Abstract
Insulin-like growth factor-1 receptor (IGF-1R) is a cell membrane receptor involved in cell proliferation and apoptosis, which is highly expressed in lung squamous cell carcinoma (SCC). The present study aimed to observe the influence of IGF-1R silencing on the radiosensitivity of SCC and investigate the potential mechanisms involved. Human lung SCC H520 cells with relatively high expression of IGF-1R were used. IGF-1R expression was silenced using short hairpin RNA. The influence of IGF-1R silencing on radiosensitivity and apoptosis was assessed using a clone formation assay and flow cytometry. The expression levels of proteins relevant in DNA damage repair and hypoxic signaling pathways were analyzed using western blotting. Decreased expression of IGF-1R led to an increase in the sensitivity of H520 cells to irradiation. Molecular analysis showed that the reduced expression of IGF-1R decreased the protein expression of ataxia-telangiectasia mutated (ATM), H2A histone family member X (H2AX) and p53 binding protein 1 (53BP1), which are associated with the DNA repair pathway. Furthermore, 53BP1 is also known to be involved in apoptosis. Proteins involved in the hypoxic pathway, including hypoxia inducible factor 1 α (HIF-1α), matrix metallopeptidase 9 (MMP-9) and vascular endothelial growth factor A (VEGFA) were also involved in the radiosensitivity. In conclusion, decreased expression of IGF-1R leads to improved radiosensitivity of SCC cells, and the underlying mechanism may be associated with the decreased expression of proteins involved in ATM/H2AX/53BP1 DNA damage repair and the HIF-1α/MMP-9 hypoxic pathway, which results in the induction of apoptosis and increased radiosensitivity. These findings suggest that targeting of IGF-1R may represent a novel approach for lung SCC radiation treatment.
Keywords: insulin-like growth factor-1 receptor, radio-sensitivity, DNA repair, lung squamous cell carcinoma
Introduction
Lung cancer is the leading cause of death worldwide (1). Radiotherapy has been applied to treat advanced non-small-cell lung cancer (NSCLC) for the past two decades. However, one major obstacle to successful radiotherapy is the existence of radio-resistance (2). Tyrosine kinase inhibitors can improve the survival of patients with radio-resistant lung adenocarcinoma. However, few treatment targets have been identified for treatment-resistant lung squamous carcinoma (3,4). Therefore, it is important to explore more effective targeting molecules for the radio-resistant lung squamous carcinoma.
Insulin-like growth factor 1 receptor (IGF-1R) is a transmembrane receptor tyrosine kinase involved in the development and progression of cancer, whereby receptor activation strongly promotes cell growth and survival (5). IGF-1R is a heterotetrameric plasma membrane glycoprotein. Once a ligand binds to the IGF-1R, it will induce activation of the intrinsic tyrosine kinase of the β-subunit, leading to receptor phosphorylation and activation of protein kinases which are associated with carcinogenesis and tumor progression (6,7). IGF-1R has been found to be over-expressed in a variety of human malignancies including lung cancer. One study showed that IGF-1R expression is significantly associated a reduction in overall survival. Median survival in lung cancer patients with high and low IGF-1R expression is 26.51 vs. 47.77 months (P=0.017), and disease-free survival is 17.44 vs. 37.65 months (P=0.045), respectively. IGF-1R is activated in NSCLC patients, particularly in those with squamous cell carcinoma (SCC) (8). IGF-1R plays an important role in lung SCC. CP-751,871 is a fully human monoclonal antibody against IGF-IR, which can increase the radio-sensitivity of NSCLC cell lines and application of this antibody has been attributed to inhibition of DNA repair and enhanced irradiation-induced apoptosis (9). However, the mechanisms underlying the association between IGF-1R over-expression and radio-resistance in human lung squamous carcinoma cells are still unknown.
Radiation induces DNA damage and destroys the structure of DNA molecules, including nucleotide excision, single strand breaks and double strand breaks (DSBs) (10,11). The DNA lesions can be recognized by DNA checkpoints which can trigger DNA repair pathways (12). However, apoptosis will be initiated if DNA damage is not repaired. In the current study, a lentiviral vector-based RNA interference expression system was used to stably suppress the expression of IGF-1R and the effects of IGF-1R knockdown on the radio-sensitivity of lung SCC and the potential mechanisms were explored.
Materials and methods
Cell culture, materials and reagents
The H520 human lung squamous carcinoma cells were purchased from the Cancer Institute of Tongji University (Shanghai, China). The cells were grown in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and maintained in a humidified 5% CO2 atmosphere at 37°C. The major sources of materials and reagents used in the present study are as follows: IGF-1R, phospho-IGF1R, phospho-H2A histone family member X (H2AX), p53 binding protein 1 (53BP1), phospho-Akt, hypoxia inducible factor 1 α (HIF-1α), and β-actin were supplied by Cell Signaling Technology, Inc., (Danvers, MA, USA). Phosphorylated ataxia-telangiectasia mutated (p-ATM) was purchased from Abcam (Cambridge, UK). An Annexin V-APC/PI apoptosis detection kit was purchased from BioLegend, Inc., (San Diego, CA, USA). TRIzol reagent was provided by Invitrogen; Thermo Fisher Scientific, Inc. Cell Counting Kit-8 was purchased from Dojindo Molecular Technologies, Inc., (Kumamoto, Japan).
X-ray irradiation
The H520 human lung squamous carcinoma cell line was irradiated using a linear accelerator (Elekta Instrument AB, Stockholm, Sweden) with an 8 MV X-ray at a dose rate of 500 cGy/min, and then cells were further incubated up to specified time points, and harvested for the subsequent experiments.
CCK-8 assay
Cells were seeded into 96-well culture plates at a density of 5,000 cells/well and allowed to adhere for 24 h. After X-ray irradiation, the cells were then incubated for different h in a humidified chamber at 37°C. Viable cells were evaluated with the CCK-8 assay according to the manufacturer's instructions. CCK-8 solution was added to the cells in 96-well plates and then incubated at 37°C for an additional 1 h, and the absorbance at 450 nm was determined at using a microplate reader (ELX800; BioTek Instruments, Inc., Winooski, VT, USA).
