Abstract
Behavioral plasticity allows for context-dependent prioritization of competing drives, such as sleep and foraging. Despite the identification of neuropeptides and hormones implicated in dual control of sleep drive and appetite, our understanding of the mechanism underlying the conserved sleep-suppressing effect of food deprivation is limited. Caenorhabditis elegans provides an intriguing model for the dissection of sleep function and regulation as these nematodes engage a quiescence program following exposure to noxious conditions, a phenomenon known as stress-induced sleep (SIS). Here we show that food deprivation potently suppresses SIS, an effect enhanced at high population density. We present evidence that food deprivation reduces the need to sleep, protecting against the lethality associated with defective SIS. Additionally, we find that SIS is regulated by both target of rapamycin and transforming growth factor-β nutrient signaling pathways, thus identifying mechanisms coordinating sleep drive with internal and external indicators of food availability.
Keywords: starvation, stress, sleep, TOR, TGF-β
SLEEP is a highly coordinated and quickly reversible state of behavioral quiescence that is characterized by reduced motor activity and lowered sensory responsiveness (Allada and Siegel 2008) . Sleep has been identified in a wide range of species and the genetic mechanisms underlying this behavioral state are highly conserved (Campbell and Tobler 1984; Allada and Siegel 2008; Cirelli 2009; Crocker and Sehgal 2010; Trojanowski and Raizen 2016). A major challenge in understanding the function and evolution of sleep lies in elucidating the mechanisms that allow for its coordination with competing needs, such as reproduction and foraging. For example, a peptide released by Drosophila males during copulation suppresses daytime sleep in females, an effect compatible with increased foraging and egg laying (Isaac et al. 2010). Additionally, food deprivation (FD) has long been known to have a sleep-suppressing effect across species (Jacobs and McGinty 1971; MacFadyen et al. 1973; Rashotte et al. 1998; Roky et al. 2003; Keene et al. 2010) and various neuropeptides, including orexin/hypocretin and neuropeptide Y, have been shown to stimulate both wakefulness and appetite in mammals (Shukla and Basheer 2016). Short sleep duration is correlated with increased levels of the appetite-stimulating hormone ghrelin, and decreased levels of the appetite-suppressing hormone leptin (Shukla and Basheer 2016). These observations indicate that the regulation of sleep and feeding are tightly linked; however, the mechanism underlying food-dependent plasticity in sleep is poorly understood. An intriguing question is whether fasting-dependent sleep suppression simply interferes with sleep drive—the engagement of signaling pathways that promote sleep—or reduces sleep need, the yet-unknown cellular debt that accrues with wakefulness.
Recently, invertebrate model organisms such as Drosophila, Caenorhabditis elegans, and the jellyfish Cassiopea have emerged as valuable systems for studying the function of sleep and the genetic and neural circuits that control it (Cirelli 2009; Crocker and Sehgal 2010; Nath et al. 2017). In C. elegans, conditions that induce cellular damage, such as exposure to noxious heat or UV light, have been shown to induce sleep (Hill et al. 2014; Nelson et al. 2014; Iannacone et al. 2017). This stress-induced sleep (SIS) is characterized by cessation of motor activity and a rapidly reversible reduction in sensory responsiveness (Hill et al. 2014), which are both hallmarks of vertebrate sleep. Additionally, SIS appears to be beneficial, conferring a survival advantage compared to sleepless animals under the same conditions (Hill et al. 2014). This supports the notion that a conserved core function of sleep is to repair cellular damage.
SIS is dependent on epidermal growth factor (EGF), which promotes sleep in mammals (Kushikata et al. 1998; Kramer et al. 2001), and on the activation of EGF receptors (EGFR) on the sleep-inducing ALA neuron (Van Buskirk and Sternberg 2007; Hill et al. 2014). The sleep-promoting function of the ALA neuron has been shown to depend on the collective action of several ALA-expressed neuropeptides that control discrete aspects of sleep behavior, such as the cessation of locomotion or feeding (Nelson et al. 2014; Nath et al. 2016). The conditions that produce SIS are damaging by nature and can have effects on behavior that are independent of ALA-mediated sleep (Hill et al. 2014; Nelson et al. 2014). However, these effects can be distinguished from ALA-dependent sleep by examination of ceh-17 mutant animals, which lack a transcription factor necessary for ALA neuron differentiation (Van Buskirk and Sternberg 2010).
