Abstract
Background
Cardiac infarction frequently leads to arrhythmia and ischemia/reperfusion (I/R) aggravates cardiac injury. Pinocembrin can resist cerebral ischemia and decrease cardiac infarction area. This study thus generated a rat myocardial I/R model to assess the effect on ventricular rhythm and expression of gap junction connexin (Cx43).
Material/Methods
Male SD rats were randomly assigned into sham, model, and pinocembrin (30 mg/kg) pretreatment groups (N=15 each). The I/R model was generated by ligation of the left anterior descending coronary artery for 30 min. The pinocembrin group received intravenous injection 10 min before surgery. Heart rate (HR), mean artery pressure (MAP), rate pressure product (RPP), and arrhythmia were observed at 10 min before ischemia, 30 min after ischemia, and at 30, 60, and 120 min after reperfusion. ELISA was used to assess serum CK-MB and cTnI levels. Na+-K+ATPase and Ca+-Mg2+ATPase levels were quantified by spectrometry, followed by HE staining, IHC approach for Cx43 expression, and Western blot for Kir2.1 protein expression.
Results
Model rats had significantly lower HR, MAP, and RPP than in the sham group, and the pinocembrin pretreatment group had higher serum indexes. Arrhythmia index, CK-MB, and cTnI were higher in the model and pinocembrin groups, while Na+-K+ATPase, Ca+-Mg2+ATPase, Cx43, and Kir2.1 proteins were lower (p<0.05).
Conclusions
Pinocembrin alleviated ventricular arrhythmia in I/R rats via enhancing Na+-K+ATPase and Ca+-Mg2+ATPase activity and upregulating Cx43 and Kir2.1 protein expression.
MeSH Keywords: Heart Diseases, Myocardial Ischemia, Reperfusion
Background
Acute myocardial infarction is an important cause of cardiac death. Early acute myocardial ischemia and myocardial damage destabilize electric activity and ventricular repolarization, leading to malignant arrhythmia or death [1,2]. An effective treatment for myocardial ischemia is to recover coronary blood flow, but reperfusion after ischemia may lead to cardiac function or cardiomyocyte injury, causing intracellular calcium over-load, cell apoptosis, and inflammation. Defining the functional mechanism is important for preventing myocardial ischemia/reperfusion (I/R) injury. Pinocembrin is a flavonoid compound originated from propolis, which has anti-oxidation and protective effects for the cardiovascular system. It can resist against I/R injury via anti-oxidation, alleviating calcium overloading, inhibiting inflammation and cardiomyocyte apoptosis. Pinocembrin can alleviate ventricular fibrillation and cardiac infarction area in I/R rats, and can increase expression of phosphorylated gap junction connexin, thus protecting myocardial tissues [3,4]. Currently, the related functional mechanism of pinocembrin in resisting against myocardial I/R injury has not been defined.
Intracellular calcium overloading is an important cause of cardiac arrhythmia. Na+-K+ATPase and Ca2+-Mg2+ATPase play important roles in maintaining intracellular calcium homeostasis, as their enzymatic activity is decreased in myocardial tissues in arrhythmic patients [5,6]. The gap junction between cardiomyocytes is the structural basis for dispersion of myocardial tissue action potential, and plays an important role in cellular electric coupling and action transmission, mainly depending on change in gap junction connexin [7,8]. The major gap junction connexin in ventricular cardiomyocytes is Cx43. Under cardiac pathological conditions, humoral factors show abnormal levels, thus affecting expression, translocation, and distribution of gap junction connexin, disrupting communication between cardiomyocytes, enhancing cardiac potential de-coupling rate, and inducing cardiac arrhythmia [9,10]. The present study generated a rat myocardial I/R model in which the effect of pinocembrin pretreatment on Cx43 expression and ventricular rhythm was observed to determine the functional mechanism involved in alleviating cardiac arrhythmia on the molecular level, and to provide evidence for clinical treatment.
