Abstract
Dendritic cells (DCs) are professional antigen-presenting cells that are pivotal in the generation and sustainability of antitumor immune responses. Whole tumor cell lysates (TCLs) have been used as sources of tumor antigens for the development of DC vaccines. However, the clinical outcomes of the use of TCL-based DC vaccines have so far been unsatisfactory because of the weak immunogenicity of tumor cells. To improve the efficacy of TCL-based DC vaccines, viruses have been used to enhance the immunity of TCLs and to further enhance the antigen delivery and antigen-presenting ability of DCs. The aim of the present study was to improve the antigen-presenting ability of DCs and to use them to effectively activate T lymphocytes. The present study demonstrated that DCs loaded with the lysate of Newcastle Disease Virus (NDV)-infected tumor cells (NDV-TCL) have increased levels of cluster of differentiation 80 (CD80), CD86, CD83 and human leukocyte antigen-antigen D-associated expression, compared with those loaded with TCL alone. The DCs loaded with the NDV-TCL promoted T-cell proliferation and antitumor cytokine secretion from T cells. These results indicated that loading DCs with NDV-TCL could enhance the antigen-presenting ability of the DCs. On the basis of the results of the present study, we hypothesize that this method of loading DCs with NDV-TCL can be used to develop novel DC vaccines for tumor immunotherapy in the future.
Keywords: newcastle disease virus, dendritic cell vaccine, T-cell activation, tumor cell lysate, antitumor immunity
Introduction
Dendritic cells (DCs) are the most important antigen-presenting cells in the body; DC-based cancer immunotherapy has been investigated in previous years (1–4). Additionally, the approval of the first DC vaccine, Provenge (generic name, sipuleucel-T) (5), by the US Food and Drug Administration for the treatment of prostate cancer in 2010 was a milestone in the immunotherapeutic application of DC vaccines. Whole-tumor cell lysates (TCLs) have been used as the source of tumor antigens for the development of DC vaccines; however, the clinical outcomes of TCL-based DC vaccines have been unsatisfactory owing to the weak immunogenicity of tumor cells (6). To improve the efficacy of TCL-based DC vaccines, viruses have been used to enhance the immunity of TCLs, to further enhance the antigen delivery and to increase the antigen-presenting ability of DCs (7).
The Newcastle Disease Virus (NDV) is a bird RNA virus of the Paramyxovirus family. Specifically, NDV belongs to the genus Avulavirus, which does not include any known natural pathogens of humans (8). Over the last 6 decades, NDV has been tested as an anticancer agent because of its oncolytic properties. Owing to the oncolytic and immunostimulatory properties of NDV, necrotic tumor cells destroyed by the virus are phagocytosed and perceived as dangerous by antigen-presenting cells. These professional antigen-presenting cells then process tumor-associated antigens (TAA), become activated and present the processed TAA peptides to T cells for cognate interaction and the induction of an immune response. Owing to NDV's properties of tumor-selective replication, oncolytic capacity and immune stimulation (9), it is considered a promising candidate for use in enhancing the efficacy of DC vaccines (10–12). Virus-induced augmentation of the antigenicity of tumor antigens had been observed in several model systems (13,14).
The aim of the present study was to determine whether DCs loaded with NDV-infected TCL (NDV-TCL) possessed a stronger antitumor ability than DCs loaded only with TCL. The results of the present study indicate the presence of a potential novel method for increasing the efficacy of DC vaccines and for the development of novel DC vaccines for immunotherapy in the future.
Materials and methods
Patient information
In the present study, 12 lung cancer patients were recruited from The First Hospital of Jilin University (Changchun, China) from August 2015 to December 2015. The age range was 45–68 years old and the mean age was 56 years old. There were eight female and four male patients. The inclusion criteria were as follows: No radiation and chemotherapy for more than 1 month prior to blood collection. Other clinical data of the patients have been listed in Table I. The present study was reviewed and approved by the ethics committee of The First Hospital of Jilin University, with written informed consent provided by all participants.
Table I.
Characteristics of the 12 lung cancer patients.
| No. | Sex | Age, years | Diagnosis | Stage |
|---|---|---|---|---|
| 1 | F | 50 | Lung cancer (adenocarcinoma) | T2N3M0, IIIB |
| 2 | F | 60 | Lung cancer (adenocarcinoma) | T3N3M1, IV |
| 3 | F | 49 | Lung cancer (adenocarcinoma) | T4N2M1, IV |
| 4 | F | 62 | Lung cancer (adenocarcinoma) | T1N3M0, IIIB |
| 5 | F | 46 | Lung cancer (squamous carcinoma) | T4N2M0, IIA |
| 6 | M | 62 | Lung cancer (squamous carcinoma) | T2N1M0, IIIA |
| 7 | M | 58 | Lung cancer (small cell) | Limited stage |
| 8 | M | 45 | Lung cancer (small cell) | Limited stage |
| 9 | M | 60 | Lung cancer (small cell) | Limited stage |
| 10 | F | 64 | Lung cancer (small cell) | Extensive stage |
| 11 | F | 48 | Lung cancer (small cell) | Extensive stage |
| 12 | F | 68 | Lung cancer (small cell) | Extensive stage |
T, tumor; N, node; M, metastasis.