We wished to take advantage of the genetic tractability of C. elegans SIS to investigate plasticity in sleep drive. We show that FD potently suppresses SIS, an effect mediated in part by PHA-4/FOXA, a transcription factor normally repressed by the nutrient-responsive target of rapamycin (TOR) kinase. We present evidence that starvation affects SIS at the level of sleep need and functions upstream of ALA neuron activation. Additionally, we show that competence to engage in SIS under well-fed conditions is dependent on the transforming growth factor-β (TGF-β)-related ligand DAF-7, and that this regulation occurs downstream of ALA activation. Our work reveals multilevel modulation of C. elegans SIS by nutrient availability, affecting both sleep need and the capacity to execute the SIS program.
Materials and Methods
Strains and cultivation
Strains, genotypes, and sources are listed in Supplemental Material, Reagent Table. Strains were grown and maintained on nematode growth media (NGM) plates and fed OP50 Escherichia coli bacteria as a food source. Unless otherwise noted, all strains were raised at 20° with the exception of daf-c strains and corresponding control animals, which were raised at 15°.
Temperature-sensitive strains
SM190: smg-1(cc546);pha-4(zu225), harboring a temperature-sensitive mutation in the nonsense-mediated decay factor smg-1, was maintained at 24° and shifted at the L2 stage to 20° to activate SMG-1, thus reducing pha-4 expression (Gaudet and Mango 2002). CB1370: daf-2(e1370) is temperature sensitive for dauer formation and was therefore raised at 15° to obtain adults for examination of SIS. Although the temperature-sensitive nature of the daf-2 allele is likely attributable to the temperature dependence of dauer formation (Golden and Riddle 1984b), we also examined SIS in daf-2(e1370) animals that had been raised at 25° from the L3 stage and obtained similar results.
Standard for all assays
Animals were assayed for SIS as prefertile young adults and were examined prior to testing for normal locomotion and feeding. Except where noted, plates were gently placed on a stereomicroscope stage ∼40 sec prior to examination of behavior. Unless otherwise stated, no more than two independent trials were performed per day, each containing 25 animals. Cessation of locomotion and feeding were scored as measures of SIS. This was achieved by examining each animal for 5 sec at 250× or 320× magnification. Cessation of locomotion was defined as complete immobility and cessation of feeding was defined as the absence of contractions of the posterior pharyngeal bulb.
Heat shock
The intensity and duration of C. elegans SIS in response to heat shock is dependent on the temperature, such that a 30 min heat shock at 35° induces approximately an hour of quiescence, while a 37° heat shock induces an additional and prolonged second bout of quiescence (Hill et al. 2014; Nelson et al. 2014). In addition to inducing ALA-dependent SIS, the 30 min, 37° heat shock causes significant ALA-independent immobility, as evidenced by quiescence in ceh-17 mutants lacking the sleep-inducing ALA neuron (Van Buskirk and Sternberg 2007; Hill et al. 2014; Nelson et al. 2014). We found that a shorter 37° heat shock eliminates the majority of ALA-independent effects (Figure S1, A and B). Therefore, we adopted a 37°, 11 min heat shock as our standard assay procedure. In this protocol, animals were transferred to small (35 × 10 mm) NGM plates seeded with OP50 E. coli. Plates were sealed with parafilm prior to placing them upright (agar side down) in a 37° water bath. Plates were subsequently placed on ice for 2 min following the heat shock to return them to room temperature (Hill et al. 2014). Cessation of locomotion was scored at 8 min after the end of the heat shock, and cessation of feeding was scored at 17 min after heat shock, as these were determined to be the peaks of ALA-dependent quiescence for each subbehavior of SIS (Figure S1, A and B). Although locomotion and feeding are both suppressed at the 8 min time point, we observed a small amount of ALA-independent suppression of feeding at this time point (Figure S1B). We found 15–17 min after the end of heat shock to be a reliable time point to judge heat-induced, ALA-dependent suppression of feeding.
UV exposure
Animals were transferred to small (35 × 10 mm) NGM plates seeded with OP50 E. coli. Plates were placed face down with the lid on (agar side up), on a UVP M-10E Mini Benchtop UV Transilluminator gel box for 45 sec. The onset of SIS in response to UV light (302nm) is delayed in comparison to heat shock, and the duration is much longer (Iannacone et al. 2017). Thus, cessation of locomotion was scored at 1 hr after UV exposure when ALA-independent immobility is minimal (Figure S1C). Cessation of feeding was not scored in UV assays because we found UV exposure induced significant ALA-independent suppression of feeding (Figure S1D).
