Abstract
Type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD) commonly exist together. It has been regarded as a manifestation of the metabolic syndrome. The presentations of NAFLD range from simple steatosis (NAFL), nonalcoholic steatohepatitis (NASH), and cirrhosis. NAFLD has a prevalence of 70% among T2DM patients. Overweight/obesity and insulin resistance (IR) have been strongly linked with NAFLD. Noninvasive assessment and staging of disease are based on clinical parameters such as age, sex, liver function test, platelet count, lipid profile, BMI, and imaging modalities such as USG, transient elastography (TE), and magnetic resonance imaging mass spectroscopy. Such clinical scoring systems and TE are useful in the early detection of NAFLD and predicting fibrosis. The principle behind the management of NAFLD with T2DM involves an indirect effect through improvement in IR and glycemia and thus is used for the treatment of T2DM as well.
Keywords: Cirrhosis, insulin resistance, metabolic syndrome, nonalcoholic fatty liver, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, statins, transient elastography, type 2 diabetes mellitus
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2DM) often coexist.[1] The prevalence of NAFLD is 59.67% in T2DM patients.[1] This results in adverse outcomes such as higher rates of mortality due to cirrhosis.[1] NAFLD includes a spectrum of pathological conditions, which range from simple steatosis (NAFL), nonalcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma.[2]
DEFINITION OF NONALCOHOLIC FATTY LIVER DISEASE
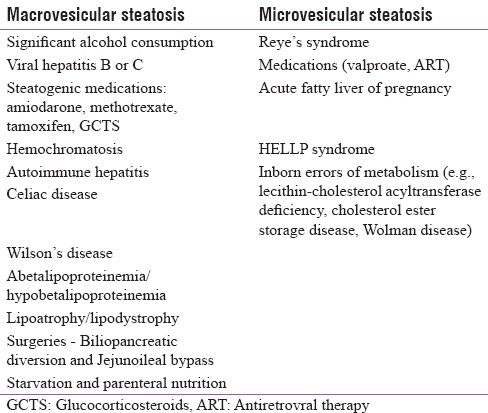
NAFLD is defined as hepatic steatosis diagnosed either by histology/imaging with macrovesicular steatosis in >5% of hepatocytes according to histological analysis or by proton density fat fraction or >5.6% as assessed by proton magnetic resonance spectroscopy (MRS) or quantitative fat/water selective magnetic resonance imaging (MRI) with no secondary cause for steatosis.[3] Secondary causes of steatosis are included in Table 1.[4]
Table 1.
Secondary causes of nonalcoholic fatty liver disease
PREVALENCE
NAFLD is a growing public health problem reaching epidemic proportions and is considered as the most common cause of chronic liver disease.[5] The prevalence of NAFL is 31% by MRS[6] and 12.2% by histology, of which 5% had NASH. The prevalence of NAFLD is 59.67% in T2DM[5] whereas biopsy-proven NASH in asymptomatic T2DM patients with normal liver function tests (LFTs) is 20%.[7] This suggests that serum liver enzymes are less indicative of the severity of intrahepatic fat accumulation and that the currently used “normal” reference values for serum liver enzymes need revision.[8] Armstrong et al. estimated the prevalence of advanced fibrosis in asymptomatic T2DM to be around 5%–7%.[8]
RISK FACTORS FOR NONALCOHOLIC FATTY LIVER DISEASE
Type 2 diabetes mellitus
Metabolic syndrome[9]
Obesity[9]
Physical inactivity[10]
A high-calorie diet, excess saturated fats, refined carbohydrates, sugar-sweetened beverages, a high fructose intake3
Obstructive sleep apnea.[3]
Since NAFLD is closely linked to metabolic syndrome, it has been regarded as the hepatic manifestation of the syndrome.[1] Evaluating the risk for NAFLD is recommended in all patients with any component of metabolic syndrome as all components of the metabolic syndrome correlate with the degree of liver fat content and also vice versa.[3]
Natural history of nonalcoholic fatty liver disease
NAFLD is a slowly progressive disease; however, in 20%, it progresses rapidly.[1] Progression in NAFL to fibrosis Stage 1 is every 14 years and every 7 years in NASH, which is further increased in the presence of arterial hypertension.[11] Cirrhosis and liver failure occurs in 11%–20% in NASH patients[11] over 10–15 years.