Generation of DCs
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (400 × g at room temperature for 30 min) using Ficoll medium (Takeda Pharmaceuticals International GmbH, Zurich, Switzerland) from whole blood samples (50 ml) of the 12 lung cancer patients. DCs were induced from adherent cells derived from PBMCs following 2 h of incubation at 37°C in a CO2 incubator. Non-adherent cells were collected and used as T cells for the cytokine secretion studies. The adherent cells were cultured in CellGenix GMP DC (CellGenix, Freiburg, Germany) with 50 ng/ml interleukin-4 (IL-4) and 50 ng/ml recombinant human granulocyte macrophage-colony stimulating factor (GM-CSF) both (Miltenyi Biotec, GmbH Bergisch Gladbach, Germany) for 5 days, and the same concentration of cytokines (IL-4 and GM-CSF) was added on day 2. On day 5, 1 µg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added into the culture medium. On day 7, the cultured cells were harvested and stained with cluster of differentiation (CD)14-FITC (cat. no. 347493), human leukocyte antigen-antigen D related (HLA-DR)-PerCP (cat. no. 347364) (both from BD Biosciences, San Jose, CA, USA), CD1a-PE (cat. no. 555807), CD80-PE (cat. no. 340294), CD86-FITC (cat. no. 555657) and CD83-APC (cat. no. 551073) (all from BD Pharmingen; BD Biosciences) at room temperature for 15 min. At the same time, the cells were stained with mouse immunoglobulin G (IgG)2b κ-FITC (cat. no. 555742), mouse IgG1 κ-PE (cat. no. 555749), mouse IgG2a κ-PerCP (553933) and mouse IgG1κ-APC (cat. no. 555751) (all from BD Biosciences) at room temperature for 15 min. These antibodies were used as control isotype-matched antibodies. All antibodies used above were ready for use and did not require dilution. The surface phenotype of the DCs was determined using FACSCalibur flow cytometer (BD Biosciences). The data were analyzed by FlowJo V7.6 software (Tree Star, Inc., Ashland, OR, USA).
Giemsa staining of the DCs
To evaluate the morphology of DCs, DCs were collected, washed with PBS once and seeded (2.4×105 cells/ml) onto a clean microscope slide. The cells were then air-dried and stained with Giemsa for morphological examination by light microscopy (magnification, ×1,000). Giemsa staining was performed using the Diff-Quik kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer's protocol.
NDV source
NDV J76 was kindly supplied by Yuanguo Li (Military Veterinary Institute, AMMS, Key laboratory of Jilin Province for Zoonosis Prevention and Control, Changchun, China). The working stock of the virus was sterilized by passing it through a 0.22-µm filter prior to using it to infect the tumor cells.
Production of TCL and NDV-TCL
For the preparation of the TCL, human lung adenocarcinoma A549 cells were obtained from Beijing Beina Chuanglian Biotechnology Institute (Beijing, China) and cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The tumor cells (1×106 cells/ml) were collected in a tube, washed with PBS twice, resuspended in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) and then used to produce the TCL through five cycles of freezing in liquid nitrogen and thawing at 37°C in a water bath, followed by low-speed centrifugation (460 × g at room temperature for 10 min) to remove subcellular particles. For preparing the NDV-TCL, the tumor cells were first incubated for 1 h at 37 in a CO2 incubator in the presence of NDV J76 (16 hemagglutinating units per 1×106 cells). The NDV-infected tumor cells were then washed with PBS to remove the unbound virus and further cultured for 48 h. The same approach as that used for preparing the TCL was then adopted for the preparation of the NDV-TCL. The protein concentrations of the TCL and NDV-TCL were then determined using the Bicinchoninic Protein Assay kit (Wuhan Boster Biological Technology, Ltd., Wuhan, China).
Loading DCs with TCL and NDV-TCL
For preparation of the DCs loaded with TCL and NDV-TCL, on day 7 following chemokine treatment, the cultured DCs (1×106 cells/ml) were loaded with TCL (100 µg/ml) or NDV-TCL (100 µg/ml); 1 µg/ml LPS was then added after 4 h. On day 8, the DCs were collected for the subsequent experiments. The surface phenotype of the different DCs (DCs, TCL-DCs, NDV-TCL-DCs) was then determined using a FACSCalibur flow cytometer (BD Biosciences) following staining with HLA-DR-PerCP, CD80-PE, CD86-FITC and CD83-APC (ready-to-use dilution, as aforementioned) at room temperature for 15 min. Appropriate isotype-matched antibodies were used as controls. The data were analyzed by FlowJo V7.6 software.
Expression of programmed death ligand 1 (PD-L1) on the different DCs
PD-L1 expression on the different DCs was also determined using a FACSCalibur flow cytometer with PD-L1-APC (dilution: ready for use; cat. no. 329708; BioLegend, Inc., San Diego, CA, USA) at room temperature for 15 min on day 8. Data were analyzed using FlowJo 7.6 software.
