Abstract
Background:
In human immunodeficiency virus (HIV)-infected men, hypogonadism is the most common endocrinological disorder, and most cases of hypogonadism are secondary. The aim of this study was to find out the hormonal abnormalities in HIV-infected males and it's correlation with CD4 cell counts.
Materials and Methods:
One hundred HIV-infected male patients were evaluated in the Department of Medicine, Postgraduate Institute of Medical Education and Research and Dr. Ram Manohar Lohia Hospital, New Delhi, India, over a period of 12 months from September 2014 to August 2015 using history, physical examination, routine baseline investigations, and CD4 counts. Free testosterone, dehydroepiandrosterone sulfate (DHEAS), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin were measured using an overnight fasting sample. Patients were divided into three groups on the basis of CD4 counts (Group A: CD4 counts ≥350/mm3, Group B: CD4 counts between 200 and 349/mm3, and Group C: CD4 counts <200/mm3). Data were analyzed using Student's t-test, ANOVA test, Chi-square test, and Pearson's test and P ≤ 0.05 was considered statistically significant.
Results:
In 100 HIV-infected males, overall prevalence of hypogonadism was found to be 66%, and 30%–35% patients had symptoms of hypoandrogenemia. Hypogonadotropic hypogonadism was found in 42% of patients. A significant association (P = 0.027) was found between prevalence of hypogonadism and the level of immunodeficiency with an increase in the prevalence of hypogonadism as CD4 counts decreased. Lower levels of free testosterone and DHEAS were found in cases of severe immunosuppression with a statistically significant correlation with CD4 counts. Correlation of other sex hormones (LH, FSH, and prolactin) with CD4 counts not statistically significant. Mean free testosterone and FSH were found to be significantly higher in patients on antiretroviral therapy (ART) than in those not on ART (P = 0.028 and P = 0.045, respectively), but no specific ART drug or their drug combination was found to have a significant correlation with levels of any sex hormone.
Conclusion:
Hypogonadism (hypogonadotropic hypogonadism) was found to be a common endocrinological disorder in HIV-infected male population, seen more commonly in association with low CD4 counts.
Keywords: CD4 counts, human immunodeficiency virus/AIDS, hypogonadism, male, sex hormones
INTRODUCTION
AIDS has evolved from a mysterious illness to a global pandemic in <20 years.[1]
The first few infections of human immunodeficiency virus (HIV) in India were reported from Chennai in 1986 and then infection started spreading throughout the country. In 1987, a National AIDS Control Programme was launched.[2] HIV infection can cause functional derangement of virtually every endocrine organ system of human body.[3] High prevalence of hypogonadism was reported in many studies conducted in HIV-infected males during preantiretroviral therapy (ART) era. Although hypogonadism is common among HIV-infected men, it's true prevalence remains poorly defined and widely ranging from 17%[4] to over 50%[5] in different studies. Androgen deficiency may result either from primary testicular failure or secondary to inadequate signaling from the pituitary or hypothalamus, but most cases of hypogonadism in HIV-infected men are secondary, as demonstrated by reduced or inappropriately normal gonadotrophin levels in the setting of decreased testosterone.[6] Disease processes affecting the hypothalamus or pituitary (stalk or gland) can suppress GnRH or gonadotropin secretion, respectively. In patients with HIV infection or immunosuppression, lymphoma or syphilis of the pituitary can precipitate or mimic apoplexy[7,8], and meningeal or pituitary infection with Mycobacterium tuberculosis, Toxoplasma gondii, Pneumocystis jirovecii, Cytomegalovirus (CMV), or candidiasis may result in fibrosis and gradual loss of function.[9] Increased prolactin levels and gynecomastia have been reported among HIV-infected patients.[10] Early recognition of clinical hypogonadism and appropriate treatment may improve clinical outcomes and quality of life for affected individuals.
Dehydroepiandrosterone sulfate (DHEAS) levels have been found to be lower in HIV-infected patients as compared to general population,[11] and also among HIV-infected patients, it has been reported to be lower in AIDS patients as compared to asymptomatic patients.[12] Few studies have suggested an intra-adrenal shunting toward cortisol synthesis due to 17.20 lyase dysfunction resulting in a reduced DHEA to cortisol ratio on cosyntropin stimulation.[13]
Very few studies have been conducted in India on the prevalence of hypogonadism in HIV-infected males.
