Abstract
The nonsense-mediated messenger RNA (mRNA) decay (NMD) pathway is a cellular quality control and post-transcriptional gene regulatory mechanism and is essential for viability in most multicellular organisms . A complex of proteins has been identified to be required for NMD function to occur; however, there is an incomplete understanding of the individual contributions of each of these factors to the NMD process. Central to the NMD process are three proteins, Upf1 (SMG-2), Upf2 (SMG-3), and Upf3 (SMG-4), which are found in all eukaryotes, with Upf1 and Upf2 being absolutely required for NMD in all organisms in which their functions have been examined. The other known NMD factors, Smg1, Smg5, Smg6, and Smg7, are more variable in their presence in different orders of organisms and are thought to have a more regulatory role. Here we present the first genetic analysis of the NMD factor Smg5 in Drosophila. Surprisingly, we find that unlike the other analyzed Smg genes in this organism, Smg5 is essential for NMD activity. We found this is due in part to a requirement for Smg5 in both the activity of Smg6-dependent endonucleolytic cleavage, as well as an additional Smg6-independent mechanism. Redundancy between these degradation pathways explains why some Drosophila NMD genes are not required for all NMD-pathway activity. We also found that while the NMD component Smg1 has only a minimal role in Drosophila NMD during normal conditions, it becomes essential when NMD activity is compromised by partial loss of Smg5 function. Our findings suggest that not all NMD complex components are required for NMD function at all times, but instead are utilized in a context-dependent manner in vivo.
Keywords: nonsense-mediated mRNA decay, NMD, Drosophila, Smg5, Smg6, pcm, Xrn1
EUKARYOTIC cells utilize a number of pathways to maintain error-free translation so as to preserve the fidelity of protein function (Adjibade and Mazroui 2014). Nonsense-mediated messenger RNA (mRNA) decay (NMD) is one such pathway, which prevents the translation of potentially harmful truncated proteins by recognizing and destroying mRNAs that contain erroneous premature-termination codons (PTCs) (Celik et al. 2015). In addition to this cellular quality control function, NMD degrades many endogenous wild-type mRNAs as a mechanism of post-transcriptional gene regulation (Peccarelli and Kebaara 2014).
While the phenomenon of NMD has been well characterized for several decades, the mechanisms initiating target recognition and degradation are still not well understood and it remains unclear if all the factors required for NMD activity have even been identified. Genes required for NMD were first found by genetic screens in yeast and C. elegans, which led to the identification of seven proteins required for NMD (Hodgkin et al. 1989; Leeds et al. 1991, 1992; Cali et al. 1999). Three of these genes, Upf1, Upf2, and Upf3, are present in every eukaryote examined, while the other four, Smg1, Smg5, Smg6, and Smg7, have variable presence across species (Siwaszek et al. 2014). In the absence of any one of these factors, PTC-containing mRNAs and endogenous targets are not efficiently degraded and instead accumulate in the cell (Gatfield et al. 2003; Rehwinkel et al. 2005). The molecular identities and biochemical characterization of the individual NMD genes have revealed clues about their roles in the NMD pathway. Upf1 is an ATP-dependent RNA helicase, and this activity is required for NMD (Czaplinski et al. 1995; Weng et al. 1996a,b). Upf3 binds mRNAs both directly and through an interaction with the exon–exon junction complex (Gehring et al. 2003). Upf2 binds both Upf1 and Upf3, bridging an interaction between these two factors (He et al. 1997; Lykke-Andersen et al. 2000), helping stabilize Upf1–mRNA interactions. Smg1 encodes a PIKK-like kinase that can phosphorylate Upf1. Loss of Smg1 leads to reduced phospho-Upf1 in all organisms examined (Page et al. 1999; Yamashita et al. 2001; Grimson et al. 2004). In contrast, Upf1 is hyper-phosphorylated in C. elegans smg-5, smg-6, or smg-7 mutants in a Smg1-dependent manner (Page et al. 1999), and RNA interference (RNAi) inhibition of Smg5, Smg6, or Smg7 in mammalian cells also results in Upf1 hyper-phosphorylation (Okada-Katsuhata et al. 2012). The finding that loss of any of the Smg genes reduces the efficiency of the NMD pathway even though they result in opposite effects on the Upf1 phosphorylation state has led to the concept that a cycle of Upf1 phosphorylation/dephosphorylation is a critical aspect of the NMD process (Ohnishi et al. 2003). The importance of Upf1 phosphorylation may be due to the 14-3-3-like domain found in Smg5, Smg6, and Smg7 proteins (Fukuhara et al. 2005). This domain binds phosphorylated residues, suggesting that Upf1 phosphorylation by Smg1 initiates binding of these factors to an NMD complex (Ohnishi et al. 2003). Smg6 is an endonuclease that cleaves targeted mRNAs near the PTC site (Huntzinger et al. 2008; Eberle et al. 2009), suggesting that Smg6 binding to Upf1 is likely required for degradation of NMD targets. The functions of Smg5 and Smg7 are less clear, but a complex of Smg5 and Smg7 has been shown to bind a subunit of the PP2A phosphatase, suggesting Upf1 dephosphorylation may be mediated by these factors, likely after Smg6-mediated cleavage occurs (Anders et al. 2003; Ohnishi et al. 2003). Overall, these findings have led to a model in which Upf1 phosphorylation is critical for the NMD pathway, with Smg1 being required to phosphorylate Upf1 to recruit Smg6 and initiate NMD target cleavage, and Smg5 and Smg7 being required to dephosphorylate Upf1 to promote complex disassembly and recycling to new target mRNAs.
However, arguing against this model, recent studies dissecting the binding of Smg6 to Upf1 suggest that Upf1 phosphorylation by Smg1 may not be key for normal NMD activity. It has been demonstrated that Smg6 can bind Upf1 through a nonphosphorylated domain in the protein, indicating that Upf1 phosphorylation is not required for complex assembly (Chakrabarti et al. 2014; Nicholson et al. 2014). Additionally, Upf1 hyper-phosphorylation has been shown to mitigate the effects of reduced Smg5, Smg6, or Smg7 function (Durand et al. 2016). These findings suggest a revised model in which Smg6 contributes to the initiation of NMD target degradation independent of or at low levels of Upf1 phosphorylation, but when the level of NMD activity is insufficient, Smg1 activity increases the phosphorylation state of Upf1 to enhance the binding of Smg5 and Smg7, thus increasing NMD efficiency (Durand et al. 2016). Supporting this model, Smg5 and Smg7 have been shown in mammalian cell culture to interact indirectly with both decapping and deadenylation complexes (Cho et al. 2013; Loh et al. 2013), and thus may promote exonucleolytic degradation of NMD targets. Indeed, both endonucleolytic and exonucleolytic degradation products of endogenous NMD targets can be detected in mammalian cells (Lykke-Andersen et al. 2014; Schmidt et al. 2015; Colombo et al. 2017; Ottens et al. 2017). However, in part because experiments have been primarily performed in divergent cell lines, using different methods of gene manipulation and mostly studying transfected NMD target genes, it is unclear to what extent phosphorylation-independent Upf1-binding and the recruitment of decapping and deadenylation complexes occur during normal NMD activity in vivo.
NMD is required for viability in most complex organisms, including plants, Drosophila, zebrafish, and mice (Medghalchi et al. 2001; Arciga-Reyes et al. 2006; Metzstein and Krasnow 2006; Yoine et al. 2006; Kerényi et al. 2008; Weischenfeldt et al. 2008; Wittkopp et al. 2009; Li et al. 2015). Drosophila lacking Upf1 or Upf2 die during early larval stages, with no animals surviving to adulthood (Metzstein and Krasnow 2006; Chapin et al. 2014). However, Drosophila lacking Upf3, Smg1, or Smg6 can survive to adulthood (Chen et al. 2005; Metzstein and Krasnow 2006; Avery et al. 2011; Frizzell et al. 2012). The viability of Upf3, Smg1, and Smg6 mutants suggests that these animals have sufficient NMD activity to survive to adulthood, and indeed, these mutants display significant residual NMD activity. In particular, Smg1 mutants show only a very small reduction in NMD activity (Chen et al. 2005; Metzstein and Krasnow 2006). Smg5 is the only known Drosophila NMD gene for which loss-of-function mutations are yet to be described [the Drosophila melanogaster genome does not contain an Smg7 ortholog (Chiu et al. 2003; Gatfield et al. 2003)]. Here we describe the first analysis of Drosophila Smg5 mutants and discover that Smg5 is essential for NMD activity in this organism. By performing double-mutant analysis of NMD genes, we have found that Smg1 becomes essential for NMD when Smg5 function is compromised, and that Smg5 functions in a Smg6-independent degradation pathway in vivo. Our findings are consistent with the model that Smg1-mediated phosphorylation is only required under conditions of abnormal NMD progression, and that Drosophila utilize multiple independent mechanisms to initiate NMD target degradation.
