Abstract
Introduction
Management of mid-portion Achilles tendinopathy is a challenge for both clinicians and researchers. Alteration in tendon structure, muscle performance and pain processing mechanisms have been suggested as mechanisms driving improvement in pain and function. However, few trials have used consistent outcome measures to track changes in pain and function.
Objectives
1) To identify all outcomes measures used in trials utilizing exercise-based interventions for mid-portion Achilles tendinopathy (AT) that assess self-reported pain and function and to report on the reliability and validity of the identified measures, and 2) Propose measures to optimally assess self-reported pain and function in patients with AT.
Design
Literature Review
Data Sources
Three major electronic databases were searched from inception until May 2016 for studies using isometric, eccentric or isotonic loading protocols for mid-portion AT.
Eligibility Criteria
Randomized and non-randomized trials of isometric, eccentric or isotonic loading in people with mid-portion AT.
Results
Forty-six studies were included and all outcome measures assessing self-reported pain and function were extracted. While a variety of outcome measures have been used, few have provided reliability data. There is evidence to suggest that the Victorian Institute of Sports Assessment- Achilles (VISA-A) is the only valid and reliable measure of self-reported pain and function for people with mid-portion AT. No other outcome measures have been validated in mid-portion AT.
Conclusion
The VISA-A remains the gold standard for assessing pain and function in mid-portion AT. However, while the validity or reliability of the Numerical Rating Scale (NRS) of pain during a functional task has not been established it may be a better measure of immediate treatment effect.
Level of evidence
5
Keywords: Achilles, outcome measures, reliability, tendinopathy, validity
INTRODUCTION
Achilles tendinopathy (AT) is one of the most common running-related injuries, with a prevalence of 6.2-9.5% in recreational runners1 and 2-18.5% in ultra-marathon runners.1 AT can present as either mid-portion or insertional tendinopathy. Mid-portion tendinopathy affects the mid-portion of the tendon approximately 2-4cm proximal to the insertion whereas insertional tendinopathy affects the tendon insertion onto the calcaneus.2 AT is characterised clinically by pain and stiffness (either mid-portion of the tendon or at the insertion) and these symptoms affect athletic function, which is a key diagnostic feature of the condition.3,4 Mid-portion and insertional Achilles tendinopathy are considered different clinical entities and thus are considered separately within the literature. Therefore, this review will purely focus on mid-portion Achilles tendinopathy.
Management of AT is a challenge for both clinicians and researchers. Exercise rehabilitation, specifically either eccentric or isotonic resistance training are effective interventions.2,5 While loading programmes have demonstrated effectiveness, symptoms can persist up to 5 years following exercise rehabilitation with some participants not reaching complete resolution and some not responding to the intervention.6 One potential explanation for this relates to our incomplete understanding of the mechanisms underpinning this therapy.7 Several mechanisms have been alluded to in the literature including 1) alterations in tendon structure8,9 2) alterations in muscle performance7 and 3) alterations in pain mechanisms.10
One challenge in understanding these mechanisms is the fact that few trials have used consistent outcome measures to track changes in pain and function. The chosen outcome measures also often lack sufficient psychometric properties. It is important that clinicians and researchers are familiar with the outcome measures that have been used in clinical trials including their validity and reliability, as poor choices in outcome measures can lead to results with questionable utility. However, while valid and reliable measures are important for researchers it is also important for clinicians to know which measures are accessible for everyday practice. This may in turn reduce barriers to implementation of these measures.11
The objectives of this review were: 1) To identify all outcomes measures that have been used in trials that involved exercise based interventions for mid-portion AT assessing self-reported pain and function, and to report on the reliability and validity of the identified measures; and 2) Recommend those measures that optimally assess self-reported pain and function in patients with AT.
METHODS
Criteria for considering studies for this review
Types of studies
Both non-randomized cohort studies and randomised controlled trials were included if a loading protocol was used to treat mid-portion AT. Case reports, clinical observations and systematic reviews were excluded.
Types of participants
Physically active and sedentary participants aged 18 years and over identified as having mid-portion AT for greater than three months were included. Studies including participants with insertional AT or other causes of pain (differential diagnoses) anywhere in the Achilles region were excluded from the review.
Types of interventions
Intervention studies using either isometric, eccentric, concentric or isotonic (eccentric and concentric) loading protocols were included. Studies that employed an isometric, eccentric, concentric or isotonic loading program in conjunction with a placebo therapy (for example sham laser treatment) were included.
Types of outcomes measures
Only studies that used a self-reported measure of pain and function in mid-portion AT were included.
Search methods for identification of studies
Electronic Searches
Searches using free text terms (Table 1) were used to identify published articles on the following electronic databases; PUBMED, CINAHL (Ovid) and CINAHL (EBSCO). Only peer reviewed, human, clinical trials and cohort studies were included.
Table 1.