There is a 2.2-fold increase in overall mortality in NAFLD with the most common cause of death being cardiovascular disease. Patients with NASH (but not NAFL) have an increased liver-related mortality rate with decompensated liver failure and HCC corresponding to 2%.[11] The mortality rate of T2DM patients due to cirrhosis is more than twice the general population and patients with both NAFLD and T2DM. Furthermore, they tend to have a poor prognosis with higher rates of cirrhosis and mortality.[12]
Pathological ectopic fat accumulation together with low-grade chronic inflammatory state in the liver, an organ that is not able to accumulate fat, is a manifestation of NAFLD.[1] The risk of T2DM increases by 5-fold in these patients as a result of IR in hepatic, muscle, and adipose tissue.[13] The risk of developing T2DM can be modified with improvement in NAFLD.[14] As of today, there are no prediction models for the development of T2DM in individuals with NAFLD, and hence, a pragmatic approach would be an annual surveillance.[5]
The individual's risk of developing NAFLD is modified or increased by the presence of T2DM.[15] One of the likely precursors for NAFLD and its further progression could be prediabetes.[16]
NONALCOHOLIC FATTY LIVER DISEASE AND DIABETES
The association
Cardiovascular events in NAFLD are increased by 1.87-fold in the presence of T2DM. NAFLD has been associated with increased carotid intima-media thickness, increased coronary artery calcium score, early left ventricular diastolic dysfunction, decreased myocardial perfusion, and reduced myocardial high-energy phosphate metabolism in patients with T2DM.[17]
NAFLD is also known to increase microvascular complications of diabetes such as chronic kidney disease and retinopathy.[18] These pathological changes occur due to the release of pro-inflammatory, procoagulant, and pro-oxidant mediators, which result in hepatic/systemic IR, atherogenic dyslipidemia, and the release of fetuin-A, fibroblast growthfactor-21, and retinol-binding protein-4 by liver.[19] In the liver and skeletal muscle, fetuin-A binds and inhibits the insulin receptor tyrosine kinase, thus inhibiting insulin signal transduction, resulting in systemic and hepatic IR.[19]
Patients with diabetes or prediabetes had progressive fibrosis when their serial biopsies were studied.[2] It has also been suggested that the advanced forms of NAFLD such as NASH, advanced fibrosis, cirrhosis, and HCC occur more commonly in these patients.[1] Those patients with worsening metabolic risk factors tend to have increased progression from NAFL to NASH and fibrosis.[20] A clinical model which predicted NASH and advanced fibrosis in NAFLD patients with T2DM was developed by Bazick et al. in 2015 which had a better accuracy than the NAFLD fibrosis score with a specificity of 90.0% and a sensitivity of 56.8%.[21] This model took the following parameters into account such as BMI, waist circumference, HbA1c, IR by homeostasis model assessment (HOMA), and serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, and ferritin.[21] Thus, it could diagnose the presence of NASH in 1249 patients.[21] Increased levels of inflammatory biomarkers and hyperinsulinemia that increased the risk of HCC[22] further strengthened the pathophysiological link between T2DM and HCC. Thus, patients with NAFLD should be screened for metabolic syndrome and T2DM[23] and vice versa. Elastography can be used as a tool for screening NAFLD in T2DM patients.[23] Although there is growing evidence that NAFLD is closely associated with CAD/CKD in T2DM, whether a causal association exists needs to be established.[1]
Pathophysiology
The main pathogenic mechanism of NAFLD is IR in the liver and extrahepatic tissues such as adipose tissue and skeletal muscle which act synergistically leading to systemic inflammation which causes the release of proatherogenic and nephrotoxic factors.[24] There is an increased flux of free fatty acids (FFAs) to ectopic tissues due to an increased rate of lipolysis in the dysfunctional adipose tissue causing the muscle and liver to develop IR and apoptosis.[25] Thus, the “lipotoxic state” in NASH results in hepatocyte necroinflammation.[25]