T-cell proliferation assays
Allogenic T cells were obtained from one healthy volunteer (male, 56 years old) and stained with 5 µM carboxy fluorescein succinimidyl ester (Molecular Probes; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The labeled cells were then washed, suspended (1×106 cells/ml) and co-cultured with the allogenic DCs, TCL-DCs, or NDV-TCL-DCs at a ratio of 5:1 in 200 µl RPMI-1640 medium with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 1X non-essential amino acid (Gibco; Thermo Fisher Scientific, Inc.), 2 mM L-glutamine (Sigma-Aldrich; Merck KGaA), 55 nM β-mercaptoethanol (Sigma-Aldrich; Merck KGaA), and 100 nM sodium pyruvate (Sigma-Aldrich; Merck KGaA) in 96-well rounded bottom plates. After 5 days, the cells were collected, washed with PBS and incubated with CD3-APC (dilution: ready for use; cat. no. 340440; BD Biosciences) for 15 min at room temperature, and the proliferating T cells were then characterized by flow cytometry, as aforementioned.
Determination of intracellular interferon (IFN)-γ and IL-2 levels
The T cells were co-cultured with autologous DCs, TCL-DCs, or NDV-TCL-DCs at a ratio of 5:1 for 24 h. The secretion of IFN-γ and IL-2 from the T cells was then determined using the BD Cytofix/Cytoperm™ kit (BD Biosciences) according to the manufacturer's protocol. In brief, the co-cultured cells were harvested and adjusted to a concentration of 1×106 cells/ml in RPMI-1640 medium containing 10% FBS and incubated with 0.1% GolgiStop (BD Biosciences) for 4 h. Next, the cells were stained with CD3-PerCP (cat. no. 347344), CD4-FITC (cat. no. 340133) or CD8-FITC (cat. no. 555366) (all BD Biosciences) at room temperature for 15 min, followed by intracellular staining with IFN-γ-APC (cat. no. 554702) or IL-2–PE (cat. no. 554428) or rat IgG2b κ-PE (556925) or mouse IgG1 κ-APC (cat. no. 555751) (all from BD Biosciences) at room temperature for 30 min to determine intracellular cytokine levels. All the antibodies used above were ready for use and did not require dilution.
Determination of soluble IFN-γ levels
The T cells (1×106 cells/ml) were co-cultured with autologous DCs, TCL-DCs, or NDV-TCL-DCs at a ratio of 5:1 at 37°C for 24 h in a humidified atmosphere of 5% CO2. The level of IFN-γ in the cell culture supernatants was then quantified using the cytometric bead array human IFN-γ Flex set kit (BD Biosciences) on the FACSCalibur flow cytometer, according to the manufacturer's protocol. The data were analyzed by FlowJo V7.6 software
Statistical analysis
The data were analyzed using non-parametric Friedman's 2-way analysis of variance by ranks test, followed by pairwise comparisons, the statistical comparison method in SPSS 23 software, and the P-values were adjusted. All statistical analyses were performed using IBM SPSS 23 software (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference. Each experiment was repeated in triplicate.
Results
Characteristics of the DCs
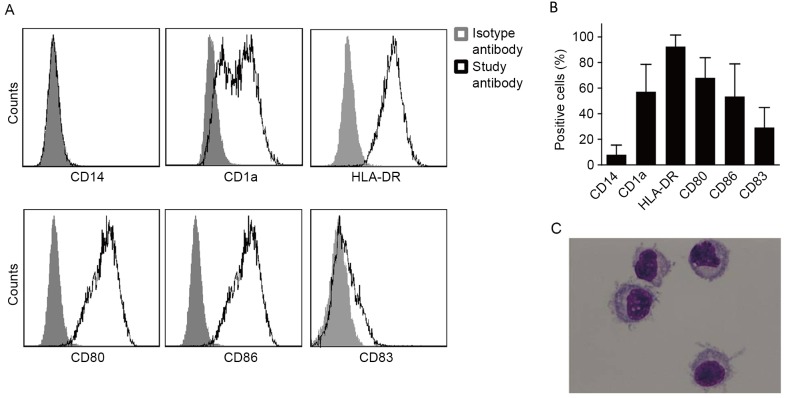
DCs induced by human PBMCs exhibit a high level of HLA-DR expression, a moderate level of CD1a, CD80 and CD86 expression, and a low level of CD83 expression. However, a low level of CD14 expression was also exhibited by the DCs (Fig. 1A and B).
Figure 1.
Characteristics of DCs induced from PBMCs of cancer patients. DCs were induced from the PBMCs of lung cancer patients in the presence of granulocyte macrophage colony stimulating factor and interleukin-4 following in vitro culture for 7 days. (A) Representative flow cytometry plots demonstrating the surface molecular expression on the DCs. (B) The percentage of surface molecular expression on DCs induced from 12 patients with lung cancer. (C) Morphology of the induced DCs stained with Giemsa (magnification, ×1,000). CD14, cluster of differentiation 14; DCs, dendritic cells; PBMCs, peripheral blood monocytes; HLA-DR, human leukocyte antigen-D related.
