Abstract
Objectives
Reports of the prognostic significance of ALK-rearrangement in resected non-small cell lung cancer (NSCLC) have been contradictory. We aimed to determine the prognosis of early-stage ALK-positive lung cancers relative to KRAS- and EGFR-mutant lung cancers.
Material and Methods
We reviewed medical records of patients with resected NSCLC harboring an ALK rearrangement (n=29) or a driver mutation in EGFR (n=255) or KRAS (n=480). Recurrence-free survival (RFS) was estimated for each genotype with the differences reported as a hazard ratio (HR).
Results
Among the 764 patients, 555 (73%), 101 (13%), and 108 (14%) had stage I, II, and III NSCLC, respectively. ALK-positive patients were distributed across all stages: 10 (34%) stage I, 6 (21%) stage II, and 13 (45%) stage III. Median RFS was not reached for EGFR-mutant patients, 24.3 months (95%CI 11.4 to 65.3) for ALK-positive patients, and 72.9 months (95%CI 59.7 to undefined) for KRAS-mutant patients. When adjusted for stage, ALK-positive NSCLC remained associated with worse RFS compared to EGFR-mutant (HR 1.8, 95%CI: 1.1–3.1), but not when compared to KRAS-mutant (HR 1.3, 95%CI: 0.8–2.1) NSCLC.
Conclusions
In this large series of resected NSCLC, ALK rearrangements were associated with a trend toward inferior disease outcomes compared to other clinically relevant genomic subsets. These data support the need for clinical trials evaluating use of ALK inhibitors among ALK-positive patients with localized or locally-advanced disease.
Keywords: ALK-rearrangement, early-stage non-small cell lung cancer
INTRODUCTION
Lung cancers driven by oncogenic rearrangements involving the anaplastic lymphoma kinase (ALK) gene represent a rare but clinically relevant subset of non-small cell lung cancer (NSCLC).1 Sequential use of an ever-expanding repertoire of highly effective ALK inhibitors has significantly improved outcomes for patients with metastatic ALK-positive NSCLC.2 However, the widespread use of ALK inhibitors has also affected our ability to study the prognostic relevance of this predictive biomarker. Despite being an established therapeutic target in the metastatic setting, the rarity of ALK rearrangements in non-metastatic disease poses a challenge to clinical studies of ALK inhibitors in the resectable or locally advanced setting. Theoretically, determining the prognostic impact of ALK status should be more straightforward in the early-stage setting given the absence of approved targeted therapies. Yet, the few published studies of resectable ALK-positive NSCLC have had conflicting results, likely due to inclusion of molecularly heterogeneous comparator groups.3–6
Given the limitations of the current literature, we undertook this pooled retrospective analysis to better understand the prognostic implications of ALK rearrangement. Outcomes of patients with resected ALK-positive tumors were compared to two other clinically relevant cohorts: patients with resected tumors harboring activating mutations in the epidermal growth factor receptor gene (EGFR) and Kirsten rat sarcoma virus gene (KRAS).
MATERIALS AND METHODS
Study Population
Patients (n=764) with surgically resected Stage I–III (American Joint Committee on Cancer 7th edition) NSCLC harboring an EGFR or KRAS mutation or an ALK rearrangement were identified from the charts of consecutive patients that underwent resection at Massachusetts General Hospital or Memorial Sloan Kettering Cancer Center between January 2009 and December 2012. This study was approved by the Institutional Review Board at both institutions.
Data Collection
Data were extracted from medical records, updated as of December 2016. Recurrence-free survival (RFS) was measured from the date of surgery to the date of death or development of relapse. Overall survival (OS) was measured from the date of surgical resection. For cases without events, RFS and/or OS were censored at the time of last follow-up.