There are three sources of triacylglycerol (TAG) which tends to accumulate in the liver: 59% of it is from circulating FFAs; de novo lipogenesis (DNL – the process in which carbohydrates are converted to lipids) contributes to 26%; and the rest 14% is from the diet.[25] FFAs entering the portal circulation has one of the three fates: either to undergo ß-oxidation; to undergo resterification to TAG and get fluxed out as very low-density lipoprotein; or to get stored in the liver after re-esterification.[25] DNL is further enhanced by IR.[24] There is reduced rate of glycogen synthesis with increased rate of gluconeogenesis in NAFLD.[25] The increase in intrahepatic glucose and resultant product of glycolysis pyruvate act as substrates for DNL which in turn increases the production of acetyl-CoA, which gets converted to malonyl-CoA contributing to DNL, instead of allowing it to enter the citric acid cycle.[25] All the above contribute to hepatic steatosis.[25] Increased FFA flux into the liver aggravates hepatic IR.[24] The mechanism contributing to hepatic IR is the translocation of protein kinase C to the membrane compartment from the cytosol causing impaired activation of insulin receptor substrate – phosphoinositol-3 kinase.[24] Increased levels of ceramide in hepatocytes repress protein kinase B activity resulting in decreased insulin signaling.[24] Oxidative stress, mitochondrial dysfunction, and circulating cytokines are the contributing factors for transition from simple steatosis to NASH, which then progresses to fibrosis.[25] In addition, there is a second “hit” that causes the failure of hepatocytes to regenerate causing an increase in fibrosis.[2]
DIAGNOSIS AND MANAGEMENT
Diagnosis
For the diagnosis of NAFLD, there should be no history of previous or ongoing significant alcohol consumption, no exposure to steatogenic medications or other causes of liver disease, such as viral hepatitis.[2]
For a precise diagnosis of NAFLD, liver biopsy is the investigation of choice[2]
-
Indications for liver biopsy in NAFLD include:[1]
- NAFLD patients at increased risk to have steatohepatitis and advanced fibrosis
- The metabolic syndrome and NAFLD fibrosis score: patients at risk for steatohepatitis and advanced fibrosis
- Suspected NAFLD in whom competing etiologies for hepatic steatosis and coexisting chronic liver diseases cannot be excluded.
Based on liver biopsy, staging of steatosis and fibrosis can be made by various scores such as NAFLD activity score (NAS), steatosis, activity, and fibrosis score.[3]
Noninvasive imaging test for steatosis is ultrasound (USG) (preferred for first-line diagnosis which shows increased echogenicity)/MRI: proton density fat fraction and proton MRS or quantitative fat/water-selective MRI/fibroscan/CT.[3] USG has a sensitivity of 84.8%, specificity of 93.6%, PPV of 77%, and NPV of 67%.[3] Transient elastography (TE) can detect steatosis >10% in contrast to MRI which can detect steatosis as low as <1%.[1] Another advantage of MRI is that it is not affected by obesity in contrast to TE whose sensitivity declines in patients with BMI >35 kg/m2. Furthermore, it can screen for HCC at the same session. There are various steatosis scores which predict the presence, not the severity, of steatosis such as fatty liver index, Steato test, and NAFLD liver fat score.[3]
However, imaging techniques are unable to differentiate between simple benign steatosis, NASH, and the degree of fibrosis, and they merely describe the presence of a “fatty liver.[1]”
Whenever imaging tools are not available or feasible (e.g., large epidemiological studies), serum biomarkers and scores can also be considered alternative for the diagnosis of steatosis with a 2- to 4-fold elevation of serum ALT and AST levels.[2] However, it can be normal in 78% of the patients.[3] Usually AST/ALT ratio is <1.[3] However, it may increase as fibrosis advances.[3] Furthermore, alkaline phosphatase and GGT levels may be elevated, but the serum bilirubin level, prothrombin time, and serum albumin level are normal, except in cirrhosis.[1]
Based on clinical, biochemical, or imaging measures, one cannot distinguish NASH from steatosis. Therefore, cytokeratin-18 fragments (CK-18) produced during cell death by caspases can be used with a 66% sensitivity and 82% specificity. However, it is not recommended in routine clinical practice. Other biomarkers for diagnosis of NASH include the following:
Other cell death markers (e.g., soluble Fas and intact CK-18)
Adipokines (e.g., adiponectin, tumor necrosis factor-alpha [TNF-α], interleukin-6, and adipocyte fatty acid-binding protein)
Metabolic markers (HOMA-IR and growth factor receptor 21)
Inflammatory markers (e.g., C-reactive protein [CRP]).
For the diagnosis of liver fibrosis, various scores can be used such as NAFLD fibrosis score, enhanced liver fibrosis score, Fibro test, BARD score (AST, ALT, BMI, Diabetes/Impaired Fasting Glucose), and FIB4 score.[1] One of the useful diagnostic techniques in NAFLD is TE which relies on measuring velocity of 50 MHz shearwave passing through the liver which is converted to a stiffness score measured in kPa.[3] It has a sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 100%, 73.9%, 77.8%, 100%, and 86.4%, respectively, in predicting fibrosis in NAFLD.[3]
Other imaging modalities for fibrosis include acoustic radiation force impulse, shear wave elastography, and magnetic resonance elastography.
