hub-and-spoke model was the preferred option, with a centralized facility to provide high-throughput sequencing for surveillance and to act as a local facility for more urgent samples in the London area combined with several lower-throughput facilities in designated regions where a local rapid response is necessary to optimize patient management (1).
Following a survey of PHE laboratories considering replacement of traditional phenotypic and genotypic testing with WGS, the estimated DNA submission numbers ranged from 30,000 to 50,000 samples per annum. It was decided that this level of throughput precluded the use of manual library preparation methods, with the appropriate workflow for the automation instrumentation platform requiring the use of liquid-handling robots. There is currently no “gold standard” for WGS methodology for either sample preparation prior to analysis (library preparation) or WGS instrumentation. Illumina's instrumentation is the current market leader, and Illumina's MiSeqDx and NextSeq 550Dx platforms and kits have been approved for in vitro diagnostic use by the U.S. Food and Drug Administration (FDA), with the MiSeqDx platform receiving premarket FDA clearance in 2013 and the NextSeq 550Dx platform receiving this clearance in November 2017. In the United States, the majority of WGS tests are currently developed in-house as laboratory-developed tests and are regulated under the Clinical Laboratory Improvement Amendments (CLIA) regulations. The Centers for Medicare & Medicaid Services (CMS) regulate all laboratory testing (except research) performed on humans in the United States through CLIA. In total, CLIA covers approximately 244,000 laboratory entities. The Division of Laboratory Services, within the Survey and Certification Group under the Center for Clinical Standards and Quality (CCSQ), has the responsibility for implementing the CLIA program. The objective of the CLIA program is to ensure quality laboratory testing (https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA). In response to this, the Centers for Disease Control and Prevention (CDC) established a national workgroup of experts to develop recommendations for ensuring the quality of WGS results and produced a document entitled “Assuring the Quality of Next-Generation Sequencing in Clinical Laboratory Practice” (2). These guidelines and workgroup recommendations were adapted for nonhuman WGS services and were used to develop a comprehensive validation plan and ongoing quality assurance plan for the setup and validation of WGS at PHE and to establish the performance specifications for the WGS test system.
Detailed instrument specifications were drawn up for both medium- and lower-throughput WGS instruments and, because manipulation of the large numbers of samples expected could not be carried out manually, associated liquid-handling robotics. Laboratory space and conditions required by the instrumentation were designed and organized, including calibrated and monitored temperature regulation for the laboratory, fridges, and freezers. Following the requisite tendering process, the following instrumentation was procured and installed in custom-built facilities based in London:
- Overall
- Two Illumina HiSeq instruments for high-throughput sequencing
- Two Illumina MiSeq instruments for lower-throughput and very-small-genome sequencing
- Two Beckman Biomek Span 8 robots for liquid handling
- Prelibrary and reagent use only
- Two Beckman Biomek fixed-head robots for liquid handling
- Prelibrary and postlibrary use
- Two PerkinElmer Sciclone G3 instruments for DNA (library) preparation prior to sequencing
- Two PerkinElmer LabChip DX instruments for DNA library preparation quality checking
- One Promega GloMax instrument for DNA concentration of diluted samples
- One Applied Biosystems ViiA7 instrument for real-time quantification of prepared libraries
AIM
The aim of the present study was to set up a next-generation sequencing workflow which delivers an average of ∼300 Mb of data per sample, with a guaranteed minimum of 150 Mb of high-quality data (>Q30; Q is a standard quality score that measures the probability that a base is called incorrectly). With sequencing-by-synthesis (SBS) technology, each base in a read is assigned a quality score by a phred-like algorithm (3, 4) similar to that originally developed for Sanger sequencing experiments. The quality score (Q) of a given base is defined by the following equation: Q = −10 log10(e), where e is the estimated probability of the base call being wrong. Thus, a higher quality score indicates a lower probability of error. As shown in Table 1, a quality score of 20 represents an error rate of 1 in 100, with a corresponding call accuracy of 99%. Sequencing would be performed using Illumina sequencing technology on a HiSeq or MiSeq platform, and a number of robotic platforms would be used to prepare customer DNA samples and for library production.
TABLE 1.
