LETTER
The incidence of syphilis has increased in the United States since 2000 (1, 2) and remains a global public health concern (3, 4). A tiered serology-based testing approach, historically starting with nontreponemal testing, is recommended by the Centers for Disease Control and Prevention (CDC) (5). To increase throughput and improve sensitivity for latent syphilis, laboratories are switching to a testing algorithm that starts with an FDA-cleared automated treponeme-specific chemiluminescent immunoassay (CLIA), followed by confirmation with a nontreponemal test and/or a different treponeme-specific test (6–11).
In this study, we compared the recently FDA-cleared Architect Syphilis TP (Architect; Abbott Laboratories, Abbott Park, IL) CLIA to the Liaison Treponema (Liaison; DiaSorin, Stillwater, MN) CLIA by testing 1,028 consecutive sera submitted for syphilis screening to the University of North Carolina Hospitals (UNCH) Clinical Immunology Laboratory. Serum was stored for no longer than 3 months at −80°C. All specimens were remnant specimens from UNCH patients over the age of 18, and approval was obtained from the University of North Carolina Institutional Review Board. Initial screening identified 976 nonreactive and 47 reactive specimens that were concordant by both methods (Table 1). Of the five discordant specimens, four were Architect reactive and Liaison nonreactive, while one specimen was Liaison reactive and Architect nonreactive, providing positive, negative, and total percent agreements of 97.9%, 99.6%, and 99.5%, respectively (kappa coefficient = 0.947; 95% confidence interval [95% CI], 0.901 to 0.993).
TABLE 1.
Liaison and Architect result concordancea
| Architect result | No. of specimens with Liaison result: |
Total | |
|---|---|---|---|
| RX | NR | ||
| RX | 47 | 4 | 51 |
| NR | 1 | 976 | 977 |
| Total | 48 | 980 | 1,028 |
NR, nonreactive; RX, reactive. The positive and negative percent agreements were 97.9% and 99.6%, respectively. The kappa coefficient was 0.947 (95% CI, 0.901 to 0.993).
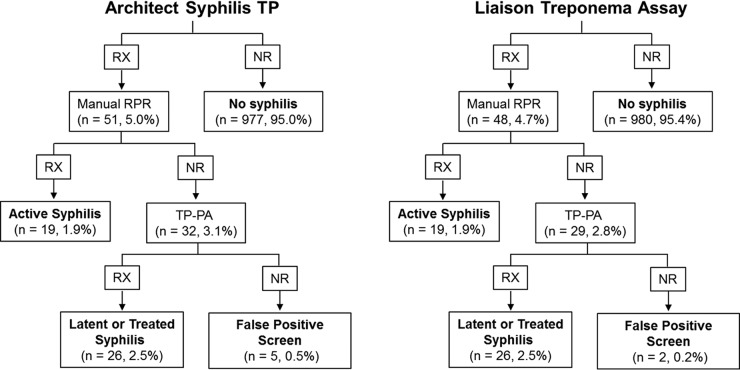
Comparing each assay's screening results to reverse-algorithm-verified positive results (see the materials and methods in the supplemental material), the Architect Syphilis TP assay identified 45 of 45 verified positive results, while five specimens were Architect reactive, ASI rapid plasma reagin (RPR) (Arlington Scientific, Inc., Springville, UT) nonreactive, and Serodia Treponema pallidum particle agglutination (TP-PA) (Fujirebio Inc., Malvern, PA) nonreactive, for a false-positivity rate of 0.5% (Fig. 1). No verified-positive specimens were missed by Architect, providing a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 100% (95% CI, 92.1 to 100%), 99.5% (95% CI, 98.8 to 99.8%), 90.0% (95% CI, 78.2 to 96.7%), and 100.0% (95% CI, 99.6 to 100.0%), respectively (Table 2). The Liaison Treponema assay also identified 45 of 45 verified positives, while two specimens were positive by the Liaison assay but RPR and TP-PA nonreactive, for a false-positivity rate of 0.2% (Fig. 1). The Liaison assay also had no false-negative specimens, for a sensitivity, specificity, PPV, and NPV of 100.0% (95% CI, 92.1 to 100.0%), 99.8% (95% CI, 99.3 to 100.0%), 95.7% (95% CI, 85.5 to 99.5%), and 100.0% (95% CI, 99.6 to 100.0%), respectively (Table 2). One specimen was falsely positive by both the Architect and Liaison assays, while an additional specimen that was positive by both assays was RPR nonreactive but TP-PA indeterminate (Table 3). The levels of inter- and intra-assay precision of both assays were comparable, with the coefficients of variation of all reactive TP-PA specimens being equal to or less than 3.5% (Table S1).