Mature DCs exhibited a laterally positioned nucleus and less cytoplasm. A number of dendritic protuberances were also observed on the cell membrane surface, which is the typical morphological characteristic of DCs (Fig. 1C).
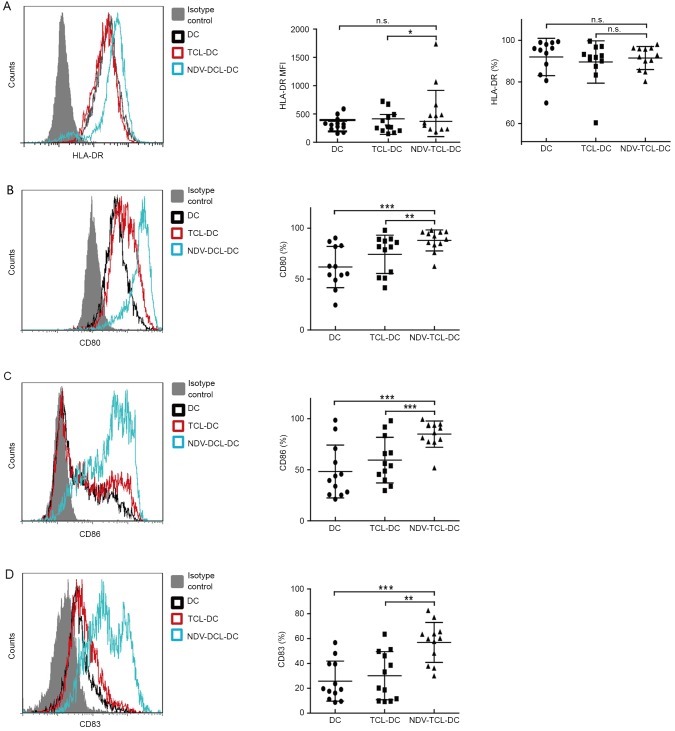
NDV-TCL-DCs exhibit increased levels of co-stimulatory molecule expression
DC activation of naive T cells requires two signals: One is initiated by the T-cell receptor (TCR) recognition of the antigen peptide presented by the major histocompatibility complex (MHC), including HLA-DR; the other is mediated by the co-stimulatory molecule CD28 on T cells, with its ligands CD80 and CD86, which are expressed on mature DCs. Nearly all of the NDV-TCL-DCs (median, 91%; range, 80–98%) expressed HLA-DR (Fig. 2A) and the expression level was increased compared with the TCL-DCs. CD80, CD83 and CD86 are important markers of mature DCs. During the process of DC maturation, the expression of CD83, CD80 and CD86 on DCs is upregulated. In the present study, the expression of CD80, CD83 and CD86 on the NDV-TCL-DCs was increased compared with that on the TCL-DCs (P<0.01, P<0.001 and P<0.01, respectively) and unloaded DCs (P<0.001; Fig. 2B-D). These data indicated that the NDV-TCL-DCs were more mature than the TCL-DCs and that they possessed a stronger potential to promote T cells to exhibit antitumor ability.
Figure 2.
Increased expression of co-stimulatory molecules on DCs loaded with the lysate of tumor cells infected with NVD. (A) HLA-DR expression on the three types of DCs. (B) CD80 expression on the three types of DCs. (C) CD86 expression on the three types of DCs. (D) CD83 expression on the three types of DCs. All error bars represent the mean ± standard deviation. *P<0.05; **P<0.01; ***P<0.001. n.s., not significant; CD80, cluster of differentiation 80; NDV-TCL-DCs, Newcastle disease virus-tumor cell lysate-dendritic cells; MFI, mean fluorescence intensity; HLA-DR, human leukocyte antigen-D related.
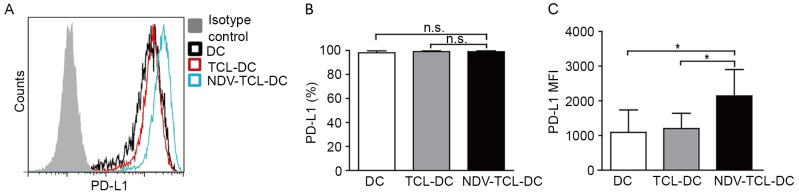
NDV-TCL-DCs demonstrate an increased PD-L1 expression compared with TCL-DCs
A number of studies have previously reported that PD-L1 is expressed at high levels on mature DCs, along with co-stimulatory molecules (15–18). In the present study, it was demonstrated that although the expression of PD-L1 on DCs did not differ significantly between the three groups, the expression of PD-L1 on the NDV-TCL-DCs tended to increase in comparison with the other two groups (Fig. 3A). These data further confirmed that the NDV-TCL-DCs may be more mature than the TCL-DCs (19).
Figure 3.