Materials and Methods
Fly genetics
Alleles used are listed in Supplemental Material, Table S1. D. melanogaster stocks were raised on standard cornmeal/dextrose food at 25°. The alleles Smg132AP and Smg6292 are null mutants (Metzstein and Krasnow 2006; Frizzell et al. 2012) and are on y w FRT19A and FRT82B chromosomes, respectively, with the Smg6 allele being balanced over TM6B, P{Dfd-EYFP} Sb1 ca1 (Le et al. 2006). All Smg5 alleles are on FRT40A chromosomes and balanced over CyO, P{Dfd-EYFP} (Le et al. 2006). Other alleles used were Gadd45F17 (Nelson et al. 2016), pcm14 (Waldron et al. 2015), Upf225G (Metzstein and Krasnow 2006), and DHR783 (Fisk and Thummel 1998). y w FRT19A was used as a control chromosome for all experiments for genes located on the X-chromosome (Smg1 and pcm alleles). FRT40A or FRT82B were respectively used as the control chromosome for the experiments using Smg5 or Smg6 alleles. For viability tests, animals containing mutant alleles over a balancer were mated for 3 days, and offspring were collected for 10 days, beginning 10 days after mating was initiated. All offspring were scored, and percent expected viable was determined by the ratio of balancer negative animals to balancer positive animals.
Molecular biology:
SV40 3′UTR constructs:
Deletion constructs of the SV40 3′UTR were made by one or two round PCR amplification with Phusion polymerase (NEB) using a pUASTeGFP::SV40 3′UTR plasmid as a template (Metzstein and Krasnow 2006). Amplicons were used to replace the full-length SV40 3′ UTR in a pUAST-eGFP plasmid modified to contain an attB site-specific recombination site (a gift from Jyoti Misra and Carl Thummel). All constructs were verified by sequencing and sent to Genetic Services (Cambridge, MA) for injection and site-specific integration into the attP16 (second chromosome) site (Venken et al. 2006). Expression levels of the modified SV40 3′UTR constructs were measured by mating transgenic males to y w FRT19A; e22c-GAL4, UAS-nlsDsRed2::SV40 3′UTR/CyO and y w FRT19A Upf225G; e22c-GAL4, UAS-nlsDsRed2::SV40 3′UTR/CyO females. Wandering third instar larvae expressing both DsRed and GFP were imaged using a Leica MZ16 fluorescence stereomicroscope.
Smg5 rescue construct:
A 6.8-kb genomic fragment (coordinates 3L:13,898,772–13,905,608; FB2018_02) was generated by PCR using a genomic DNA template with Phusion polymerase. PCR products were cloned into P[acman]-ApR (Venken et al. 2006) and transformed by site-specific integration into the attP2 (third chromosome) site (Venken et al. 2006).
Screens for NMD-defective alleles
The codon changes of all Smg5 alleles can be found in Table S2. For the mosaic genetic screen, males with an isogenized FRT40A second chromosome were starved for 8 hr and then fed on sucrose containing 1% ethyl methanesulfonate overnight. Mutagenized males were then mated with FRT40A; P{da-GAL4 w+} P{UAS-FLP} P{UAS-eGFP::SV40 3′UTR} females (Wodarz et al. 1995), and F1 wandering L3 larvae were collected in glycerol and scored for mosaic enhanced GFP fluorescence using a Leica MZ 16F microscope equipped with epifluorescent illumination. Mutant mosaic animals were cleaned in PBS and placed in vials with food to continue development. After eclosion, candidate mutant lines were established and retested to confirm an NMD defect. Candidate alleles were tested for complementation with Df(2L)BSC345 (Cook et al. 2012), which deletes the Smg5 locus, and lines that failed to complement this deficiency for lethality or fluorescence enhancement were balanced over CyO, P{Dfd:eYFP w+} (Le et al. 2006).
The screen for embryos with enhanced reporter expression is described in Förster et al. (2010). Briefly, flies carrying NMD-sensitive UAS-GFP and UAS-Verm-mRFP reporters expressed in the tracheal system were mutagenized with EMS, and F2 lines were established. F3s embryos were examined for reporter expression in tracheal cells.
RNA isolation and quantification
For qRT-PCR analyses, we collected 5–10 larvae from 0 to 4 hr after the L2–L3 molt and froze them in liquid nitrogen. We isolated total RNA using TRIzol reagent (Invitrogen, Carlsbad, CA) and Phase-Lock tubes (5-Prime), and the RNeasy mini kit (QIAGEN, Valencia, CA) . We used on-column RNase-free DNase treatment (QIAGEN) to reduce genomic contamination. We determined RNA concentration by spectrophotometer and normalized concentration for reverse transcription. For reverse transcription, we used random decamers and MMLV8 reverse transcriptase (Retroscript Kit; Thermo Fisher Scientific, Waltham, MA). We performed qRT-PCR analysis using the SYBR Green qPCR Supermix (Bio-Rad, Hercules, CA) and the Bio-Rad iCycler thermocycler. All experimental reactions were performed using three technical replicates and a minimum of three biological replicates per condition, and the expression level of all experimental assays was normalized to RpL32 mRNA expression. For all qRT-PCR analyses we also measured samples that had been made without reverse transcriptase to ensure that signal was not due to contamination with genomic DNA.
Primer sequences used were RpL32_1 (ATGCTAAGCTGTCGCACAAA), RpL32_2 (CGATGTTGGGGCATCAGATAC), Gadd45_5′_1 (CATCAACGTGCTCTCCAAGTC), Gadd45_5′_2 (CGTAGATGTCGTTCTCGTAGC), Gadd45_3′_1 (ACAGCCAGATGTCACAGAATT), and Gadd45_3′_2 (CCAGCAACTGGTTTCCATTAG). All Gadd45 qPCR analysis was done using the Gadd45_5′ primer pair, unless otherwise noted.
Analysis of dHR783 PTC allele stability
We collected adult Smg5+/G115 or Smg5C391/G115 males that were also heterozygous for dHR783, a PTC-containing allele that has lower expression than the wild-type allele and is stabilized in Upf225G mutants, and thus is presumably degraded by NMD (Fisk and Thummel 1998; Nelson et al. 2016). At least three biological replicates were collected for each condition. We isolated RNA and generated complementary DNA (cDNA), as described above, and used this cDNA as a template for PCR amplification of the dHR78 transcript with the DRH78_F3/DHR78_R3 primers (TGGGGCTTATTCAGAGTTCG/ATTAATGCTGGCCACACTCC), which flank the nonsense mutation. To compare the relative abundance of the dHR783 allele to the wild-type allele, PCR products from heterozygous animals were Sanger sequenced, and the relative peak intensity for a thymine (dHR783 allele) was compared to a cytosine (wild-type allele) at nucleotide 1063. Note, this assay determines the relative expression between the wild-type and mutant alleles within an individual sample, but not absolute expression levels.
Lethal phase and larval development analysis
For lethal phase and larval development analysis, first-instar larvae were collected 20–24 hr after egg lay. Every 24 hr, animals were examined to record their developmental stage and transferred to fresh food. Larval stage was determined based on physical characteristics of the mouth hooks. Once animals entered pupariation, pupae were transferred to vials and scored for eclosion 5 days later.
Statistical analysis
All viability assay figures represent the proportion of animals of the indicated genotypes that survive to adulthood; error bars for these figures represent the 95% confidence interval of the binomial distribution, and the test of equal or given proportions was used to determine significant difference in these proportions between genotypes. For each individual experiment, conditions were compared directly to the control, so no P-value correction was applied. All other figures represent the mean value of multiple replicates and display error bars representing ±2 SEM. For tests between two variable measures, a two-sided paired Student’s t-test was used to determine significance difference between mean value data. Those qPCR experiments that compared a condition to the control, which was set to a constant of 1, were performed with a one-sided Student’s t-test.
Data availability
Drosophila strains are available upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6494774.