What's Known
Hypogonadism was recognized as being relatively common early in the HIV epidemic and most cases of hypogonadism in HIV-infected men are secondary
Low testosterone concentrations are associated with lower CD4 cell count, advanced stage of illness, medication use, and weight loss.
What's New
The prevalence of hypogonadism in our study population (66%) is much higher than reported in previous studies (17%–50%)
Androgen deficiency has been found to occur at a much younger age in HIV-infected men as compared to that in normal healthy individuals
24% of cases (one-third of all patients with hypogonadism) had primary hypogonadism, i.e., testicular failure.
Aim
Hence, we undertook the present study to find the predictors and prevalence of hypogonadism in HIV-infected men and the effect of CD4 counts and other factors on the severity of male hypogonadism.
MATERIALS AND METHODS
This cross-sectional observational study was conducted on HIV-positive men attending the ART center at the Department of Medicine, Postgraduate Institute of Medical Education and Research (PGIMER) and Dr. Ram Manohar Lohia (RML) Hospital, New Delhi, India, over a period of 12 months from September 2014 to August 2015 after satisfying all inclusion and exclusion criteria. During this period, the samples were collected from 100 diagnosed HIV-positive male patients in ART center at PGIMER, Dr. RML Hospital, New Delhi. The biochemical parameters were done in the department of biochemistry.
Inclusion criteria
HIV-positive male 18–50 years of age (the positive cases were confirmed by a series of 3 tests as per the National AIDS Control Organization [NACO] Guidelines) were included in the study.
Exclusion criteria
History suggestive of orchitis/orchidectomy (bilateral or unilateral)/trauma to testis/any local pathology or patients who have received sex hormones or hormonal analogs in the previous 6 months
Patients with a history of use of drugs known to affect sex hormone levels, for example, ketoconazole, megestrol acetate, spironolactone, chemoradiotherapy, antipsychotics, barbiturate, anticonvulsant, clomiphene, tacrolimus, cyclosporine A, and cimetidine
Substance abuse-opiates (including heroin and methadone) or marijuana
Patients with a history of concomitant comorbidities such as diabetes mellitus, chronic kidney disease, i.e., serum creatinine >1.5 mg% or chronic liver disease or past history of meningitis/stroke/cryptococcal infection
Patients with Stage 3 and 4 active opportunistic infections.
All the patients were evaluated as per the standard protocol of history, physical examination, and underwent routine baseline investigations including complete blood count, kidney function test, liver function test, plasma glucose (both fasting and postprandial), lipid profile, serum proteins, venereal disease research laboratory, anti-HCV, HbsAg, and CD4 counts. Free testosterone, DHEAS, luteinizing hormone (LH), follicle-stimulating hormone (FSH) and prolactin were measured in overnight fasting pooled sample.
History and examination were done with emphasis on symptoms related to hypogonadism and the answer was recorded as yes or no.
-
History taking – Presenting symptoms with duration
- History of weight loss
-
History of symptoms of hypogonadism yes/no
-
a)Erectile dysfunction (ED)
-
b)Infertility
-
c)Decreased/impaired beard and body hair growth (decreased shaving frequency)
-
d)Decrease in muscle mass
-
e)Development of breast tissue
-
f)Fatigue
-
g)Decreased sex drive (lack of desire for sexual activity)
-
h)Difficulty concentrating
-
i)Hot flushes
-
j)Excessive growth of arms and legs in relation to the trunk of the body
-
k)Impaired growth of the penis and the testicles.
-
a)
-
History of past illness–
- History of trauma to the testes
- Any testicular surgery
- Local pathology – orchitis, varicocele, hydrocele, testicular torsion, testicular tumors
- History of diabetes mellitus.
-
Drug history–
- Use of sex hormones/sex hormonal analogs in the previous 6 months
- Substance abuse
- Chemo/radiotherapy
- Anticonvulsants, barbiturates, clomiphene, spironolactone, megestrol acetate, ketoconazole, antipsychotics, cyclosporine A, cimetidine, and tacrolimus.
-
Definition of ED–
ED is defined as the persistent inability to achieve or maintain penile erection sufficient for satisfactory sexual performance.[14]
-
Examination
- General physical examination including secondary sexual characters
- Anthropometry including arm span and height in cm
-
Local examination was done as follows
- Inspection – any visible swelling, atrophy, redness, ulcers, and presence of both the testes in the scrotum
- Palpation – surface of testes, varicosities, and tenderness.