Clonogenic assay for radio-sensitivity
Different numbers of cells (0 Gy with 1×103 cells, 2 Gy with 2×103 cells, 4 Gy with 4×103 cells, 6 Gy with 6×103 cells, 8 Gy with 8×103 cells, and 10 Gy with 1×104 cells) were plated in 60-mm dishes depending on the dose of irradiation, and cultured overnight. After X-Ray irradiation with 0, 2, 4, 6, 8, or 10 Gy, the cells were cultured for two weeks at 37°C. Cells were washed three times using phosphate buffered saline (PBS) and fixed using ice-cold methanol for 15 min, and then stained using 1% crystal violet dye for 15 min and rinsed in distilled water to wash away excess dye. Plates were allowed to dry before being scanned. Only colonies consisting of 50 or more cells were counted.
Flow cytometry
The cells were cultured at a density of 5×104 cells per well in growth medium for 12 h in 24-well plates, and irradiated at the indicated doses. Apoptotic cells were quantified using an Annexin-APC/PI apoptosis detection kit and FACS Cali-bur flow cytometry, as described previously. The cells were harvested through centrifugation after irradiation and washed twice using cold PBS. Cells were then re-suspended in 100 µl of Annexin-V binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM Cacl2, pH 7.4), incubated with 5 µl of Annexin-V-APC for 15 min at room temperature, and counterstained using propidium iodide (final concentration: 1 µg/ml). After the incubation period, the cell suspension was diluted with 190 µl of Annexin-V binding buffer. Ten thousand cells were acquired per sample, and data were acquired using flow cytometry with a Becton-Dickinson FACS can flow cytometer with Cell Quest software (BD Biosciences, Franklin Lakes, NJ, USA). Cells in the early stages of apoptosis were Annexin-positive, whereas cells that were Annexin-positive and PI-positive were in the late stages of apoptosis.
Western blot analysis
The treated cells (1×107 cells/6 ml DMEM with 10% FBS in a 90-mm dish) were collected and washed twice using cold PBS. Cells were lysed in 200 µl of lysis buffer. The lysate was incubated on ice for 30 min, vortexed and centrifuged at 14,000 × g for 15 min at 4°C. The supernatant was collected and protein concentration was determined using the Bradford Assay. After the addition of sample loading buffer, the protein samples underwent electrophoresis using a 10% SDS-polyacrylamide gel and were then transferred to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). After blocking for 4 h at room temperature in a solution of 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20, the membranes were incubated overnight at 4°C with primary antibody (against phospho-IGF-1R, IGF-1R, phospho-ATM, phospho-AKT, phospho-H2AX, 53BP1, HIF-1α, matrix metallopeptidase 9 (MMP-9) and VEGFA) at a concentration of 1:1,000 in Tris-buffered saline with 0.1% Tween-20 containing 5% non-fat dry milk. After washes four times, the membranes were incubated with a horseradish peroxidase-conjugated secondary anti-rabbit IgG anti-body (1:5,000 dilution) at room temperature for 1 h and were then washed six times. The blots were developed using an Immobilon™ Western Chemiluminescent detection reagent (EMD Millipore) and the results were recorded using the ChemiDox™ XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Short hairpin RNA (shRNA) infection
The lentiviral vectors expressing shRNA were successfully constructed by Shanghai GenePharma Co., Ltd, Shanghai, China. Cells were seeded at 2×105 per well in 6-well plates and incubated overnight, and the cells were then infected using the following sequences: IGF-1R shRNA-1 (5′-GCAACCTGAGTTACTACATTG-3′), IGF-1R shRNA-2 (5′-GCACCATCTTCAAGGGCAATT-3′), IGF-1R shRNA-3 (5′-GCCCAACACCTACAGGTTTGA-3′), IGF-1R shRNA-4 (5′-GCAAAGTCTTTGAGAATTTCC-3′) or negative control shRNA (5′-TTCTCCGAACGTGTCACGT-3′). Then the cells were selected using Puromycin (2 µg/ml), whereby puromycin-resistant colonies were picked, expanded, and analyzed separately.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells were seeded in 6-well plates and allowed to grow in DMEM supplemented with 10% FBS until semi-confluent, before being irradiated. After irradiation, total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the recommendations provided by the manufacturer. cDNA was generated from sample RNA using the RevertAid First strand cDNA Cynthesis kit (no. 1622). Subsequently, 2 µl of cDNA and Taq PCR Master Mix (Qiagen GmbH, Hilden, Germany) were used for PCR. The primer sequences used for the RT-qPCR were as follows: IGF-1R forward primer: CCTGAAAGGAAGCGGAGAG, reverse primer: GGGTCGGTGATGTTGTAGGT; GAPDH forward primer: ATGACATCAAGAAGGTGGTG, reverse primer: CATACCAGGAAATGAGCTTG. PCR amplifications were performed as follows: 5 min at 94°C followed by 25 cycles of [30 sec at 94°C, 30 sec at 55°C, 30 sec at 72°C] and a final extension at 72°C for 5 min.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Each experiment was performed three times. All data are expressed as mean ± standard deviation (SD) unless otherwise specified. Comparison of data between two groups was statistically analyzed using a two-tailed Student's t-test. P<0.05 was considered to indicate a statistically significant difference. Levels of statistical significance are denoted as *P<0.05, **P<0.01 and OL-0-0-8705P<0.001, as shown in the Figures.
Results
Lentivirus-mediated shRNA efficiently inhibited the expression of IGF-1R in H520 lung squamous carcinoma cells
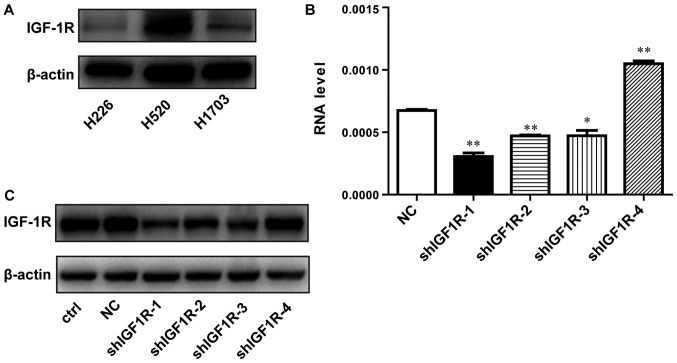
To examine whether IGF-1R expression is associated with the radio-resistance of SCC cells, we detected the expression of IGF-1R in several SCC cells by western blotting. As shown in Fig. 1A, the level of IGF-1R was relatively higher in H520 cells compared with H1703 and H226 cells. Therefore, H520 cells was selected for the following experiments. Lentivirus-mediated shRNA was applied to knockdown the IGF-1R gene. The shRNA infection efficiency was evaluated using RT-qPCR and western blotting. The IGF-1R mRNA levels were reduced by 55% in shIGF1R-1-treated cells compared with cells in the negative control (NC) group, in which no gene was targeted (Fig. 1B). As shown in Fig. 1C, western blotting was used to examine the interference efficiency of different shRNA sequences. Compared with the NC group, IGF-1R expression was also significantly decreased by approximately 69% in shIGF1R-1-treated H520 cells. The knockdown IGF1R-1 cell line was called shIGF-1R H520 cells. Therefore, this sequence (shIGF1R-1) was selected for its effective knockdown of IGF-1R expression and was used for subsequent experiments.