Results
Isolation of Smg5 mutant alleles
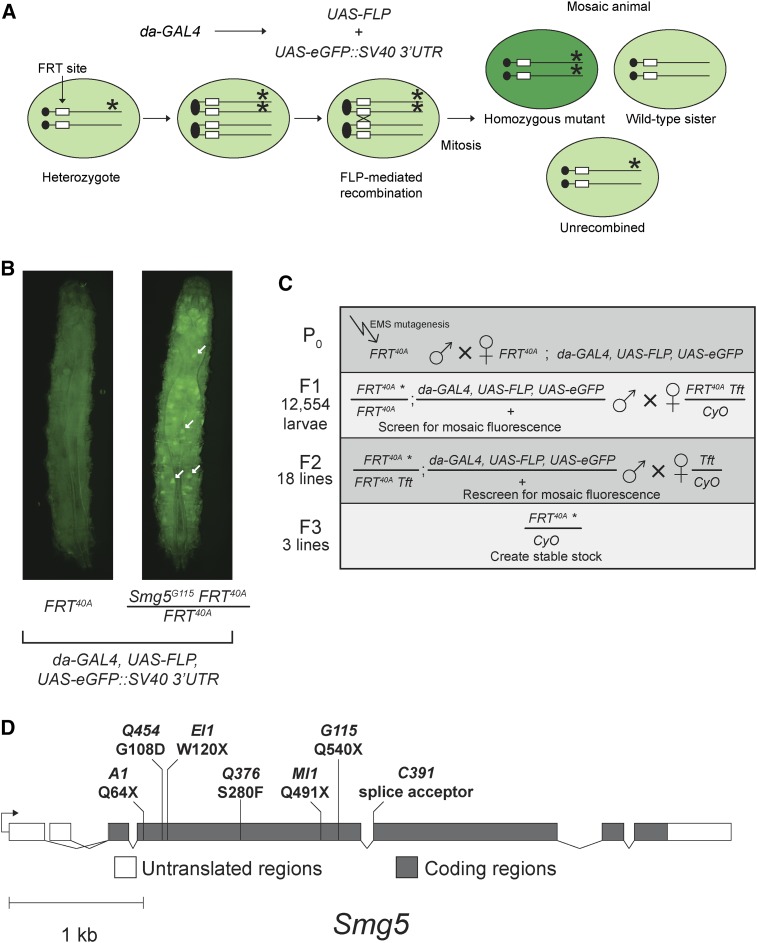
To identify Drosophila Smg5 mutant alleles, we used two different genetic screens. First, we performed an EMS-mutagenesis screen in mosaic animals expressing an NMD-sensitive GFP reporter, a method similar to one we previously used to recover Smg6 alleles (Frizzell et al. 2012). This reporter expresses GFP from a pUAST construct (Brand and Perrimon 1993) bearing a UAS promoter and an NMD-sensitive SV40 3′UTR (Metzstein and Krasnow 2006). We generated mosaics using the da-GAL4 driver to ubiquitously express FLP recombinase (Figure 1A). Individual homozygous mutant cells with defective NMD activity show increased reporter expression and GFP fluorescence (Figure 1A). The mosaic enhanced fluorescence phenotype was easy to distinguish in late L3 larvae (Figure 1B), and mosaic animals remain viable and fertile, so even lethal alleles can be recovered from individual mutants. An added benefit of this approach is that by mutagenizing animals that have an FRT site located near the centromere on the left arm of the second chromosome (FRT40A), we could specifically isolate mutations only on this chromosome arm. Since Smg5 is located on the left arm of the second chromosome, mutations identified from the screen would likely include Smg5 alleles. Using this approach, we screened 12,554 larvae and identified three mutants with mosaic enhancement of GFP fluorescence (Figure 1C). We found each of these three mutants were homozygous lethal. We crossed each allele to a deficiency that deletes Smg5 and found that all three failed to complement for lethality, suggesting that they had mutations in Smg5.
Figure 1.
Mosaic screen for novel NMD-defective mutations on the second chromosome identifies Smg5 alleles. (A) Scheme to generate mosaics and detect mutants with defective NMD. The GAL4 transcription factor is ubiquitously expressed under a daughterless (da) promoter and activates transcription of FLP recombinase and the NMD-sensitive eGFP::SV40 3′UTR fluorescent reporter, both under UAS control. The reporter mRNA is usually degraded by NMD, and thus cells lacking NMD activity due to a homozygous mutation in a gene required for NMD activity show increased green fluorescence. (B) Example of mosaic GFP-reporter fluorescence phenotype detected in our screen. Late L3 larvae expressing the NMD-sensitive eGFP::SV40 3′UTR fluorescent reporter in animals with a wild-type FRT40A chromosome (left) or an FRT40A Smg5G115 chromosome (right). Individual homozygous mutant cells with increased GFP fluorescence in the Smg5 mutant animal are indicated by white arrows. Overall increased fluorescence is due to other out-of-focus mutant cells. Dorsal view; anterior at top. (C) Scheme for recovering mutations identified in the screen. The total number of candidate mutants scored in each generation is shown on the left side of each row. Genotypes on the left in the F1 and F2 generations are the offspring from the previous mating. (D) Molecular identity of isolated Smg5 mutations. Four alleles (A1, EI1, MI1, and G115) are nonsense mutations. C391 is a mutation in a splice acceptor site. Q454 and Q376 are missense mutations. The codon changes for all Smg5 alleles are listed in Table S2.
Our second screen was of animals expressing a GFP::SV40 3′UTR reporter in the embryonic tracheal system (Förster et al. 2010). This screen identified four mutants that showed increased fluorescence (Figure 1D and Figure S1). All four of these alleles failed to complement a Smg5 deficiency using increased fluorescence signal as an assay (data not shown), indicating they contained mutations in Smg5. As expected for mutations disrupting NMD-pathway function (Metzstein and Krasnow 2006), the increase in fluorescence was independent of the fluorescent reporter examined, with both GFP and mRFP showing similar increases in expression in a homozygous mutant background (Figure S1F).
Sequencing of the Smg5 locus in the seven candidate lines revealed they all contained mutations in Smg5, including nonsense mutations (G115, A1, EI1, and MI1), an altered splice acceptor site (C391), and missense mutations in highly conserved α-helices of the Smg5 14-3-3-like domain (Q454 and Q376) (Fukuhara et al. 2005) (Figure 1D and Table S2).
Smg5 is an essential NMD factor in Drosophila
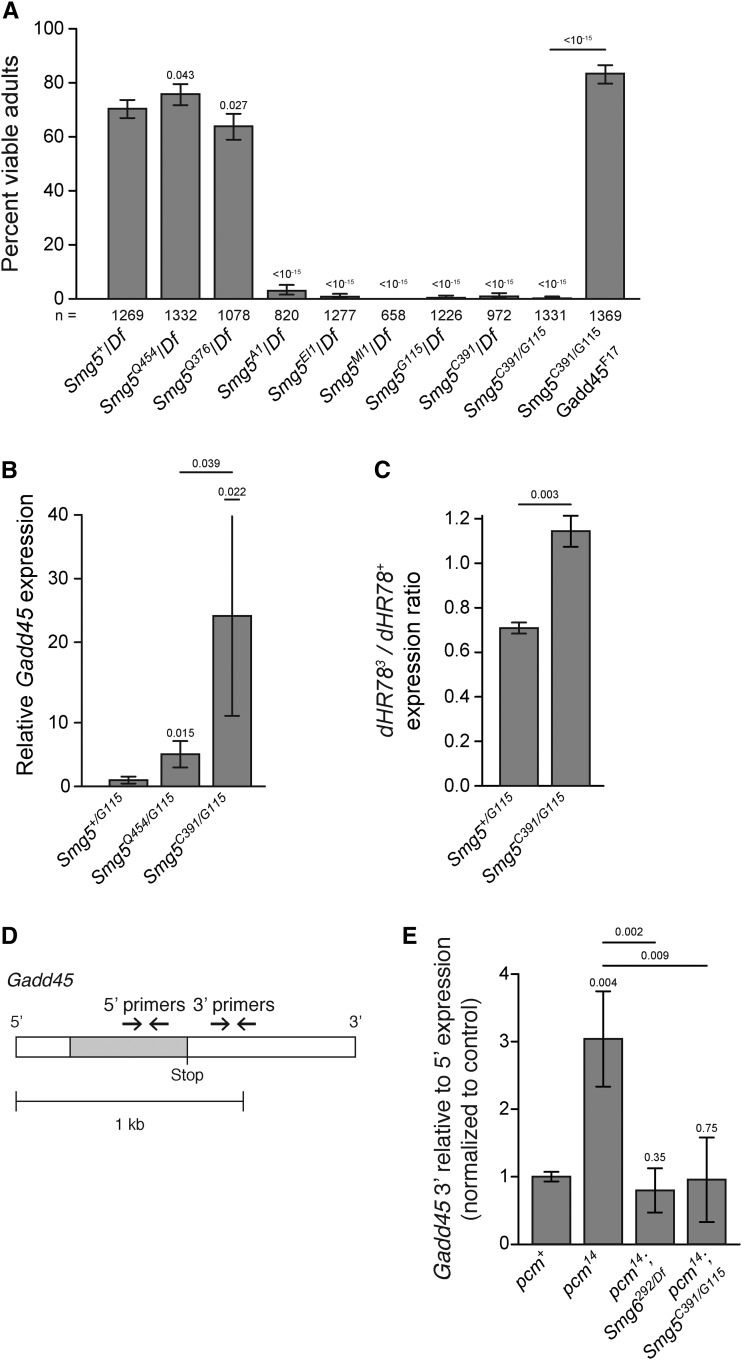
Drosophila lacking any functional NMD activity, such as Upf1 and Upf2 null mutants, fail to develop to adulthood, dying primarily during early larval stages (Chapin et al. 2014). We found that the Smg5 nonsense alleles A1, EI1, MI1, and G115, and splice acceptor site allele C391 all failed to survive to adulthood when over a deficiency that deletes Smg5, or as Smg5C391/G115 trans-heterozygotes (Figure 2A). The lethality of these alleles combined with their molecular aberrations suggested that they are complete loss-of-function mutations. We found that Smg5C391/G115 mutants have developmental delays, with Smg5 mutants spending almost twice as long in larval stages as control animals (Figure S2A), and most Smg5C391/G115 mutants die during pupariation (Figure S2B). This developmental delay and lethal phase is similar to, but somewhat weaker than, the developmental defects of null Upf1 and Upf2 mutants (Chapin et al. 2014). Conversely, the missense alleles Q454 and Q376 were viable over the deficiency and each other (Figure 2A), suggesting that these are likely hypomorphic alleles. We found that a 6.8-kb genomic fragment encompassing the Smg5 locus could rescue Smg5C391/G115 mutants to adult viability (Figure S3), indicating the lethality in this genotype is due to loss of Smg5.