Eunuchoid proportion.
Eunuchoid proportions are defined as an arm span >2 cm greater than height and suggest that androgen deficiency occurred before epiphyseal fusion.[15]
Principle
BECTON-DICKINSON FACS flow cytometer was used to obtain CD4 T-cell count.
Free testosterone and DHEAS were done by enzyme-linked immunosorbent assay (ELISA) technique using BioRad EVOLIS Twin Plus fully automated ELISA workstation.
LH, FSH, and prolactin were done by enhanced chemiluminescence immunoassay technique using VITROS ECI from Johnson and Johnson.
Distribution according to CD4 counts
The subjects were divided into three groups depending on the CD4 counts. There were 48 patients in group C with CD4 <200/mm3 (marked immunodeficiency), 27 patients in Group B with CD4 count of 200–349/mm3 (moderate immunodeficiency), and 25 patients in Group A having CD4 counts ≥350/mm3 (mild immunodeficiency). This classification according to CD4 counts was done as per prevailing 2014–2015 NACO working guidelines for medical officers at ART centers in India. During 2014–2015, ART was started at CD4 counts <350/mm3 (NACO national guidelines).
-
Normal ranges for hormones:
- LH = 1.8–7.8 mIU/mL
- FSH = 1.55–9.74 mIU/mL
- Free testosterone = 3.84–34.17 pg/mL
- PROLACTIN = 3–18.6 ng/mL
- DHEAS = 0.39–4.63 ug/mL.
Samples for hormone analysis were collected after overnight fasting between 8 and 9 a. m. The samples were drawn three times at 15 min interval.
Definition of hypogonadism
Hypogonadism is a syndrome associated with impaired androgen production or action. Androgen deficiency can result from abnormalities of testicular function (primary hypogonadism), hypothalamic or pituitary regulation of testicular function (secondary hypogonadism), or impairment of androgen action at the target tissue (androgen insensitivity)[16]
Low testosterone levels were defined as value <3.84 pg/mL
Definition of primary hypogonadism[17]–Low free testosterone + Elevated LH and/or FSH
Definition of hypogonadotropic (secondary) hypogonadism[17]–
Low free testosterone + low/inappropriately normal LH and FSH
Statistical analysis
The analysis was carried out in Microsoft Excel 2007 and SPSS software (IBM Corp. Statistics for Windows, Version 20.0, Released 2011, Armonk, NY, USA). The quantitative variables were reported as mean ± standard deviation and t-test was to determine the difference in the means of two groups. Statistical significance of outcomes with different variables was determined by t-test, ANOVA, test and Chi-square test. Pearson's test was used to calculate the correlation. P ≤ 0.05 was taken as level of statistical significance.
RESULTS
Demographic characteristics of the patients studied
The details of the demographic profile of the patients are depicted in Table 1.
Table 1.
Characteristics of the patients studied
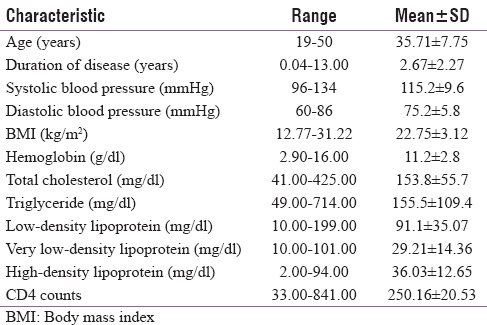
Age of the subjects ranged from 19 to 50 years with a mean value of 35.71 ± 7.75 years. Various sources of infection were heterosexual in 96 subjects, blood transfusion in 2, and intravenous drug abuse in two patients. Among patients with prior opportunistic coinfections (n = 27), most common opportunistic infection was pulmonary tuberculosis in ten patients, oral candidiasis in eight, CMV retinitis in one patient, and extrapulmonary tuberculosis in eight patients (all had abdominal tuberculosis). HBV coinfection and HCV coinfection were found in eight patients and in one patient, respectively. One patient had both pulmonary tuberculosis and hepatitis B infection.