Tumor Pathology and Genetic Analysis
Tumor histology was defined using World Health Organization criteria. ALK rearrangements were identified using a dual-color break-apart fluorescence in situ hybridization assay. Mutations in EGFR and KRAS were detected by one of two next-generation sequencing platforms: MSK-IMPACT, a hybridization capture-based assay,7 and SNaPshot, an anchored multiplex polymerase chain reaction-based assay.8
Statistical Analysis
Fisher’s exact test was used to compare categorical characteristics between genotypes, while age was analyzed by Wilcoxon rank-sum test. The Kaplan-Meier method was used to estimate the distributions of RFS and OS. The proportional hazards model was used to estimate the hazard ratio (HR) for assessing RFS and OS differences between genotypes adjusting for stage at diagnosis, with the 95% confidence interval constructed by the Wald method. Data analysis was performed using SAS 9.4 (SAS Inst Inc, Cary, NC), with all p-values based on a two-sided hypothesis.
RESULTS
Patient Characteristics
We identified 764 patients, the baseline characteristics of which are summarized in Table 1. Of the 764 patients, 29 (4%) were ALK-positive, whereas 480 (63%) and 255 (33%) were KRAS- and EGFR-mutant, respectively. Patients with ALK-positive tumors were distributed across all stages: 10 (34%) stage I, 6 (21%) stage II, and 13 (45%) stage III. Patients with ALK-positive NSCLC were younger with a lesser smoking history compared to those with KRAS-mutant NSCLC (p= < 0.001), and had a more even gender distribution compared to patients with EGFR-mutant NSCLC (p= 0.013).
Table 1.
Clinicopathological Characteristics and Treatment Histories
| Clinical Characteristics | ALK (n = 29) | EGFR (n = 255) | KRAS (n = 480) | ALK vs. EGFR p-value |
ALK vs. KRAS p-value |
|---|---|---|---|---|---|
|
| |||||
| Age at Diagnosis (years) | 0.002 | 0.001 | |||
| Median | 63 | 67 | 68 | ||
| Range | 29–78 | 37–90 | 41–89 | ||
|
| |||||
| Sex—number (%) | 0.013 | 0.325 | |||
| Male | 13 (45) | 58 (23) | 171 (36) | ||
| Female | 16 (55) | 197 (77) | 309 (64) | ||
|
| |||||
| Smoking History—number (%) | 0.186 | <0.001 | |||
| Never | 17 (59) | 131 (51) | 23 (5) | ||
| Light (≤10 pack years) | 8 (28) | 50 (20) | 27 (6) | ||
| Heavy (> 10 pack years) | 4 (14) | 73 (29) | 430 (90) | ||
| Unknown | 0 (0) | 1 (<1) | 0 (0) | ||
|
| |||||
| Histology-number (%) | 0.117* | 0.252* | |||
| Adenocarcinoma | 27 (93) | 251 (98) | 465 (97) | ||
| Adenosquamous | 1 (3) | 3 (1) | 10 (2) | ||
| Spindle Cell | 1 (3) | 0 (0) | 0 (0) | ||
| Pleomorphic Carcinoma | 0 (0) | 1 (<1) | 2 (<1) | ||
| Not Otherwise Specified | 0 (0) | 0 (0) | 3 (<1) | ||
|
| |||||
| Stage - number (%) | <0.001 | <0.001 | |||
| Stage I | 10 (34) | 194 (76) | 351 (73) | ||
| Stage II | 6 (21) | 25 (10) | 70 (15) | ||
| Stage III | 13 (45) | 36 (14) | 59 (12) | ||
|
| |||||
| Treatment Stage I -number (%) | 1.000 | 1.000 | |||
| Surgery alone | 10 (100) | 189 (97) | 337 (97) | ||
| Adjuvant Chemotherapy | 0 (0) | 5 (3) | 14 (3) | ||
|
| |||||
| Treatment Stage II - number (%) | 0.394 | 0.692 | |||
| Surgery alone | 2 (33) | 14 (56) | 31 (44) | ||
| Adjuvant Chemotherapy | 4 (67) | 11 (44) | 39 (56) | ||
|
| |||||
| Treatment Stage III - number (%) | 1.000 | 1.000 | |||
| Surgery alone | 3 (23) | 7 (19) | 13 (22) | ||
| Neoadjuvant Chemoradiation | 6 (46) | 17 (47) | 26 (44) | ||
| Neoadjuvant Chemotherapy | 4 (31) | 12 (33) | 16 (27) | ||
| Adjuvant Radiation | 0 (0) | 0 (0) | 4 (7) | ||
|
| |||||
| EGFR TKI—number (%) | |||||
| Stage I | 0 (0) | 16 (8) | 0 (0) | ||
| Stage II | 0 (0) | 9 (36) | 0 (0) | ||
| Stage III | 0 (0) | 16 (44) | 0 (0) | ||
Adenocarcinoma versus other histologies
Note: All patients underwent surgical resection.