Systematic Review Search Strategy
| Number | Combiners | Terms |
|---|---|---|
| 1 | Problem of Interest | Achilles tend* |
| 2 | Intervention | exercise OR eccentric OR isotonic OR resistance OR strength* |
| 3 | #1 AND #2 | |
| Limitations | Peer reviewed, human, clinical trials written in English were included. |
Searching other Resources
Reference lists from reviews and retrieved articles were checked and citation searches on key articles performed. The list of included studies were evaluated by content experts to help identify any additional relevant studies.
Data collection and analysis
Selection of Studies
One review author (MM) independently searched and assessed the titles and abstracts of potential studies identified by the search strategy for their eligibility. Studies were exported to reference management software EndNote X8.0.2 (Clarivate Analytics, 2017) and duplicates were removed. If the eligibility of a study was unclear from the title and abstract the full paper was assessed. Studies that did not match the inclusion criteria for this review were excluded. Studies were not anonymised prior to assessment.
A PRISMA study flow diagram12 was used to document the screening process as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions.13
Data abstraction and management
One review author (MM) independently extracted data from all included studies using a standardised and piloted data extraction form on Microsoft Excel (Microsoft, 2016). The following information was recorded; primary author, year of publication, study design, study population (diagnosis), sample size, loading intervention (e.g. heavy eccentric calf training), outcome measures used, number of follow up points and time (weeks) at each follow up point.
Data synthesis
Reliability, validity, minimally clinically important difference (MCID) and the minimal detectable change (MDC) were reported if provided by the study using the outcome measure. If the study provided a reference to psychometric properties, the referenced study was used to extract the data.
RESULTS
Selection of Studies
A total of 46 studies were included and are presented in a Prisma Flow Chart (Figure 1).
Figure 1.
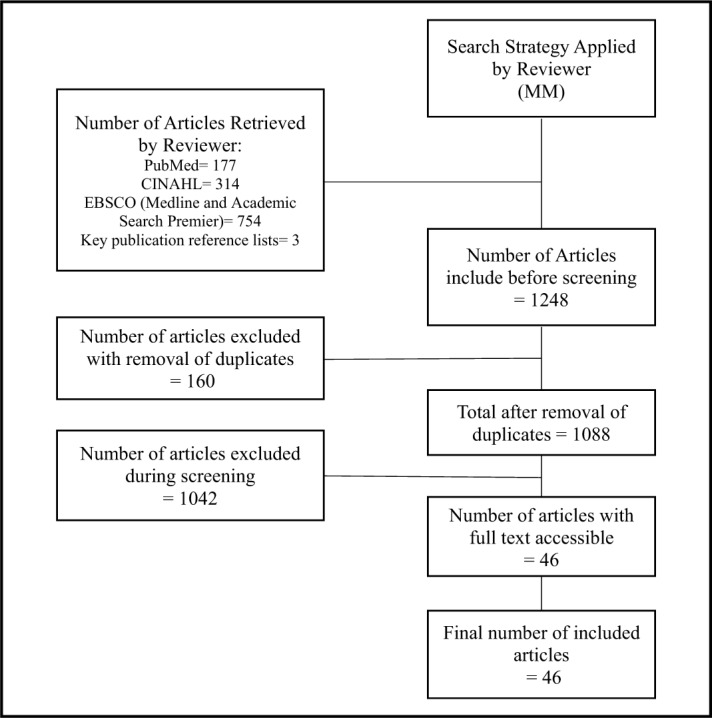
Prisma Flow Chart.
Data Extraction
All studies using a loading intervention for mid-portion AT used a measure of self-reported pain and function. The characteristics of the included studies are presented in Appendix A.
Data Synthesis
The outcome measures used to assess pain and function in the interventional clinical trials are presented in Table 2. Most outcome measures in this domain did not report their reliability and have not been validated in mid-portion AT.
Table 2.