Figure 2.
Smg5 is required for viability and NMD activity in Drosophila. (A) Adult viability of Smg5 mutant alleles trans-heterozygous to either a deficiency removing the Smg5 locus (Df) or other Smg5 mutant alleles. Error bars represent 95% confidence interval of the binomial distribution. P-value listed compared to Smg5+/Df condition or between indicated conditions determined by the test of equal or given proportions. n = total number of animals scored. (B) Expression of the endogenous NMD target Gadd45, as measured by qRT-PCR. Error bars represent 2 SEM. P-value listed for each condition compared to controls or between indicated conditions, determined by two-sided Student’s t-test. n ≥ 3 for all conditions. qPCR primers used were located 5′ to the Gadd45 termination sequence. (C) Relative abundance of PTC-containing dHR783 allele (Fisk and Thummel 1998) mRNA compared to wild-type dHR78 allele mRNA in animals heterozygous for dHR783 in each indicated genotype. Error bars represent 2 SEM. P-value listed for each condition compared to +/Smg5G115, determined by two-sided Student’s t-test. n = 3 for all conditions. (D) Diagram of the endogenous NMD target Gadd45 transcript and 5′ and 3′ qRT-PCR primer pairs. Open boxes indicate UTRs; gray boxes indicate coding regions. 5′ primer pair is located 5′ to the stop codon and the 3′ primer pair is 3′ to the stop codon. (E) Gadd45 expression measured with the 3′ primer pair relative to the 5′ primer pair. The 3′ region is preferentially stabilized in pcm14 mutants. This preferential stabilization is lost when either Smg6 or Smg5 are lost. Error bars represent 2 SEM. P-value listed for each condition compared to the pcm+ condition or between indicated conditions, determined by two-sided Student’s t-test. n ≥ 3 for all conditions.
Lethal mutations in Drosophila NMD genes generally have severe defects in NMD function, as measured by increased expression of endogenous NMD targets (Metzstein and Krasnow 2006; Avery et al. 2011; Frizzell et al. 2012). To test if lethal Smg5 mutant alleles also have strong defects in NMD activity, we used qRT-PCR to measure the expression of the endogenous NMD target Gadd45 (Chapin et al. 2014; Nelson et al. 2016). Since Gadd45 is directly targeted by NMD, the amount of Gadd45 mRNA in mutants serves as a measure of the decrease in NMD activity. We measured Gadd45 mRNA levels in early third instar larvae and found that Smg5C391/G115 mutants had a large increase in Gadd45 mRNA expression. In contrast, viable Smg5Q454/G115 mutants showed a much smaller increase in Gadd45 (Figure 2B). Increased Gadd45 expression is a major factor contributing to the death of Upf1 and Upf2 mutants, and loss of Gadd45 can suppress Upf1 and Upf2 mutant lethality (Nelson et al. 2016). We found that loss of Gadd45 also suppresses the lethality of Smg5C391/G115 mutants (Figure 2A), indicating that these animals are dying due to a similar loss of NMD function as Upf1 or Upf2 mutants. These results strongly suggest that Smg5 mutant lethality is specifically due to a loss of NMD activity and not due to loss of any NMD-independent Smg5 function.
Smg5 null mutants lack most, if not all, detectable NMD activity
To directly test if Drosophila Smg5 mutants have any residual NMD activity, we measured the stability of PTC-containing mRNAs in Smg5 mutants by examining the relative expression of mutant to wild-type allele expression in animals heterozygous for a PTC-containing allele (Chen et al. 2005; Frizzell et al. 2012; Nelson et al. 2016). In Smg5+/G115 ; dHR783/+ control animals, we found levels of the PTC-containing dHR783 transcript to be significantly reduced compared to the wild-type dHR78+ transcript (Figure 2C), indicative of NMD acting on the mutant allele. By contrast, we found that in Smg5C391/G115 ; dHR783/+ animals, the levels of the PTC-containing dHR783 transcript were equal to the levels of wild-type dHR78+ transcript (Figure 2C), indicating NMD-mediated degradation of PTC-containing transcripts is absent in Smg5 mutants.
Smg6-mediated cleavage is a known mechanism for degradation of NMD targets in Drosophila, so we tested if Smg5 mutants still retain this endonuclease activity. NMD target cleavage can be observed through measuring the relative abundance of NMD target mRNA fragments 5′ to the stop codon in relation to fragments 3′ to the stop codon (Figure 2D) in animals lacking the only cytoplasmic 5′-to-3′ exonuclease, Xrn1, which in Drosophila is encoded by the gene pacman (pcm) (Till et al. 1998). Null pcm mutants have no 5′-to-3′ exonuclease activity (Waldron et al. 2015), and thus mRNAs cleaved by Smg6 near the stop codon in such mutants accumulate mRNA fragments 3′ to the stop codon while still degrading the 5′ fragments through exosome complex activity. Thus, a change in relative levels of 3′ and 5′ regions of an mRNA when measured in pcm mutants relative to pcm+ animals is indicative of NMD-mediated cleavage (Nelson et al. 2016). As we showed previously (Nelson et al. 2016), the ratio of the 3′ fragment to 5′ fragment of the Gadd45 mRNA is increased in pcm null mutants compared to pcm+ animals, and this effect is dependent on Smg6 function (Figure 2E), even though in both cases overall Gadd45 expression is increased (Figure S4A). Interestingly, we found that this change in relative 3′ to 5′ ratios of Gadd45 is also lost in double mutants of null alleles of pcm and Smg5 (Figure 2E), again despite an overall increase in both 5′ and 3′ Gadd45 expression (Figure S4A). These data reveal that Smg5 is required for Smg6 endonuclease activity, further indicating that Drosophila Smg5 mutants lack any NMD activity.