Recurrence-Free Survival
With median follow-up of 60.5 months (range: 0.1 to 89.7), 235 (31%) patients relapsed. Eighteen (8%) patients experienced locoregional recurrence and 217 (92%) patients had distant metastasis. The distribution of relapse sites, including the frequency of intracranial metastases, did not differ significantly between molecular subgroups (ALK vs. EGFR, p=0.54; ALK vs. KRAS, p=1.00). Fourteen of the 29 (48%) patients with ALK-positive NSCLC developed recurrent disease during surveillance, including 2 of 10 patients (20%) with stage I NSCLC, 2 of 6 (33 %) with Stage II NSCLC, and 10 of 13 (77%) patients with stage III NSCLC. Among the 255 patients with EGFR-mutant NSCLC, 63 (25%) relapsed, while 158 of the 480 (33%) patients with KRAS-mutant NSCLC relapsed during the surveillance period.
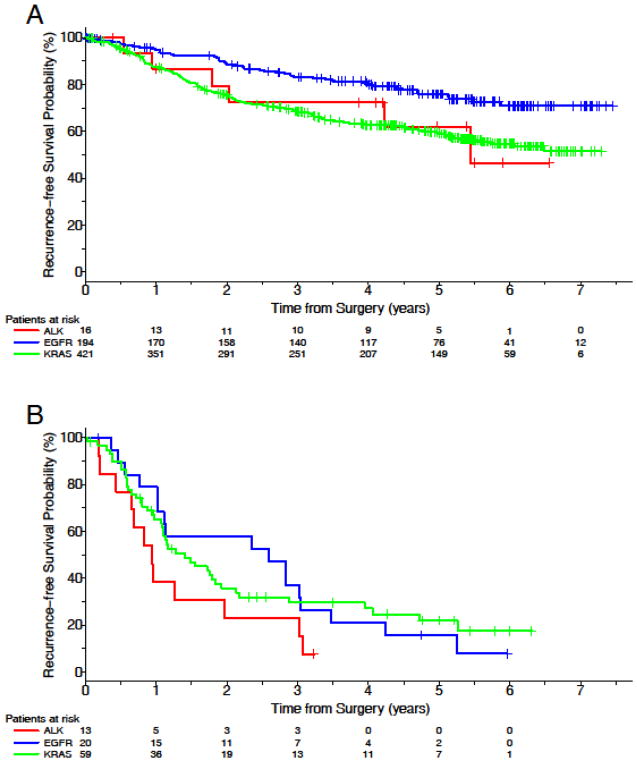
Median RFS was 24.3 months (95% CI: 11.4–65.3) for patients with ALK-positive NSCLC but not reached for patients with EGFR-mutant NSCLC and 72.9 months (95% CI: 59.7 to undefined) for patients with KRAS-mutant NSCLC. There was no RFS difference between patients with ALK-positive and KRAS-mutant tumors when adjusted for stage (HR=1.3 ALK vs. KRAS, 95% CI: 0.8–2.1). When adjusted for stage at diagnosis, ALK-positive NSCLC was associated with inferior RFS compared to EGFR-mutant NSCLC (HR 1.8, 95% CI: 1.1–3.1). Of note, 41 (16%) patients with EGFR-mutant cancer received adjuvant EGFR inhibitors. A subgroup analysis was performed to determine if the difference in RFS was influenced by TKI exposure. When patients treated with adjuvant EGFR TKIs were excluded and stage controlled, RFS for patients with ALK-positive NSCLC was numerically shorter than those with EGFR-mutant tumors, but the trend was not statistically significant (HR=1.6, 95% CI: 0.9–2.8, p=0.104). RFS by genotype (excluding patients who received an adjuvant EGFR inhibitor) is presented in Table 2 and Figures 1A and 1B. An RFS analysis which includes patients who received an adjuvant EGFR inhibitor is presented in Supplemental Figure 1.