Outcome measures assessing self-reported pain and function
| Outcome Measure | Number of Times used in Clinical Trials | Follow up Times (weeks) | Validity | Reliability |
|---|---|---|---|---|
| Visual Analogue Scale of Pain at Rest | 618,24,31,45,50,55 | 2, 3, 4, 6, 8, 12, 26 | Assessed against the Numerical Rating Scale in Rheumatoid Disease: r = 0.62-0.9156 Assessed against a Simple Descriptive Scale in Rheumatic Disease: r = 0.73-0.7856 | Test Retest Reliability in Mid-portion Achilles Tendinopathy: r = 0.4545 |
| Visual Analogue Scale of Pain with various functional tasks | 115, 14, 15, 18, 24, 32, 34, 40, 45, 46, 49, | 2, 4, 6, 8, 12, 26, 52 | Not Reported | Test Retest Reliability in Mid-portion Achilles Tendinopathy with Jumping: r = 0.6945 Test Retest Reliability in Mid-portion Achilles Tendinopathy with Heel Raise: r = 0.6145 |
| 100mm VAS of Pain with 1kg Squeeze of the Achilles Tendon | 317,29,45 | 2, 6, 12, 26 | Not Reported | Not Reported |
| 4 Point Scale of Pain with 1kg Squeeze of the Achilles Tendon | 136 | 1, 3, 6, 12, 39 | Not Reported | Not Reported |
| Numerical Rating Scale of Pain at Rest | 252, 53, | 4, 12, 52 | Assessed against the Visual Analogue Scale in Rheumatoid Disease: r = 0.61-0.9156 Assessed against a Simple Descriptive Scale in Rheumatic Disease: r = 0.68-0.8856 | Test Retest Reliability in Rheumatoid Arthritis: r = 0.95-0.9657 |
| Numerical Rating Scale of Pain over time | 541,42,52-54 | 4, 12, 16, 26, 52 | Not Reported | Not Reported |
| 5 Point Likert Scale of Difficulty in Sport | 143 | 6, 12, 26, 52 | Not Reported | Not Reported |
| Victorian Institute of Sport Assessment - Achilles | 285,6,16,17,20-28,30,33,35,39,41,42,44,46-48,50-54 | 2, 3, 4, 6, 8, 12, 16, 24, 26, 36, 52, 2.2 years | Assessed against the Percy and Conochie's grade of severity in Achilles Tendinopathy: p<0.0158 Assessed against the Curwin and Stanish grade of severity in Achilles Tendinopathy: p<0.00144 Assessment of severity in pre-surgical Achilles Tendinopathy, non-surgical Achilles Tendinopathy and two control groups: p<0.00158 | Test Retest Reliability in Achilles Tendinopathy: r = 0.93-0.9858 Intra-rater Reliability in Achilles Tendinopathy: r = 0.9058 Inter-rater Reliability in Achilles Tendinopathy: r = 0.90-0.9758 |
| Modified Curwin and Stanish Six Level Pain Scale | 28,9 | 12, 60, 220 | Not Reported | Not Reported |
| Functional Index of the Leg and Lower Limb (FILLA) | 118 | 2, 4, 6, 12 | Not Reported | Not Reported |
| American Orthopedic Foot and Ankle Score (AOFAS) Hindfoot Scale | 140 | 6, 12 | Assessed against the Foot Function Index in Rheumatoid Arthritis Hallux Pain: p<0.0559 | Test Retest Reliability in Rheumatoid Arthritis Hallux Pain: ICC = 0.9559 Intra-Rater Reliability in Rheumatoid Arthritis: ICC = 0.9560 Inter-Rater Reliability in Rheumatoid Arthritis: ICC = 0.9160 |
| Short Form-36 (SF-36) | 235,40 | 4, 6, 12, 26, 52 | Assessed against the Visual Analogue Scale of Pain in Rheumatoid Arthritis: r = -0.4861 | Test Retest Reliability in General Practice: α = 0.7862 |
| EuroQoL | 218,30 | 2, 4, 6, 12, 26 | Assessed against the Western Ontario and McMaster Universities Osteoarthritis Index in Knee Osteoarthritis: Spearman's rho = 0.20-0.6063 Assessed against the SF-36 in Knee Osteoarthritis: Spearman's rho = 0.20-0.6063 | Test Retest Reliability in Knee Osteoarthritis: 0.70-0.7363 |
| Foot and Ankle Outcome Score (FAOS) | 331,36,43 | 3, 6, 12, 39 | Assessed against the Karlsson Score in Foot and Ankle Osteoarthritis: r = 0.58-0.6764 | Test Retest Reliability in Foot and Ankle Osteoarthritis: ICC = 0.70-0.9264 |
| Numerical Scale of Physical Activity | 143 | 6, 12, 26, 52 | Not Reported | Test Retest Reliability: “satisfactory”65 |
| Numerical Scales of Improvement | 89,16,29,36,41,42,47,54 | 4, 12, 16, 26, 52 | Not Reported | Not Reported |
| Treatment Satisfaction | 720,23,24,34,37,38,50 | 3, 6, 12, 52, 112, 200 | Not Reported | Not Reported |
| Patient Global Impression of Change (PGIC) | 154 | 12, 26, 52 | Assessed against the Self-Assessment of Treatment Scale in Postherpic neuralgia: r = 0.68-0.9066 | Not Reported |
Victorian Institute of Sport- Achilles
The Victorian Institute of Sports Assessment- Achilles (VISA-A) has been used in 28 clinical trials and was the most frequently used outcome measure to assess pain and function. The VISA-A has excellent reliability (test-retest r = 0.93-0.98)58 and is the only outcome measure used in clinical trials that has been validated for AT; it was validated against two tendon pain rating scales, the Percy and Conochie's grade of severity (Spearmans r = 0.58, p = <0.01)58 and that of Curwin and Stanish (Spearmans r = -0.57, p = <0.001).58 No MDC has been reported.
The MCID of an outcome measure is important both for study design (e.g. power calculations), as well as measuring whether or not an intervention reflects a meaningful improvement for the patient.67 The majority of outcome measures reported in the literature to assess pain and function have not yet had the MCID calculated for mid-portion AT. The VISA-A has had the MCID reported for insertional AT with an improvement of 6.5 points reflecting a meaningful improvement for the patient.68 The MCID of the VISA-A in mid-portion AT has only once been reported in one pilot study, with an MCID of 16 points.68 However, this study did not provide any information on how the MCID was calculated and it is unlikely using the study design they would have been able to complete calculations required for determining a MCID.67 However, most clinical trials reviewed here used other scores, with 10 points5,16,35,44,46,47 being the most common MCID reported (Table 3).