Increased PD-L1 expression on DCs loaded with the lysate of tumor cells infected with NDV. (A) A representative flow cytometry plot exhibiting PD-L1 expression on the three types of DCs. (B) Graph demonstrating the MFI of PD-L1 on the three types of DCs induced from 12 patients with lung cancer. (C) Graph illustrating the percentage of PD-L1 expression on the three types of DCs induced from peripheral blood monocytes of 12 patients with lung cancer. *P<0.05. n.s., not significant; NDV-TCL-DCs, Newcastle Disease Virus-tumor cell lysate-dendritic cells; PD-L1, programmed death ligand 1; MFI, mean fluorescence intensity.
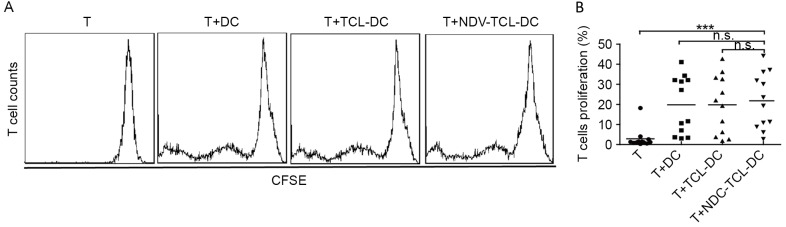
NDV-TCL-DCs promote allogeneic T-cell proliferation
A mixed lymphocyte reaction is an ex vivo cellular immune reaction that occurs between two allogeneic lymphocyte populations. This assay was used to evaluate the ability of the NDV-TCL-DCs to promote T-cell proliferation in vitro. As depicted in Fig. 4, incubation with the NDV-TCL-DCs significantly increased the proliferation of T cells compared with the T cells alone (P<0.001). However, there were no significant differences between the increase in T-cell proliferation caused by the NDV-TCL-DCs and that caused by TCL-DCs, which indicated that NDV-TCL-DCs had the same potential to stimulate allogeneic T-cell proliferation as TCL-DCs.
Figure 4.
DCs loaded with the lysate of tumor cells infected with NDV promoted allogeneic T-cell proliferation. (A) A representative flow cytometry plot demonstrating the proliferation of T cells co-cultured with the three types of DCs. (B) Graph demonstrating the proliferation of T cells co-cultured with the three types of DCs induced from peripheral blood monocytes of 12 patients with lung cancer. ***P<0.001. n.s., not significant; NDV-TCL-DCs, Newcastle Disease Virus-tumor cell lysate-dendritic cells; CSFE, carboxy fluorescein succinimidyl ester; T, T cell.
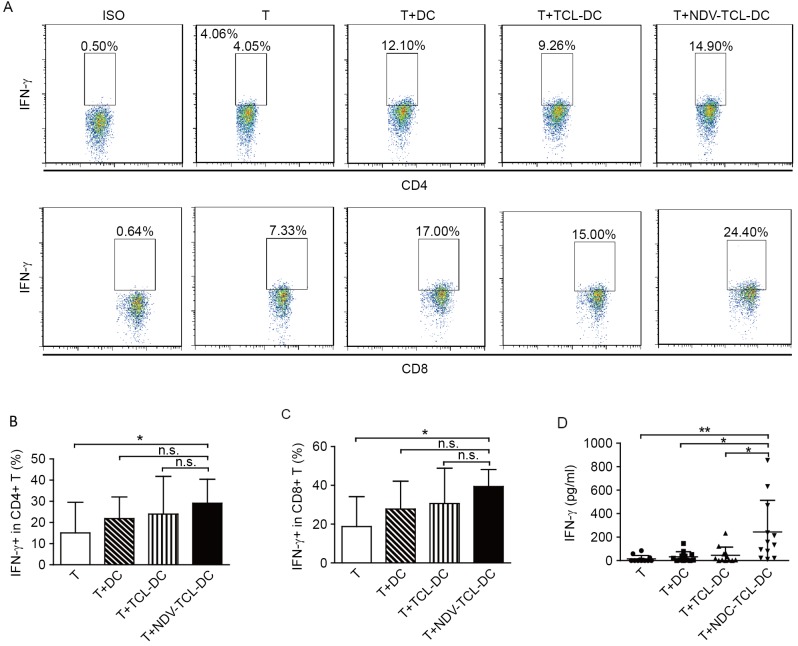
NDV-TCL-DCs enhance the IFN-γ secretion of autologous CD4+ and CD8+ T cells
The secretion of IFN-γ is regarded as an index of T-cell activation. The results of the present study demonstrated that incubation with NDV-TCL-DCs significantly increased the intracellular IFN-γ production in both CD4+ and CD8+ T cells compared with T cells alone (P<0.05; Fig. 5A-C). The increase in IFN-γ production by the T cells indicates that stimulation with NDV-TCL-DCs can effectively activate CD4+ and CD8+ T cells. Simultaneously, IFN-γ release in the supernatants of the T cells co-cultured with NDV-TCL-DCs was measured using the cytometric bead array technique. As depicted in Fig. 5D, there was a negligible level of IFN-γ in the supernatants of the T cells cultured alone and those co-cultured with TCL-DCs. However, the level of IFN-γ in the supernatant of T cells co-cultured with NDV-TCL-DCs was significantly increased (P<0.01; Fig. 5D). These experiments revealed significant increases in the activation of CD4+ T cells and CD8+ cytotoxic T lymphocytes in response to co-culture with NDV-TCL-DCs. Additionally, the results support our hypothesis that immunization with NDV-TCL-DCs may activate specific CD4+ and CD8+ T cells in cancer patients.