Table 2.
Recurrence-Free Survival (RFS) By Genotype
| Molecular Driver | Median RFS Months (95% CI) |
ALK vs. EGFR Hazard Ratio (95% CI) |
ALK vs. KRAS Hazard Ratio (95% CI) |
|---|---|---|---|
| ALK | 24.3 months (11.4 to 65.3) | 1.6 0.9–2.8 |
1.3 0.8–2.1 |
| EGFR* | Not reached | ||
| KRAS | 72.9 months (59.7 to not reached) |
Patients who received EGFR tyrosine kinase inhibitors are excluded; CI: confidence interval
Figure 1.
Recurrence free survival by genotype when patients treated with an adjuvant EGFR tyrosine kinase inhibitor were excluded. (A) Stages I and II. (B) Stage III.
Overall Survival
201 (26%) patients died during follow-up, including 11 patients with ALK-positive, 42 patients with EGFR-mutant, and 148 patients with KRAS-mutant NSCLC. In patients with Stage I–II NSCLC, 5-year OS rates were 84% of ALK-positive, 88% of EGFR-mutant, and 72% of KRAS-mutant patients and OS was not significantly different (ALK vs EGFR p=0.353; ALK vs KRAS p=0.472). Among patients with Stage III NSCLC, the 5-year OS rate was 29% in the ALK-positive, 47% and 38% in the EGFR- and KRAS-mutant groups, respectively. There was no statistical difference in OS in Stage III (ALK vs EGFR p=0.190; ALK vs KRAS p=0.844). When patients who received adjuvant EGFR TKIs were excluded, the 5-year survival rate for EGFR-positive patients was 87% for stage I–II and 39% for Stage III. Table 3 and Supplemental Figures 2A and 2B depict OS of patients who did not receive adjuvant genotype-guided therapy. Supplemental Figure 3 illustrates the results of the same analysis, but includes patients who received an EGFR inhibitor in the adjuvant setting.
Table 3.
Overall Survival (OS) By Genotype
| Molecular Driver | 5-Year OS (%) |
ALK vs. EGFR P-value |
ALK vs. KRAS P-Value |
|---|---|---|---|
| Stage I & II Patients | |||
| ALK | 84 | 0.383 | 0.472 |
| EGFR* | 87 | ||
| KRAS | 72 | ||
| Stage III Patients | |||
| ALK | 29 | 0.545 | 0.844 |
| EGFR* | 39 | ||
| KRAS | 38 | ||
Patients who received EGFR tyrosine kinase inhibitors are excluded
DISCUSSION
The diagnosis and treatment of patients with metastatic NSCLC has been revolutionized by the detection of driver alterations and development of personalized therapies targeting these alterations.9 Although widespread implementation of molecular testing for NSCLC has been critical to characterizing these molecular subsets, the speed of drug development has confounded our ability to discern the prognostic implications of these molecular drivers. The same is not true in early-stage disease where studies of targeted therapies lag years behind approvals in advanced disease.10 The current lack of approved adjuvant/neo-adjuvant targeted therapies offers a unique opportunity to determine whether specific molecular alterations influence the natural history of resected NSCLC. Arguably, establishing prognostic relevance may be most valuable in early- stage disease where rational design of perioperative clinical trials may lead to cures.