FIG 1.
Reverse syphilis testing algorithm. RX, reactive; NR, nonreactive; RPR, rapid plasma reagin.
TABLE 2.
Reverse-algorithm comparisona
| Test and result | No. (%) of indicated positive specimens that were verified to be: |
Total (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| RX | NR | ||||||
| Liaison | |||||||
| RX | 45 (4.4) | 2 (0.2) | 47 (4.6) | ||||
| NR | 0 (0.0) | 980 (95.4) | 980 (95.4) | ||||
| Totals | 45 (4.4) | 982 (95.6) | 1,027 | 100.0 | 99.8 | 95.7 | 100.0 |
| Architect | |||||||
| RX | 45 (4.4) | 5 (0.5) | 50 (4.9) | ||||
| NR | 0 (0.0) | 977 (95.1) | 977 (95.1) | ||||
| Totals | 45 (4.4) | 982 (95.6) | 1,027 | 100.0 | 99.5 | 90.0 | 100.0 |
RX, reactive; NR, nonreactive; PPV, positive predictive value; NPV, negative predictive value.
TABLE 3.
Discordant-specimen resultsa
| Specimen | Liaison result | Architect result | ASI RPR result | TP-PA result | Interpretation |
|---|---|---|---|---|---|
| 610 | NR | RX | NR | NR | Architect false positive |
| 948 | NR | RX | NR | NR | Architect false positive |
| 1002 | NR | RX | NR | NR | Architect false positive |
| 1025 | RX | RX | NR | NR | Dually false positive |
| 1128 | NR | RX | NR | NR | Architect false positive |
| 1282 | RX | NR | NR | NR | Liaison false positive |
| 1493 | RX | RX | NR | IND | Undefined |
NR, nonreactive; RX, reactive; IND, indeterminate.
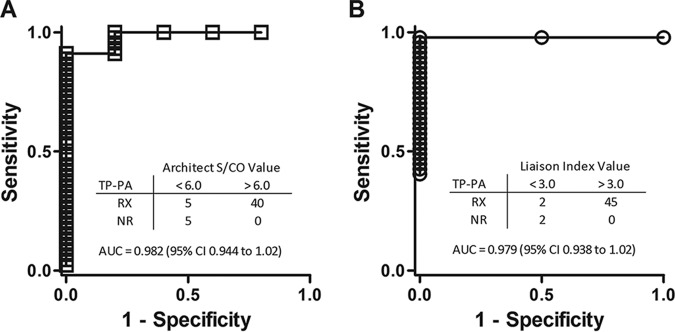
Considering that the reverse-testing algorithm involves one additional test compared to the traditional algorithm, strategies to identify potential false positives and reduce unnecessary tests have been employed by using chemiluminescent signal strengths to set a cutoff for confirmatory testing (12–14). The optimal Architect assay signal to cutoff (S/CO) and Liaison assay index values used to predict TP-PA confirmatory testing results were determined using receiver operating characteristic (ROC) curves. An S/CO value that provided 100% specificity was determined for the Architect Syphilis TP assay, as all specimens with an S/CO value greater than 6.0 (40/40; P = 0.0001) were confirmed by TP-PA testing (Fig. 2A). For the Liaison Treponema assay, an index value greater than 3.0 provided 100% specificity, as all specimens testing higher than 3.0 (45/45) were confirmed by TP-PA testing (Fig. 2B) (P = 0.005). Adopting a testing approach where subsequent testing is based on CLIA signal strengths might significantly reduce costs for a laboratory (13); however, a larger sample size which includes more CLIA-reactive but RPR- and TP-PA-nonreactive specimens will be needed to determine reliable S/CO and index values to use clinically.
FIG 2.
ROC analysis to predict truly positive (TP-PA-confirmed) specimens. Signal/cutoff (S/CO) and index values providing 100% specificity were selected for the Architect Syphilis TP assay (manufacturer's reactive S/CO, ≥1.00) (A) and Liaison Treponema assay (manufacturer's equivocal index, 0.90 < 1.10, and positive index, ≥1.10) (B) for which all specimens with higher values resulted in reactive TP-PA results. P was 0.0001 (A) and P was 0.005 (B) by Fisher's exact test. AUC, area under the curve.