Table 2 shows distribution of patients in three groups of CD4 counts according to baseline characteristics, symptoms and treatment. Among 100 HIV-infected male patients, 83 were on ART from ART center and 17 were ART naïve. (P = 0.00 ; significantly higher number of patients on ART in group C).
Table 2.
Distribution according to baseline characteristics, symptoms, and treatment
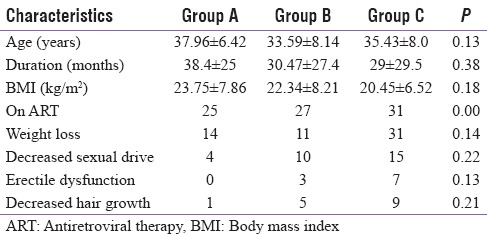
Twenty-three patients had a history of significant (>10%) weight loss and 33 had weight loss but <10%. Twenty-nine patients complained of decreased sexual drive. The number of patients complaining of ED and decreased hair growth was 10 and 15, respectively. None of the patients had eunuchoid proportions on examination. Gynecomastia was found in four patients.
Hypogonadism in human immunodeficiency virus-infected males
We found that 66% patients had low testosterone levels and the prevalence of androgen deficiency or hypogonadism increased as the CD4 counts decreased. The results are depicted in Table 3.
Table 3.
Number of patients in each group according to the type of hypogonadism
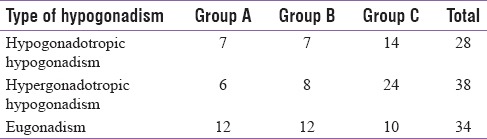
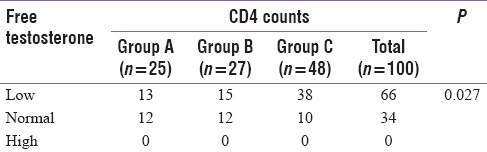
There was a significant association (P = 0.027) between prevalence of low testosterone levels and the level of immunodeficiency as shown in Table 4.
Table 4.
Number of patients in each group according to free testosterone levels
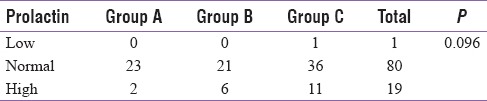
As shown in Table 5, only one patient had low prolactin levels, eighty patients had normal levels, and 19 patients had high prolactin levels suggestive of hyperprolactinemia. High levels of serum prolactin were found in 2 out of 25 (8%) in Group A, 6 out of 27 (22.2%) in Group B, and 11 out of 48 (22.9%) in Group C, showing increased prevalence of hyperprolactinemia in patients with decreasing CD4 counts although the difference was not statistically significant (P = 0.096).
Table 5.
Number of patients in each group according to prolactin levels
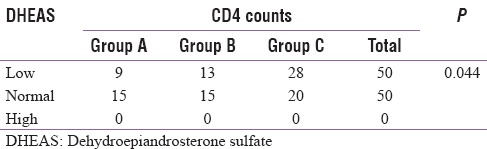
As per Table 6, fifty out of 100 subjects had low DHEAS levels and 50 had normal levels. In Group A, serum DHEAS levels were low in 9 out of 25 (36%) of the patients, 13 out of 27 (48.1%) in Group B, and 28 out of 48 (58.3%) patients in Group C. As the CD4 counts decreased, the percentage of patients having low DHEAS increased and the association between prevalence of low DHEAS levels and the level of immunodeficiency was statistically significant (P = 0.044)
Table 6.
Number of patients in each group according to dehydroepiandrosterone sulfate levels
Mean values of sex hormones in various groups of CD4 counts
The mean values of sex hormones in various groups of HIV-infected patients depending on the CD4 counts have been depicted in Table 7.
Table 7.
Mean values of sex hormones in various groups of CD4 counts (ANOVA test)
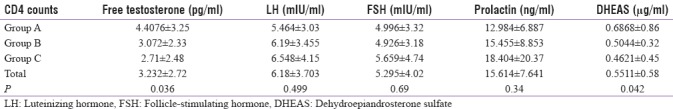
The difference in mean levels of free testosterone among the three groups with different CD4 counts was statistically significant (P = 0.036). The testosterone levels were significantly lower in Group C than in Group A (P = 0.00756) and significantly lower in Group B as compared to Group A (P = 0.046). The levels of free testosterone progressively decreased with decreasing CD4 counts (i.e., with more immunosuppression).