Figure 1.
The effect of lentivirus-mediated shRNA targeting IGF-1R on IGF-1R mRNA levels and protein expression. (A) The expression of IGF-1R in different SCC cells by western blotting. (B) Reverse transcription-quantitative polymerase chain reaction analysis of IGF-1R mRNA in negative control or infected H520 (NC or shIGF-1R) cells. (C) Western blot analysis of IGF-1R protein expression in negative control or infected H520 (NC or shIGF-1R) cells. *P<0.05 and **P<0.01 vs. the NC group. Data are presented as the mean ± SD. IGF-1R, insulin-like growth factor-1 receptor; SCC, squamous cell carcinoma; NC, negative control.
Knockdown of IGF-1R suppression cell growth, increased apoptosis and enhanced the radio-sensitivity of H520 cells
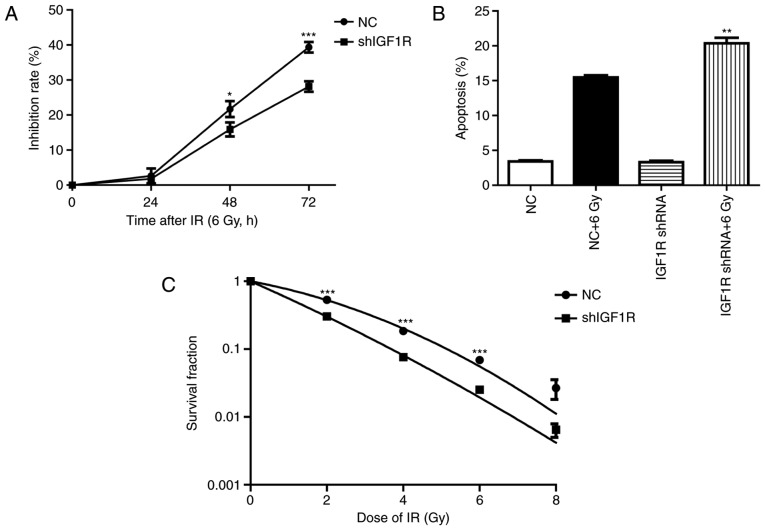
To investigated the effect of IGF1R knockdown on the H520 cells after irradiation. Cell growth was assessed by CCK-8 assay (13). As shown in Fig. 2A, the Inhibition rate of IGF1R knockdown cells at 48 h were (21.7±1.3) and (15.89±1.2%) in the NC group cells (P<0.05, n=3). The Inhibition rate of IGF1R knockdown cells at 72 h were (39.3±0.9) and (28.14±0.9%) in the NC group cells (P<0.001, n=3). Though the proliferation inhibition rate were no significant difference between IGF1R knockdown cells and NC cells after irradiation for 24 h, it has shown increase at 48 h and obviously increased at 72 h in the IGF1R knockdown cells compared with the NC cells.
Figure 2.
The effect of IGF-1R knockdown combined with irradiation on apoptosis and radio-sensitivity in H520 cells. (A) CCK-8 assay analysis of proliferation in NC and stably transfected H520 cells treated using irradiation. *P<0.01 vs. the NC + irradiated (6 Gy) group. ***P<0.001 vs. the NC + irradiated (6 Gy) group. Data are presented as the mean ± SD. (B) Flow cytometric analysis of apoptosis rates in NC and stably infectedH520 cells treated using irradiation. **P<0.01 vs. the NC + irradiated (6 Gy) group. Data are presented as the mean ± SD. (C) Radiation dose-survival curves in NC and stably infected H520 cells treated using irradiation. ***P<0.001 vs. the NC + irradiated (6 Gy) group. Data are presented as the mean ± SD. IGF-1R, insulin-like growth factor-1 receptor; NC, negative control; SD, standard deviation.
To test the effect of IGF-1R knockdown on the apoptosis induced by irradiation, flow cytometry was used to investigate the percentage of apoptotic cells. After an irradiation of 6 Gy for 72 h, the percentage of early apoptosis increased by 78% in the NC group. However, apoptosis increased by 84% after irradiation in the shIGF-1R H520 group. This result indicates that knockdown of IGF-1R increased the early apoptosis of H520 cells after irradiation (P<0.01, n=3; Fig. 2B).
The radio-sensitivity of H520 cells after IGF-1R knockdown was investigated by clone formation assay (12). Compared with the negative control group, IGF-1R knockdown significantly enhanced inhibition of clone formation in H520 cells, which was dependent on the dose of delivered radiation. These survival fraction results illustrate that IGF-1R knockdown increased the radio-sensitivity of H520 cells (P<0.05, compared with NC group; Fig. 2C).
Irradiation induced the activation of IGF-1R signaling pathways in H520 cells
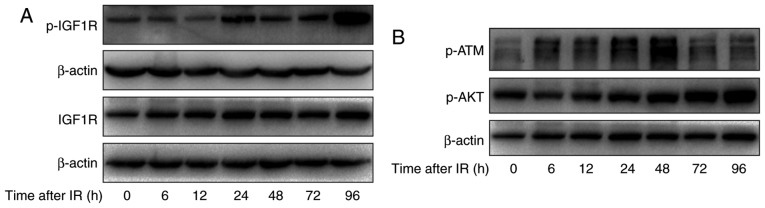
To study the relationship between IGF-1R and irradiation, the effect of irradiation on the activation of IGF-1R and total levels of IGF-1R in H520 cells was assessed. Cells were treated with irradiation of 6 Gy and harvested at different times after irradiation (0, 6, 12, 24, 48, 72 and 96 h). As shown in Fig. 3A, increased levels of phosphorylated IGF-1R were apparent at 12 h and this was sustained to 72 h and increased further at 96 h. Meanwhile, the total levels of IGF-1R were increased at 12 h and this was sustained to 96 h. This finding further indicated that IGF-1R signaling pathways may affect radio-sensitivity in H520 cells.