FD
Animals were synchronized by egg bleach and removed from food 18–24 hr prior to the young adult stage (as early L4 animals). This was achieved by rinsing plates with M9 into a microfuge tube and spinning briefly, followed by three rinses with M9. Rinsed animals were pipetted either to new plates seeded with OP50 E. coli or to unseeded NGM plates and placed back at 20° to develop to the young adult stage. Prior to testing for SIS, food-deprived animals were examined to ensure that they were moving and pumping. With the exception of assays testing the effect of complete absence of food (Figure 1, A and B), food-deprived animals were transferred to fresh food immediately before heat shock or UV-light exposure.
Figure 1.
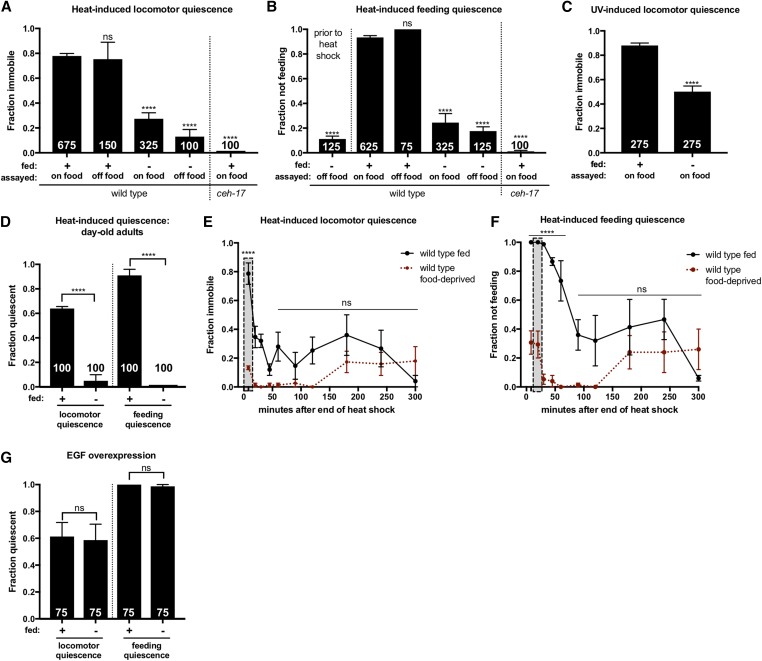
Food-deprived animals are severely impaired for SIS. SIS was measured by examination of immobility and cessation of feeding after a 37°, 11 min heat shock or 45 sec UV exposure (indicated above each graph). (A and B) FD suppresses SIS and this effect is not dependent on access to food. Food-deprived animals were examined for pharyngeal pumping prior to heat shock (B) to ensure that animals continue to pump in the absence of food. (C) FD inhibits UV-induced sleep. (D) Sleep suppression is not specific to the L4-to-adult FD protocol. Animals were deprived of food as prefertile young adults and assayed for SIS 24 hr later. (A–D) One-way ANOVA with Dunnett’s multiple comparisons test. The total number of animals examined is indicated at the bottom of each bar. Error bars represent SEM (25 animals per trial). (E and F) In the hours following heat shock, food-deprived animals show a loss rather than a delay of (E) locomotor and (F) feeding quiescence. The vertical box indicates the peak of ALA-dependent quiescence, examined in single time point SIS assays. Error bars represent SEM of three trials (n = 25 each). Student’s t-test with Holm–Sidak correction. (G) FD does not affect EGF-induced quiescence. Mild heat exposure (33° for 10 min) was used to induce EGF-OE. Two hours after EGF induction, plates were gently moved to the microscope stage for examination of quiescence. One-way ANOVA with Tukey’s multiple comparison test. The total number of animals examined is indicated at the bottom of each bar. Error bars represent SEM of trials (n = 25 each). (See also Figures S1 and S2). **** P ≤ 0.0001. ns, not significant vs. control.
Crowding
Animals were synchronized by egg bleach and grown at either high or low population density. Crowded but well-fed conditions were achieved by adding 150 µl of OP50 to plates each day to ensure that the animals at high population density had continuous food. To assess the impact of crowding on starved animals, populations were monitored until animals at high density ran out of food. At this point, animals from the low-density group were removed from food using the FD protocol described above and both groups were allowed to grow to adulthood. Representative images of the population density of animals are pictured in Figure 2, C and D. In addition to scoring animals for cessation of locomotion, moving animals were scored according to whether they were full-body active, defined as any forward or backward movement, or exhibited solely head movement, defined as dorsal–ventral displacement of the head.