There was a linear increase in prolactin levels with decreasing CD4 counts, but the difference was not statistically significant. Mean DHEAS levels significantly increased with increasing CD4 counts (P = 0.042) and lower DHEAS levels significantly predicted a more immunosuppressive state.
Correlation between sex hormones and CD4 counts
There was a direct correlation between CD4 count and free testosterone values (r = 0.232, P = 0.027) and DHEAS levels (r = 0.18, P = 0.044). Free testosterone and DHEAS values were significantly decreased in the patients who had low CD4 cell count. Correlation of LH (r = −0.146, P = 0.147), FSH (r = −0.092, P = 0.488), and prolactin levels (r = −0.039, P = 0.096) with CD4 counts was not significant.
Mean levels of sex hormones and CD4 counts in human immunodeficiency virus-infected antiretroviral therapy-naïve male patients versus patients on antiretroviral therapy
The mean free testosterone in patients on ART (3.49 ± 2.79 pg/ml) was significantly higher (P = 0.016) than those not on ART (1.96 ± 1.94 pg/ml). Mean levels of FSH were also significantly higher (P = 0.0227) in patients on ART (5.60 ± 4.183 mIU/ml) as compared to ART-naïve patients (3.52 ± 2.51 mIU/ml). Mean levels of LH in patients on ART (6.42 ± 3.74 mIU/ml) were found to be higher as compared to ART-naïve patients (4.97 ± 3.34 mIU/ml), but the difference between two groups was not statistically significant (P = 0.071). Likewise mean levels of prolactin were also higher in patients on ART (16.3 ± 16.44 ng/ml) as compared to ART-naïve patients (13.2 ± 6.57 ng/ml), but the difference was not statistically significant (P = 0.224). Mean levels' DHEAS were higher (although statistically not significant) in patients on ART (0.6153 ± 0.667 mcg/ml) as compared to ART-naïve patients (0.5879 ± 0.564 mcg/ml) (P = 0.07). Anemia (Hb <12 gm%) was seen significantly more (74.2%) in patients with androgen deficiency. Similarly, mean levels of hemoglobin in patients with diagnosed androgen deficiency were 10.85 gm% as compared to 11.98 gm% in patients with no deficiency and the difference was statistically significant (P = 0.034).
DISCUSSION
HIV affects almost all the endocrine organs, and our study shows that sex hormone dysfunction is frequent in HIV infection, and with the progression of disease, there is an increased prevalence of hypogonadism that almost proportionately increases in patients with advancing HIV infection.
Among 100 HIV-infected males, various sources of infection were sexual (in 96%) being the most common, followed by blood transfusion (in 2%) and intravenous drug use (in 2%). It is in accordance with the data obtained from NACO which states that unprotected sex (87.4% heterosexual and 1.3% homosexual) is the major route of HIV transmission, followed by transmission from parent to child (5.4%) and use of infected blood and blood products (1.0%).[18]
In the current study, 35%–40% of patients had symptoms of hypoandrogenemia. In a study conducted by Dobs et al. in 1998, around 67% of male patients with advanced HIV disease complained of loss of libido and 33% of them complained of impotence.[5] Crum-Ciamflone has reported 61.4% prevalence of ED in HIV-positive men, which had no correlation with hypogonadism,[4] and was found to be more associated with mental health and same might hold true for our findings. Another reason for this low symptomatic prevalence of hypogonadism in our study as compared to Western population areas may be the social inhibition, especially in medium-to-low socioeconomic groups regarding the discussion of sexual dysfunction which is very common in this part of the world.
In the current study, 66% patients had low testosterone levels suggestive of hypogonadism and 34% had normal levels. Among patients with hypogonadism (n = 66), 63.6% patients had hypogonadotropic hypogonadism and 36.3% patients had primary hypogonadism. Nevertheless, 36% prevalence of primary hypogonadism points toward a direct endocrinopathy due to the HIV virus on the testicular apparatus which is mainly regarded as a neurotropic virus with minimal effects on glands. As our patients were in the age group of 18–50 years, we can infer that hypogonadism seems to occur earlier in HIV-infected males.