Relationships between quality scores and base call accuracies
| Quality score | Probability of incorrect base call | Inferred base call accuracy (%) |
|---|---|---|
| Q10 | 1:10 | 90 |
| Q20 | 1:100 | 99 |
| Q30 | 1:1,000 | 99.9 |
Performance characteristics to be measured during validation, comprising the entire end-to-end process, including automation and sequencing components, were as follows.
Accuracy.
Accuracy represents the depth of coverage and was calculated from the average number of reads of Q30 and above for organisms with a range of GC contents and genome sizes.
Precision.
Precision is the degree of agreement between replicate measurements of the same material.
Repeatability.
Repeatability is a measure of within-run precision.
Reproducibility.
Reproducibility is a measure of between-run precision. A custom similarity measure between the sequence reads and a representative set of reference genomes was provided by the NCBI. The kmer identification (kmer ID; a kmer is defined as a motif [or small word] of length k observed more than once in a genomic or sequenced sequence) is the one most similar to the reads sequenced, based on our custom similarity value (a percentage). The kmer ID also tries to assess whether there is DNA from more than one bacterial sample (i.e., if the sample is mixed). A mismatched kmer ID and workflow can identify transposition of a sample on the sequencing plate.
WORKFLOW
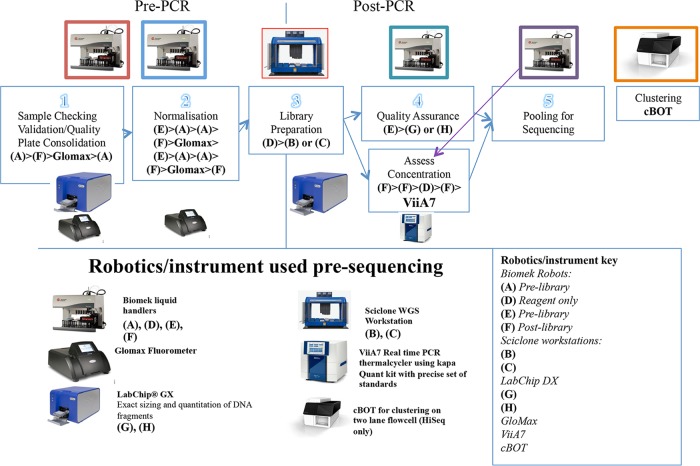
With Illumina technology, whole-genome sequencing can be described as the ability to sequence hundreds of millions of short sequences that represent the entire genome. The multipart process is summarized by the following steps: (i) DNA validation/quality check, (ii) DNA dilution, (iii) library preparation, (iv) quality assurance, and (v) sequencing. Each of these steps requires the different instruments listed for different parts of the sequencing process, as shown in more detail in Fig. 1.
FIG 1.
Robotic workflow. Automated liquid handlers and other instruments used to prepare libraries and provide QC. Their sequential use at each of the five stages is outlined.
VALIDATION WITH MIXED-PLATE RUNS
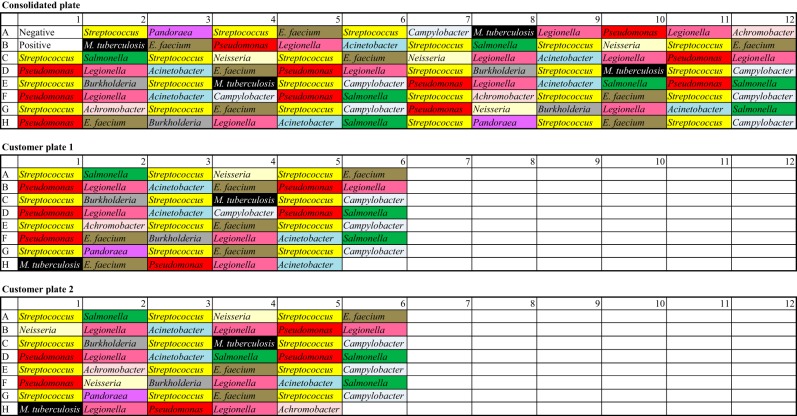
A test plate comprising 10 distinct genera of differing genome sizes and GC contents was chosen to validate the entire workflow from start (DNA quantitation) to sequencing on the HiSeq and MiSeq platforms. The DNA extracts (total of 1 ng per individual sample library preparation), prepared by the submitting laboratories by use of a range of methods, including automated extraction on a QiaSymphony platform (Qiagen), were also divided into two partial plates which were to be combined robotically into a full plate to represent incomplete plate submissions from service users to test automated liquid handling consolidation programs. The validation plate(s) was arranged in such a way that no samples of the same genera were situated in adjacent wells and two negative controls (nuclease-free water) and two positive controls (commercially sourced Escherichia coli K-12 DNA) were included. Multiple replicates of these plates were then stored frozen to ensure one freeze-thaw cycle for all validation testing of the different instruments used in the process. The plate layout is shown in Fig. 2, and the genome sizes and GC contents are shown in Table 2. For MiSeq validation, the first 22 samples from the consolidation plate were processed and sequenced on each of the two MiSeq instruments.