Figure 3.
The effect of irradiation on the IGF-1R downstream signaling pathway in H520 cells. (A) H520 cells were irradiated at 6 Gy for 0, 6, 12, 24, 48, 72 and 96 h, and their protein content was harvested. Western blot analysis of the expression of p-IGF1R and IGF1R was conducted. (B) H520 cells were irradiated at 6 Gy for 0, 6, 12, 24, 48, 72 and 96 h, and their protein content was harvested. Western blot analysis of the expression of p-ATM and p-AKT was conducted. IGF-1R, insulin-like growth factor-1 receptor.
To explore the mechanism of IGF-1R in the regulation of apoptosis, first, apoptosis-related signaling pathways were investigated using western blotting. As shown in Fig. 3B, irradiation induced the activation of ATM in H520 cells at 6 h and this was sustained to 48 h. Moreover, the phosphorylation of AKT was significantly increased after irradiation, in a time-dependent manner.
Irradiation induced DNA damage repair-related protein expression in H520 cells
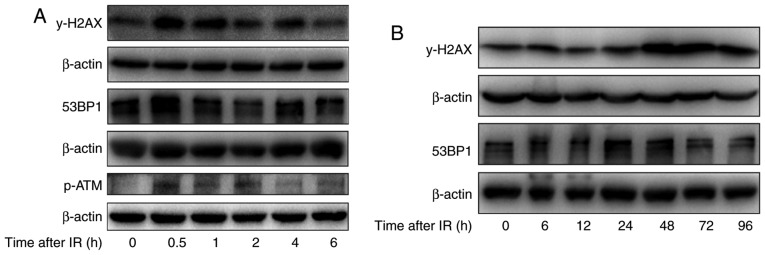
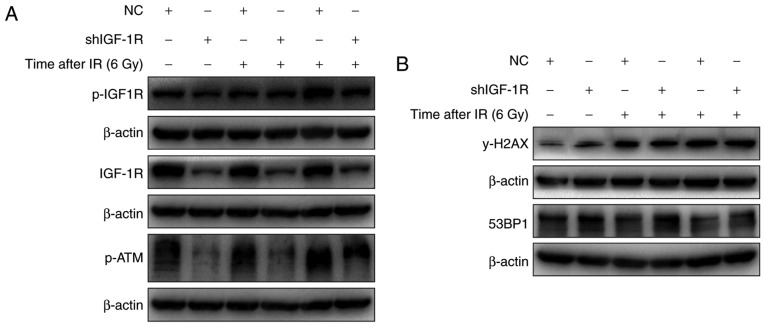
To further study the effect of irradiation on DNA damage repair in H520 cells after irradiation with 6 Gy, the levels of γ-H2AX, the phosphorylated form of H2AX, which can indicate DNA damage repair, were investigated. As shown in Fig. 4A, after short durations of irradiation (0–6 h), the levels of γ-H2AX were first increased at 0.5 h, were reduced substantially by 2 h and were reduced further by 6 h. However, after irradiation for longer durations (0–96 h), the γ-H2AX levels were substantially increased from 24 to 96 h, and remained high at 48 to 96 h. Meanwhile, the levels of phosphorylation ATM were obviously increase at 30 min after irradiation. In addition, the levels of 53BP1, another protein associated with the DNA damage response were also determined, and the expression of 53BP1 increased at 0.5 h and returned to basal levels at 6 h after irradiation in H520 cells, which is similar to the trend of H2AX activation after irradiation. These results suggest that irradiation induced DNA damage repair at 30 min after irradiation in H520 cells by inducing phosphorylation of H2AX and recruiting 53BP1 to the DNA damage sites.
Figure 4.
The effect of irradiation on DNA damage repair-related proteins in H520 cells. (A) H520 cells were irradiated at 6 Gy for 0, 0.5, 1, 2, 4 and 6 h, and their protein content was harvested. Western blot analysis of the expression of 53BP1, p-ATM and γ-H2AX was conducted. (B) H520 cells were irradiated at 6 Gy for 0, 6, 12, 24, 48, 72 and 96 h, and their protein content was harvested. Western blot analysis of the expression of 53BP1 and γ-H2AX was conducted. p-ATM, phosphorylated ataxia-telangiectasia mutated.
In addition to its role in DNA damage repair after a short duration of irradiation, H2AX is also associated with apoptosis after exposure to irradiation. The western blotting analysis indicates that irradiation induced the phosphorylation of H2AX in a time-dependent manner, especially at 48 and 72 h (Fig. 4B).
IGF-1R knockdown blocked the activation of ATM and decreased γ-H2AX levels
The role of ATM activation in the radio-sensitivity of H520 cells after IGF-1R knockdown was investigated. As shown in Fig. 5A, irradiation induced the phosphorylation of IGF-1R in both IGF-1R knockdown and NC cells. And the levels of phosphorylated IGF-1R were lower in the IGF-1R knockdown cells compared with the NC cells. Similarly, the activation of ATM also occurred after irradiation, and the activation trend was similar to that of IGF-1R phosphorylation. These results confirm that IGF-IR knockdown regulates the activation of ATM after irradiation.
Figure 5.
The effect of IGF-1R knockdown on γ-H2AX levels in irradiated H520 cells. (A) IGF-1R shRNA and NC cells were irradiated at 6 Gy for 48 and 72 h, and their protein content was harvested. Western blot analysis of the expression of p-IGF1R, IGF1R and p-ATM was conducted. (B) IGF-1R shRNA and NC cells were irradiated at 6 Gy for 48 and 72 h, and their protein content was harvested. Western blot analysis of the expression of 53BP1 and γ-H2AX was conducted. IGF-1R, insulin-like growth factor-1 receptor; NC, negative control.
The expression of γ-H2AX, which is associated with apoptosis, was increased in H520 cells after irradiation. To evaluate the effect of IGF-1R on apoptosis induced by irradiation, the effect of irradiation on the levels of γ-H2AX after IGF-1R knockdown was determined using western blotting. As shown in Fig. 5B, the levels of γ-H2AX were increased after IGF-1R knockdown in H520 cells, and irradiation also up-regulated the levels of γ-H2AX after irradiation for 48 and 72 h. However, compared with the NC cells, the levels of γ-H2AX demonstrated a smaller increase in the IGF-1R knockdown cells after irradiation. These results illustrate that IGF-1R knockdown attenuated irradiation-induced apoptosis by up-regulating levels of γ-H2AX.