Figure 2.
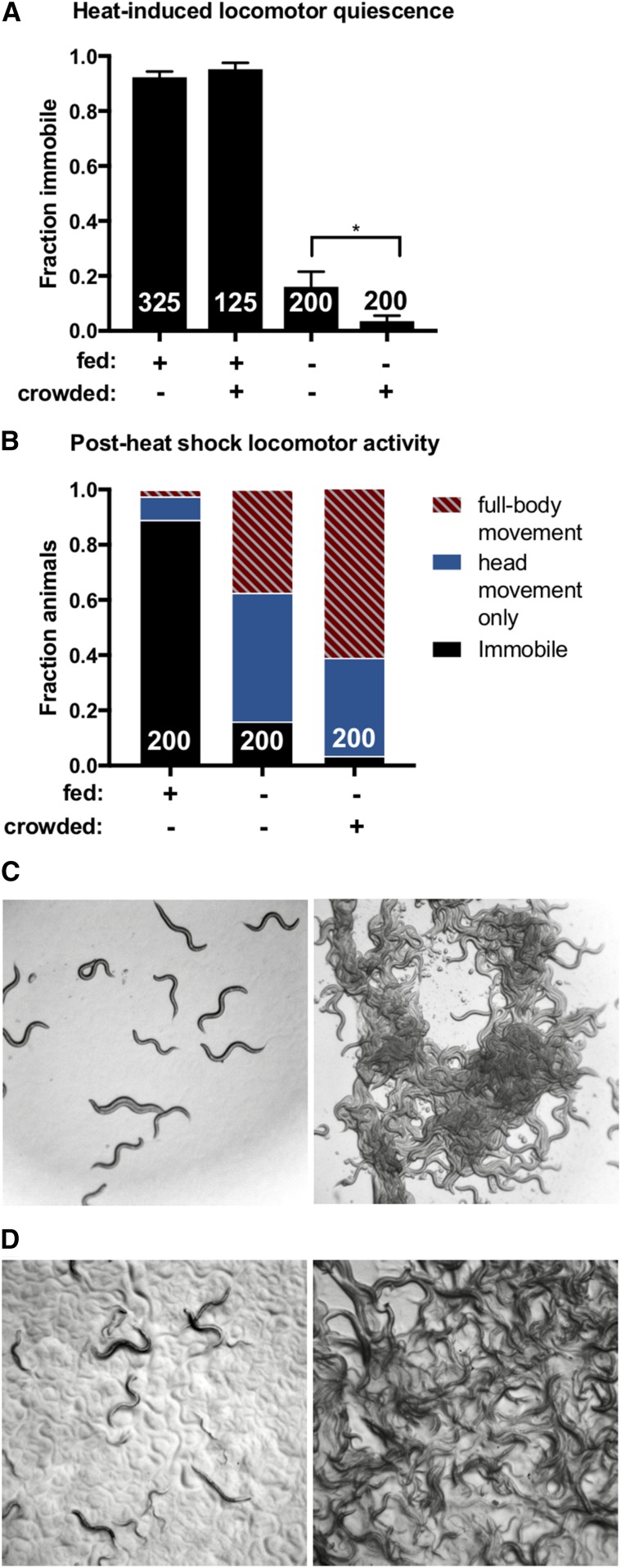
Increasing population density enhances starvation-induced sleep suppression. (A and B) Animals grown at high or low population density were heat shocked at 37° for 11 min and examined at low density for immobility as a measure of SIS. (A) Crowding enhances the inhibitory effect of FD on SIS, but has no impact when food is abundant. One-tailed Student’s t-test. The total number of animals examined is indicated at the bottom of each bar. Error bars represent SEM (25 animals per trial). * P = 0.027. (B) Nonquiescent, food-deprived animals grown at high population density show significantly more full-body activity compared to food-deprived animals grown at low population density. Two-way ANOVA with Sidak’s multiple comparisons test. The total number of animals examined is indicated at the bottom of each bar. P < 0.0001. (C) Representative images of food-deprived animals grown at low (left) or high (right) population density. (D) Representative images of well-fed animals grown at low (left) or high (right) population density. (See also Figures S2 and S3.)