FIG 2.
Plate layout.
TABLE 2.
Genome sizes and GC contents of organisms examined in this study
| Species | Avg (range) genus genome length (Mbp) | Avg (range) % GC | Gram stain resulta |
|---|---|---|---|
| Acinetobacter baumannii | 3.84 (2.67–5.02) | 40.07 (36.67–45.87) | Negative |
| Achromobacter xylosoxidans | 6.69 (6.15–7.36) | 66.36 (64.7–68.2) | Negative |
| Burkholderia cepacia | 7.78 (6.31–10.64) | 63.43 (61.4–67.2) | Negative |
| Escherichia coli | 5.09 (4.64–5.44) | 50.7 (50.6–50.8) | Negative |
| Enterobacter cloacae | 4.78 (4.32–5.16) | 54.54 (52.9–55.5) | Negative |
| Enterococcus faecalis | 2.99 (2.35–3.64) | 40.15 (37.8–42.5) | Positive |
| Klebsiella pneumoniae | 5.9 (5.46–6.34) | 56.25 (55–57.5) | Negative |
| Pandoraea apista | 5.405 (5.04–5.77) | 62.4 (62.5–62.3) | Negative |
| Pseudomonas aeruginosa | 6.27 (5.34–7.2) | 62.85 (59.3–66.4) | Negative |
| Neisseria gonorrhoeae | 2.11 (4,153 nt–2.24) | 52.36 (47.95–52.70) | Negative |
| Chlamydia trachomatis | 1.17 (1.08–1.35) | 40.12 (38.3–41.35) | NA |
| Streptococcus pyogenes | 1.89 (1.83–1.94) | 38.4 (38.3–38.6) | Positive |
| Streptococcus pneumoniae | 2.05 (1.77–2.33) | 39.3 (37.2–41.4) | Positive |
| Legionella pneumophila | 3.52 (3.4–3.64) | 38.4 (38.3–38.5) | Negative |
| Streptococcus agalactiae | 2.12 (2.03–2.21) | 35.45 (35.3–35.6) | Positive |
| Mycobacterium tuberculosis | 4.395 (4.39–4.4) | 65.6 | NA |
| Campylobacter jejuni | 1.76 (1.59–2.14) | 34.1 (1.59–2.14) | Negative |
NA, not available.
Following consolidation, plates were processed using the robotic workflow depicted in Fig. 1. Each instrument used in the process was tested, and following processing, each plate was loaded onto the sequencers, with checking of all flow cell positions and lanes on all four sequencers.
Automated analysis.
The outputs of sequencing were streamed directly to a local high-performance computing (HPC) infrastructure, where they entered a series of automated processes, with the completion of each triggering the next until reports were delivered back to the service user. The computing resource for sequence analysis was an HPC cluster comprised of 26 nodes, each with 16 computing cores and approximately 130 Gb of RAM. Job scheduling via the Univa grid engine allowed for analyses to be distributed across all available nodes. Once it had been detected that sequencing was complete, the raw output went through a demultiplexing stage to convert the binary data into analyzable FASTQ files (https://support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.html). These files were then assessed for quality in terms of read counts and were trimmed with Trimmomatic (5) to remove regions of low-quality sequence and reads that were too short to usefully contribute to analysis. A further quality assessment removed samples with insufficient reads for analysis (<10,000) and used FASTQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) to determine several basic quality measures. The previous steps were applied to all samples. Samples submitted to bacterial (usually species specific) workflows then went through a kmer-based identification step in which the presumptive organism must match the bioinformatics workflow requested by the service user (i.e., if a Salmonella workflow was requested for a sample, then the top kmer match must be a Salmonella organism). If the kmer identification did not match the requested workflow, the sample did not proceed to analysis.