IGF-1R knockdown attenuated irradiation-induced HIF-1α signaling in H520 cells
HIF-1 is a critical gene in tumor resistance and is the downstream of IGF-1R. To explore the mechanism of IGF-1R in radio-resistance of H520 cells, the effect of irradiation on HIF-1α expression was assessed. As shown in Fig. 6, after IGF-1R knockdown, the expression of HIF-1α was decreased in H520 cells. In addition, irradiation caused a significant increase in HIF-1α expression. Compared with the NC cells, the expression of HIF-1α was significantly down-regulated in IGF-1R knockdown cells after irradiation. Furthermore, the proteins derived from downstream genes of HIF-1α, such as MMP-9 and VEGFA, also showed a similar decrease in IGF-1R knockdown cells. After irradiation for 48 h, the decrease was more obvious in the IGF-1R knockdown cells compared with the NC cells.
Figure 6.
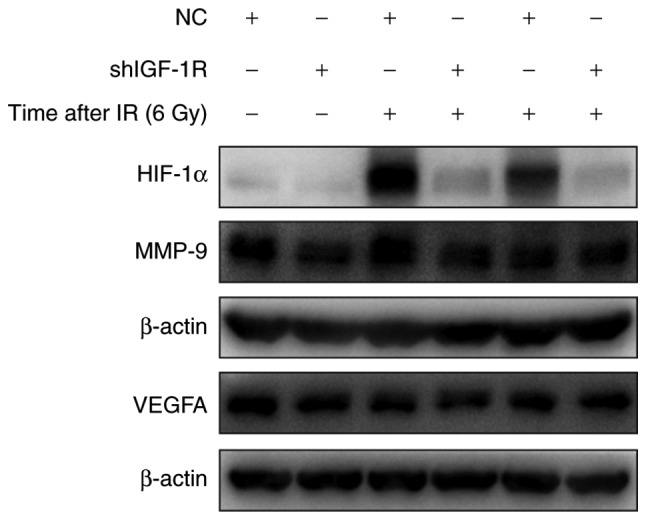
The effect of IGF-1R knockdown on HIF-1α, MMP-9 and VEGFA levels in H520 cells. IGF-1R shRNA and NC cells were irradiated at 6 Gy for 48 and 72 h, and their protein content was harvested. Western blot analysis of the expression of HIF-1α, MMP-9 and VEGFA was conducted. IGF-1R, insulin-like growth factor-1 receptor; HIF-1α, hypoxia inducible factor 1 α; MMP-9, matrix metallopeptidase 9; VEGFA, vascular endothelial growth factor A; NC, negative control.
Discussion
Radiotherapy is frequently applied for the treatment of lung cancer. However, for many patients, the results are still unsatisfactory because of radio-resistance. Therefore, exploring the mechanism underlying treatment resistance is important. Novel therapeutic approaches, such as up-regulating tumor suppressive genes or down-regulating oncogenes, which can enhance the radio-sensitivity or chemo-sensitivity of tumor cells, are urgently required.
IGF-1R is a transmembrane receptor tyrosine kinase, which is frequently over-expressed in tumors, and involved in cell proliferation, protection against apoptosis, cell invasion and metastasis. IGF-1R is over-expressed in lung squamous carcinoma and patients with such tumors demonstrate resistance to conventional anti-cancer treatments including radiotherapy and chemotherapy. Therefore, IGF-1R has attracted increased attention as an anti-cancer treatment target (14). Various strategies have been employed to inhibit IGF-1R expression and function, including anti-sense oligonucleotides, specific IGF-1R inhibitors, antibodies to IGF-IR, or dominant negative IGF-1R mutants, which have been shown to inhibit in vitro or in vivo growth of these tumors, enable reversal of the transformed phenotype and induce apoptosis (15–17). Some researchers have shown that the activation of IGF-1R signaling pathways induces chemo-resistance in a variety of malignancies (18). It has been reported that siRNA, antisense or monoclonal antibody-mediated inhibition of IGF-1R can enhance the chemo-sensitivity of human esophageal squamous carcinoma and breast cancer cells (19,20). However, there have been no reports concerning the correlation between IGF-1R expression and the radio-resistance of lung squamous carcinoma cells. In the present study, the therapeutic potential of a lentivirus-mediated shRNA targeting IGF-1R, combined with radiotherapy, for the treatment of human lung squamous carcinoma was investigated. RNA interference with shRNA was used to stably target IGF-1R, and can effectively down-regulate the expression of IGF-1R in human H520 lung squamous carcinoma cells.
Exposure to irradiation immediately triggers DSBs, which results in recruitment of phosphorylated H2AX to the DSB sites. This is the early response to irradiation-induced DSBs. Subsequently, DNA damage repair pathways are activated to prevent cell division and repair the damage to promote cell survival. The pathways are initiated within a few h after irradiation, and the phosphorylation of H2AX is crucial for the repair process. However, cell apoptosis will be induced if the DNA damage is unrepaired. DSBs are the most important lesion in the induction of apoptosis. In the current study, it was found that H2AX is phosphorylated at 30 min after irradiation, and returns to basal levels at 4 h after irradiation. The results indicate that DNA damage repair occurs at 30 min after irradiation in H520 cells, and the phosphorylation of H2AX at Ser 139 is an early response to DSBs induced by irradiation. Meanwhile, we found that the levels of phosphorylation ATM were obviously increase at 30 min after irradiation. In addition, the levels of 53BP1, another important protein associated with the DNA damage response were also determined, and the expression of 53BP1 increased at 30 min and returned to basal levels at 6 h after irradiation in H520 cells, which is similar to the trend of H2AX activation after irradiation. Furthermore, it was demonstrated that the levels of phosphorylated H2AX reached a second peak at 48 h after irradiation for 6 Gy in H520 cells, and this process was sustained to 72 h. Phosphorylation of ATM was activation simultaneously at 48 h after exposuring to irradiation. These results indicated that the DNA damage repair may evoked at 30 min, and once repair process are failure, the cell will occur cell cycle arrest and/or apoptosis. Therefor, it will make sense that H520 cells appear first peak of gamma H2AX at 30 min after irradiation. When DNA damage exceeds repair, cell will die, but this process will not be actively accepted by dying cell, we think residual cell may activate some survival signals to resist death, such as p-AKT pathway (21). Our results have also shown that the levels of p-AKT highly increased at 48 h after irrdiation and may explain the levels of phosphorylated H2AX reaching a second peak at 48 h after irradiation. And this hypothesis needs us to further investigated. In IGF-1R knockdown cells, it was found that the levels of phosphorylated H2AX were up-regulated compared with NC cells. After irradiation, the levels of phosphorylated H2AX increased both in the IGF-1R knockdown cells and NC cells, and the increase was sustained to 72 h. The apoptosis analyses confirmed that greater levels of apoptosis were induced in IGF-1R knockdown cells compared with NC cells after irradiation. These results suggest that the levels of phosphorylated H2AX induced by irradiation can be attributed to cell apoptosis after IGF-1R knockdown in H520 cells.