Material and Methods
Experimental animals and grouping
Healthy male SD rats (10 weeks age, body weight 350–400 g) were provided by the Laboratory Animal Center of Fudan University (Certificate No. SYXK-2013-0025) and were kept in an SPF-grade animal house with standard food and water. Rats were randomly assigned into a sham (A) group, model (B) group, and 30 mg/kg pinocembrin pretreatment (C) group (N=15 each). Group C received intravenous injection of pinocembrin 10 min before ischemia, while groups A and B received an equal volume of saline.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Zhongshan Hospital, Fudan University.
Drugs and reagents
Pinocembrin (Beisite Chem, China), urethane (Sigma, US), test kits for CK-MB, and cTnI and rat Cx43 immunohistochemistry (IHC) stain were purchased from Zhongshan Jinqiao (China). Rabbit anti-rat Kir2.1 monoclonal antibody, secondary antibody, and DAB kit were from Boster Bio, China.
K+ATPase, Ca2+-Mg2+ATPase test kits were from Jiancheng Bio, China.
Animal model preparation
The rat I/R model was generated following previous methods [11]. Rats were anesthetized by intraperitoneal injection of 5% urethane and were fixed in supine position. By EKG electrode recording, rats with abnormal cardiac function were excluded. A small-animal respiration machine (tidal volume 8 ml/kg, respiratory rhythm 70 per min, exhalation/inhalation ratio 1: 2) was connected to branchial intubation. The left common carotid artery was separated for insertion of the epidural anesthesia tube, which was connected to an electrophysiology recorder. The descending branch of the left coronary artery was ligated. In the sham group, vessels were not ligated. After 30-min ischemia treatment, 2-h reperfusion was performed to record the EKG pattern. The ischemia model was verified as elevated ST segment or higher magnitude of QRS wave group, plus wider waves and T wave fusion, and more than 20 mmHg decrease of artery pressure, with cyanosis on ventricular walls at the ligated region. The reperfusion model was verified as the disappearance of cyanosis, more than 50% decrease of ST segment elevation at 30 min after reperfusion, and exclusion of severe atrioventricular block before surgery, with lower than 60 mmHg mean artery pressure (MAP). Rat heart rate (HR), MPA, and rate pressure product (RPP) were measured at 10 min before ischemia (T0), 30 min after ischemia (T1), 30 min after reperfusion (T2), 60 min after reperfusion (T3), and 120 min after reperfusion (T3), along with observation of cardiac arrhythmia.
Spectrometry for myocardial Na+-K+ATPase and Ca2+-Mg2+ATPase
Myocardial tissues were homogenized and quantified for protein using Coomassie brilliant blue and spectrometry phosphorus determination. Na+-K+ATPase and Ca2+-Mg2+ATPase were determined following instructions of the test kit.
ELISA for measuring serum CK-MB and cTnI level
Following manual instructions, absorbance (A) values at 45 0nm wavelength were measured using a microplate reader.
HE staining and observation
Myocardial tissues were fixed in paraformaldehyde and were prepared for paraffin-based slices (5 μm). After hematoxylin-eosin staining, slices were observed under a light-field microscope.
IHC approach to quantify myocardial Cx43 expression
Rats were sacrificed to collect myocardial tissues, which were immersed in paraformaldehyde and were prepared for paraffin-based slices (5 μm). After de-waxing and antigen retrieval, hydrogen peroxide was added for primary antibody (Cx43 at 1: 100) incubation, followed by adding polymer enhancer, and enzyme-labelled anti-rabbit/rat polymers. DAB staining was performed, followed by counter-staining, dehydration, and mounting. Image-pro plus software was used for analysis. Five different fields were selected around the tumor tissue under a 40× scope to record number of positive cells for calculating average values.
Western blot for determining Kir2.1 protein expression
Total proteins were extracted from cells, separated by SDS-PAGE, and were transferred to PVDF membranes. After blocking in defatted milk powder, Kir2.1 protein monoclonal antibody (1: 500) was added, in parallel with GAPDH antibody (1: 1000) for overnight incubation. After TBST rinsing, secondary antibody (1: 2000) was added for 1-h incubation, followed by substrate development in the dark. Bands were analyzed by Quantity One imaging software.