Table 3.
Frequency of the MCID reported for the VISA-A in mid-portion Achilles Tendinopathy in clinical trials using loading protocols
| MCID | Frequency |
|---|---|
| 10 | 65,16,35,44,46,47 |
| 12 | 421,25,27,30 |
| 15 | 242,50 |
| 16 | 351-53 |
| 17 | 139 |
| 20 | 154 |
In addition to the MCID another psychometric property commonly used is the minimal detectable change. However, none of the papers included in this review which used the VISA-A made any reference to this.
Visual Analogue Scale and Numerical Rating Scale
Variations of the Visual Analogue Scale (VAS) (Table Two) and Numerical Rating Scale (NRS) using average pain, worst pain, pain at rest or during functional tasks have been used in sixteen and five clinical trials, respectively. The VAS has been shown to have poor test-reliability at rest in AT (r = 0.45)45 however this is marginally better when used to assess pain during functional tasks (r = 0.61-0.69).45 Whilst the VAS has been shown to be valid when tested against a variety of pain rating scales in other conditions, such as rheumatoid disease, total knee replacement and acute abdominal pain,56,69,70 it has yet to be validated in AT.
The NRS has been shown to be highly reliable in other musculoskeletal pain conditions, such as rheumatoid arthritis,57 but it has yet to be determined in patients with AT. While the NRS has been shown to be valid when tested against a variety of pain rating scales in other conditions (e.g. rheumatoid disease, chronic low back pain, osteoarthritis )56,71,72 it has yet to be validated in AT.
A variety of other self-reported pain and improvement scales have been used in clinical trials however none of these scales have been validated in AT (Table 2).
None of the papers included in this review which used the NRS or VAS made any reference to either the MCID or MDC for these measures in mid-portion AT pain.
DISCUSSION
Pain and function have been measured with VISA-A and pain scales including NRS and VAS. However, the timing and instructions of implementing the VAS and NRS differs vastly between trials; for example worst pain today, current resting pain or pain during loading task such as hopping. It is unclear in both a research and clinical setting when these pain and function outcome measures should be used.
The results of this review indicate that the VISA-A is the only validated and reliable measure of pain and function for mid-portion AT. It is therefore recommended as currently being the best primary outcome measure to assess these clinical domains. However, problems do remain when considering utilisation of the VISA-A in clinical practice; firstly, completion of the VISA-A may not be practical for assessing immediate response to treatment. Furthermore, the process used to develop and validate the VISA-A does not conform to current recommendations in developing a self-reported outcome measure and is missing components suggested to be vital in developing a self-reported outcome measure.73 Given that the VAS and NRS of pain are easily applied and have been validated56,69–72 for musculoskeletal pain in other conditions, they may be more appropriate for assessing immediate treatment effect; however, further research is of course required to establish reliability and validity. Specifically, given that the NRS of pain has been shown to be more reliable, valid and responsive than the VAS of pain in other musculoskeletal pain conditions57,74 it may be the preferred choice. Immediate treatment effects have been measured in patellar tendinopathy by comparing the NRS of pain during a provocative functional test (single leg decline squat) before and immediately after a loading program.75,76 By mirroring this investigational methodology in AT, further information on the immediate effect of loading on tendon pain can be gathered. However, caution must be taken given that the validity and reliability of the NRS of pain in AT remains unknown.
Improvements have been observed in VISA-A scores in as early as two weeks following commencement of a loading protocol.17 For both clinicians and researchers, completing a VISA-A at multiple time points during the intervention may help determine the rate of change, and provide insight into the mechanisms relating to improvements based on the temporal response.
While the MCID of the VISA-A has been reported in insertional AT it has not been formally reported in mid-portion AT. No clear consensus exists with the only study which has reported an MCID not providing any information on how the MCID was calculated. Without clear information on how the MCID was calculated we cannot be confident in the results. Currently the authors would suggest that two different methods can be utilized for choosing the MCID for sample size calculations when using the VISA-A as the primary outcome measure in a clinical trial; 1) using the MCID of insertional AT (6.5 points);68 or 2) using the most commonly reported MCID in clinical trials (10 points).5,16,35,44,46,47 Either of these methods of selecting a MCID for power calculations to estimate sample sizes are appropriate, however they are potential sources of error given the true MCID for mid-portion AT has not been calculated.
The quality of the studies using an exercise intervention for mid-portion AT was very low. A mix of randomized and non-randomized studies existed, each containing small sample sizes. Only four out of 46 studies identified in this paper have a sample size of greater than 50 participants. When considering that the quality of evidence is low, and that 18 out of 46 studies failed to use a reliable or validated measure of self-reported pain and function clinicians must be wary when drawing conclusions based on study efficacy from this body of work.