MANAGEMENT
There is a limitation for the use of therapeutic disease-modifying options in NAFLD.[2]
The interventions for the management have an indirect effect through improvement in IR and glycemia and thus are used for the treatment ofT2DM as well.[1] Pharmacotherapy has to be reserved for those with highest risk for disease progression in NAFLD.[1] Definitive clinical trials are limited.[1] Some of the drugs such as ursodeoxycholic acids are not recommended for the treatment of NASH/NAFLD.[26] In this discussion, only the treatment options useful in NAFLD and T2DM are included:
Lifestyle Modification
It has been recommended that 500–1000 kcal energy deficit is required to induce a weight loss of 500–1000 g/week. Mediterranean diet with high-protein intake reduces liver fat on H1-MRS, when compared with a low-fat, high-carbohydrate diet.[5] Thus, a weight loss ≥7% over 12 months causes NASH regression in 25% and steatosis regression in 40%. However, rapid weight loss can worsen LFT in NAFLD. Fructose-containing beverages and foods and saturated fatty acids should be avoided.[5] It is also stated that alcohol intake should be below the risk threshold (i.e., <30 g for men; <20 g for women).[5] Regular coffee consumption of 2–3 cups daily also is known to decrease the risk of hepatic fibrosis. A 150–200 min/week of moderate intensity aerobic physical activities in 3–5 sessions such as brisk walking, stationery cycling, and 3 times/week of 45 min of resistance training also have been mentioned to be useful.[5] Exercise alone can reduce hepatic steatosis, but its ability to improve other aspects of liver histology however remains unknown.[5]
STATINS
The GREACE trial showed the safety of statins in NAFLD/NASH.[27] In dyslipidemia, statins and other lipid-lowering agents are considered safe in NAFLD and NASH with improvement in histology.[27] Although statin use is warranted in NASH cirrhosis, it should be avoided in decompensated cirrhosis.[26]
NAFLD is considered as one of the CVD risk factors.[1] In T2DM, cardiovascular risk tends to be underestimated.[1] Algorithms used for traditional CVD risk calculation usually tend to underrate cardiovascular risk in T2DM and NAFLD.[1] Since there is no available evidence to the contrary, a statin is required if estimated 10-year cardiovascular risk is >15%. In addition to the benefits statins have on lipids, there is an improvement in insulin sensitivity, decrease in production of advanced glycation endproducts (AGEs), and anti-inflammatory effects, which might reduce steatosis and inflammation associated with NASH.[28] However, statin should not be used in NASH alone without the association of dyslipidemia till there is proven histological improvements as clinical trials on statins as treatment for NASH are limited and have shown inconsistent results.[4]
OMEGA-3 POLYUNSATURATED FATTY ACID
Hypertriglyceridemia, which often coexists in NAFLD and T2DM, can be treated with high-dose omega-3 polyunsaturated fatty acid (PUFAs).[5] Their mechanism of action involves the activation of FGF21 which in turn activates peroxisome proliferator-activated receptor alfa (PPAR alfa) α, resulting in activation of several genes involved in fatty acid oxidation.[29] However, PUFAs cannot be considered to have a specific role in the treatment of NAFLD/NASH[4] unless there is coexisting hypertriglyceridemia.[26]
VITAMIN E
Oxidative stress occurs in both NAFLD and T2DM.[1] According to PIVENS trial, 800 IU/day of Vitamin E for 96 weeks improved liver enzymes, steatosis, inflammation, and ballooning (except fibrosis) and induced resolution of NASH in 42% of patients.[30] Consequently, it has been considered as a first-line pharmacotherapy at dose of 800 IU/day for nondiabetic adults with biopsy-proven NASH.[3] Nevertheless, it has not been recommended in T2DM with NASH, NAFLD without liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis unless further data supporting its effectiveness become available.[4]
PENTOXIFYLLINE
Pentoxifylline, a nonselective phosphodiesterase inhibitor, has a role in decreasing the inflammatory pathways such as TNF-α.[31] Mixed results on plasma aminotransferases and hepatic steatosis on imaging have been established with some studies showing improvement while some showing no improvement.[32]
INSULIN
Although IR with hyperinsulinemia is deleterious to the liver, exogenous insulin in T2DM patients can be worthwhile.[33] As NAFLD is closely linked with IR, there is an increased requirement of insulin, which has the potential for weight gain.[34] T2DM patients inadequately controlled on oral antidiabetic drugs when put on 12 weeks of insulin glargine therapy had hepatic fat reduction on MRS by 12.6 % to 9.9% with an improvement in HbA1c from 7.9% to 7.2%.[33] While insulin enhances lipogenesis with decrease in lipid oxidation in vitro,[35] human studies show paradoxical improvement in liver fat, which could be attributed to increased TAG secretion, improved hepatic insulin sensitivity, and reduced gluconeogenesis.[36,37] Thus, in T2DM and across all the stages of NAFLD, insulin is effective in optimizing glycemic control.[36] Insulin has a profibrotic effect, causing proliferation of hepatic stellate cells and accumulation of type 1 collagen.[38] Ryysy et al. concluded that intrahepatic triglyceride content was associated with high daily insulin dose and linked with IR.[39]