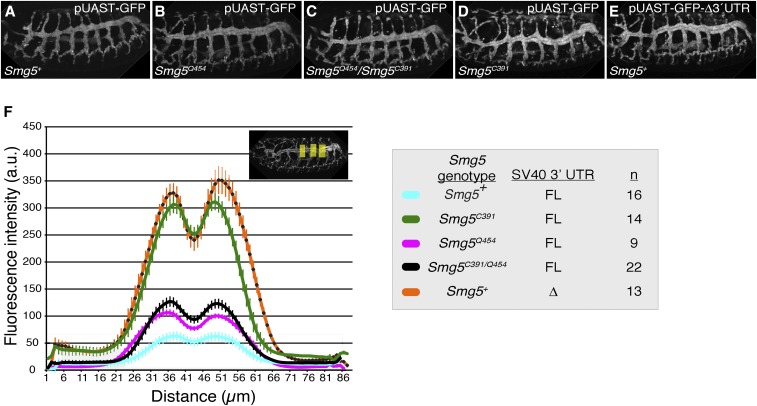
As an additional gauge of NMD activity in Smg5 mutants, we directly measured fluorescence levels of the NMD-sensitive SV40 3′UTR GFP reporter in homozygous mutant embryos (Figure 3). We found that homozygous Smg5C391 embryos exhibited ∼fivefold increase in fluorescent signal compared to Smg5+ embryos (Figure 3, D and F), comparable to the increase in GFP mRNA levels observed in the strongest previously measured NMD mutant, Upf225G (Metzstein and Krasnow 2006). As expected, embryos homozygous for the hypomorphic allele Smg5Q454 showed a smaller increase in fluorescent signal (Figure 3, B and F), while the trans-heterozygous combination Smg5Q454/C391 showed an intermediate signal, close to the Smg5Q454 signal (Figure 3, C and F). To identify the severity of NMD disruption in Smg5 mutants, we sought to directly compare the increase in fluorescent intensity of the NMD-sensitive SV40 3′UTR in Smg5 mutants to the increase observed when removing the NMD-sensitive element in the SV40 3′UTR. To identify this element, we generated a series of deletion constructs of the SV40 3′UTR and tested the relative increase in observed fluorescence when NMD is disrupted for each construct (Figure S5). Interestingly, we found that baseline reporter expression in a background with functional NMD was, in general, increased at each iteration of shortening the SV40 3′UTR fragment (Figure S5, middle column). Comparing the relative fluorescence increase of each reporter when NMD is disrupted revealed that the shortest SV40 3′UTR fragments failed to have increased reporter expression upon NMD loss, revealing that these UTRs are not targeted by NMD (Figure S5, right column). However, for the intermediate length UTR constructs, the magnitude of expression enhancement was dependent on the exact location of the deletion. These results are consistent with a model in which 3′UTR length is a major determinant of NMD sensitivity (Boehm et al. 2014), though there is likely a contribution of specific sequence elements in modulating sensitivity of intermediate length UTRs. Most importantly, we found that the shortest construct was entirely insensitive to NMD, and that this construct had a fivefold increase in fluorescence intensity compared to the full-length SV40 3′UTR, almost exactly the same as the increase observed in the Smg5 null mutant background (Figure 3, E and F). Hence, we conclude that loss of Smg5 phenocopies loss of NMD sensitivity, suggesting Smg5 is required for all NMD activity in embryos.
Figure 3.
Loss of Smg5 enhances NMD-sensitive reporter expression in embryos. (A–E) Embryos of indicated genotypes carrying GFP reporter transgene with full-length NMD-sensitive SV40 3′UTR (pUAST-GFP) or NMD-insensitive SV40 3′UTR deletion construct (pUAST-GFP-∆3′UTR). (F) Scan of GFP intensity averaged across three areas of embryo (shown in inset) in animals of the indicated genotype. FL, full-length SV40 3′UTR; ∆, SV40 3′UTR deletion construct; n, number of embryos scored.
Smg5 mutant lethality is not Smg1-dependent
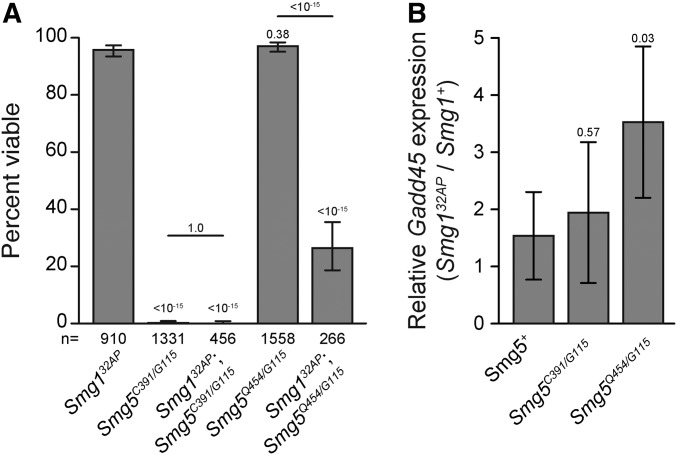
The phosphorylation of Upf1 by Smg1 has been proposed to be a critical step in the NMD process, at least in part by recruiting Smg6 to the NMD complex to initiate target degradation (Hug et al. 2016). Dephosphorylation of Upf1 is thought to be mediated by Smg5, which interacts with the PP2A phosphatase; this activity may be required for complex disassembly after target degradation has been initiated (Ohnishi et al. 2003). This model predicts that the necessity of Smg5 for NMD activity requires Upf1 phosphorylation by Smg1, and thus it is expected that null Smg132AP mutants (henceforth just referred to as Smg1 mutants), which are fully viable and have robust NMD activity (Chen et al. 2005; Metzstein and Krasnow 2006; Frizzell et al. 2012), would suppress Smg5 mutant lethality. In contrast to this prediction, we found that Smg1; Smg5 double mutants were in fact no more viable than Smg5 mutants (Figure 4A), and Smg1 mutants had no effect on the developmental delay or lethal stage of Smg5 mutants (Figure S2, A and B). These findings suggest that a failure to dephosphorylate Upf1 is not responsible for Smg5 mutant lethality; however, the lethality of Smg1; Smg5 double mutants may also be explained by unknown factors that phosphorylate Upf1 in the absence of Smg1.
Figure 4.
Smg5 mutant lethality is not dependent on Smg1. (A) Adult viability of Smg1 and Smg5 hypomorph single and double mutants. Error bars represent 95% confidence interval of the binomial distribution. P-value listed for each condition compared to Smg132AP or between indicated conditions determined by the test of equal or given proportions. n = total number of animals scored. Data for Smg5C391/G115 is duplicated from Figure 2A and displayed here for comparison between relevant genotypes. (B) Relative expression of the endogenous NMD target Gadd45 in Smg132AP mutants compared to Smg1+ controls in Smg5+ and mutant backgrounds as measured by qRT-PCR. Error bars represent 2 SEM. P-value listed for each condition compared to Smg5+ determined by two-sided Student’s t-test. n ≥ 3 for all conditions. qPCR primers used were located 5′ to the Gadd45 termination sequence.
If failure to dephosphorylate Upf1 causes lethality in both Smg5 mutants and Smg1; Smg5 double mutants, we would expect loss of Smg1 to have no effect on the viability of hypomorphic Smg5 mutants, since these alleles are viable (Figure 2A), and so should have sufficient Upf1-dephosphorylation activity. Surprisingly, we found that double mutants for the Smg1 null allele and a hypomorphic Smg5 allele show significant lethality, even though each mutation on its own is viable (Figure 4A). This result further indicates that Smg5 mutant lethality is not due to failure to dephosphorylate Upf1. An alternative model has been proposed, in which Upf1 phosphorylation by Smg1 is not required for NMD under normal conditions, but serves to enhance Smg6 and Smg5 efficiency upon stress conditions to reinforce pathway activity (Durand et al. 2016). To test this model genetically, we examined the requirement for Smg1 function when Smg5 function is impaired, by observing the relative increase in NMD target expression in Smg1 and Smg5 mutant combinations. The contribution of Smg1 that is independent of Smg5 activity can be assessed by examining Gadd45 expression levels in Smg1; Smg5 mutant conditions. We found that Smg1; Smg5+ mutants show only a small increase in Gadd45 expression (Figure 4B and Figure S4B), consistent with previous observations that loss of Smg1 normally has little impact on NMD function (Chen et al. 2005; Metzstein and Krasnow 2006). In Smg1; Smg5null double mutants, a similar small increase in relative Gadd45 expression is observed (Figure 4B and Figure S4B), indicating that Smg1 contributes to degrade an equal portion of NMD targets when Smg5 is active or absent. Interestingly, we found that the relative increase in Gadd45 expression upon loss of Smg1 is greater in the Smg5 hypomorphic background than in Smg5+ animals (Figure 4B and Figure S4B). This result indicates that when Smg5 activity is reduced, but still functional, Smg1 has a much larger contribution to NMD activity than under normal conditions or when Smg5 function is completely removed. Together, these findings suggest that there is some Smg1 activity that normally is responsible for a small amount of NMD activity, independent of Smg5, but when Smg5 NMD function is impaired, Smg1 can enhance Smg5 activity to contribute to this NMD function as well.