CONCLUSION
Many different outcome measures have been used to assess pain and function in clinical trials that study the treatment of mid-portion AT with exercise rehabilitation. To assess pain and function the VISA-A appears to be the most valid and reliable tool. However, the NRS of pain during a functional task is possibly a simpler tool to assess immediate effect post-treatment or short-term effects of interventions as it may be more responsive to change. It is important for clinicians and researchers to be aware of the outcome measures that have been used as well as the reliability and validity of these measures. By identifying the best measures, rehabilitation professionals can optimize clinical assessment and improve clinical trials, as well as identify areas that require further research.
Appendix A. Individual Study Characteristics
| Study Name | Study Design | Cohort Size (n) | Loading Intervention in Exercise Arm of Study |
|---|---|---|---|
| Alfredson et al. (1998)14 | Cohort | 15 | Heavy Eccentric Calf Training |
| Alfredson & Lorentzon. (2003)15 | Cohort | 6 | Heavy Eccentric Calf Training |
| Bell et al. (2013)16 | RCT | 27 | Heavy Eccentric Calf Training |
| Beyer et al. (2015)5 | RCT | 25 | Heavy Eccentric Calf Training |
| 22 | Heavy Slow Resistance Training | ||
| Brown et al. (2006)17 | RCT | 18 | Heavy Eccentric Calf Training |
| Chester et al. (2008)18 | RCT | 8 | Heavy Eccentric Calf Training |
| Crill et al. (2014)19 | Cohort | 25 | Heavy Eccentric Calf Training |
| De Jonge et al. (2010)20* | Cohort | 32 | Heavy Eccentric Calf Training |
| De Jonge et al. (2011)21* | RCT | 27 | Heavy Eccentric Calf Training |
| De Jonge et al. (2015)22* | RCT | 54 | Heavy Eccentric Calf Training |
| De Vos et al. (2007)23 | RCT | 32 | Heavy Eccentric Calf Training |
| De Vos et al. (2007)24* | Cohort | 58 | Heavy Eccentric Calf Training |
| De Vos et al. (2010)25* | RCT | 27 | Heavy Eccentric Calf Training |
| De Vos et al. (2011)26* | RCT | 27 | Heavy Eccentric Calf Training |
| De Vos et al. (2012)27* | Cohort | 24 | Heavy Eccentric Calf Training |
| Gardin et al. (2010)8* | Cohort | 24 | Heavy Eccentric Calf Training |
| Herrington et al. (2007)28 | RCT | 13 | Heavy Eccentric Calf Training |
| Horstmann et al. (2013)29 | RCT | 19 | Heavy Eccentric Calf Training |
| Kearney et al. (2013)30 | RCT | 10 | Heavy Eccentric Calf Training |
| Knobloch et al. (2008)31 | RCT | 59 | Heavy Eccentric Calf Training |
| Langberg et al. (2007)32 | Cohort | 6 | Heavy Eccentric Calf Training |
| Maffuli et al. (2008)33 | Cohort | 45 | Heavy Eccentric Calf Training |
| Mafi et al. (2001)34 | RCT | 22 | Heavy Eccentric Calf Training |
| 22 | Concentric Calf Training | ||
| Munteneau et al. (2015)35 | RCT | 54 | Heavy Eccentric Calf Training |
| Norregaard et al. (2007)36 | RCT | 21 | Heavy Eccentric Calf Training |
| Ohberg & Alfedson (2004)37 | Cohort | 30 | Heavy Eccentric Calf Training |
| Ohberg et al. (2004)38 | Cohort | 25 | Heavy Eccentric Calf Training |
| Pearson et al. (2012)39 | RCT | 18 | Heavy Eccentric Calf Training |
| Peterson et al. (2007)40 | RCT | 37 | Modified Heavy Eccentric Calf Training |
| Rompe et al. (2007)41 | RCT | 23 | Heavy Eccentric Calf Training |
| Rompe et al. (2009)42 | RCT | 30 | Heavy Eccentric Calf Training |
| Roos et al. (2004)43 | RCT | 16 | Modified Heavy Eccentric Calf Training |
| Sayana & Maffuli (2007)44 | Cohort | 34 | Heavy Eccentric Calf Training |
| Shalabi et al. (2004)9 | Cohort | 25 | Heavy Eccentric Calf Training |
| Silbernagel et al. (2001)45 | RCT | 22 | Eccentric Overload |
| Silbernagel et al. (2007)46 | RCT | 26 | Eccentric Overload with Active Rest |
| 24 | Eccentric Overload | ||
| Silbernagel et al. (2011)47* | Cohort | 34 | Eccentric Overload |
| Stasinopoulos & Manias (2013)48 | RCT | 20 | Heavy Eccentric Calf Training |
| 21 | Stanish Protocol | ||
| Stergioulas et al. (2008)49 | RCT | 20 | Heavy Eccentric Calf Training |
| Stevens & Tan (2014)50 | RCT | 14 | Heavy Eccentric Calf Training |
| 12 | Modified Heavy Eccentric Calf Training | ||
| Tumilty et al. (2008)51 | RCT | 10 | Heavy Eccentric Calf Training |
| Tumilty et al. (2012)52 | RCT | 17 | Heavy Eccentric Calf Training |
| Tumilty et al. (2016)53 | RCT | 13 | Heavy Eccentric Calf Training |
| 19 | Modified Heavy Eccentric Calf Training | ||
| Van der Plas et al. (2012)6* | Cohort | 46 | Heavy Eccentric Calf Training |
| Yelland et al. (2011)54 | RCT | 15 | Heavy Eccentric Calf Training |
| Yu et al. (2013)55 | RCT | 16 | Heavy Eccentric Calf Training |
| 16 | Concentric Calf Training |
The results of this study are a follow up of an included study or present different components of data from another included study.