Figure 5.
DCs loaded with the lysate of tumor cells infected with the NDV enhanced the secretion of IFN-γ from CD4+ and CD8+ T cells. (A) A representative flow cytometry plot demonstrating the percentage of IFN-γ-positive cells among CD4+ and CD8+ T cells co-cultured with the three types of DCs. The percentage of IFN-γ-positive cells among (B) CD4+ and (C) CD8+ T cells co-cultured with the three types of DCs induced from peripheral blood monocytes of 12 patients with lung cancer. (D) Soluble IFN-γ release in the supernatant of T cells co-cultured with the three types of DC. All error bars represent the mean ± standard deviation. *P<0.05; **P<0.01; n.s., not significant; IFN-γ, interferon-γ; CD4, cluster of differentiation 4; NDV-TCL-DCs, Newcastle Disease Virus-tumor cell lysate-dendritic cells; T, T cell.
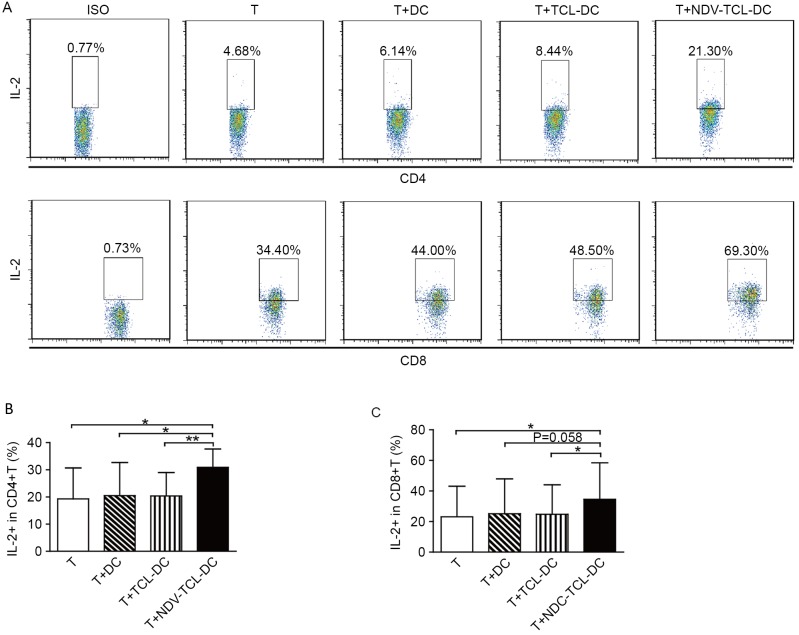
NDV-TCL-DCs enhances IL-2 secretion by autologous CD4+ and CD8+ T cells
IL-2 serves a critical role in effector T-cell development and antitumor functions. Therefore, the secretion of intracellular IL-2 from CD4+ and CD8+ T cells co-cultured with the NDV-TCL-DCs was also measured; it was demonstrated that co-culture with the NDV-TCL-DCs significantly enhanced the percentage of IL-2-positive CD4+ and CD8+ T cells (P<0.05; Fig. 6). However, this effect was not observed upon co-culture with TCL-DCs or DCs alone. These results indicated that the NDV-TCL-DCs had the ability to enhance the percentage of IL-2-positive T cells.
Figure 6.
DCs loaded with the lysate of tumor cells infected with the NDV-TCL-DCs induced the production of IL-2 from CD4+ and CD8+ T cells. (A) A representative fluorescence activated cell sorting plot demonstrating the percentage of IL-2-positive cells among CD4+ and CD8+ T cells co-cultured with the three types of DCs. The percentage of IL-2-positive cells among (B) CD4+ and (C) CD8+ T cells co-cultured with the three types of DCs induced from peripheral blood monocytes of 12 patients with lung cancer. All error bars represent the mean ± standard deviation. *P<0.05; **P<0.01. CD4, cluster of differentiation 4; NDV-TCL-DCs, Newcastle Disease Virus-tumor cell lysate-dendritic cells; IL-2, interleukin-2; T, T cell.
Discussion
The present study attempted to develop a novel DC vaccine using DCs loaded with NDV-infected TCL. NDV-TCL-DCs promoted T-cell proliferation and antitumor cytokine secretion. The results of the present study indicated that loading DCs with NDV-TCL could enhance the antigen-presenting ability of DCs. We hypothesize that this novel DC-loading strategy could aid the development of novel DC vaccines for tumor immunotherapy in the future.