Smg1 is not required for Smg6 activity
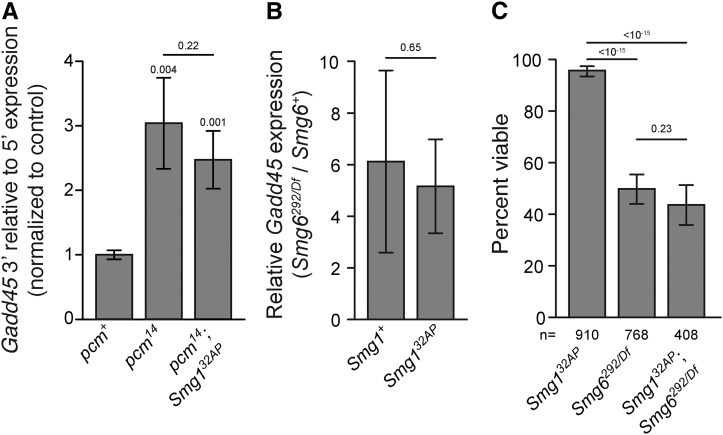
Smg6 has been shown to bind Upf1 at residues phosphorylated by Smg1 (Fukuhara et al. 2005; Okada-Katsuhata et al. 2012), leading to the model that Smg1 is required for Smg6 complex entry and cleavage of NMD targets. However, Drosophila Smg6 mutants have much stronger NMD defects than Smg1 mutants (Frizzell et al. 2012), and Smg6 has recently been shown to be capable of binding nonphosphorylated Upf1 (Chakrabarti et al. 2014; Nicholson et al. 2014). These data suggest an alternative model in which Smg6 can cleave NMD targets even in the absence of Smg1 kinase activity. In support of this latter model, we found that using the 3′-to-5′ Gadd45 expression ratio is the same in Smg1, pcm double mutants compared to pcm single mutants (Figure 5A), even though overall Gadd45 expression is increased in the double mutants (Figure S6). This result reveals that Gadd45 mRNA is cleaved in the absence of Smg1 just as efficiently as in animals with wild-type Smg1. We also found the same relative increase in Gadd45 mRNA levels upon loss of Smg6 in animals with or without functional Smg1 (Figure 5B), indicating that Smg6 functions equally to NMD activity with or without Smg1 activity. Consistent with these findings, double mutants between null Smg1 and Smg6 alleles do not have reduced viability or enhanced developmental delay compared to Smg6 single mutants (Figure 5C and Figure S2C). Together, these data suggest that Smg1 does not contribute to NMD-independent Smg6 function, and that Smg1is not required for normal Smg6 activity in vivo.
Figure 5.
Smg1 is not required for Smg6 activity and Smg5 is required for exonucleolytic NMD activity. (A) Gadd45 3′ expression relative to 5′ expression in indicated genotypes, measured by qRT-PCR. The 3′ region is preferentially stabilized in pcm14 mutants with or without functional Smg1. Error bars represent 2 SEM. P-value listed for each condition compared to pcm+ or between indicated conditions determined by two-sided Student’s t-test. n ≥ 3 for all conditions. Data for pcm+ and pcm14 conditions are duplicated from Figure 2E and displayed here for comparison between relevant genotypes. (B) Relative expression of the endogenous NMD target Gadd45 measured by qRT-PCR using the 5′ primer pair in Smg6292/Df mutants compared to Smg6+ controls in either Smg1+ or Smg132AP mutant backgrounds. Error bars represent 2 SEM. P-value between conditions determined by two-sided Student’s t-test. n ≥ 3 for all conditions. (C) Adult viability of Smg132AP and Smg6292/Df null mutants, and Smg132AP; Smg6292/Df double mutants. Error bars represent 95% confidence interval of the binomial distribution. P-value listed between indicated conditions determined by the test of equal or given proportions. n displays total number of animals scored. Data for Smg132AP are duplicated from Figure 4A and displayed here for comparison between relevant genotypes. Primers for all qPCR experiments were located 5′ to the Gadd45 termination sequence.
Discussion
The degradation of both specific normal and many kinds of erroneous mRNAs by the NMD pathway is a crucial gene regulatory mechanism and arose in the ancestors of all eukaryotes. While many factors required for NMD have been biochemically characterized, the individual contribution of each factor to the recognition and degradation of NMD targets is not fully understood. Through our genetic analysis of Smg5 in Drosophila, we have found that NMD utilizes multiple mechanisms to promote target degradation in vivo. One of our main findings is that Smg5 null mutants have as severe defects as either Upf1 and Upf2 null mutants, indicating that Smg5 is a critical factor for promoting NMD target recognition and/or decay. In support of this interpretation, we found that Smg5 is required for both Smg6-mediated endonucleolytic cleavage of NMD targets and a separate, Smg6-independent, decay process. Our findings are surprising, given that Smg5 has primarily been thought to promote NMD complex recycling, but with only a secondary requirement to stimulate decay activity (Ohnishi et al. 2003). Instead, we propose that Smg5 is a critical NMD factor necessary for at least two, independent NMD degradation mechanisms.
In contrast to Smg5 having a critical role in target degradation, our data are less supportive for a Smg5 function in NMD complex recycling. The phosphorylation of Upf1 at multiple residues by Smg1 (Yamashita et al. 2001; Grimson et al. 2004) is thought to be required to initiate, or at least stimulate, NMD-mediated degradation (Anders et al. 2003; Ohnishi et al. 2003). Subsequent Smg5-mediated recruitment of the PP2A phosphatase to the NMD complex is thought to lead to Upf1 dephosphorylation to promote complex disassembly and recycling (Anders et al. 2003; Ohnishi et al. 2003). While Smg1, and thus Upf1 phosphorylation, does not seem to play a major role in NMD in Drosophila (Chen et al. 2005; Metzstein and Krasnow 2006), failure to dephosphorylate Upf1 could still be the cause of the strong NMD defect in Drosophila Smg5 mutants. This model predicts that since Smg5 mutant lethality is due to lack of Upf1 dephosphorylation, the loss of Smg1 should be epistatic to the loss of Smg5, since Upf1 dephosphorylation would no longer be required in Smg1 mutant animals. However, we found just the opposite: Smg1 mutations do not suppress Smg5 mutations at all, and Smg1 mutants actually enhance the defect of Smg5 hypomorphic alleles. These data are instead consistent with a more recently proposed model in which Upf1 phosphorylation is only required when the NMD process is “stalled” or is otherwise impaired (Durand et al. 2016), such as occurs in hypomorphic Smg5 mutants. Based on our findings, we propose that NMD functions under two states: nonphosphorylated Upf1, which requires Smg5 to stimulate both Smg6-mediated and Smg6-independent decay, and phosphorylated Upf1, which enhances the interaction of Smg6 with Upf1 and stimulates Smg6-mediated decay independently of Smg5. For most substrates under normal conditions, the former mechanism predominates, with the latter only occurring when the first process does not efficiently occur. Additionally, some specific substrates may require Smg1-stimulated NMD by default. For instance, while loss of Smg1 leads to barely detectable stabilization of a PTC-containing substrate (Chen et al. 2005; Metzstein and Krasnow 2006), there is a significant (>twofold) increase in endogenous substrates, such as Gadd45, and reporters using the NMD-sensitive SV40 3′UTR (Metzstein and Krasnow 2006; Frizzell et al. 2012). The difference in targeting between these alternative substrates is not yet known, but there is indication of differential pathway usage also in mammalian cells (Chan et al. 2007; Ottens et al. 2017).
Global analysis of NMD targets in intact Drosophila (Frizzell et al. 2012) and in cell culture (Lykke-Andersen et al. 2014; Schmidt et al. 2015; Colombo et al. 2017; Ottens et al. 2017) has suggested that the degradation of NMD substrates occurs mainly through Smg6-mediated endonucleolytic cleavage. Substrates bypassing this decay process appear to go through an alternative, decapping-mediated process (Lykke-Andersen et al. 2014). The stronger NMD defects observed in Smg5 null mutants as compared to Smg6 null mutants, shown here, suggest that Smg5 is required for Smg6-independent decay activity, but also by measuring differential decay of 5′ and 3′ NMD target fragments, we have obtained evidence that Smg5-dependent, Smg6-independent decay processes do indeed occur in vivo. While the mechanism of this decay remains unclear, there is in vitro evidence that Smg5 can interact with the Dcp decapping complex (Cho et al. 2009, 2013; Loh et al. 2013), recruiting the complex to the NMD target and eventually leading to exonucleolytic decay initiated at the 5′ end of the message. There is also evidence that Smg5 may also initiate decay via deadenylation (Loh et al. 2013). While we show that degradation of an NMD substrate 5′ to the predicted Smg6-cleavage site is dependent on Smg5, we cannot yet distinguish whether this activity is due to a role in decapping, deadenylation, or both. It is also possible that the preference for which decay mechanism degrades NMD targets may differ among individual NMD targets. Furthermore, the choice between decay mechanisms may differ in tissue-specific or developmental contexts. It will be important to parse the relative contribution of each decay pathway to the degradation of NMD targets to understand the mechanism underlying the bias in decay.