Previous studies have shown that IGF-1R may enhance DNA repair through activation of IGF-1R/p38 signaling after irradiation in mouse embryo fibroblasts and breast cancer cells (22,23). Irradiation can induce DSBs and activate related kinases, such as ATM, ATR and DNA protein kinase, which can phosphorylate histone H2AX (24). Furthermore, phosphorylated-H2AX is an important marker of radiation-induced DSBs and recruits other DNA repair proteins including 53BP1, MDC1 (mediator of DNA damage check point protein1) and BRCA1 (breast cancer type 1 susceptibility protein) to DSB sites (25). 53BP1 is regulated by ATM after DNA damage. ATM-deficient cells show no 53BP1 hyperphosphorylation and reduced 53BP1 foci formation in response to radiation compared with cells expressing wild-type ATM. 53BP1 is an ATM substrate that is involved early in the DNA damage-signaling pathways and is regulated by ATM after irradiation (26). ATM has a critical role in the response to DNA damage (27). However, little research has focused on the association between IGF-1R and ATM activation. This study is the first to show that IGF-IR knockdown results in significantly decreased levels of phosphorylated ATM in lung squamous carcinoma cells. The levels of phosphorylated ATM in lung squamous carcinoma cells were increased following irradiation. Furthermore, though the increase was more obvious in IGF-1R knockdown cells after irradiation, the levels of phosphorylated ATM were lower in IGF-1R knockdown cells compared with NC cells. These results suggest that IGF-1R signaling has a distinct effect on ATM activation, and IGF-1R knockdown enhances radio-sensitivity by attenuating the activation of ATM/H2AX/53BP1 signaling.
Hypoxia, a tumor-specific microenvironment, is associated with radiation resistance, because the depletion of oxygen affects the radiolysis of H2O and reduces the production of reactive and cytotoxic species, and radiation-induced DNA damage is fixed under normoxia. HIF-1 has been reported to play a key role in hypoxia-related radio-resistance (28). HIF-1 is a heterodimeric transcription factor, including an α-subunit (HIF-1α) and a β-subunit (HIF-1β), and its activity is mainly dependent on the HIF-1α subunit. HIF-1 binds to its cognate DNA sequence, the hypoxia-responsive element, and induces the expression of several factors, such as MMP-9 and VEGF. MMP-9 is involved in the breakdown of extracellular matrix in physiological processes, including angiogenesis, and tumor cell migration. Previous studies have reported that EGF/EGFR signaling activates downstream PI3K/Akt to induce FoxO1 nuclear exclusion, which activates MMP-9 to promote the invasiveness and metastasis of NSCLC (29). VEGF has been reported to not only induce angiogenesis but also protect endothelial cells from the cytotoxic effects of irradiation and consequently increase tumor radio-resistance (30). The hypoxic microenvironment of the tumor is one of the main reasons underlying radio-resistance (31,32).
Hypoxia can induce the expression of several genes including HIF-1, VEGF and MMP-9, which regulate the survival and invasiveness of tumor cells (33–35). However, the association between IGF-1R and HIF-1α in radio-resistance has not been fully elucidated. In the current study, to assess the mechanism of IGF-1R in the radio-sensitivity of H520 cells, the effect of IGF-1R down-regulation and hypoxia on the radio-sensitivity of H520 cells was investigated. The results show that irradiation has an effect on the microenvironment of tumor cells, in terms of increasing the expression of the hypoxia-related gene, HIF-1α, after irradiation in H520 cells. However, the expression of HIF-1α was decreased after IGF-1R knockdown. Irradiation induced HIF-1α expression, but the increase was inhibited in IGF-1R knockdown cells compared with NC cells. These results illustrate that HIF-1α plays an important role in the response to irradiation, and IGF-1R knockdown increases radio-sensitivity through decreasing the expression of HIF-1α.
The levels of MMP-9 and VEGF were analyzed using western blotting: The expression of MMP-9 in H520 cells after treatment with radiation and IGF-1R knockdown is shown in Fig. 6. The cells showed a decrease in the expression of MMP-9 upon inhibition of HIF-1α expression. However, there was no obvious change in the expression of VEGFA because of IGF-1R silencing. The results reveal that the expression of MMP-9 is influenced by HIF-1α levels, whereby MMP-9 levels appear to decrease upon a decrease in HIF-1α levels. Previous studies have reported that both MMP-9 and VEGFA can be influenced by different levels of HIF-1α. It is possible that the radio-sensitivity of H520 cells is influenced by the hypoxic pathway.
Radioresistance is considered to be a majoy obstacles of ineffective treatment. Resistance to apoptosis shown in the presented study maybe one of the reasons. Meanwhile, the other mechanisms, such as autophagic cell death, may also considered as a new way resulting to radioresistance. As shown in various studies, autophagic cell death was considered as the second type of programmed cell death (36–38). The occurrence of apoptosis in the presented study does not exclude the chance of autophagy incidence. Moreover, these two processes may exist simultaneously (39,40). Some of our results (Data not shown) have indicated that irradiation induces the occurrence of autophapy, which was confirmed by an increase expression of autophagic marker LC3-II. However, more investigations are needed to further clarify the mechanisms.