In a study conducted by Meena et al.[19] in 2011, hypogonadism was observed in 33% of male HIV patients compared to 66% in our study. This can be explained by the fact that most patients (48%) in the current study were in the group of severe immunosuppression, whereas only 33% patients in the study by Meena et al. were severely immunosuppressed. In contrast to our findings, they observed primary hypogonadism to be more common (55.4%) among patients with hypogonadism. There was a significant association between the prevalence of hypogonadism and level of CD4 counts as the prevalence of hypogonadism increased with a decrease in CD4 counts. A significant correlation between free testosterone levels and CD4 counts (r = 0.232, P = 0.027) similar to that reported by Meena et al.[19] (r = 0.175, P = 0.037) was found. There was a significant difference between the mean levels of free testosterone among the three groups of patients with different CD4 counts (P = 0.0364). It was also reported in a study by Meena et al. in which levels of total testosterone reduced as the level of immune suppression increased.[19]
Among the study population, majority of the patients had normal LH and FSH levels in each group of CD4 counts. LH levels were low in 7% and high in 27% of the total study population; FSH levels were low in 7% and high in 10% of the patients, respectively. Similar results have been reported in other studies.[19]
In the current study, highest mean levels of LH and FSH were found in patients with severe immunodeficiency and the lowest levels of LH were found in patients with mild or no immunodeficiency in patients with advanced immunodeficiency, but the levels of LH and FSH did not differ significantly between the groups. The reason for this high LH and FSH in Group C may be positive feedback by low testosterone levels in this group which may be because of direct deleterious effect of increased viral load on testicular apparatus and so causing primary hypogonadism.
There was no statistically significant correlation between LH levels and CD4 counts (r = −0.146, P = 0.147) and FSH levels and CD4 counts (r = −0.072, P = 0.488) in our study. Meena et al., however, reported a significant inverse correlation between LH and CD4 counts (r = −0.228, P = 0.006) and FSH was also found to be inversely proportional to the total CD4 count, but the difference was not statistically significant.[19] We also got a similar inverse relationship between LH levels and CD4 counts.
One-fifth of our patients had high prolactin levels suggestive of hyperprolactinemia. Mean prolactin levels were found to be highest in patients with severe immunosuppression (17.4042 ± 20.3703 ng/ml) and lowest (12.984 ± 6.887 ng/ml) in patients with mild or no immunosuppression, but the difference was not statistically significant (P = 0.096).
These findings are similar to the prevalence of hyperprolactinemia (21.4%) formed by Collazos et al. in HIV-infected patients in 2002.[20] Whether hyperprolactinemia in our patients with low CD4 counts is because of some external factor or ART or virus per se (HIV being a neurotropic virus) is a matter of debate.
Kandathil et al. in India in 2005 concluded that mean DHEAS levels in the normal healthy individuals, in HIV-infected individuals, among the asymptomatic individuals and that in the symptomatic individuals were 207 ± 123 μg/dl, 68 ± 47 μg/dl, 83.5 ± 52 μg/dl, and 53 ± 33 μg/dl, respectively. The study clearly showed a significantly low level of DHEAS among the Indian HIV-1-infected individuals compared to that of the age- and sex-matched healthy individuals.[11] Similar findings were reported by Kana et al. in 2010 in HIV-1 infected versus HIV-1 seronegative Thai military conscripts.[21] In recent studies, DHEAS levels have shown promise as a prognostic marker in monitoring HIV infection.[22] We also found mean CD4 counts to be significantly lower in patients with low DHEAS levels (209.5 ± 168.74/mm3) than in patients having normal DHEAS levels (290.94 ± 230.89/mm3) (P = 0.023). ART does not seem to have an effect on DHEAS levels.
In the present study, 50% of patients had low DHEAS levels and the majority of these (64.3%) had severe immunodeficiency. The CD4 counts significantly showed an increasing trend with increasing DHEAS levels.
Mean free testosterone levels in patients on ART (3.49 ± 2.89 pg/ml) were significantly higher than in those not on ART (1.96 ± 1.94 pg/ml) (P = 0.016). Mean levels of FSH also differed significantly between ART-naïve patients (3.52 ± 2.51 mIU/ml) and patients on ART (5.658 ± 4.1835 mIU/ml) (P = 0.022). Mean of LH, DHEAS, and prolactin was higher in patients on ART than ART-naïve patients, but the mean values did not differ significantly between the two groups. This may be due to the fact that the population size between two groups was markedly different (83 vs. 17).