Rate of body bending
The rate of body bending was obtained by counting the number of body bends completed by each individual animal for 1 min. A body bend was defined as the completion of a full sinusoidal curve in either the forward or backward direction. For body bending measured on food, plates were thinly seeded with OP50 in the afternoon of the day prior to testing. After being transferred to scoring plates, animals were allowed to settle for 5 min prior to the start of the assay. Crowding and FD were performed as described above.
Satiety quiescence and ALA ablation
Young adults were deprived of food for 18 hr, transferred singly to NGM plates seeded with high-quality food (HB101 E. coli), and allowed to refeed for 3 hr to induce satiety quiescence (You et al. 2008) prior to observation under a stereomicroscope. The duration of quiescence was defined as the time from identification of a quiescent animal until either locomotion or pharyngeal pumping was observed. For laser ablation, the ALA neuron cell body was identified in wild-type L2 animals by its position in the dorsal ganglion, as previously described (Van Buskirk and Sternberg 2007).
EGF overexpression
To induce EGF overexpression (EGF-OE), young adult hsp-16.41::lin-3c animals were transferred to standard (60 × 15 mm) NGM plates and subjected to a 33° heat shock for 10 min. This is a very mild heat exposure and does not induce a prolonged SIS response, but does trigger prolonged transgene-dependent (EGF-induced) sleep, allowing us to differentiate between SIS and EGF-induced sleep by examination of behavior 2 hr after heat shock. To examine the effects of FD, animals were deprived of food as described above and transferred to food 90 min after EGF-OE induction (30 min prior to examination of behavior). Per our standard assay procedure, plates were gently moved to a stereomicroscope stage 40 sec prior to examination of quiescence.
Survival
Well-fed and food-deprived animals were transferred to standard (60 × 15 mm) plates seeded with E. coli immediately prior to a severe heat shock of 40° for 20 min (Hill et al. 2014). Each trial contained 50–70 animals. Survival was scored for 4 days following heat shock. Animals were scored as dead if unresponsive after three prods: one to the tail, one to the midbody, and one to the head. Animals that crawled off the plate were censored on the day of their disappearance and were thus only included in statistical analysis until the day of the censoring event.
Rapamycin
Young adult animals were placed on NGM plates containing 200 µM rapamycin for 6 hr prior to testing for SIS. As a rapamycin stock solution of 200 mM in DMSO was used, control plates incorporated 0.1% DMSO.
RNA-mediated interference
RNA-mediated interference (RNAi) was performed by feeding using the strain VH624, which is generally RNAi hypersensitized and neuronally RNAi enhanced by lin-15B and nre-1 mutations, respectively. Two-generation RNAi was used to inactivate LET-363 and DAF-15. RNAi against LET-363 was performed by growing HT115 bacteria expressing let-363 double-stranded RNA (dsRNA) (Ahringer library) or an empty vector overnight at 37° in 25 µg/ml carbenicillin. The following day, the bacteria were seeded onto plates containing 1 mM IPTG and 25 µg/ml carbenicillin and left at 37° overnight. The next morning, after being cooled to room temperature, L4 animals were placed on the RNAi plates and incubated at 15° to lay eggs, as the VH624 strain is sterile above 15°. L1 animals were moved to fresh RNAi plates, shifted to 20° and allowed to grow to adulthood. To perform RNAi against DAF-15, HT115 bacteria expressing daf-15 dsRNA (gift of S. Mango) were grown overnight at 37° in 50 µg/ml kanamycin and seeded onto plates containing 1 mM IPTG and 50 µg/ml kanamycin. The remainder of the procedure was performed exactly as described above. As two-generation RNAi against PHA-4 resulted in sickly or arrested animals, one-generation RNAi was used to inactivate PHA-4. This was performed by growing HT115 bacteria expressing pha-4 dsRNA (Ahringer library) or an empty vector overnight at 37° in 25 µg/ml carbenicillin. The following day, the bacteria were seeded onto plates containing 1 mM IPTG and 25 µg/ml carbenicillin and left at 37° overnight. The next morning, after being cooled to room temperature, eggs were placed on the RNAi plates and placed at 20° to grow to adulthood. For RNAi experiments, pUC19 (single T7 promoter) or L4440 (double T7 promoter) were used as vector-only controls, which were found to behave similarly.