Each of the pipelines provided by the service is composed of a set of analytical components, where the component list, analysis settings, and reference data are defined specifically for that pipeline. Samples were directed to the pipeline that matched the workflow selected by the service user, whereupon sequential execution of analytical components on the HPC cluster provided one or more results for final reporting.
Upon completion of analyses, results were aggregated into custom PDF documents, which were provided along with the quality-trimmed FASTQ files to the service user via secure FTP.
Results.
The raw data from all sequencing runs were analyzed and broken down into total yields, giving the number of genomes with <30× coverage. Among the genomes with <30× coverage, the genomes which were >5 Mbp long were excluded, and those with high GC contents were identified. The results showed that although a percentage (<5%) of the DNA extracts did not reach 30× coverage, it was as a direct result of large genome size (>5 Mb) and/or markedly high GC content.
To confirm that the plates were consolidated in the correct orientation and that the robotic processes were being performed correctly, the identity following sequencing was ascertained using the kmer ID. The kmer ID calculates the similarity between the set of WGS reads and a predefined set of published reference genomes by using kmer vectors based on our custom similarity value (a percentage) and can assess whether there is DNA from more than one bacterial sample (i.e., if the sample is mixed).
The kmer ID analysis was performed for all runs, carried out twice for each sequencing instrument, and showed that the majority of sequencing identifications were in concordance with the known identities. The only exception was that the Achromobacter samples were identified as Bordetella parapertussis, which was because Achromobacter (an uncommonly identified organism) was not included in the kmer ID database. The other exception was two DNA samples identified as Acinetobacter from the sending lab but identified following sequencing as a mixture of E. coli and Klebsiella pneumoniae. Subsequent investigation suggested that there was a sending lab labeling error.
Conclusions.
Analysis of the mixed-plate runs demonstrated that the workflow, from plate consolidation to sequencing, was working as expected. The sequencing yield, however, was affected by genome size and GC content, as expected, and thus consideration has to be given to which samples to put onto mixed plates. Plate consolidation is an important part of the workflow which allows smaller-number submissions (i.e., incomplete plates) to be combined and maximizes the number of samples processed on each of the flow cells. By successfully combining customer samples by use of automated processes, costs are reduced and turnaround times minimized. The mixed-plate analysis also demonstrated that the two HiSeq machines, two MiSeq machines, and two SciClone liquid handlers performed comparably to each other.
In summary, the mixed-plate analysis demonstrated, in terms of high kmer identification accuracy, precision, repeatability, and reproducibility, that the WGS workflow is robust and fit for its purpose.
POSTIMPLEMENTATION SURVEILLANCE AND VERIFICATION
Internal QC.
All positive and negative controls are routinely monitored for performance and verification of the workflow. Positive and negative controls are included in each run. The negative control is molecular biology-grade water, and the positive control is commercially available E. coli K-12 DNA. Both are processed through the same workflow at the same time, as the samples and their positions are not replicated in sequential plates. E. coli K-12 was chosen because it represents a relatively large genome with 50% GC content. Other positive controls or a known mixture of GC content/genome size controls might be appropriate, depending on the species being sequenced. Automatic pass and release of data to customers occur in the event of the following quality control (QC) metrics: for the positive control (positional), >99% kmer ID for E. coli K-12 and >150-Mb, Q30+ posttrim yield; for the negative control (positional), fewer than 100,000 reads; and a kmer mismatch rate of <7%. However, manual release of data occurs if the positive control has a yield of <150 Mb but is still K-12 (>99% kmer ID) and ≥90% of samples have posttrim yields of ≥150 Mb (Q30+) or if there are more than 100,000 negative-control reads but the kmer ID is an environmental/reagent contaminant that is not normally sequenced.
Internal quality assurance.
Blinded samples are routinely resequenced and analyzed to ensure that identical results are obtained for separate runs.
EQA.
Global Microbial Identifier's proficiency test (PT) program facilitates the production of reliable laboratory results of consistently good quality within the area of WGS to facilitate harmonization and standardization in whole-genome sequencing and data analysis. In the absence of formally accredited external quality assurance (EQA) schemes, the proficiency test represents an important tool for the evaluation and production of reliable laboratory results of consistently good quality within the areas of DNA preparation, sequencing, and analysis (e.g., clustering).
Results for Salmonella enterica.