In a similar study by Ezeugwunne et al.,[23] results showed a significant rise in FSH, LH, in HIV seropositive participants on ART compared, respectively, to HIV seropositive subjects not on ART. The testosterone levels significantly decreased in both symptomatic HIV-infected subjects on ART and those not on ART compared with the HIV-seronegative controls (P < 0.05). On the other hand, prolactin was raised in symptomatic HIV seropositive participants, not on ART compared, respectively, to the symptomatic HIV seropositive on ART.[23] In contrast to our findings, Rietschel et al.[24] observed no difference in testosterone or free testosterone levels between groups of patients classified by whether they were receiving highly active ART (HAART). These data suggested that hypogonadism remains relatively common in men with AIDS wasting, despite treatment with HAART.[24] However, this study had some limitations. Since it was an observational (and not prospective study) and that too with 100 cases, so a causal relationship of low CD4 counts on male hypogonadism cannot be established. Second, no special investigations were done to rule out occult active opportunistic infections such as tuberculosis/fungal infections which are known to make an impact on testicular and adrenal functions. Finally, the effect of individual drugs on gonadal functions cannot be ruled out because in India HAART is given as either single tablet fixed-dose combination tenofovir + lamivudine + efavirenz or zidovudine + lamivudine + nevirapine.
CONCLUSION
Our research concludes that hypogonadism is a very common endocrinological disorder in HIV-infected men (seen in two-third of all HIV-infected males) and associated with low CD4 counts. Although two-third of patients with hypogonadism had a secondary cause, the 36% prevalence of primary hypogonadism points toward testicular failure which may be caused by HIV virus itself. Increasing immunodeficiency directly correlates with decreasing levels of testosterone and DHEAS. Although serum FSH and LH levels decrease proportionately with decreasing CD4 counts (or with increasing immunodeficiency), the association does not reach statistical significance. Since ART seems to protect this complication, so early initiation of ART (as against the national practice of late initiation of ART in India) should be advocated. With ART, the longevity is much increased, and so a sex life questionnaire followed by hormone therapy (if required) should be offered to these patients so that they can live a normal healthy life.
Financial support and sponsorship
This study was financially supported by G.G.S.I.P.U.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fauci AS, Lane HC. Harrison's Principal of Internal Medicine. In: Kasper A, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson GL, editors. Human immunodeficiency virus disease: AIDS and related disorders. 18th ed. New York: Mc Graw Hill; 2012. pp. 1506–87. [Google Scholar]
- 2.Overview of HIV and AIDS in India. 2012. [Last accessed on 2015 Mar 22]. Available at: http://www.avert.org/aidsindia.htm .
- 3.Sinha U, Sengupta N, Mukhopadhyay P, Roy KS. Human immunodeficiency virus endocrinopathy. Indian J Endocrinol Metab. 2011;15:251–60. doi: 10.4103/2230-8210.85574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crum-Cianflone NF, Bavaro M, Hale B, Amling C, Truett A, Brandt C, et al. Erectile dysfunction and hypogonadism among men with HIV. AIDS Patient Care STDS. 2007;21:9–19. doi: 10.1089/apc.2006.0071. [DOI] [PubMed] [Google Scholar]
- 5.Dobs AS, Dempsey MA, Ladenson PW, Polk BF. Endocrine disorders in men infected with human immunodeficiency virus. Am J Med. 1988;84:611–6. doi: 10.1016/0002-9343(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 6.Mylonakis E, Koutkia P, Grinspoon S. Diagnosis and treatment of androgen deficiency in human immunodeficiency virus-infected men and women. Clin Infect Dis. 2001;33:857–64. doi: 10.1086/322695. [DOI] [PubMed] [Google Scholar]
- 7.Quintero Wolfe S, Hood B, Barker J, Benveniste RJ. Primary central nervous system lymphoma mimicking pituitary apoplexy: Case report. Pituitary. 2009;12:76–9. doi: 10.1007/s11102-008-0084-8. [DOI] [PubMed] [Google Scholar]