METFORMIN
Metformin is the first-line therapeutic agent in the treatment of T2DM.[2] Metformin decreases body fat with an improvement in hepatic insulin sensitivity.[40] Fatty acid oxidation is enhanced, and de novo lipogenesis is reduced secondary to activation of protein kinase without significant histological improvement in hepatic steatosis or inflammation.[41] In NAFLD without diabetes, there is no license for the use of metformin.[2] It has been stated that there is improved survival in cirrhosis and HCC even though definitive improvement in steatosis or histological features of NASH has not been established.[42]
SULFONYLUREA
Role of sulfonylurea in NAFLD with diabetes[2] has still not been established by prospective studies. On the contrary, retrospective data have suggested that T2DM with NAFLD treated with sulfonylureas has a higher risk of fibrosis due to the profibrotic effect of insulin.[43]
THIAZOLIDINEDIONES
Glitazones cause adipose tissue sensitization to insulin through activation of PPARϒ resulting in fatty acid uptake and storage.[44] There is also an increase in adiponectin with amelioration of pro-inflammatory adipokines, thus reducing gluconeogenesis and fatty acid influx improving insulin sensitivity.[45] They also cause restoration of normal adipose tissue biology and result in an improvement in hepatic steatosis.[46] PIVENS trial compared low-dose pioglitazone versus Vitamin E versus placebo for 2 years in patients without overt diabetes and concluded that pioglitazone (improved all histological features [except for fibrosis]) and resolution of NASH was achieved more than placebo.[30] Cusi et al. in a double-blind randomized placebo-controlled study concluded that reduction in NAS (incorporating the sum of improvement of hepatic steatosis, inflammation, and ballooning without worsening of fibrosis) was achieved with pioglitazone in NASH with prediabetes or T2DM.[46] Improvement in fibrosis, insulin sensitivity in liver, skeletal muscle, and adipose tissue was also present;[46] the positive outcomes were maintained even after 36 months of treatment.[46] To sum up, it has a role in biopsy-proven NASH with T2DM.[44] Nevertheless, NASH can recur off-therapy and thus continued treatment is mandatory.[47] However, in nondiabetics with NAFLD, there is no approval by FDA for its use.[4]
DIPEPTIDYL PEPTIDASE IV INHIBITORS
NASH patients have higher serum dipeptidyl peptidase IV (DDP-IV) as compared to controls with liver staining for DDP-IV correlating with histopathological grade.[48] In animal models of diet-induced obesity, DDP-IV inhibitors result in activating 5′-Adenosine monophosphate-activated protein kinase (AMPK) with downregulation of genes involved in lipogenesis, thus attenuating lipogenesis.[49] In animal models of liver injury, amelioration of liver inflammation due to an improvement in insulin sensitivity and hepatic steatosis prevented the progression to fibrosis.[48] DDP-IV inhibition resulted in an improved glycemic control with reduced AST and ALT in a small, nonrandomized study of individuals with ultrasonographic steatosis.[50] Treatment with DDP-IV inhibitors for 6 months resulted in reduced hepatic triglyceride as measured by MRS in a prospective blinded randomized controlled study.[50] At present, evidence to discriminate the use of different DDP-IV inhibitors for patients with coexistent NAFLD with diabetes[2] is still lacking. It has to be used cautiously in patients with severe hepatic impairment.[49]
GLUCAGON-LIKE PEPTIDE-1 ANALOGS
Glucagon-like peptide-1 (GLP-1) analogs can result in an improved hepatic steatosis and steatohepatitis by weight loss and by the expression of GLP-1 receptor as seen in animal studies.[49] GLP-1agonists have a direct action to inhibit lipogenesis in hepatocytes resulting in an improvement in insulin action in hepatocytes and adipose tissue since the pathology in NAFLD involves DNL.[50]
Incubation of hepatocytes with NASH with exenatide resulted in an increased peroxisome PPARδ expression causing reduced c-Jun N-terminal kinase phosphorylation.[49] This causes an increased insulin sensitization.[49] In addition, there was an increased protein kinase A activity, Akt and AMPK phosphorylation, which resulted in protein kinase a-dependent increase in PPAR alfa activity.[46] Liraglutide is found to be useful for those with NASH both with diabetes.[51] In a placebo-controlled study, 39% of patients who received liraglutide had resolution of NASH compared to only 9% in the placebo arm at 52 weeks.[51] However it is premature to consider GLP1 analogs to specifically treat NAFLD/NASH.