The workflow identified each S. enterica topology correctly, assigning specific samples to cluster1 and cluster2. Overall, 97% of labs correctly clustered cluster1 samples, and 86% correctly clustered cluster2 samples.
Results for Escherichia coli.
The workflow identified each E. coli topology correctly, assigning specific samples to cluster1 and cluster2. Overall, 97% of labs correctly clustered cluster1 samples, and 91% correctly clustered cluster2 samples.
Results for Staphylococcus aureus.
The workflow identified each S. aureus topology correctly, assigning specific samples to cluster1, cluster2, and cluster3. Overall, 93% of labs correctly clustered cluster1 samples, 93% correctly clustered cluster2 samples, and 97% correctly clustered cluster3 samples.
Insert sizes are also routinely monitored, as an average insert size of <∼150 bp adversely affects assembly.
LEARNING AND BENEFITS
The present report provides the following recommendations and benefits for WGS laboratories:
Make sure you know your throughput and expected turnaround time, and manage expectations
Design the system from end to end and to be sequencing platform agnostic
The kmer ID gives a definitive confirmation of species and indications of mixtures/transposition
WGS allows migration of certain current reference microbiology activities to WGS-based approaches (forensic, rapid, cost-effective)
WGS allows a rapid response to outbreaks (including epidemics/pandemics)
WGS provides improved, more accurate information to inform public health outbreak management and policy
Near-real-time generation of genomic data on all strains (for certain organisms) generates a significant resource for research and development
Experiences with initial projects are very positive, including good correlations with inferred drug susceptibilities
WGS data are more reliable than or comparable to data obtained by traditional methods
Multilocus sequence typing (MLST) (and/or single nucleotide polymorphism [SNP] typing) can give added value
ACCREDITATION
A centralized WGS service for PHE is operational in London, United Kingdom, to provide medium-throughput capability and has produced over 100,000 genomes, to date (April 2014 to October 2017), with a turnaround time of 5 days. The service was recently accredited under United Kingdom Accreditation Service (UKAS) standard ISO15189.
OUTCOMES
A centralized WGS service is used by the National Infection Service laboratories of PHE to generate information for improved outbreak investigations (5–11; https://support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.html; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), local and national public health surveillance (12–17), genotypic drug resistance inferences (18–20), and molecular characterization for diversity and evolution analyses (21–28) to further develop and inform infection control using WGS.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Arnold C. 2016. Considerations in centralizing whole genome sequencing for microbiology in a public health setting. Expert Rev Mol Diagn 16:619–621. doi: 10.1586/14737159.2016.1164039. [DOI] [PubMed] [Google Scholar]
- 2.Gargis AS, Kalman L, Berry MW, Bick DP, Dimmock DP, Hambuch T, Lu F, Lyon E, Voelkerding KV, Zehnbauer BA, Agarwala R, Bennett SF, Chen B, Chin EL, Compton JG, Das S, Farkas DH, Ferber MJ, Funke BH, Furtado MR, Ganova-Raeva LM, Geigenmüller U, Gunselman SJ, Hegde MR, Johnson PL, Kasarskis A, Kulkarni S, Lenk T, Liu CS, Manion M, Manolio TA, Mardis ER, Merker JD, Rajeevan MS, Reese MG, Rehm HL, Simen BB, Yeakley JM, Zook JM, Lubin IM. 2012. Assuring the quality of next-generation sequencing in clinical laboratory practice. Nat Biotechnol 30:1033–1036. doi: 10.1038/nbt.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ewing B, Hillier L, Wendl MC, Green P. 1998. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 4.Ewing B, Green P. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 8:186–194. doi: 10.1101/gr.8.3.186. [DOI] [PubMed] [Google Scholar]