REFERENCES
- 1.Lopes AD, Hespanhol LC, Yeung SS, et al. What are the main running-related musculoskeletal injuries? Sports Med. 2012;42(10):891-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25(1):3-15. [DOI] [PubMed] [Google Scholar]
- 3.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409-16. [DOI] [PubMed] [Google Scholar]
- 4.Cook JL, Rio E, Purdam CR, et al. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research? Br J Sports Med. 2016;50(19):1187-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer R, Kongsgaard M, Hougs Kjaer B, et al. Heavy slow resistance versus eccentric training as treatment for achilles tendinopathy: A randomized controlled trial. Am J Sports Med. 2015;43(7):1704-11. [DOI] [PubMed] [Google Scholar]
- 6.van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson's heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46(3):214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill S, Watson PJ, Barry S. Why are eccentric exercises effective for achilles tendinopathy? Int J Sport Phys Ther. 2015;10(4):552-62. [PMC free article] [PubMed] [Google Scholar]
- 8.Gardin A, Movin T, Svensson L, et al. The long-term clinical and MRI results following eccentric calf muscle training in chronic Achilles tendinosis. Skeletal Radiol. 2010;39(5):435-42. [DOI] [PubMed] [Google Scholar]
- 9.Shalabi A, Kristoffersen-Wilberg M, Svensson L, et al. Eccentric training of the gastrocnemius-soleus complex in chronic Achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am J Sports Med. 2004;32(5):1286-96. [DOI] [PubMed] [Google Scholar]
- 10.Rio E, Kidgell D, Moseley GL, et al. Tendon neuroplastic training: changing the way we think about tendon rehabilitation: a narrative review. Br J Sports Med. 2016;50(4):209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jette DU, Halbert J, Iverson C, et al. Use of standardized outcome measures in physical therapist practice: perceptions and applications. Phys Ther. 2009;89(2):125-35. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. In: Collaboration TC, ed. Available from www.handbook.cochrane.org, 2011.
- 14.Alfredson H, Pietila T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26(3):360-6. [DOI] [PubMed] [Google Scholar]
- 15.Alfredson H, Lorentzon R. Intratendinous glutamate levels and eccentric training in chronic Achilles tendinosis: a prospective study using microdialysis technique. Knee Surg Sports Traumatol Arthrosc. 2003;11(3):196-9. [DOI] [PubMed] [Google Scholar]
- 16.Bell KJ, Fulcher ML, Rowlands DS, et al. Impact of autologous blood injections in treatment of mid-portion Achilles tendinopathy: double blind randomised controlled trial. BMJ (Clinical research ed) 2013;346:f2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown R, Orchard J, Kinchington M, et al. Aprotinin in the management of Achilles tendinopathy: a randomised controlled trial. Br J Sports Med. 2006;40(3):275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chester R, Costa ML, Shepstone L, et al. Eccentric calf muscle training compared with therapeutic ultrasound for chronic Achilles tendon pain--a pilot study. Man Ther. 2008;13(6):484-91. [DOI] [PubMed] [Google Scholar]
- 19.Crill MT, Berlet G, Hyer C. Plantar flexor muscle architecture changes as a result of eccentric exercise in patients with Achilles tendinosis. Foot Ankle Spec. 2014;7(6):460-5. [DOI] [PubMed] [Google Scholar]
- 20.de Jonge S, de Vos RJ, Van Schie HT, et al. One-year follow-up of a randomised controlled trial on added splinting to eccentric exercises in chronic midportion Achilles tendinopathy. Br J Sports Med. 2010;44(9):673-7. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39(8):1623-9. [DOI] [PubMed] [Google Scholar]
- 22.de Jonge S, Tol JL, Weir A, et al. The tendon structure returns to asymptomatic values in nonoperatively treated Achilles tendinopathy but is not associated with symptoms: A prospective study. Am J Sports Med. 2015;43(12):2950-8. [DOI] [PubMed] [Google Scholar]
- 23.de Vos RJ, Weir A, Visser RJ, et al. The additional value of a night splint to eccentric exercises in chronic midportion Achilles tendinopathy: a randomised controlled trial. Br J Sports Med. 2007;41(7):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Vos RJ, Weir A, Cobben LP, et al. The value of power Doppler ultrasonography in Achilles tendinopathy: a prospective study. Am J Sports Med. 2007;35(10):1696-701. [DOI] [PubMed] [Google Scholar]
- 25.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-9. [DOI] [PubMed] [Google Scholar]
- 26.de Vos RJ, Weir A, Tol JL, et al. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-92. [DOI] [PubMed] [Google Scholar]