- 8.Spinner C, Noe S, Schwerdtfeger C, Todorova A, Gaa J, Schmid RM, et al. Acute hypophysitis and hypopituitarism in syphilitic meningitis in HIV infected patient. BMC Infect Dis. 2013;13:481. doi: 10.1186/1471-2334-13-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husain N, Husain M, Rao P. Pituitary tuberculosis mimicking idiopathic granulomatous hypophysitis. Pituitary. 2008;11:313–5. doi: 10.1007/s11102-007-0068-0. [DOI] [PubMed] [Google Scholar]
- 10.Biglia A, Blanco JL, Martínez E, Domingo P, Casamitjana R, Sambeat M, et al. Gynecomastia among HIV-infected patients is associated with hypogonadism: A case-control study. Clin Infect Dis. 2004;39:1514–9. doi: 10.1086/425363. [DOI] [PubMed] [Google Scholar]
- 11.Kandathil AJ, Kannangai R, David S, Selvakumar R, Job V, Abraham OC, et al. Human immunodeficiency virus infection and levels of dehydroepiandrosterone sulfate in plasma among Indians. Clin Diagn Lab Immunol. 2005;12:1117–8. doi: 10.1128/CDLI.12.9.1117-1118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christeff N, Nunez EA, Gougeon ML. Changes in cortisol/DHEA ratio in HIV-infected men are related to immunological and metabolic perturbations leading to malnutrition and lipodystrophy. Ann N Y Acad Sci. 2000;917:962–70. doi: 10.1111/j.1749-6632.2000.tb05463.x. [DOI] [PubMed] [Google Scholar]
- 13.Grinspoon S, Corcoran C, Stanley T, Rabe J, Wilkie S. Mechanisms of androgen deficiency in human immunodeficiency virus-infected women with the wasting syndrome. J Clin Endocrinol Metab. 2001;86:4120–6. doi: 10.1210/jcem.86.9.7843. [DOI] [PubMed] [Google Scholar]
- 14.NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 15.Bhasin S, Jameson JL. Disorders of the testes and male reproductive system. In: Kasper A, Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, editors. Harrison's Principal of Internal Medicine. 18th ed. New York: Mc Graw Hill; 2012. pp. 3010–27. [Google Scholar]
- 16.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in adult men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 17.Bhasin S. William Textbook of Endocrinology. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Testicular disorders. 11th ed. Philadelphia, PA: Saunders Elsevier; 2008. pp. 645–80. [Google Scholar]
- 18.Annual Report 2010-2011, National Aids Control Organization, Department Of AIDS Control Ministry of Health and Family Welfare, Government of India. [Last accessed on 2014 Dec 24]. Available from: http://www.nacoonline.org .
- 19.Meena LP, Rai M, Singh SK, Chakravarty J, Singh A, Goel R, et al. Endocrine changes in male HIV patients. J Assoc Physicians India. 2011;59:365–6. 371. [PubMed] [Google Scholar]
- 20.Collazos J, Ibarra S, Martínez E, Mayo J. Serum prolactin concentrations in patients infected with human immunodeficiency virus. HIV Clin Trials. 2002;3:133–8. doi: 10.1310/QAQQ-XTCJ-8AL4-6F5P. [DOI] [PubMed] [Google Scholar]
- 21.Kana K, Tabprasit S, Songprasom K, Chuenchitra T, Hiransuthikul N. Comparative study of serum dehydroepiandrosterone sulfate levels in HIV-1 infected and HIV-1-seronegative royal Thai army conscripts. R Thai Army Med J. 2010;63:71–80. [Google Scholar]
- 22.Kannangai R, Kandathil AJ, Ebenezer DL, Mathai E, Prakash AJ, Abraham OC, et al. Usefulness of alternate prognostic serum and plasma markers for antiretroviral therapy for human immunodeficiency virus Type 1 infection. Clin Vaccine Immunol. 2008;15:154–8. doi: 10.1128/CVI.00193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezeugwunne I, Onyenekwe C, Ahaneku J, Ifeanyichukwu M, Meludu S, Onwurah O, et al. Serum hormonal levels in HIV/AIDS infected male subjects on antiretroviral therapy (ART) in Nnewi, Nigeria. Int J Biol Chem Sci. 2012;6:1409–18. [Google Scholar]
- 24.Rietschel P, Corcoran C, Stanley T, Basgoz N, Klibanski A, Grinspoon S, et al. Prevalence of hypogonadism among men with weight loss related to human immunodeficiency virus infection who were receiving highly active antiretroviral therapy. Clin Infect Dis. 2000;31:1240–4. doi: 10.1086/317457. [DOI] [PubMed] [Google Scholar]