SODIUM GLUCOSE COTRANSPORTER 2 INHIBITORS
In animal models of NAFLD with sodium glucose cotransporter 2 inhibitors, a protective effect on steatosis, inflammation, and fibrosis was seen.[52] Glycosuria by causing negative energy balance and substrate switching toward lipids as a source of energy expenditure can cause attenuation of steatosis–fibrosis progression.[2] No human studies of SLGT2 inhibitors and NAFLD are available.[2]
BARIATRIC SURGERY
Indication for bariatric surgery is noncirrhotic NASH unresponsive to lifestyle changes and pharmacotherapy.[53] Clearance of NASH was seen in 85% of patients, and inflammation and fibrosis in 37% and 20%, respectively.[53] This was attributed to weight loss. The prevalence of metabolic syndrome reduced from 70% to 14%,[54] i.e., there was a resolution of hypertension, dysglycemia, and dyslipidemia in 85%, 93.8%, and 95.6% of patients, respectively. Portal hypertension should be excluded before attempting surgery.[54]
Mechanisms by which bariatric surgery improves NAFLD are as follows:[53]
Decreasing ghrelin and increasing GLP-1, Pancreatic polypeptide y (PPY), and oxyntomodulin, thereby enhancing insulin sensitivity and decreasing appetite
Decreasing inflammation by decreasing IL1, IL8, CRP, and TNFα
Improving dyslipidemia
Improving adiponectin level and decreasing the expression of hepatic factors involved in the progression of fibrosis and inflammation
Decreasing IR
Promoting weight loss.
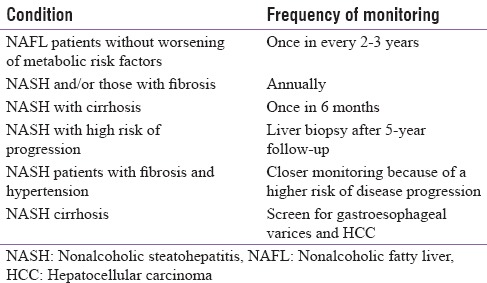
Guidelines for follow-up in NAFLD patients[4] are shown in Table 2.
Table 2.
Follow-up guidelines of nonalcoholic fatty liver disease
FUTURE DIRECTIONS
The FLINT study showed that treatment with obetichoic acid which is a farsenoid X receptor activator resulted in modest benefit by suppressing the cholesterol 7 alfa hydroxylase, a rate limiting enzyme in bile acid synthesis from cholesterol. It also causes stimulation of PPAR gamma resulting in decreased levels of triglycerides.[55] But until further safety and efficacy data are available, it is recommended not to use as an off-label drug to treat NASH.[26] In GOLDEN 505 trial, elafibranor, a PPAR α/δ agonist, at a dose 120 mg for 1 year, resulted in combined benefits of lipid metabolism of a PPARα and insulin sensitivity of PPARδ agonist, but there was no resolution of NASH. However, histological benefits in patients with a higher NAS score (≥4) were reported with an improvement in cardiometabolic profile[56] with a mild reversible increase in serum creatinine.[26]
Other drugs that are in development are:
CONCLUSION
T2DM and NAFLD have a common association. The increasing prevalence makes it a public health problem. They influence the course of the other. In the presence of steatosis, immediate clinical investigation for features of metabolic syndrome, IR, and T2DM is required because of its close association with IR. Vice versa in patients with T2DM, evaluation of NAFLD or NASH is mandated. T2DM needs to be controlled effectively to reduce IR which can show an amelioration in the NAFL. The diagnosis and management of NAFLD in T2DM has many challenges that need to be addressed. The current gold standard of care involves tailoring a treatment strategy to optimize the metabolic control with the goal to improve liver phenotype. The causative mechanism driving NAFLD progression in T2DM and evaluating the results of newer antidiabetic treatments and identification of additional novel targets require future research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Anstee QM, McPherson S, Day CP. How big a problem is non-alcoholic fatty liver disease? BMJ. 2011;343:d3897. doi: 10.1136/bmj.d3897. [DOI] [PubMed] [Google Scholar]
- 2.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.aEuropean Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Byrne CD. Clinical review: Nonalcoholic fatty liver disease: A novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab. 2013;98:483–95. doi: 10.1210/jc.2012-3093. [DOI] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 7.Kotronen A, Juurinen L, Hakkarainen A, Westerbacka J, Cornér A, Bergholm R, et al. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care. 2008;31:165–9. doi: 10.2337/dc07-1463. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong MJ, Hazlehurst JM, Parker R, Koushiappi E, Mann J, Khan S, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014;107:33–41. doi: 10.1093/qjmed/hct198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillenbrand A, Kiebler B, Schwab C, Scheja L, Xu P, Henne-Bruns D, et al. Prevalence of non-alcoholic fatty liver disease in four different weight related patient groups: Association with small bowel length and risk factors. BMC Res Notes. 2015;8:290. doi: 10.1186/s13104-015-1224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu S, Chang Y, Jung HS, Yun KE, Kwon MJ, Choi Y, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63:1229–37. doi: 10.1016/j.jhep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R, et al. Fibrosis progression in nonalcoholic fatty liver vs. nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–540. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt KN, Pranav V, Dipika Y, Dharmesh N, Radhika N, Arvind S. Prevalaence of nonalcoholic fatty liver disease in type 2 diabetes mellitus and its relation with insulin resistance in South Gujarat region. J Mahatma Gandhi Inst Med Sci. 2017;22:8–11. [Google Scholar]
- 13.Jäger S, Jacobs S, Kröger J, Stefan N, Fritsche A, Weikert C, et al. Association between the fatty liver index and risk of type 2 diabetes in the EPIC-potsdam study. PLoS One. 2015;10:e0124749. doi: 10.1371/journal.pone.0124749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care. 2015;38:1673–9. doi: 10.2337/dc15-0140. [DOI] [PubMed] [Google Scholar]
- 15.Jun DW, Kim HJ, Bae JH, Lee OY. The clinical significance of HbA1c as a predictive factor for abnormal postprandial glucose metabolism in NAFLD patients with an elevated liver chemistry. Hepatogastroenterology. 2011;58:1274–9. doi: 10.5754/hge10729. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto N, Arase Y, Kawamura Y, Ohmoto-Sekine M, Amakawa K, Ogawa K, et al. Significance of oral glucose tolerance tests in non-alcoholic fatty liver disease patients with a fasting plasma glucose level of < 126 mg/dL and HbA1c level of ≤ 6.4% in Japan. Intern Med. 2015;54:875–80. doi: 10.2169/internalmedicine.54.3437. [DOI] [PubMed] [Google Scholar]
- 17.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–21. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 18.Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14:99–114. doi: 10.1038/nrendo.2017.173. [DOI] [PubMed] [Google Scholar]
- 19.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: The roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406–12. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550–6. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, et al. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: Guidelines for referral in NAFLD. Diabetes Care. 2015;38:1347–55. doi: 10.2337/dc14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aleksandrova K, Boeing H, Nöthlings U, Jenab M, Fedirko V, Kaaks R, et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60:858–71. doi: 10.1002/hep.27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abenavoli L, Beaugrand M. Transient elastography in non-alcoholic fatty liver disease. Ann Hepatol. 2012;11:172–8. [PubMed] [Google Scholar]
- 24.Lipina C, Hundal HS. Sphingolipids: Agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011;54:1596–607. doi: 10.1007/s00125-011-2127-3. [DOI] [PubMed] [Google Scholar]
- 25.du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, et al. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology. 2015;149:635–48e14. doi: 10.1053/j.gastro.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 26.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the study of liver diseases. Hepatology. 2018;67:328–57. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 27.Cohen DE, Anania FA, Chalasani N. National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Mills EP, Brown KPD, Smith JD, Vang PW, Trotta K. Treating nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A review of efficacy and safety. Ther Adv Endocrinol Metab. 2018;9:15–28. doi: 10.1177/2042018817741852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hawkins C, Sargent R, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137–44. doi: 10.1097/MCG.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, Vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, et al. Pentoxifylline improves nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Hepatology. 2011;54:1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: A randomized controlled trial. Ann Hepatol. 2011;10:277–86. [PubMed] [Google Scholar]