Statistical methods
SPSS 19.0 was used for statistical analysis. Comparison of ratios was performed by chi-square analysis. Measurement data were first tested for normality and are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to compare means among multiple groups, followed by LSD test between 2 groups. A statistical significance was defined when p<0.05.
Results
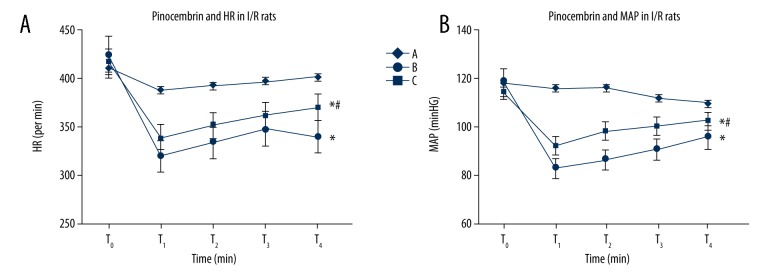
Effects of pinocembrin pretreatment on hemodynamic and arrhythmia in I/R rats
At T1, T2, T3, and T4, group B and group C had lower HR, MAP, and RPP. Group B had significantly lower HR, MAP, and RPP than group A at T1, T2, T3, and T4. Group C had higher HR, MAP, and RPP than group B (p<0.05). Group B and C had higher cardiac arrhythmia scores than group A, while group C had lower scores than group B (p<0.05, Figures 1, 2).
Figure 1.
Effects of pinocembrin on HR and MAP of I/R rats. T0 – 10 min before ischemia; T1 – 30 min after ischemia; T2 – 30 min after reperfusion; T3 – 60 min after reperfusion; T4 – 120 min re-perfusion. HR – heart rate; MAP – mean artery pressure. A – Sham group; B – model group; C – pinocembrin group. * p<0.05 compared to group A; # p<0.05 compared to group B.
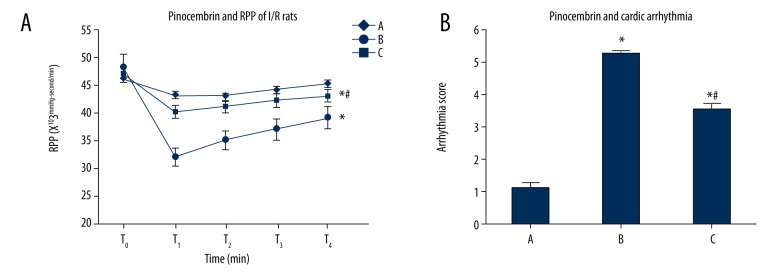
Figure 2.
Effects of pinocembrin on I/R rat RPP and cardiac arrhythmia. T0 – 10 min before ischemia; T1 – 30 min after ischemia; T2 – 30 min after reperfusion; T3 – 60 min after re-perfusion; T4 – 120 min reperfusion. RPP – rate pressure product. A – sham group; B – model group; C – pinocembrin group. * p<0.05 compared to group A; # p<0.05 compared to group B.
Effect of pinocembrin pretreatment on CK-MB and cTnI expression in I/R rats
Group B and group C and higher CK-MB and cTnI levels than group A, and group C had lower CK-MB and cTnI levels than group B (p<0.05, Figure 3).
Figure 3.
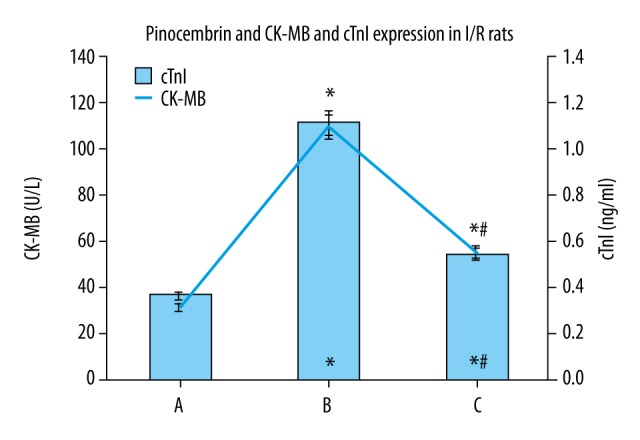
Effects of pinocembrin on CK-MB and cTnI expression in I/R rats. CK-MB – creatine phosphate kinase isoforms; cTnI – troponin. A – sham group; B – model group; C – pinocembrin group. * p<0.05 compared to group A; # p<0.05 compared to group B.