- 27.de Vos RJ, Heijboer MP, Weinans H, et al. Tendon structure's lack of relation to clinical outcome after eccentric exercises in chronic midportion Achilles tendinopathy. J Sports Rehab. 2012;21(1):34-43. [DOI] [PubMed] [Google Scholar]
- 28.Herrington L, McCulloch R. The role of eccentric training in the management of Achilles tendinopathy: A pilot study. Phys Ther Sport. 2007;8(4):191-96. [Google Scholar]
- 29.Horstmann T, Jud HM, Frohlich V, et al. Whole-body vibration versus eccentric training or a wait-and-see approach for chronic Achilles tendinopathy: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43(11):794-803. [DOI] [PubMed] [Google Scholar]
- 30.Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-richplasma injection with an eccentric loading programme. Bone Joint. 2013;2(10):227-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knobloch K, Schreibmueller L, Longo UG, et al. Eccentric exercises for the management of tendinopathy of the main body of the Achilles tendon with or without the AirHeel brace. A randomized controlled trial. A: effects on pain and microcirculation. Disabil Rehabil. 2008;30(20-22):1685-91. [DOI] [PubMed] [Google Scholar]
- 32.Langberg H, Ellingsgaard H, Madsen T, et al. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand J Med Sci Sports. 2007;17(1):61-6. [DOI] [PubMed] [Google Scholar]
- 33.Maffulli N, Walley G, Sayana MK, et al. Eccentric calf muscle training in athletic patients with Achilles tendinopathy. Disabil Rehabil. 2008;30(20-22):1677-84. [DOI] [PubMed] [Google Scholar]
- 34.Mafi N, Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):42-7. [DOI] [PubMed] [Google Scholar]
- 35.Munteanu SE, Scott LA, Bonanno DR, et al. Effectiveness of customised foot orthoses for Achilles tendinopathy: a randomised controlled trial. Br J Sports Med. 2015;49(15):989-94. [DOI] [PubMed] [Google Scholar]
- 36.Norregaard J, Larsen CC, Bieler T, et al. Eccentric exercise in treatment of Achilles tendinopathy. Scand J Med Sci Sports. 2007;17(2):133-8. [DOI] [PubMed] [Google Scholar]
- 37.Ohberg L, Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg Sports Traumatol Arthrosc. 2004;12(5):465-70. [DOI] [PubMed] [Google Scholar]
- 38.Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med. 2004;38(1):8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson J, Rowlands D, Highet R. Autologous blood injection to treat achilles tendinopathy? A randomized controlled trial. J Sports Rehab. 2012;21(3):218-24. [DOI] [PubMed] [Google Scholar]
- 40.Petersen W, Welp R, Rosenbaum D. Chronic Achilles tendinopathy: a prospective randomized study comparing the therapeutic effect of eccentric training, the AirHeel brace, and a combination of both. Am J Sports Med. 2007;35(10):1659-67. [DOI] [PubMed] [Google Scholar]
- 41.Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35(3):374-83. [DOI] [PubMed] [Google Scholar]
- 42.Rompe JD, Furia J, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2009;37(3):463-70. [DOI] [PubMed] [Google Scholar]
- 43.Roos EM, Engstrom M, Lagerquist A, et al. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy -- a randomized trial with 1-year follow-up. Scand J Med Sci Sports. 2004;14(5):286-95. [DOI] [PubMed] [Google Scholar]
- 44.Sayana MK, Maffulli N. Eccentric calf muscle training in non-athletic patients with Achilles tendinopathy. J Sci Med Sport. 2007;10(1):52-8. [DOI] [PubMed] [Google Scholar]
- 45.Silbernagel KG, Thomee R, Thomee P, et al. Eccentric overload training for patients with chronic Achilles tendon pain--a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports. 2001;11(4):197-206. [DOI] [PubMed] [Google Scholar]
- 46.Silbernagel KG, Thomee R, Eriksson BI, et al. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: a randomized controlled study. Am J Sports Med. 2007;35(6):897-906. [DOI] [PubMed] [Google Scholar]
- 47.Silbernagel KG, Brorsson A, Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: a 5-year follow-up. Am J Sports Med. 2011;39(3):607-13. [DOI] [PubMed] [Google Scholar]
- 48.Stasinopoulos D, Manias P. Comparing two eccentric exercise programmes for the management of Achilles tendinopathy. A pilot trial. J Bodyw Mov Ther. 2013;17(3):309-15. [DOI] [PubMed] [Google Scholar]
- 49.Stergioulas A, Stergioula M, Aarskog R, et al. Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic achilles tendinopathy. Am J Sports Med. 2008;36(5):881-7. [DOI] [PubMed] [Google Scholar]
- 50.Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44(2):59-67. [DOI] [PubMed] [Google Scholar]
- 51.Tumilty S, Munn J, Abbott JH, et al. Laser therapy in the treatment of achilles tendinopathy: a pilot study. Photomed Laser Surg. 2008;26(1):25-30. [DOI] [PubMed] [Google Scholar]