Strain construction
A daf-7(e1372);EGF-OE strain (CVB20) was generated by crossing PS5970 syIs197[hs:lin-3c, myo-2:dsRED], him-5 to CB1372 daf-7(e1372) and confirmed by the presence of dsRED along with the increased fat accumulation (Greer et al. 2008) and constitutive dauer formation phenotypes of daf-7 (Ren et al. 1996).
Quantification and statistical analysis
GraphPad Prism software was used to compile data and perform statistical analyses. The specific statistical tests used for each assay are listed in the corresponding figure legends.
Data availability
Requests for strains and reagents will be fulfilled by the lead contact, cheryl.vanbuskirk@csun.edu. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and supplemental material. Supplemental material includes Figures S1–S8 and the Reagent Table, available at Figshare: https://doi.org/10.25386/genetics.6513068.
Results
FD suppresses SIS
During our studies of C. elegans SIS, we noted that animals from food-depleted plates were SIS defective. To more precisely characterize the impact of FD on SIS, we deprived L4 animals of food for 24 hr and examined them for SIS at the young adult stage. We examined the behavior of all food-deprived animals only after the reintroduction of food, as this is known to rapidly suppress starvation-induced hyperactivity in C. elegans (Sawin et al. 2000). In these studies, SIS was quantified by examination of behavioral quiescence—defined as complete immobility and cessation of feeding—that is dependent on the function of the sleep-inducing ALA neuron. We chose to examine both heat-induced sleep (Hill et al. 2014) and UV light-induced sleep (Iannacone et al. 2017), and the time points chosen for examination of ALA-dependent quiescence following each type of stressor was determined by examination of time courses of wild-type and ALA-defective ceh-17 mutant animals (Figure S1, A–D). As we found that UV exposure causes significant ALA-independent suppression of pharyngeal pumping throughout the recovery time course (Figure S1D), only locomotor quiescence was examined in UV-induced sleep assays. We found that FD potently suppresses SIS following both heat and UV exposure (Figure 1, A–C), and does not cause hyperactivity in mock-treated animals (Figure S2). This indicates that, similar to Drosophila and mammals, the regulation of sleep and feeding in C. elegans is tightly linked.
As FD during the L4 stage is known to induce a variety of morphological changes and affect development, leading to adult reproductive diapause (Angelo and Van Gilst 2009), we reasoned that the life stage during which an animal experiences FD may be an important determinant of starvation-induced sleep suppression. To examine this possibility, we varied our FD protocol such that young adults, rather than L4 animals, were deprived of food for 24 hr and examined for SIS as day-old adults. We found that FD during adulthood similarly suppresses SIS (Figure 1D), suggesting that starvation-induced sleep suppression is not the result of developmental changes induced by FD during the L4 stage.
We speculated that the drive to forage may override the drive to sleep only when food is present. We therefore wished to examine whether the reintroduction of food is required for starvation-induced sleep suppression. To this end, food-deprived animals were heat shocked and examined for SIS in the absence of food. We found that even in the absence of food, food-deprived animals are severely impaired for SIS (Figure 1, A and B), indicating that starvation-induced sleep suppression does not require access to food.
We wished to determine if suppression of SIS by FD represents a delay rather than a loss of quiescence, an effect that might be visible at a later time point after animals are fed. To investigate this possibility, food-deprived animals were heat shocked immediately after their reintroduction to food and monitored for SIS for 5 hr. We found that well-fed animals experience an initial robust bout of SIS followed by a less penetrant bout peaking at ∼3 hr after the end of heat shock, whereas food-deprived animals lack the initial bout of SIS and show a second bout similar in magnitude to that of well-fed animals (Figure 1, E and F). Thus, food-deprived animals show a reduction, rather than a delay, of quiescence.
We next wished to determine where in the SIS pathway FD acts to suppress sleep. As ALA-dependent sleep can be triggered by overexpression of the EGF homolog LIN-3/EGF (Van Buskirk and Sternberg 2010; Cho and Sternberg 2014), we were able to investigate whether FD is likely to affect sleep upstream or downstream of ALA activation. In this assay, a lin-3c complementary DNA under the control of the hsp-16.41 promoter drives inducible EGF-OE in response to a mild heat exposure, triggering very brief, mild SIS followed by prolonged and robust EGF-induced sleep. We found that FD has no effect on EGF-induced sleep (Figure 1G), indicating that FD likely exerts its sleep-suppressing effect upstream of ALA neuron activation.