Effects of pinocembrin pretreatment on Na+-K+ATPase, Ca2+-Mg2+ATPase levels in I/R rats
Group B and C had lower Na+-K+ATPase, Ca2+-Mg2+ATPase than group A (p<0.05). Group C had higher Na+-K+ATPase, Ca2+-Mg2+ATPase levels than group B (p<0.05, Figure 4).
Figure 4.
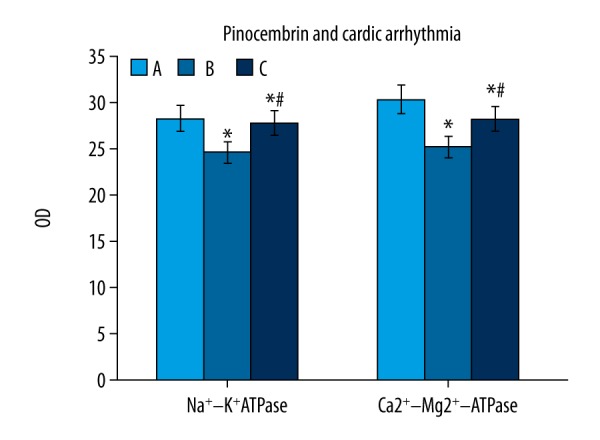
Effects of pinocembrin pre-treatment on Na+-K+ATPase and Ca2+-Mg2+ATPase levels in I/R rats. A – sham group; B – model group; C – pinocembrin group. * p<0.05 compared to group A; # p<0.05 compared to group B.
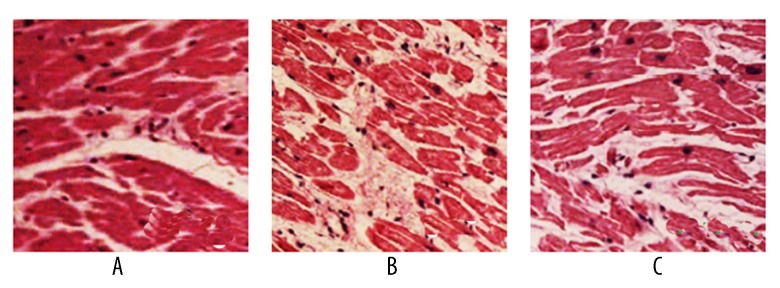
Myocardial morphology of rats
The sham group had normal myocardial morphology and regular arrangement of myocardial fibers. The model group had irregular arrangement of myocardial fibers, with inflammatory cell infiltration in myocardial mesenchymal. Stained myocardial fibers showed platelet-like distribution. The pinocembrin group showed significant improvement of myocardial tissues (Figure 5).
Figure 5.
Myocardial tissue morphology change (HE staining, ×400). A – sham group; B – model group; C – pinocembrin group.
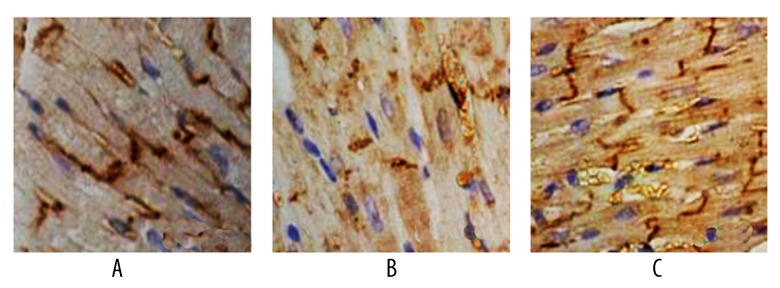
Effects of pinocembrin pretreatment on Cx43 and Kir2.1 protein expression in I/R rats
Cx43 protein was strongly positively expressed in the sham group, with regular band distribution. The model group had lower Cx43 expression with dispersed dotted distribution without regular patterns. Group B and C had lower Cx43 and Kir2.1 levels than group A (p<0.05). Group C had higher Cx43 and Kir2.1 protein expression than group B (p<0.05, Figures 6–8).