In conclusion, the results of the present study indicated that IGF-1R may play an important role in the radioresistance of SCC cells. And the underlying mechanism is probably related to the decreased expression of proteins involved in ATM/H2AX/53BP1 DNA damage repair and the HIF-1α/MMP-9 hypoxic pathway, which results in the induction of apoptosis and increased radio-sensitivity. These findings suggest that targeting of IGF-1R may represent a new approach for lung SCC radiation treatment.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- IGF-1R
insulin-like growth factor-1 receptor
- SCC
lung squamous cell carcinoma
- ATM
ataxia-telangiectasia mutated
- H2AX
H2A histone family member X
- 53BP1
p53 binding protein 1
- HIF-1α
hypoxia inducible factor 1 alpha
- MMP-9
matrix metallopeptidase 9
- NSCLC
non-small-cell lung cancer
- DSBs
double strand breaks
- MDC1
mediator of DNA damage check point protein1
- BRCA1
breast cancer type 1 susceptibility protein
- shRNA
Short hairpin RNA
Funding
This work was funded by a seed fund of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University (grant no. RJZZ13-026) and the clinical study with combined traditional Chinese medicine and western medicine of Shanghai Municipal Committee for health and family planning (grant no. ZHYY-ZXYJHZX-201610).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XXL performed the experiments, drafted the manuscript and analyzed the data. HYC assisted with the data analysis. XX and MY assisted with the experiments. HBC participated in design of irradiation experiments. LX participated in the cell irradiation experiments. YLH, JMT and DZ assisted with the experiments. YRB participated in the study design and coordination and helped to revised the manuscript. XMM was responsible for the study design and final approval of the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Dong JC, Gao H, Zuo SY, Zhang HQ, Zhao G, Sun SL, Han HL, Jin LL, Shao LH, Wei W, Jin SZ. Neuropilin 1 expression correlates with the Radio-resistance of human non-small-cell lung cancer cells. J Cell Mol Med. 2015;19:2286–2295. doi: 10.1111/jcmm.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juchum M, Gunther M, Laufer SA. Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors. Drug Resist Updat. 2015;20:12–28. doi: 10.1016/j.drup.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC) Respirology. 2015;20:370–378. doi: 10.1111/resp.12490. [DOI] [PubMed] [Google Scholar]
- 5.Valenciano A, Henríquez-Hernández LA, Moreno M, Lloret M, Lara PC. Role of IGF-1 receptor in radiation response. Transl Oncol. 2012;5:1–9. doi: 10.1593/tlo.11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao H, Dong W, Shen H, Xu J, Zhu L, Liu Q, Du J. Combinational therapy enhances the effects of anti-IGF-1R mAb figitumumab to target small cell lung cancer. PLoS One. 2015;10:e0135844. doi: 10.1371/journal.pone.0135844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T, Uehara H, Ogawa H, Umemoto H, Bando Y, Izumi K. Inhibition of EP2/EP4 signaling abrogates IGF-1R-mediated cancer cell growth: Involvement of protein kinase C-θ activation. Oncotarget. 2015;6:4829–4844. doi: 10.18632/oncotarget.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JS, Kim ES, Liu D, Lee JJ, Behrens C, Lippman SM, Hong WK, Wistuba II, Lee E, Lee HY. Activation of insulin-like growth factor 1 receptor in patients with non-small cell lung cancer. Oncotarget. 2015;6:16746–16756. doi: 10.18632/oncotarget.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasa T, Okamoto I, Suzuki M, Hatashita E, Yamada Y, Fukuoka M, Ono K, Nakagawa K. Inhibition of insulin-like growth factor 1 receptor by CP-751,871 radiosensitizes non-small cell lung cancer cells. Clin Cancer Res. 2009;15:5117–5125. doi: 10.1158/1078-0432.CCR-09-0478. [DOI] [PubMed] [Google Scholar]
- 10.Morgan MA, Lawrence TS. Molecular pathways: Overcoming radiation resistance by targeting DNA damage response pathways. Clin Cancer Res. 2015;21:2898–2904. doi: 10.1158/1078-0432.CCR-13-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakasugi M, Sasaki T, Matsumoto M, Nagaoka M, Inoue K, Inobe M, Horibata K, Tanaka K, Matsunaga T. Nucleotide excision repair-dependent DNA double-strand break formation and ATM signaling activation in mammalian quiescent cells. J Biol Chem. 2014;289:28730–28737. doi: 10.1074/jbc.M114.589747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears CR, Cooney SA, Chin-Sinex H, Mendonca MS, Turchi JJ. DNA damage response (DDR) pathway engagement in cisplatin radiosensitization of non-small cell lung cancer. DNA Repair (Amst) 2016;40:35–46. doi: 10.1016/j.dnarep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Lu P, Liang Z, Zhang Z, Shi W, Cai X, Chen C. Increased insulin-like growth factor 1 receptor (IGF1R) expression in small cell lung cancer and the effect of inhibition of IGF1R expression by RNAi on growth of human small cell lung cancer NCI-H446 cell. Growth Factors. 2015;33:337–346. doi: 10.3109/08977194.2015.1088533. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, Dong W, Qu X, Shen H, Xu J, Zhu L, Liu Q, Du J. Metformin enhances the therapy effects of anti-IGF-1R mAb figitumumab to NSCLC. Sci Rep. 2016;6:31072. doi: 10.1038/srep31072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa J, Wraight CJ, Freier SM, Peralta E, Atley LM, Monia BP, Gleave ME, Cox ME. Antisense oligonucleotide targeting of insulin-like growth factor-1 receptor (IGF-1R) in prostate cancer. Prostate. 2010;70:206–218. doi: 10.1002/pros.21054. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Huang F, Bai Z, Chi B, Wu J, Chen X. Curcumol inhibits growth and induces apoptosis of colorectal cancer LoVo cell line via IGF-1R and p38 MAPK pathway. Int J Mol Sci. 2015;16:19851–19867. doi: 10.3390/ijms160819851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Shen F, Ma P, Hui H, Pei S, Chen M, Wang Z, Zhou W, Jin B. GSK1838705A, an IGF-1R inhibitor, inhibits glioma cell proliferation and suppresses tumor growth in vivo. Mol Med Rep. 2015;12:5641–5646. doi: 10.3892/mmr.2015.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramcharan R, Aleksic T, Kamdoum WP, Gao S, Pfister SX, Tanner J, Bridges E, Asher R, Watson AJ, Margison GP, et al. IGF-1R inhibition induces schedule-dependent sensitization of human melanoma to temozolomide. Oncotarget. 