Increasing population density exacerbates starvation-induced sleep suppression
As access to limited resources quickly decreases as competition increases, we reasoned that the drive to sleep might be further suppressed by increasing population density. To test this hypothesis, food-deprived animals grown at either low or high population density were examined for SIS. We found that crowding enhances starvation-induced sleep suppression at the level of locomotor activity (Figure 2, A and B), but does not produce hyperactivity in mock-treated animals (Figure S2). Interestingly, pharyngeal pumping was not affected to the same extent as locomotion (Figure S3), suggesting that high population density during periods of FD (Figure 2C, right) may decrease competence to respond to a subset of the neuropeptides that function coordinately to effect SIS (Nath et al. 2016). To determine if crowding affects sleep drive in the absence of FD, animals grown at either high or low population density with abundant food were examined for SIS. Surprisingly, we found that even when grown at extremely high population density, well-fed animals exhibit normal SIS (Figure 2, A and D, right). This suggests that crowding inhibits sleep only when resources are limited.
DAF-7/TGF-β but not DAF-2/insulin signaling is required for SIS
We sought to determine the mechanisms responsible for the sleep-suppressing effects of FD and crowding. In C. elegans, the TGF-β and insulin signaling pathways play major roles in transducing environmental cues about food availability into metabolic and behavioral decisions (Fielenbach and Antebi 2008). Additionally, TGF-β signaling is indicative of low population density, with daf-7 expression reduced at high concentrations of secreted pheromones (Ren et al. 1996; Schackwitz et al. 1996). As both TGF-β and insulin signal food abundance, we reasoned that these signaling pathways may confer competence to engage in SIS when resources are ample, and that TGF-β may play an additional role in mediating the effects of population density. To test this, we examined SIS in animals with reduction-of-function (rf) mutations in daf-2, encoding the C. elegans insulin/IGF receptor (Kimura et al. 1997), and daf-7, encoding a secreted ligand in the TGF-β family (Ren et al. 1996). We found that mutations in daf-7, but not daf-2, interfere with SIS (Figure 3, A–C, and Figure S4, A–E); indicating that TGF-β, but not insulin signaling, is required. An SIS defect was also observed in animals harboring an rf mutation in the TGF-β type-1 coreceptor, encoded by daf-1 (Georgi et al. 1990) (Figure 3, A–C).
Figure 3.
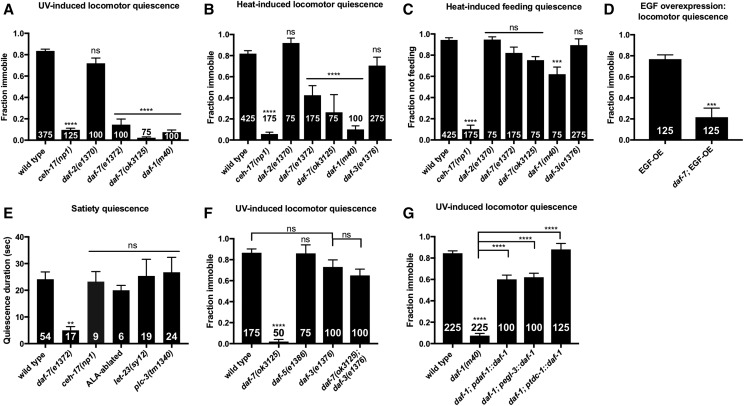
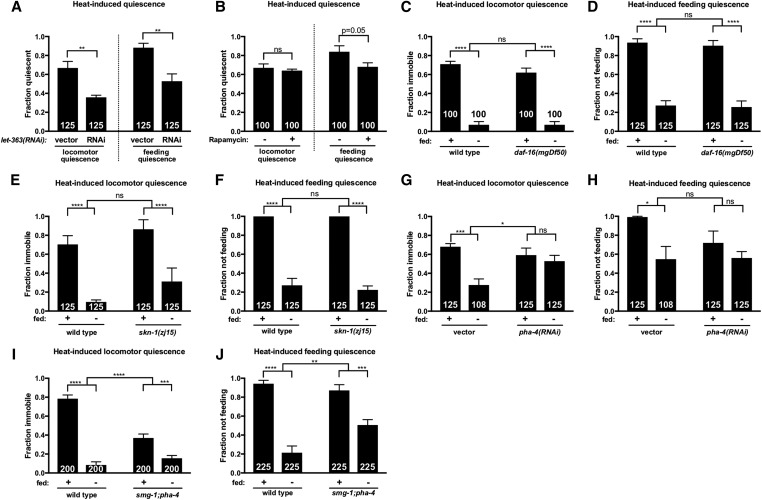
TGF-β signaling is required in the RIM/RIC interneurons to promote SIS. (A) daf-2(rf) mutants exhibit normal UV-induced sleep, while daf-7(rf) and daf-1(rf) mutants are severely impaired. (B) rf mutations in daf-2/Insulin signaling display wild-type, heat-induced locomotor quiescence while (rf) mutations in daf-7 and daf-1, components of TGF-β signaling, are severely impaired for locomotor quiescence during SIS. daf-3/Co-SMAD(rf) mutants do not show a significant SIS defect. (C) Only daf-1(rf) mutants show a significant defect in heat-induced feeding quiescence. (D) daf-7 is required downstream of ALA activation for EGF-induced sleep. Mild heat exposure (33° for 10 min) was used to induce EGF-OE and 2 hr later animals were examined for immobility as a measure of EGF-induced