Figure 6.
IHC staining for Cx43 in myocardial tissues. A – sham group; B – model group; C – pinocembrin group.
Figure 7.
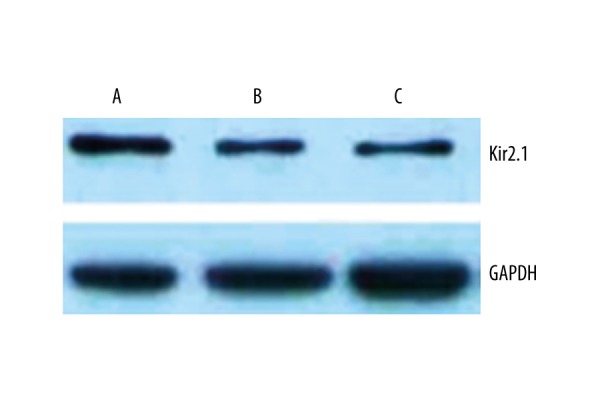
Kir2.1 protein expression in myocardial tissues. A – sham group; B – model group; C – pinocembrin group.
Figure 8.
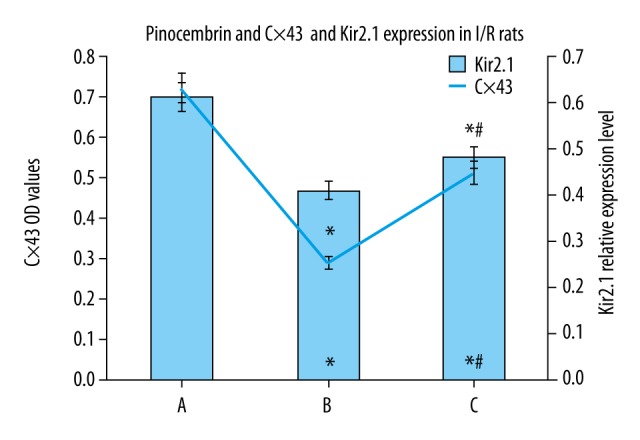
Effects of pinocembrin pre-treatment on Cx43 and Kir2.1 protein expression in I/R rats. A – sham group; B – model group; C – pinocembrin group.
Discussion
Cardiomyocytes may be denatured and undergo necrosis and lysis during ischemia. A multitude of factors are involved in the occurrence and development of heart disease [12]. Reperfusion injury potentiates membrane permeability of cardiomyocytes and elevates myocardial injury marker levels in serum. The severity of myocardial tissue injury is correlated with CK-MB and cTnI levels [13,14]. Recent findings show that a variety of components from plants, such as malvidin, contribute to cardioprotective effects against isoproterenol-induced myocardial infarction in rats [15]. In the present study, I/R model rats had significantly higher serum CK-MB and cTnI levels than in the control group, and the pinocembrin pretreatment group had remarkably lower CK-MB or cTnI levels. HE staining also confirmed alleviated myocardial tissue injury in the pinocembrin pretreatment group, indicating that pinocembrin pretreatment can alleviate rat myocardial I/R injury. The pathogenesis mechanism of cardiac arrhythmia has not been fully defined. Some studies showed that it might be related with degeneration of transmission fibers and pacemaker cells, abnormal ion channels, abnormal tension of the vagus nerve, or bilateral transmembrane ion transport in myocardial cells [16,17]. As the common ion driving pump in membranes, Na+-K+-ATPase is responsible for transmembrane transport of Na+ and K+. It can maintain the concentration gradient of Na+ and K+ across membrane and resist electrochemical gradients. A major function of Ca2+-Mg2+-ATPase is to actively transport Ca2+ out of cells and to take Ca2+ into the sarcoplasmic reticulum. Due to disordered Ca2+ release from the repository, the sarcoplasmic reticulum has lower permeability of Ca2+ and suppressed Ca2+ inflow, causing slow auto-depolarization of sinus node and inducing chronic cardiac arrhythmia. Both Na+-K+-ATPase and Ca2+-Mg2+-ATPase participate in ion channel and ion transport of cardiomyocytes [16,17]. Bilateral ion transport is the basis for action potential formation. Due to the effect of membrane permeability, abnormal ion channel of myocardial tissues is the major cause of chronic cardiac arrhythmia [18,19]. Our study shows that pinocembrin pretreatment can significantly elevate Na+-K+-ATPase and Ca2+-Mg2+-ATPase activity in I/R rat myocardial tissues, maintain normal ion concentration gradient and electrochemical gradient inside and outside of cells, and maintain normal cellular energy metabolism.