To date, defining the prognostic impact of molecular drivers of early-stage NSCLC has been challenging due to the relative rarity of these subsets and the redefinition of comparator arms over time as understanding of the molecular drivers of NSCLC evolved. Indeed, use of molecularly heterogeneous comparator arms likely obscures interpretation of findings from the previously published, early-stage ALK studies. For example, the Lungscape project, a European multi-institutional effort that analyzed prevalence and disease outcomes of resected ALK-positive NSCLC through study of a large biobank of lung adenocarcinomas, reported superior RFS and OS for patients with ALK-positive early-stage NSCLC.3 In contrast, two separate studies observed an association between ALK-positivity and inferior RFS 4,6. These latter two studies exclusively evaluated outcomes among never-smokers, whereas the comparator population in the Lungscape study was predominantly comprised of smokers. Furthermore, these studies assessed ALK-positive and ROS1-postive cancers as a single group and included a mix of genotypes in the comparator cohort. Considering that most lung cancer arises in smokers and the biology of lung cancer among never-smokers is influenced by genotype, establishing the true prognostic relevance of ALK status in early-stage NSCLC will ultimately depend on assessment of disease outcomes of patients with ALK-positive lung cancer relative to other molecularly-defined cohorts, including cohorts that include smokers.
Here, we present results from a large multi-institution study that included three molecularly-defined NSCLC subsets encompassing a spectrum of tobacco exposure. We did not observe a statistical difference when RFS of patients with resected ALK-positive tumors was compared to that of patients with EGFR- mutant NSCLC who had not received an EGFR inhibitor or patients with KRAS-mutant tumors. However, there were numerical differences favoring the non-ALK groups. Our findings are intriguing, but they must be interpreted within the limitations of our study, including the retrospective nature of the analysis, differences in stage distribution across oncogenic drivers, and the small number of ALK-positive patients studied. The frequency of relapse across molecular cohorts despite curative intent therapy highlights the importance of enrolling patients with ALK-positive or EGFR-mutant lung cancer on studies of targeted therapies.
Supplementary Material
Recurrence free survival by genotype, including patients who received an adjuvant EGFR tyrosine kinase inhibitor. (A) Stages I and II. (B) Stage III.
Overall survival by genotype when patients treated with an adjuvant EGFR tyrosine kinase inhibitor were excluded. (A) Stages I and II. (B) Stage III.
Overall survival by genotype, including patients who received an adjuvant EGFR tyrosine kinase inhibitor. (A) Stages I and II. (B) Stage III.
Highlights.
One-third of resected ALK-, KRAS- or EGFR-positive lung cancer patients relapsed within 5 years.
ALK-positive patients had a numerically shorter relapse-free survival than other groups.
Relapse-free survival was not statistically different when molecular subgroups were compared.
There was no statistical difference in overall survival across molecular groups.
Acknowledgments
Funding: NIH P30 CA008748
Footnotes
Conflicts of Interest
JEC has served as a compensated consultant or received honoraria from Bristol Myers Squibb, AstraZeneca, Genentech, and Merck. IDJ has served as a compensated consultant or received honoraria from Boehringer-Ingelheim and Foundation Medicine. ATS has served as a compensated consultant or received honoraria from Pfizer, Novartis, Genentech/Roche, Ariad/Takeda, Loxo, Blueprint, Foundation Medicine, Ignyta, and KSQ Therapeutics. JFG has served as a compensated consultant or received honoraria from Pfizer, Genentech/Roche, Novartis, Bristol-Myers Squibb, Merck, Incyte, Ariad, Loxo, and Clovis Oncology. The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov. 2017;7(2):137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: results from the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol. 2014;32(25):2780–2787. doi: 10.1200/JCO.2013.54.5921. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Shim HS, Kang DR, et al. Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer. 2014;83(3):389–395. doi: 10.1016/j.lungcan.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84(2):121–126. doi: 10.1016/j.lungcan.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Kulig K, Boland JM, et al. Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol. 2012;7(1):90–97. doi: 10.1097/JTO.0b013e31823c5c32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2(5):146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. Jama. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST Trials: A Golden Opportunity to Transform Outcomes in Early-Stage Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21(24):5439–5444. doi: 10.1158/1078-0432.CCR-15-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recurrence free survival by genotype, including patients who received an adjuvant EGFR tyrosine kinase inhibitor. (A) Stages I and II. (B) Stage III.
Overall survival by genotype when patients treated with an adjuvant EGFR tyrosine kinase inhibitor were excluded. (A) Stages I and II. (B) Stage III.
Overall survival by genotype, including patients who received an adjuvant EGFR tyrosine kinase inhibitor. (A) Stages I and II. (B) Stage III.