- 5.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonnell J, Dallman T, Atkin S, Turbitt DA, Connor TR, Grant KA, Thomson NR, Jenkins C. 2013. Retrospective analysis of whole genome sequencing compared to prospective typing data in further informing the epidemiological investigation of an outbreak of Shigella sonnei in the UK. Epidemiol Infect 141:2568–2575. doi: 10.1017/S0950268813000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallman TJ, Byrne L, Launders N, Glen K, Grant KA, Jenkins C. 2015. The utility and public health implications of PCR and whole genome sequencing for the detection and investigation of an outbreak of Shiga toxin-producing Escherichia coli serogroup O26:H11. Epidemiol Infect 143:1672–1680. doi: 10.1017/S0950268814002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins C, Dallman TJ, Launders N, Willis C, Byrne L, Jorgensen F, Eppinger M, Adak GK, Aird H, Elviss N, Grant KA, Morgan D, McLauchlin J. 2015. Public health investigation of two outbreaks of Shiga toxin-producing Escherichia coli O157 associated with consumption of watercress. Appl Environ Microbiol 81:3946–3952. doi: 10.1128/AEM.04188-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butcher H, Elson R, Chattaway MA, Featherstone CA, Willis C, Jorgensen F, Dallman TJ, Jenkins C, McLauchlin J, Beck CR, Harrison S. 2016. Whole genome sequencing improved case ascertainment in an outbreak of Shiga toxin-producing Escherichia coli O157 associated with raw drinking milk. Epidemiol Infect 144:2812–2823. doi: 10.1017/S0950268816000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashton PM, Nair S, Peters TM, Bale JA, Powell DG, Painset A, Tewolde R, Schaefer U, Jenkins C, Dallman TJ, de Pinna EM, Grant KA, Salmonella Whole Genome Sequencing Implementation Group. 2016. Identification of Salmonella for public health surveillance using whole genome sequencing. PeerJ 4:e1752. doi: 10.7717/peerj.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowell S, King C, Jenkins C, Dallman TJ, Decraene V, Lamden K, Howard A, Featherstone CA, Cleary P. 2016. An outbreak of Shiga toxin-producing Escherichia coli serogroup O157 linked to a lamb-feeding event. Epidemiol Infect 144:2494–2500. doi: 10.1017/S0950268816001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne L, Adams N, Glen K, Dallman TJ, Kar-Purkayastha I, Beasley G, Willis C, Padfield S, Adak G, Jenkins C. 2016. Epidemiological and microbiological investigation of an outbreak of severe disease from Shiga toxin-producing Escherichia coli O157 infection associated with consumption of a slaw garnish. J Food Prot 79:1161–1168. doi: 10.4315/0362-028X.JFP-15-580. [DOI] [PubMed] [Google Scholar]
- 13.Garvey MI, Pichon B, Bradley CW, Moiemen NS, Oppenheim B, Kearns AM. 2016. Improved understanding of an outbreak of meticillin-resistant Staphylococcus aureus in a regional burns centre via whole-genome sequencing. J Hosp Infect 94:401–404. doi: 10.1016/j.jhin.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Gordon NC, Pichon B, Golubchik T, Wilson DJ, Paul J, Blanc DS, Cole K, Collins J, Cortes N, Cubbon M, Gould FK, Jenks PJ, Llewelyn M, Nash JQ, Orendi JM, Paranthaman K, Price JR, Senn L, Thomas HL, Wyllie S, Crook DW, Peto TEA, Walker AS, Kearns AM. 2017. Whole-genome sequencing reveals the contribution of long-term carriers in Staphylococcus aureus outbreak investigation. J Clin Microbiol 55:2188–2197. doi: 10.1128/JCM.00363-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallman TJ, Byrne L, Ashton PM, Cowley LA, Perry NT, Adak G, Petrovska L, Ellis RJ, Elson R, Underwood A, Green J, Hanage WP, Jenkins C, Grant K, Wain J. 2015. Whole-genome sequencing for national surveillance of Shiga toxin-producing Escherichia coli O157. Clin Infect Dis 61:305–312. doi: 10.1093/cid/civ318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chattaway MA, Dallman TJ, Gentle A, Wright MJ, Long SE, Ashton PM, Perry NT, Jenkins C. 2016. Whole genome sequencing for public health surveillance of Shiga toxin-producing Escherichia coli other than serogroup O157. Front Microbiol 7:258. doi: 10.3389/fmicb.