Major gap junction connexins in human tissues include Cx40, Cx43, and Cx45. Cx43 is the major connexin between cardiomyocytes. Cx43 functional gene locates on chromosome 6 and is mainly coded by GJA1 gene. Cx43 protein is distributed in groups at cardiomyocyte junctions to maintain cellular communication, energy exchange, and chemical information transmission. Cx43 down-regulation can de-couple cell electricity, elongate action potential duration and process of repolarization, causing transmission arrest. Cx43 thus plays an important role in maintaining normal development of the heart, synchrony of whole cardiac electrical activity, and coordination or secretion [19,20]. Abnormal expression and distribution of Cx43 can cause weakened electrical transmission coupling and intracellular transmission and induce reentrant arrhythmia. Homozygous Cx43 gene knockout mice had normal heart structure and constriction function but died at 2 months of age due to spontaneous ventricular arrhythmia; gene therapy to enhance Cx43 gene expression in those mice can protect against those lethal arrhythmia [21,22]. Gap junction structure and function play important roles in onset and progression of cardiac arrhythmia. Major gap junction protein in myocardial tissues Cx43 has close correlation with cardiac arrhythmia. Kir2.1 protein is mainly encoded by KCNJ2 gene on chromosome 17. Kir2.1 protein-induced IK1 current can maintain stable membrane resting potential. Decreased Kir2.1 protein expression or function can decrease phase 3 rapid repolarization current magnitude of action potential, and elongate QT intermittent period or action potential duration, causing cardiac arrhythmia [22,23]. Our study shows decreased Cx43 and Kir2.1 protein expression in model rat myocardial tissues, and elevated Cx43 and Kir2.1 protein expression after pinocembrin pretreatment, probably forming a single functional mechanism of pinocembrin to alleviate cardiac arrhythmia in I/R rats. Up-regulation of Cx43 and Kir2.1 protein expression is correlated with GJA1 and KCNJ2 gene expression for Cx43 and Kir2.1 protein coding [24,25]. Opening/closure of gap junction channel is correlated with multiple factors such as intracellular Ca2+ level, cytoplasmic pH, transmembrane potential, and phosphorylation status of connexin, along with lower intracellular Ca2+ level or decreased electric transmission in gap junction, in a dose-dependent manner. Pinocembrin can enhance Ca2+-Mg2+-ATPase to maintain cardiac ion channel, and can up-regulate Cx43 protein expression, thus exerting anti-arrhythmia effects. Moreover, as a major component of propolis, pinocembrin was reported to induce cytoprotective effects in human cells [26]. Pinocembrin can also function as an antioxidant and anti-inflammatory molecule, and counteract the deleterious effects elicited by reactive species/free radicals and attenuates inflammation [27]. Future research based on in vivo data are necessary to evaluate the cytoprotective effect of pinocembrin in clinical practice.
Conclusions
Pinocembrin pretreatment can alleviate the functional mechanism of cardiac arrhythmia in I/R rats via enhancing Na+-K+-ATPase and Ca2+-Mg2+-ATPase and upregulating Cx43 and Kir2.1 protein expression level. It can restore cellular gap junction connexin function and IK1 current via enhancing gap junction- or ion channel-related gene or protein expression, and correct action potential, P-R intermittent period, QRS duration, intracellular transmission velocity, and suppress cardiac arrhythmia. The mechanism regulating Cx43 and Kir2.1 protein expression might involve GJA1 and KCNJ2 genes, but this requires further studies.