Here we characterize the first Drosophila Smg5 mutants, identifying that Smg5 is critical for NMD function and viability, similar to Upf1 and Upf2, and providing genetic evidence for an essential role of Smg5 function. We also find that NMD utilizes multiple decay mechanisms to destroy its targets in vivo. All of these pathways depend on Smg5, indicating that Smg5 plays more fundamental roles in NMD than has previously been appreciated. More closely characterizing the molecular mechanisms of Smg5 function in NMD may reveal novel key features of NMD activity that have thus far escaped detection.
Acknowledgments
We are very grateful to Sarah Newbury for providing fly stocks prior to publication and Alex Chapin for performing Smg5 rescue experiments. Fly stocks were obtained from the Bloomington Drosophila Stock Center. K.A.F. was supported by University of Utah Developmental Biology Training Grant 5T32-HD07491. Work in S.L.’s laboratory was supported by the Swiss National Science Foundation (SNF_31003A_141093/1), the University of Zurich, the “Cells-in-Motion” Cluster of Excellence (EXC 1003-CiM), the Deutsche Forschungsgemeinschaft (DFG_LU 1398/2-1), and the University of Münster. Work in M.M.M.’s laboratory was supported by National Institutes of Health (NIH) grant 1R01GM084011 and a March of Dimes Award 5-FY07-664.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6494774.
Communicating editor: E. Tran
Literature Cited
- Adjibade P., Mazroui R., 2014. Control of mRNA turnover: implication of cytoplasmic RNA granules. Semin. Cell Dev. Biol. 34: 15–23. 10.1016/j.semcdb.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Anders K. R., Grimson A., Anderson P., 2003. SMG-5, required for C. elegans nonsense-mediated mRNA decay, associates with SMG-2 and protein phosphatase 2A. EMBO J. 22: 641–650. 10.1093/emboj/cdg056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciga-Reyes L., Wootton L., Kieffer M., Davies B., 2006. UPF1 is required for nonsense-mediated mRNA decay (NMD) and RNAi in Arabidopsis. Plant J. 47: 480–489. 10.1111/j.1365-313X.2006.02802.x [DOI] [PubMed] [Google Scholar]
- Avery P., Vicente-Crespo M., Francis D., Nashchekina O., Alonso C. R., et al. , 2011. Drosophila Upf1 and Upf2 loss of function inhibits cell growth and causes animal death in a Upf3-independent manner. RNA 17: 624–638. 10.1261/rna.2404211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm V., Haberman N., Ottens F., Ule J., Gehring N. H., 2014. 3′ UTR length and messenger ribonucleoprotein composition determine endocleavage efficiencies at termination codons. Cell Rep. 9: 555–568. 10.1016/j.celrep.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Cali B. M., Kuchma S. L., Latham J., Anderson P., 1999. smg-7 is required for mRNA surveillance in Caenorhabditis elegans. Genetics 151: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik A., Kervestin S., Jacobson A., 2015. NMD: at the crossroads between translation termination and ribosome recycling. Biochimie 114: 2–9. 10.1016/j.biochi.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Bonneau F., Schüssler S., Eppinger E., Conti E., 2014. Phospho-dependent and phospho-independent interactions of the helicase UPF1 with the NMD factors SMG5–SMG7 and SMG6. Nucleic Acids Res. 42: 9447–9460. 10.1093/nar/gku578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. K., Huang L., Gudikote J. P., Chang Y. F., Imam J. S., et al. , 2007. An alternative branch of the nonsense-mediated decay pathway. EMBO J. 26: 1820–1830. 10.1038/sj.emboj.7601628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin A., Hu H., Rynearson S. G., Hollien J., Yandell M., et al. , 2014. In vivo determination of direct targets of the nonsense-mediated decay pathway in Drosophila. G3 (Bethesda) 4: 485–496. 10.1534/g3.113.009357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Smith K. R., Batterham P., Robin C., 2005. Smg1 nonsense mutations do not abolish nonsense-mediated mRNA decay in Drosophila melanogaster. Genetics 171: 403–406. 10.1534/genetics.105.045674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Serin G., Ohara O., Maquat L. E., 2003. Characterization of human Smg5/7a: a protein with similarities to Caenorhabditis elegans SMG5 and SMG7 that functions in the dephosphorylation of Upf1. RNA 9: 77–87. 10.1261/rna.2137903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Kim K. M., Kim Y. K., 2009. Human proline-rich nuclear receptor coregulatory protein 2 mediates an interaction between mRNA surveillance machinery and decapping complex. Mol. Cell 33: 75–86. 10.1016/j.molcel.2008.11.022 [DOI] [PubMed] [Google Scholar]
- Cho H., Han S., Choe J., Park S. G., Choi S. S., et al. , 2013. SMG5-PNRC2 is functionally dominant compared with SMG5–SMG7 in mammalian nonsense-mediated mRNA decay. Nucleic Acids Res. 41: 1319–1328. 10.1093/nar/gks1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M., Karousis E. D., Bourquin J., Bruggmann R., Mühlemann O., 2017. Transcriptome-wide identification of NMD-targeted human mRNAs reveals extensive redundancy between SMG6- and SMG7-mediated degradation pathways. RNA 23: 189–201. 10.1261/rna.059055.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. K., Christensen S. J., Deal J. A., Coburn R. A., Deal M. E., et al. , 2012. The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome Biol. 13: R21 10.1186/gb-2012-13-3-r21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaplinski K., Weng Y., Hagan K. W., Peltz S. W., 1995. Purification and characterization of the Upf1 protein: a factor involved in translation and mRNA degradation. RNA 1: 610–623. [PMC free article] [PubMed] [Google Scholar]
- Durand S., Franks T. M., Lykke-Andersen J., 2016. Hyperphosphorylation amplifies UPF1 activity to resolve stalls in nonsense-mediated mRNA decay. Nat. Commun. 7: 12434 10.1038/ncomms12434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle A. B., Lykke-Andersen S., Mühlemann O., Jensen T. H., 2009. SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat. Struct. Mol. Biol. 16: 49–55. 10.1038/nsmb.1530 [DOI] [PubMed] [Google Scholar]
- Fisk G. J., Thummel C. S., 1998. The DHR78 nuclear receptor is required for ecdysteroid signaling during the onset of Drosophila metamorphosis. Cell 93: 543–555. 10.1016/S0092-8674(00)81184-8 [DOI] [PubMed] [Google Scholar]
- Förster D., Armbruster K., Luschnig S., 2010. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr. Biol. 20: 62–68. 10.1016/j.cub.2009.11.062 [DOI] [PubMed] [Google Scholar]
- Frizzell K. A., Rynearson S. G., Metzstein M. M., 2012. Drosophila mutants show NMD pathway activity is reduced, but not eliminated, in the absence of Smg6. RNA 18: 1475–1486. 10.1261/rna.032821.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Ebert J., Unterholzner L., Lindner D., Izaurralde E., et al. , 2005. SMG7 is a 14–3-3-like adaptor in the nonsense-mediated mRNA decay pathway. Mol. Cell 17: 537–547. 10.1016/j.molcel.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Gatfield D., Unterholzner L., Ciccarelli F. D., Bork P., Izaurralde E., 2003. Nonsense-mediated mRNA decay in Drosophila: at the intersection of the yeast and mammalian pathways. EMBO J. 22: 3960–3970. 10.1093/emboj/cdg371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring N. H., Neu-Yilik G., Schell T., Hentze M. W., 2003. Y14 and hUpf3b form an NMD-activating complex. Mol. Cell 11: 939–949. 10.1016/S1097-2765(03)00142-4 [DOI] [PubMed] [Google Scholar]