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aanensen DM, Feil EJ, Holden MT, Dordel J, Yeats CA, Fedosejev A, Goater R, Castillo-Ramírez S, Corander J, Colijn C, Chlebowicz MA, Schouls L, Heck M, Pluister G, Ruimy R, Kahlmeter G, Åhman J, Matuschek E, Friedrich AW, Parkhill J, Bentley SD, Spratt BG, Grundmann H, European SRL Working Group. 2016. Whole-genome sequencing for routine pathogen surveillance in public health: a population snapshot of invasive Staphylococcus aureus in Europe. mBio 7:e00444-. doi: 10.1128/mBio.00444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallman TJ, Chattaway MA, Mook P, Godbole G, Crook PD, Jenkins C. 2016. Use of whole-genome sequencing for the public health surveillance of Shigella sonnei in England and Wales. J Med Microbiol 65:882–884. doi: 10.1099/jmm.0.000296. [DOI] [PubMed] [Google Scholar]
- 19.Chattaway MA, Greig DR, Gentle A, Hartman HB, Dallman TJ, Jenkins C. 2017. Whole-genome sequencing for national surveillance of Shigella flexneri. Front Microbiol 8:1700. doi: 10.3389/fmicb.2017.01700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashton PM, Owen SV, Kaindama L, Rowe WPM, Lane CR, Larkin L, Nair S, Jenkins C, de Pinna EM, Feasey NA, Hinton JCD, Dallman TJ. 2017. Public health surveillance in the UK revolutionises our understanding of the invasive Salmonella typhimurium epidemic in Africa. Genome Med 9:92. doi: 10.1186/s13073-017-0480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon NC, Price JR, Cole K, Everitt R, Morgan M, Finney J, Kearns AM, Pichon B, Young B, Wilson DJ, Llewelyn MJ, Paul J, Peto TE, Crook DW, Walker AS, Golubchik T. 2014. Prediction of Staphylococcus aureus antimicrobial resistance by whole-genome sequencing. J Clin Microbiol 52:1182–1191. doi: 10.1128/JCM.03117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadouki Z, Day MR, Doumith M, Chattaway MA, Dallman TJ, Hopkins KL, Elson R, Woodford N, Godbole G, Jenkins C. 2017. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Shigella sonnei isolated from cases of diarrhoeal disease in England and Wales, 2015. J Antimicrob Chemother 72:2496–2502. doi: 10.1093/jac/dkx170. [DOI] [PubMed] [Google Scholar]
- 23.Day MR, Doumith M, Do Nascimento V, Nair S, Ashton PM, Jenkins C, Dallman TJ, Stevens FJ, Freedman J, Hopkins KL, Woodford N, De Pinna EM, Godbole G. 2018. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Salmonella enterica serovars Typhi and Paratyphi. J Antimicrob Chemother 73:365–372. doi: 10.1093/jac/dkx379. [DOI] [PubMed] [Google Scholar]
- 24.Dallman T, Smith GP, O'Brien B, Chattaway MA, Finlay D, Grant KA, Jenkins C. 2012. Characterization of a verocytotoxin-producing enteroaggregative Escherichia coli serogroup O111:H21 strain associated with a household outbreak in Northern Ireland. J Clin Microbiol 50:4116–4119. doi: 10.1128/JCM.02047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashton PM, Baker KS, Gentle A, Wooldridge DJ, Thomson NR, Dallman TJ, Jenkins C. 2014. Draft genome sequences of the type strains of Shigella flexneri held at Public Health England: comparison of classical phenotypic and novel molecular assays with whole genome sequence. Gut Pathog 6:7. doi: 10.1186/1757-4749-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dallman TJ, Chattaway MA, Cowley LA, Doumith M, Tewolde R, Wooldridge DJ, Underwood A, Ready D, Wain J, Foster K, Grant KA, Jenkins C. 2014. An investigation of the diversity of strains of enteroaggregative Escherichia coli isolated from cases associated with a large multi-pathogen foodborne outbreak in the UK. PLoS One 9:e98103. doi: 10.1371/journal.pone.0098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowley LA, Dallman TJ, Fitzgerald S, Irvine N, Rooney PJ, McAteer SP, Day M, Perry NT, Bono JL, Jenkins C, Gally DL. 2016. Short-term evolution of Shiga toxin-producing Escherichia coli O157:H7 between two food-borne outbreaks. Microb Genom 2:e000084. doi: 10.1099/mgen.0.000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox A, Pichon B, Wilkinson H, Doumith M, Hill RL, McLauchlin J, Kearns AM. 2017. Detection and molecular characterization of livestock-associated MRSA in raw meat on retail sale in North West England. Lett Appl Microbiol 64:239–245. doi: 10.1111/lam.12709. [DOI] [PubMed] [Google Scholar]