- 52.Tumilty S, McDonough S, Hurley DA, et al. Clinical effectiveness of low-level laser therapy as an adjunct to eccentric exercise for the treatment of Achilles' tendinopathy: a randomized controlled trial. Arch Phys Med Rehabil. 2012;93(5):733-9. [DOI] [PubMed] [Google Scholar]
- 53.Tumilty S, Mani R, Baxter GD. Photobiomodulation and eccentric exercise for Achilles tendinopathy: a randomized controlled trial. Lasers Med Sci. 2016;31(1):127-35. [DOI] [PubMed] [Google Scholar]
- 54.Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45(5):421-8. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, Park D, Lee G. Effect of eccentric strengthening on pain, muscle strength, endurance, and functional fitness factors in male patients with achilles tendinopathy. Am J Phys Med Rehabil. 2013;92(1):68-76. [DOI] [PubMed] [Google Scholar]
- 56.Downie WW, Leatham PA, Rhind VM, et al. Studies with pain rating scales. Ann Rheum Dis. 1978;37(4):378-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferraz MB, Quaresma MR, Aquino LR, et al. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol. 1990;17(8):1022-4. [PubMed] [Google Scholar]
- 58.Robinson JM, Cook JL, Purdam C, et al. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35(5):335-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baumhauer JF, Nawoczenski DA, DiGiovanni BF, et al. Reliability and validity of the American Orthopaedic Foot and Ankle Society clinical rating scale: a pilot study for the hallux and lesser toes. Foot Ankle Int. 2006;27(12):1014-9. [DOI] [PubMed] [Google Scholar]
- 60.Conceicao CS, Neto MG, Neto AC, et al. Analysis of the psychometric properties of the American Orthopaedic Foot and Ankle Society Score (AOFAS) in rheumatoid arthritis patients: application of the Rasch model. Rev Bras Rheumat Eng Ed. 2016;56(1):8-13. [DOI] [PubMed] [Google Scholar]
- 61.Koh ET, Leong KP, Tsou IY, et al. The reliability, validity and sensitivity to change of the Chinese version of SF-36 in oriental patients with rheumatoid arthritis. Rheumatology (Oxford). 2006;45(8):1023-8. [DOI] [PubMed] [Google Scholar]
- 62.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ (Clinical research ed). 1992;305(6846):160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fransen M, Edmonds J. Reliability and validity of the EuroQol in patients with osteoarthritis of the knee. Rheumatology (Oxford). 1999;38(9):807-13. [DOI] [PubMed] [Google Scholar]
- 64.Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-94. [DOI] [PubMed] [Google Scholar]
- 65.Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index--additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Osteoarthritis Cartilage. 1999;7(2):216-21. [DOI] [PubMed] [Google Scholar]
- 66.Wyrwich KW, Kawata AK, Thompson C, et al. Validation of the self-assessment of treatment questionnaire among patients with postherpetic neuralgia. Pain Res Treat. 2012;2012:621619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook CE. Clinimetrics Corner: The minimal clinically important change score (MCID): A necessary pretense. J Man Manip Ther. 2008;16(4):E82-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCormack J, Underwood F, Slaven E, et al. The minimum clinically important difference on the VISA-Aand LEFS for patients with insertional Achilles tendinopathy. Int J Sports Phys Ther. 2015;10(5):639-44. [PMC free article] [PubMed] [Google Scholar]
- 69.Boeckstyns ME, Backer M. Reliability and validity of the evaluation of pain in patients with total knee replacement. Pain. 1989;38(1):29-33. [DOI] [PubMed] [Google Scholar]
- 70.Gallagher EJ, Bijur PE, Latimer C, et al. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am J Emerg Med. 2002;20(4):287-90. [DOI] [PubMed] [Google Scholar]
- 71.Farrar JT, Young JP, Jr., LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-58. [DOI] [PubMed] [Google Scholar]
- 72.Page MG, Katz J, Stinson J, et al. Validation of the numerical rating scale for pain intensity and unpleasantness in pediatric acute postoperative pain: sensitivity to change over time. J Pain. 2012;13(4):359-69. [DOI] [PubMed] [Google Scholar]
- 73.Artino AR, Jr., La Rochelle JS, Dezee KJ, et al. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399-404. [DOI] [PubMed] [Google Scholar]
- 75.Rio E, Kidgell D, Purdam C, et al. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br J Sports Med. 2015;49(19):1277-83. [DOI] [PubMed] [Google Scholar]
- 76.van Ark M, Cook JL, Docking SI, et al. Do isometric and isotonic exercise programs reduce pain in athletes with patellar tendinopathy in-season? A randomised clinical trial. J Sci Med Sport. 2016;19(9):702-6. [DOI] [PubMed] [Google Scholar]