- 33.Tang A, Rabasa-Lhoret R, Castel H, Wartelle-Bladou C, Gilbert G, Massicotte-Tisluck K, et al. Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes: A Randomized trial. Diabetes Care. 2015;38:1339–46. doi: 10.2337/dc14-2548. [DOI] [PubMed] [Google Scholar]
- 34.Bae JC, Cho YK, Lee WY, Seo HI, Rhee EJ, Park SE, et al. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol. 2010;105:2389–95. doi: 10.1038/ajg.2010.275. [DOI] [PubMed] [Google Scholar]
- 35.Topping DL, Mayes PA. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972;126:295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juurinen L, Tiikkainen M, Häkkinen AM, Hakkarainen A, Yki-Järvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E829–35. doi: 10.1152/ajpendo.00133.2006. [DOI] [PubMed] [Google Scholar]
- 37.Zammit VA. Use of in vivo and in vitro techniques for the study of the effects of insulin on hepatic triacylglycerol secretion in different insulinaemic states. Biochem Soc Trans. 2000;28:103–9. doi: 10.1042/bst0280103. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: A systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–91. doi: 10.1038/ajg.2013.5. [DOI] [PubMed] [Google Scholar]
- 39.Ryysy L, Häkkinen AM, Goto T, Vehkavaara S, Westerbacka J, Halavaara J, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49:749–58. doi: 10.2337/diabetes.49.5.749. [DOI] [PubMed] [Google Scholar]
- 40.Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H, et al. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–76. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 41.Ford RJ, Fullerton MD, Pinkosky SL, Day EA, Scott JW, Oakhill JS, et al. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem J. 2015;468:125–32. doi: 10.1042/BJ20150125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:858–65. doi: 10.1111/j.1440-1746.2011.06664.x. [DOI] [PubMed] [Google Scholar]
- 43.Goh G, Pagadala M. Diabetes mellitus, insulin, sulfonylurea and advanced fibrosis in non-alcoholic fatty liver disease. J Diabetes Metab. 2014;5:1–5. [Google Scholar]
- 44.Yki-Järvinen H. Thiazolidinediones and the liver in humans. Curr Opin Lipidol. 2009;20:477–83. doi: 10.1097/MOL.0b013e3283321d37. [DOI] [PubMed] [Google Scholar]
- 45.Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2010;12:365–83. doi: 10.1111/j.1463-1326.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 46.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A Randomized trial. Ann Intern Med. 2016;165:305–15. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 47.Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: Current issues and novel treatment approaches. Drugs. 2013;73:1–14. doi: 10.1007/s40265-012-0004-0. [DOI] [PubMed] [Google Scholar]
- 48.Trevaskis JL, Griffin PS, Wittmer C, Neuschwander-Tetri BA, Brunt EM, Dolman CS, et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G762–72. doi: 10.1152/ajpgi.00476.2011. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–23. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 50.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–97. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 51.Armstrong M, Gaunt P, Aithal G, Parker R. Liraglutide is Effective in the Histological Clearance of Non-Alcoholic Steatohepatitis in a Multicentre, Doubleblinded, Randomised, Placebocontrolled Phase. Abstr G01, EASL. 2015 [Google Scholar]
- 52.Hayashizaki-Someya Y, Kurosaki E, Takasu T, Mitori H, Yamazaki S, Koide K, et al. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur J Pharmacol. 2015;754:19–24. doi: 10.1016/j.ejphar.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Bower G, Toma T, Harling L, Jiao LR, Efthimiou E, Darzi A, et al. Bariatric surgery and non-alcoholic fatty liver disease: A systematic review of liver biochemistry and histology. Obes Surg. 2015;25:2280–9. doi: 10.1007/s11695-015-1691-x. [DOI] [PubMed] [Google Scholar]
- 54.Clark JM, Alkhuraishi AR, Solga SF, Alli P, Diehl AM, Magnuson TH, et al. Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes Res. 2005;13:1180–6. doi: 10.1038/oby.2005.140. [DOI] [PubMed] [Google Scholar]
- 55.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and-δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–59e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 57.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, et al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: Evidence from a case-control study. Am J Gastroenterol. 2007;102:1251–1258. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]