- Grimson A., O’Connor S., Newman C. L., Anderson P., 2004. SMG-1 is a phosphatidylinositol kinase-related protein kinase required for nonsense-mediated mRNA decay in Caenorhabditis elegans. Mol. Cell. Biol. 24: 7483–7490. 10.1128/MCB.24.17.7483-7490.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Brown A. H., Jacobson A., 1997. Upf1p, Nmd2p, and Upf3p are interacting components of the yeast nonsense-mediated mRNA decay pathway. Mol. Cell. Biol. 17: 1580–1594. 10.1128/MCB.17.3.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., Papp A., Pulak R., Ambros V., Anderson P., 1989. A new kind of informational suppression in the nematode Caenorhabditis elegans. Genetics 123: 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug N., Longman D., Cáceres J. F., 2016. Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic Acids Res. 44: 1483–1495. 10.1093/nar/gkw010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntzinger E., Kashima I., Fauser M., Saulière J., Izaurralde E., 2008. SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoan. RNA 14: 2609–2617. 10.1261/rna.1386208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerényi Z., Mérai Z., Hiripi L., Benkovics A., Gyula P., et al. , 2008. Inter-kingdom conservation of mechanism of nonsense-mediated mRNA decay. EMBO J. 27: 1585–1595. 10.1038/emboj.2008.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T., Liang Z., Patel H., Yu M. H., Sivasubramaniam G., et al. , 2006. A new family of Drosophila balancer chromosomes with a w- dfd-GMR yellow fluorescent protein marker. Genetics 174: 2255–2257. 10.1534/genetics.106.063461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds P., Peltz S. W., Jacobson A., Culbertson M. R., 1991. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 5: 2303–2314. 10.1101/gad.5.12a.2303 [DOI] [PubMed] [Google Scholar]
- Leeds P., Wood J. M., Lee B. S., Culbertson M. R., 1992. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol. Cell. Biol. 12: 2165–2177. 10.1128/MCB.12.5.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Shi Y., Wang P., Guachalla L. M., Sun B., et al. , 2015. Smg6/Est1 licenses embryonic stem cell differentiation via nonsense-mediated mRNA decay. EMBO J. 34: 1630–1647. 10.15252/embj.201489947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh B., Jonas S., Izaurralde E., 2013. The SMG5–SMG7 heterodimer directly recruits the CCR4-NOT deadenylase complex to mRNAs containing nonsense codons via interaction with POP2. Genes Dev. 27: 2125–2138. 10.1101/gad.226951.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen J., Shu M. D., Steitz J. A., 2000. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103: 1121–1131. 10.1016/S0092-8674(00)00214-2 [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen S., Chen Y., Ardal B. R., Lilje B., Waage J., et al. , 2014. Human nonsense-mediated RNA decay initiates widely by endonucleolysis and targets snoRNA host genes. Genes Dev. 28: 2498–2517. 10.1101/gad.246538.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medghalchi S. M., Frischmeyer P. A., Mendell J. T., Kelly A. G., Lawler A. M., et al. , 2001. Rent1, a trans-effector of nonsense-mediated mRNA decay, is essential for mammalian embryonic viability. Hum. Mol. Genet. 10: 99–105. 10.1093/hmg/10.2.99 [DOI] [PubMed] [Google Scholar]
- Metzstein M. M., Krasnow M. A., 2006. Functions of the nonsense-mediated mRNA decay pathway in Drosophila development. PLoS Genet. 2: e180 10.1371/journal.pgen.0020180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. O., Moore K. A., Chapin A., Hollien J., Metzstein M. M., 2016. Degradation of Gadd45 mRNA by nonsense-mediated decay is essential for viability. eLife 5: e12876 10.7554/eLife.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson P., Josi C., Kurosawa H., Yamashita A., Mühlemann O., 2014. A novel phosphorylation-independent interaction between SMG6 and UPF1 is essential for human NMD. Nucleic Acids Res. 42: 9217–9235. 10.1093/nar/gku645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Yamashita A., Kashima I., Schell T., Anders K. R., et al. , 2003. Phosphorylation of hUPF1 induces formation of mRNA surveillance complexes containing hSMG-5 and hSMG-7. Mol. Cell 12: 1187–1200. 10.1016/S1097-2765(03)00443-X [DOI] [PubMed] [Google Scholar]
- Okada-Katsuhata Y., Yamashita A., Kutsuzawa K., Izumi N., Hirahara F., et al. , 2012. N- and C-terminal Upf1 phosphorylations create binding platforms for SMG-6 and SMG-5:SMG-7 during NMD. Nucleic Acids Res. 40: 1251–1266. 10.1093/nar/gkr791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottens F., Boehm V., Sibley C. R., Ule J., Gehring N. H., 2017. Transcript-specific characteristics determine the contribution of endo- and exonucleolytic decay pathways during the degradation of nonsense-mediated decay substrates. RNA 23: 1224–1236. 10.1261/rna.059659.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. F., Carr B., Anders K. R., Grimson A., Anderson P., 1999. SMG-2 is a phosphorylated protein required for mRNA surveillance in Caenorhabditis elegans and related to Upf1p of yeast. Mol. Cell. Biol. 19: 5943–5951. 10.1128/MCB.19.9.5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccarelli M., Kebaara B. W., 2014. Regulation of natural mRNAs by the nonsense-mediated mRNA decay pathway. Eukaryot. Cell 13: 1126–1135. 10.1128/EC.00090-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J., Letunic I., Raes J., Bork P., Izaurralde E., 2005. Nonsense-mediated mRNA decay factors act in concert to regulate common mRNA targets. RNA 11: 1530–1544. 10.1261/rna.2160905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. A., Foley P. L., Jeong D. H., Rymarquis L. A., Doyle F., et al. , 2015. Identification of SMG6 cleavage sites and a preferred RNA cleavage motif by global analysis of endogenous NMD targets in human cells. Nucleic Acids Res. 43: 309–323. 10.1093/nar/gku1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwaszek A., Ukleja M., Dziembowski A., 2014. Proteins involved in the degradation of cytoplasmic mRNA in the major eukaryotic model systems. RNA Biol. 11: 1122–1136. 10.4161/rna.34406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till D. D., Linz B., Seago J. E., Elgar S. J., Marujo P. E., et al. , 1998. Identification and developmental expression of a 5′–3′ exoribonuclease from Drosophila melanogaster. Mech. Dev. 79: 51–55. 10.1016/S0925-4773(98)00173-7 [DOI] [PubMed] [Google Scholar]
- Venken K. J. T., He Y., Hoskins R. A., Bellen H. J., 2006. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314: 1747–1751. 10.1126/science.1134426 [DOI] [PubMed] [Google Scholar]
- Waldron J. A., Jones C. I., Towler B. P., Pashler A. L., Grima D. P., et al. , 2015. Xrn1/Pacman affects apoptosis and regulates expression of hid and reaper. Biol. Open 4: 649–660. 10.1242/bio.201410199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischenfeldt J., Damgaard I., Bryder D., Theilgaard-Mönch K., Thoren L. A., et al. , 2008. NMD is essential for hematopoietic stem and progenitor cells and for eliminating by-products of programmed DNA rearrangements. Genes Dev. 22: 1381–1396. 10.1101/gad.468808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y., Czaplinski K., Peltz S. W., 1996a Identification and characterization of mutations in the UPF1 gene that affect nonsense suppression and the formation of the Upf protein complex but not mRNA turnover. Mol. Cell. Biol. 16: 5491–5506. 10.1128/MCB.16.10.5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y., Czaplinski K., Peltz S. W., 1996b Genetic and biochemical characterization of mutations in the ATPase and helicase regions of the Upf1 protein. Mol. Cell. Biol. 16: 5477–5490. 10.1128/MCB.16.10.5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp N., Huntzinger E., Weiler C., Saulière J., Schmidt S., et al. , 2009. Nonsense-mediated mRNA decay effectors are essential for zebrafish embryonic development and survival. Mol. Cell. Biol. 29: 3517–3528. 10.1128/MCB.00177-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A., Hinz U., Engelbert M., Knust E., 1995. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82: 67–76. 10.1016/0092-8674(95)90053-5 [DOI] [PubMed] [Google Scholar]
- Yamashita A., Ohnishi T., Kashima I., Taya Y., Ohno S., 2001. Human SMG-1, a novel phosphatidylinositol 3-kinase-related protein kinase, associates with components of the mRNA surveillance complex and is involved in the regulation of nonsense-mediated mRNA decay. Genes Dev. 15: 2215–2228. 10.1101/gad.913001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoine M., Nishii T., Nakamura K., 2006. Arabidopsis UPF1 RNA helicase for nonsense-mediated mRNA decay is involved in seed size control and is essential for growth. Plant Cell Physiol. 47: 572–580. 10.1093/pcp/pcj035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Drosophila strains are available upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6494774.