Footnotes
Source of support: This project supported by the Shanghai Municipal Commission of Health and Family Planning (No. 20134177) and the Shanghai Medical Key Specialty Construction Projects(Class A) (No. ZK2015A10)
Conflict of interest
None.
References
- 1.Fanton Y, Robic B, Rummens JL, et al. Cardiac atrial appendage stem cells engraft and differentiate into cardiomyocytes in vivo: A new tool for cardiac repair after MI. Int J Cardiol. 2015;201:10–19. doi: 10.1016/j.ijcard.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 2.Horvat D, Vincelj J. Impact of reperfusion therapy and infarct localization on frequency of premature ventricular beats in acute myocardial infarction. Med Glas (Zenica) 2015;12:139–43. doi: 10.17392/820-15. [DOI] [PubMed] [Google Scholar]
- 3.Lungkaphin A, Pongchaidecha A, Palee S, et al. Pinocembrin reduces cardiac arrhythmia and infarct size in rats subjected to acute myocardial ischemia/reperfusion. Appl Physiol Nutr Metab. 2015;40:1031–37. doi: 10.1139/apnm-2015-0108. [DOI] [PubMed] [Google Scholar]
- 4.Saad MA, Abdel Salam RM, Kenawy SA, Attia AS. Pinocembrin attenuates hippocampal inflammation, oxidative perturbations and apoptosis in a rat model of global cerebral ischemia reperfusion. Pharmacol Rep. 2015;67:115–22. doi: 10.1016/j.pharep.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Aiba T, Noda T, Hidaka I, et al. Acetylcholine suppresses ventricular arrhythmias and improves conduction and Connexin-43 properties during myocardial ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2015;26:678–85. doi: 10.1111/jce.12663. [DOI] [PubMed] [Google Scholar]
- 6.Ni M, Ruan L, Zhang C. Antiarrhythmic peptide AAP10 prevents arrhythmias induced by protein kinase C activation in rabbit left ventricular wedges. Int Heart J. 2015;56:234–38. doi: 10.1536/ihj.14-234. [DOI] [PubMed] [Google Scholar]
- 7.Zhao G, Zhang W, Li L, et al. Pinocembrin protects the brain against ischemia-reperfusion injury and reverses the autophagy dysfunction in the penumbra area. Molecules. 2014;19:15786–98. doi: 10.3390/molecules191015786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J, Zhao Y, Wang Y, et al. Anti-arrhythmic effect of acupuncture pretreatment in the rats subjected to simulative global ischemia and reperfusion – involvement of intracellular Ca2+ and connexin 43. BMC Complement Altern Med. 2015;15:5. doi: 10.1186/s12906-015-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohl P. Structural and functional recoupling of atrial and ventricular myocardium: New conduits for electrical flow. J Am Coll Cardiol. 2014;64:2586–88. doi: 10.1016/j.jacc.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 10.Rampazzo A, Calore M, van Hengel J, van Roy F. Intercalated discs and arrhythmogenic cardiomyopathy. Circ Cardiovasc Genet. 2014;7:930–40. doi: 10.1161/CIRCGENETICS.114.000645. [DOI] [PubMed] [Google Scholar]
- 11.Patil KD, Halperin HR, Becker LB. Cardiac arrest: Resuscitation and reperfusion. Circ Res. 2015;116:2041–49. doi: 10.1161/CIRCRESAHA.116.304495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasilowska-Barud A, Zapolski T, Barud M, Wysokinski A. Overt and covert anxiety as a toxic factor in ischemic heart disease in women: The link between psychological factors and heart disease. Med Sci Monit. 2017;23:751–58. doi: 10.12659/MSM.902544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Promsan S, Jaikumkao K, Pongchaidecha A, et al. Pinocembrin attenuates gentamicin-induced nephrotoxicity in rats. Can J Physiol Pharmacol. 