2015;6:39877–39890. doi: 10.18632/oncotarget.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Zhu C, Peng Z, Dai Y, Gu Y. Lentivirus-mediated short-hairpin RNA targeting IGF-1R inhibits growth and lymphangiogenesis in breast cancer. Oncol Rep. 2012;28:1778–1784. doi: 10.3892/or.2012.1964. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Gu X. Silencing of insulin-like growth factor-1 receptor enhances the radiation sensitivity of human esophageal squamous cell carcinoma in vitro and in vivo. World J Surg Oncol. 2014;12:325. doi: 10.1186/1477-7819-12-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahlberg SH, Gustafsson AS, Pendekanti PN, Glimelius B, Stenerlöw B. The influence of AKT isoforms on radiation sensitivity and DNA repair in colon cancer cell lines. Tumour Biol. 2014;35:3525–3534. doi: 10.1007/s13277-013-1465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Héron-Milhavet L, Karas M, Goldsmith CM, Baum BJ, LeRoith D. Insulin-like growth factor-I (IGF-I) receptor activation rescues UV-damaged cells through a p38 signaling pathway. Potential role of the IGF-I receptor in DNA repair. J Biol Chem. 2001;276:18185–18192. doi: 10.1074/jbc.M011490200. [DOI] [PubMed] [Google Scholar]
- 23.Héron-Milhavet L, LeRoith D. Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J Biol Chem. 2002;277:15600–15606. doi: 10.1074/jbc.M111142200. [DOI] [PubMed] [Google Scholar]
- 24.Osipov AN, Pustovalova M, Grekhova A, Eremin P, Vorobyova N, Pulin A, Zhavoronkov A, Roumiantsev S, Klokov DY, Eremin I. Low doses of X-rays induce prolonged and ATM-independent persistence of γH2AX foci in human gingival mesenchymal stem cells. Oncotarget. 2015;6:27275–27287. doi: 10.18632/oncotarget.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurashige T, Shimamura M, Nagayama Y. Differences in quantification of DNA double-strand breaks assessed by 53BP1/γH2AX focus formation assays and the comet assay in mammalian cells treated with irradiation and N-acetyl-L-cysteine. J Radiat Res. 2016;57:312–317. doi: 10.1093/jrr/rrw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldock RA, Day M, Wilkinson OJ, Cloney R, Jeggo PA, Oliver AW, Watts FZ, Pearl LH. ATM localization and heterochromatin repair depend on direct interaction of the 53BP1-BRCT2 domain with γH2AX. Cell Rep. 2015;13:2081–2089. doi: 10.1016/j.celrep.2015.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima M, Bouzid H, Soares DG, Selle F, Morel C, Galmarini CM, Henriques JA, Larsen AK, Escargueil AE. Dual inhibition of ATR and ATM potentiates the activity of trabectedin and lurbinectedin by perturbing the DNA damage response and homologous recombination repair. Oncotarget. 2016;7:25885–25901. doi: 10.18632/oncotarget.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghattass K, Assah R, El-Sabban M, Gali-Muhtasib H. Targeting hypoxia for sensitization of tumors to radio- and chemotherapy. Curr Cancer Drug Targets. 2013;13:670–685. doi: 10.2174/15680096113139990004. [DOI] [PubMed] [Google Scholar]
- 29.Pei J, Lou Y, Zhong R, Han B. MMP9 activation triggered by epidermal growth factor induced FoxO1 nuclear exclusion in non-small cell lung cancer. Tumour Biol. 2014;35:6673–6678. doi: 10.1007/s13277-014-1850-z. [DOI] [PubMed] [Google Scholar]
- 30.Miyasaka A, Oda K, Ikeda Y, Sone K, Fukuda T, Inaba K, Makii C, Enomoto A, Hosoya N, Tanikawa M, et al. PPI3K/mTOR pathway inhibition overcomes radioresistance via suppression of the HIF1-α/VEGF pathway in endometrial cancer. Gynecol Oncol. 2015;138:174–180. doi: 10.1016/j.ygyno.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Harada H. Hypoxia-inducible factor 1-mediated characteristic features of cancer cells for tumor radioresistance. J Radiat Res. 2016;57(Suppl 1):i99–i105. doi: 10.1093/jrr/rrw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helbig L, Koi L, Brüchner K, Gurtner K, Hess-Stumpp H, Unterschemmann K, Pruschy M, Baumann M, Yaromina A, Zips D. Hypoxia-inducible factor pathway inhibition resolves tumor hypoxia and improves local tumor control after single-dose irradiation. Int J Radiat Oncol Biol Phys. 2014;88:159–166. doi: 10.1016/j.ijrobp.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 33.Fotia C, Massa A, Boriani F, Baldini N, Granchi D. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology. 2015;67:1073–1084. doi: 10.1007/s10616-014-9731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Z, Chen D, Cheng H, Wang F. Hypoxia-inducible factor-1α protects cervical carcinoma cells from apoptosis induced by radiation via modulation of vascular endothelial growth factor and p53 under hypoxia. Med Sci Monit. 2015;21:318–325. doi: 10.12659/MSM.893265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Q, Guo M, Zeng W, Wang Y, Yang L, Pang X, Li H, Suo Y, Jiang X, Yu C. Matrix metalloproteinase 9 secreted by hypoxia cardiac fibroblasts triggers cardiac stem cell migration in vitro. Stem Cells Int. 2015;2015:836390. doi: 10.1155/2015/836390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ondrej M, Cechakova L, Durisova K, Pejchal J, Tichy A. To live or let die: Unclear task of autophagy in the radiosensitization battle. Radiother Oncol. 2016;119:265–275. doi: 10.1016/j.radonc.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 37.Milczarek M, Wiktorska K, Mielczarek L, Koronkiewicz M, Dąbrowska A, Lubelska K, Matosiuk D, Chilmonczyk Z. Autophagic cell death and premature senescence: New mechanism of 5-fluorouracil and sulforaphane synergistic anticancer effect in MDA-MB-231 triple negative breast cancer cell line. Food Chem Toxicol. 2018;111:1–8. doi: 10.1016/j.fct.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 38.Jo GH, Bögler O, Chwae YJ, Yoo H, Lee SH, Park JB, Kim YJ, Kim JH, Gwak HS. Radiation-induced autophagy contributes to cell death and induces apoptosis partly in malignant glioma cells. Cancer Res Treat. 2015;47:221–241. doi: 10.4143/crt.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan F, Khodagholi F, Dargahi L, Minai-Tehrani D, Ahmadiani A. Temporal pattern and crosstalk of necroptosis markers with autophagy and apoptosis associated proteins in ischemic hippocampus. Neurotox Res. 2018. Jan 8. (Epub ahead of print) [DOI] [PubMed]
- 40.Suzuki R, Kang Y, Li X, Roife D, Zhang R, Fleming JB. Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014;34:4685–4692. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.