DC vaccines emerged in an effort to avoid possible interference with therapeutic efficacy due to the dysfunction of endogenous DCs, which were commonly observed in cancer patients (20,21). However, it is important that the DCs used in these vaccines are presented in a ‘mature’ state to activate an antigen-specific immune response upon T-cell encounter. This differentiated state is characterized by the expression of several co-stimulatory molecules, which are necessary to activate secondary signals in the immunological synapse (22). These molecules include CD80, CD86, CD40, CD70 and inducible T-cell co-stimulator ligand (ICOS-L), which interact with their counterparts CD28, CD40L, CD27, and ICOS, respectively, which are expressed by T cells. In addition, mature DCs should exhibit elevated levels of antigen-presenting molecules, including MHC class I, MHC class II and CD1 (20,23). The present study demonstrated that co-culture with the NDV-TCL-DCs upregulated the expression of HLA-DR, CD80, CD86 and CD83, which further facilitated the process of T-cell proliferation, and activation. Without such co-stimulation, T-cell anergy or immune tolerance could occur.
In addition to the aforementioned molecules, PD-L1 is constitutively expressed at low levels on hematopoietic cells, including resting T cells, B cells, myeloid cells and immature DCs, as well as on non-hematopoietic cells, including those in the lungs, heart and other organs (24–26). Previous studies have reported that PD-L1 is also expressed at high levels on mature DCs, along with other co-stimulatory molecules (17,27). PD-L1 expression on DCs is necessary for maintaining immune tolerance in humans (28,29). PD-L1 expression on antigen presenting cells may inhibit the induction of cytotoxic T lymphocytes by transducing negative signals onto T cells; however, it has also been reported that the expression of PD-L1 on antigen presenting cells, along with CD80 and CD86, enhances the induction of antigen-specific cytotoxic T lymphocytes, an effect probably depends on the fine-tuning of the excessive stimulation of CD80 and CD86 (27). In the present study, the NDV-TCL-DCs demonstrated an increasing trend of PD-L1 expression, which was accompanied by a higher level of CD80 and CD86 expression; therefore, NDV-TCL could increase the PD-L1, CD80 and CD86 expression in DCs. This may be the reason why the NDV-TCL-DCs were demonstrated to have the strongest ability to induce T-cell proliferation and cytokine secretion.
The ability to secrete cytokines is critical in determining whether T cells are activated when they recognize an alloantigen (30). Monitoring the secretion of major cytokines, including IFN-γ and IL-2 by CD4+ and CD8+ T cells, could be useful in determining the immune response to stimulation with DCs. The experiments were conducted accordingly and it was demonstrated that the secretion of IFN-γ and IL-2 by the CD4+ and CD8+ T cells was significantly higher when they were co-cultured with the NDV-TCL-DCs compared with that when co-cultured with the TCL-DCs. Activated CD4+ and CD8+ T cells are necessary for the antitumor activity of the immune system (31). Therefore, the results of the present study indicated that the NDV-TCL-DCs had the ability to promote the secretion of antitumor cytokines from T cells and that these DCs may therefore be a potential candidate for use in tumor vaccines.
In conclusion, NDV-TCL-DCs were successfully produced and it was demonstrated that they possess the potential ability to promote T-cell proliferation and antitumor cytokine secretion. However, the underlying mechanisms were not elucidated. Future work should focus on investigation of these mechanisms. Nevertheless, the results of the present study will be helpful in developing novel DC-based tumor vaccines in the future.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- DCs
dendritic cells
- NDV
Newcastle Disease Virus
- TCL
tumor cell lysate
- NDV-TCL
lysate of tumor cells infected with NDV
- TCL-DCs
DCs loaded with the TCL
- NDV-TCL-DCs
DCs loaded with NDV-TCL
- TAA
tumor associate antigens
- PBMCs
Peripheral blood mononuclear cells
- FBS
fetal bovine serum
- MHC
major histocompatibility complex
- ICOS-L
inducible T-cell co-stimulator ligand
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 31470798, 31700764 and 81402436), the Provincial Science Fund of Jilin Provincial Department of Finance (grant nos. 2014N147, 2017C022, 20150204027YY, 20170622011JC, 20140414014GH and 20150520155JH), the Norman Bethune Program of Jilin University (grant no. 2012202) and the Youth Fund of the First Hospital of Jilin University (grant no. JDYY72016003).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HJ and WL conceived and designed the research. LZ, CN and ML performed the experiments and analyzed the data. DX, JX, XS and JC contributed to sample collection. CN, LZ, and XS wrote the manuscript. All authors reviewed the manuscript.
Ethics approval and consent to participate
A total of 12 lung cancer patient were recruited from the First Hospital of Jilin University (Changchun, China). All of them provided written informed consent for the use of biospecimens for research purposes according to the Declaration of Helsinki. The present study was permitted by the Ethics Committee of the First Hospital of Jilin University and carried out in accordance with the approved guideline ‘Use of experimental animals and human subjects.’
Consent for publication
The patients provided written informed consent for the publication of all the associated data in this article. The consent forms are available on reasonable request and will be treated confidentially.
Competing interests
The authors declare that there was no competing interests.