2016;94:808–18. doi: 10.1139/cjpp-2015-0468. [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Wu D, Wu Q, et al. Effect and mechanism of Irbesartan on occurrence of ventricular arrhythmias in rats with myocardial ischemia through connexin43 (cx43) Asian Pac J Trop Med. 2016;9:1007–12. doi: 10.1016/j.apjtm.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Wei H, Li H, Wan SP, et al. Cardioprotective effects of malvidin against isoproterenol-induced myocardial infarction in rats: A mechanistic study. Med Sci Monit. 2017;23:2007–16. doi: 10.12659/MSM.902196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Shan J, Yang Q, et al. Antiarrhythmic efficacy of CPUY102122, a multiple ion channel blocker, on rabbits with ischemia/reperfusion injury. Pharmacol Rep. 2014;66:1022–30. doi: 10.1016/j.pharep.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Seki A, Nishii K, Hagiwara N. Gap junctional regulation of pressure, fluid force, and electrical fields in the epigenetics of cardiac morphogenesis and remodeling. Life Sci. 2015;129:27–34. doi: 10.1016/j.lfs.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Dhein S, Seidel T, Salameh A, et al. Remodeling of cardiac passive electrical properties and susceptibility to ventricular and atrial arrhythmias. Front Physiol. 2014;5:424. doi: 10.3389/fphys.2014.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos-Almeida FM, Girao H, da Silva CA, et al. Cholinergic stimulation with pyridostigmine protects myocardial infarcted rats against ischemic-induced arrhythmias and preserves connexin43 protein. Am J Physiol Heart Circ Physiol. 2015;308:H101–7. doi: 10.1152/ajpheart.00591.2014. [DOI] [PubMed] [Google Scholar]
- 20.Siragam V, Cui X, Masse S, et al. TMEM43 mutation p.S358L alters intercalated disc protein expression and reduces conduction velocity in arrhythmogenic right ventricular cardiomyopathy. PLoS One. 2014;9:e109128. doi: 10.1371/journal.pone.0109128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin SY, Jo WM, Min TJ, et al. Gap junction remodelling by chronic pressure overload is related to the increased susceptibility to atrial fibrillation in rat heart. Europace. 2015;17:655–63. doi: 10.1093/europace/euu294. [DOI] [PubMed] [Google Scholar]
- 22.Chang Y, Yu T, Yang H, Peng Z. Exhaustive exercise-induced cardiac conduction system injury and changes of cTnT and Cx43. Int J Sports Med. 2015;36:1–8. doi: 10.1055/s-0034-1384545. [DOI] [PubMed] [Google Scholar]
- 23.Grippo AJ, Moffitt JA, Henry MK, et al. Altered Connexin 43 and Connexin 45 protein expression in the heart as a function of social and environmental stress in the prairie vole. Stress. 2015;18:107–14. doi: 10.3109/10253890.2014.979785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubkemeier I, Bosen F, Kim JS, et al. Human Connexin43E42K mutation from a sudden infant death victim leads to impaired ventricular activation and neonatal death in mice. Circ Cardiovasc Genet. 2015;8:21–29. doi: 10.1161/CIRCGENETICS.114.000793. [DOI] [PubMed] [Google Scholar]
- 25.Prudat Y, Kucera JP. Nonlinear behaviour of conduction and block in cardiac tissue with heterogeneous expression of connexin 43. J Mol Cell Cardiol. 2014;76:46–54. doi: 10.1016/j.yjmcc.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Wang Y, Zhao L, et al. Pinocembrin attenuates MPP(+)-induced neurotoxicity by the induction of heme oxygenase-1 through ERK1/2 pathway. Neurosci Lett. 2016;612:104–9. doi: 10.1016/j.neulet.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 27.Gu X, Zhang Q, Du Q, Shen H, Zhu Z. Pinocembrin attenuates allergic airway inflammation via inhibition of NF-kappaB pathway in mice. Int Immunopharmacol. 2017;53:90–95. doi: 10.1016/j.intimp.2017.10.005. [DOI] [PubMed] [Google Scholar]