Similar studies performed outside the United States have evaluated the Architect Syphilis TP assay, with reported sensitivities of 99.5 to 100.0% and specificities of 54.5 to 100.0%, depending on patient population (15, 16).
In conclusion, the Architect Syphilis TP assay exhibited sensitivity and specificity that were as high as those of the Liaison Treponema assay and is suitable as a syphilis screening assay in a clinical laboratory setting.
Supplementary Material
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/JCM.00214-18.
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00215-18.
REFERENCES
- 1.Patton ME, Su JR, Nelson R, Weinstock H, Centers for Disease Control and Prevention. 2014. Primary and secondary syphilis—United States, 2005–2013. MMWR Morb Mortal Wkly Rep 63:402–406. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2016. Sexually transmitted disease surveillance 2015. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 3.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2016. Report on global sexually transmitted infection surveillance 2015. World Health Organization, Geneva, Switzerland. http://apps.who.int/iris/bitstream/10665/249553/1/9789241565301-eng.pdf?ua=1 Accessed 20 April 2017.
- 5.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137. doi: 10.15585/mmwr.rr6404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binnicker MJ, Jespersen DJ, Rollins LO. 2012. Direct comparison of the traditional and reverse syphilis screening algorithms in a population with a low prevalence of syphilis. J Clin Microbiol 50:148–150. doi: 10.1128/JCM.05636-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gratrix J, Plitt S, Lee BE, Ferron L, Anderson B, Verity B, Prasad E, Bunyan R, Zahariadis G, Singh AE. 2012. Impact of reverse sequence syphilis screening on new diagnoses of late latent syphilis in Edmonton, Canada. Sex Transm Dis 39:528–530. doi: 10.1097/OLQ.0b013e31824e53f7. [DOI] [PubMed] [Google Scholar]
- 8.Loeffelholz MJ, Binnicker MJ. 2012. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J Clin Microbiol 50:2–6. doi: 10.1128/JCM.06347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra S, Boily MC, Ng V, Gold WL, Okura T, Shaw M, Mazzulli T, Fisman DN. 2011. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the Greater Toronto Area. Sex Transm Dis 38:190–196. doi: 10.1097/OLQ.0b013e3181f07e91. [DOI] [PubMed] [Google Scholar]
- 10.Rhoads DD, Genzen JR, Bashleben CP, Faix JD, Ansari MQ. 2017. Prevalence of traditional and reverse-algorithm syphilis screening in laboratory practice: a survey of participants in the College of American Pathologists Syphilis Serology Proficiency Testing Program. Arch Pathol Lab Med 141:93–97. doi: 10.5858/2016-0110-CP. [DOI] [PubMed] [Google Scholar]
- 11.Rourk AR, Nolte FS, Litwin CM. 2016. Performance characteristics of the reverse syphilis screening algorithm in a population with a moderately high prevalence of syphilis. Am J Clin Pathol 146:572–577. doi: 10.1093/ajcp/aqw182. [DOI] [PubMed] [Google Scholar]
- 12.Dai S, Chi P, Lin Y, Zheng X, Liu W, Zhang J, Zeng Q, Wu X, Liu W, Wang J. 2014. Improved reverse screening algorithm for Treponema pallidum antibody using signal-to-cutoff ratios from chemiluminescence microparticle immunoassay. Sex Transm Dis 41:29–34. doi: 10.1097/OLQ.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 13.Berry GJ, Loeffelholz MJ. 2016. Use of treponemal screening assay strength of signal to avoid unnecessary confirmatory testing. Sex Transm Dis 43:737–740. doi: 10.1097/OLQ.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 14.Yen-Lieberman B, Daniel J, Means C, Waletzky J, Daly TM. 2011. Identification of false-positive syphilis antibody results using a semiquantitative algorithm. Clin Vaccine Immunol 18:1038–1040. doi: 10.1128/CVI.05066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malm K, Andersson S, Fredlund H, Norrgren H, Biague A, Mansson F, Ballard R, Unemo M. 2015. Analytical evaluation of nine serological assays for diagnosis of syphilis. J Eur Acad Dermatol Venereol 29:2369–2376. doi: 10.1111/jdv.13237. [DOI] [PubMed] [Google Scholar]
- 16.Marangoni A, Nardini P, Foschi C, Moroni A, D'Antuono A, Bacchi Reggiani L, Cevenini R. 2013. Evaluation of the BioPlex 2200 syphilis system as a first-line method of reverse-sequence screening for syphilis diagnosis. Clin Vaccine Immunol 20:1084–1088. doi: 10.1128/CVI.00316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.