References
- 1.Sehgal K, Dhodapkar KM, Dhodapkar MV. Targeting human dendritic cells in situ to improve vaccines. Immunol Lett. 2014;162:59–67. doi: 10.1016/j.imlet.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM. Some interfaces of dendritic cell biology. APMIS. 2003;111:675–697. doi: 10.1034/j.1600-0463.2003.11107802.x. [DOI] [PubMed] [Google Scholar]
- 3.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 4.Schirrmacher V, Ahlert T, Pröbstle T, Steiner HH, Herold-Mende C, Gerhards R, Hagmüller E, Steiner HH. Immunization with virus-modified tumor cells. Semin Oncol. 1998;25:677–696. [PubMed] [Google Scholar]
- 5.Thara E, Dorff TB, Pinski JK, Quinn DI. Vaccine therapy with sipuleucel-T (Provenge) for prostate cancer. Maturitas. 2011;69:296–303. doi: 10.1016/j.maturitas.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Cohn L, Delamarre L. Dendritic cell-targeted vaccines. Front Immunol. 2014;5:255. doi: 10.3389/fimmu.2014.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillerme JB, Boisgerault N, Roulois D, Ménager J, Combredet C, Tangy F, Fonteneau JF, Gregoire M. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin Cancer Res. 2013;19:1147–1158. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- 8.Maamary J, Array F, Gao Q, García-Sastre A, Steinman RM, Palese P, Nchinda G. Newcastle disease virus expressing a dendritic cell-targeted HIV gag protein induces a potent gag-specific immune response in mice. J Virol. 2011;85:2235–2246. doi: 10.1128/JVI.02036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournier P, Schirrmacher V. Oncolytic Newcastle Disease Virus as cutting edge between tumor and host. Biology (Basel) 2013;2:936–975. doi: 10.3390/biology2030936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melchjorsen J, Jensen SB, Malmgaard L, Rasmussen SB, Weber F, Bowie AG, Matikainen S, Paludan SR. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J Virol. 2005;79:12944–12951. doi: 10.1128/JVI.79.20.12944-12951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Fournier P, Arnold A, Schirrmacher V. Polarization of human monocyte-derived dendritic cells to DC1 by in vitro stimulation with Newcastle Disease Virus. J BUON. 2009;14(Suppl 1):S111–S122. [PubMed] [Google Scholar]
- 13.Austin FC, Boone CW. Virus augmentation of the antigenicity of tumor cell extracts. Adv Cancer Res. 1979;30:301–345. doi: 10.1016/S0065-230X(08)60900-8. [DOI] [PubMed] [Google Scholar]
- 14.Sinkovics JG, Horvath JC. Virus therapy of human cancers. Melanoma Res. 2003;13:431–432. doi: 10.1097/00008390-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 17.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3 - potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 19.Williams EH, Williams CA, McLeod JD. Identification of PDL-1 as a novel biomarker of sensitizer exposure in dendritic-like cells. Toxicol In Vitro. 2010;24:1727–1735. doi: 10.1016/j.tiv.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Kalinski P, Edington H, Zeh HJ, Okada H, Butterfield LH, Kirkwood JM, Bartlett DL. Dendritic cells in cancer immunotherapy: Vaccines or autologous transplants? Immunol Res. 2011;50:235–247. doi: 10.1007/s12026-011-8224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao F, Liu C, Guo J, Sun W, Xian L, Bai D, Liu H, Cheng Y, Li B, Cui J, et al. Radiation-driven lipid accumulation and dendritic cell dysfunction in cancer. Sci Rep. 2015;5:9613. doi: 10.1038/srep09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichardt P, Dornbach B, Gunzer M. APC, T cells, and the immune synapse. Curr Top Microbiol Immunol. 2010;340:229–249. doi: 10.1007/978-3-642-03858-7_12. [DOI] [PubMed] [Google Scholar]
- 23.Linette GP, Carreno BM. Dendritic cell-based vaccines: Shining the spotlight on signal 3. Oncoimmunology. 2013;2:e26512. doi: 10.4161/onci.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talay O, Shen CH, Chen L, Chen J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci USA. 2009;106:2741–2746. doi: 10.1073/pnas.0813367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 26.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 27.Goto T, Nishida T, Takagi E, Miyao K, Koyama D, Sakemura R, Hanajiri R, Watanabe K, Imahashi N, Terakura S, et al. Programmed death-ligand 1 on antigen-presenting cells facilitates the induction of antigen-specific cytotoxic T lymphocytes: Application to adoptive T-cell immunotherapy. J Immunother. 2016;39:306–315. doi: 10.1097/CJI.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006;177:8291–8295. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 29.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Millán O, Rafael-Valdivia L, Torrademé E, López A, Fortuna V, Sánchez-Cabus S, López-Púa Y, Rimola A, Brunet M. Intracellular IFN-gamma and IL-2 expression monitoring as surrogate markers of the risk of acute rejection and personal drug response in de novo liver transplant recipients. Cytokine. 2013;61:556–564. doi: 10.1016/j.cyto.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Shimoda M, Tomimaru Y, Charpentier KP, Safran H, Carlson RI, Wands J. Tumor progression-related transmembrane protein aspartate-β-hydroxylase is a target for immunotherapy of hepatocellular carcinoma. J Hepatol. 2012;56:1129–1135. doi: 10.1016/j.jhep.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.