sleep. (E) Satiety quiescence is not mediated by components of the SIS pathway. Animals lacking a functional ALA neuron (ceh-17 mutants and ALA laser-ablated animals) show wild-type satiety quiescence, as do let-23/EGFR(rf) and plc-3/PLC-γ null mutant animals. (F) daf-3(e1376) fully suppresses the SIS defect of daf-7(ok3125). (G) daf-1 expression under the endogenous daf-1, pan-neuronal egl-3, and RIM/RIC-specific tdc-1 promoters rescues the SIS defect of daf-1(m40) mutants. All daf-c mutants and corresponding controls were raised at 15°. Cessation of locomotion and feeding were examined as measures of SIS after a 45 sec exposure to UV light or an 11 min, 37° heat shock as indicated above each graph. The total number of animals examined is indicated at the bottom of each bar. Error bars represent SEM [25 animals per trial with the exception of (F)]. One-way ANOVA with Dunnett’s (A–C and E), Tukey’s (F–G), or Student’s t-test (D). (See also Figure S4.) ** P ≤ 0.001, *** P ≤ 0.001, **** P ≤ 0.0001. ns, not significant vs. wild type (unless otherwise indicated by connecting bars on graph).
Interestingly, the disruption of SIS in daf-7 mutants is greater for locomotor quiescence (Figure 3, A and B, and Figure S3, A and B) than for feeding quiescence (Figure 3C and Figure S4C). This phenotype is distinct from the effect of FD and suggests that TGF-β signaling may act downstream of ALA activation. To determine whether DAF-7 acts upstream or downstream of ALA activation in the SIS pathway, we overexpressed LIN-3/EGF in daf-7 mutants. Consistent with a role for DAF-7 downstream of ALA activation, we found that daf-7(rf) mutants are impaired for EGF-induced quiescence (Figure 3, D and E). We noted, however, that when left completely undisturbed for an hour these animals did become quiescent (data not shown), an effect fully reversed by the minor perturbations caused by our standard scoring procedure. This suggests that daf-7(rf) greatly reduces the robustness of EGF-induced sleep. While a role for DAF-7 upstream of ALA activation cannot be ruled out, these data point to a role for DAF-7 in modulating SIS by acting on targets downstream of ALA activity.
TGF-β signaling is also required for satiety quiescence, a brief and easily perturbed quiescent state that is observed following fasting and refeeding on high-quality food (You et al. 2008). While satiety quiescence is phenotypically distinct from SIS, the requirement for TGF-β signaling in both processes led us to investigate whether other components of SIS are required for satiety quiescence. We examined ALA-defective ceh-17 mutants as well as ALA-ablated animals and found the ALA neuron to be dispensable for satiety quiescence (Figure 3F). Similarly, we found components of EGF signaling that are required for SIS to be dispensable for satiety quiescence (Figure 3F). Thus, while TGF-β signaling is required for both satiety quiescence and SIS, only the latter is mediated by EGF signaling in the sleep-inducing ALA neuron.
The DAF-7 signaling pathway in C. elegans differs from canonical TGF-β signaling in that it antagonizes, rather than activates, SMAD transcriptional activity (Patterson et al. 1997). For example, mutations in the co-SMAD DAF-3, which forms a complex with DAF-5/SNO-SKI (da Graca et al. 2004), are epistatic to the dauer-constitutive phenotype of daf-7 mutants (Patterson et al. 1997). We sought to determine whether DAF-7 promotes SIS by antagonizing SMAD transcriptional activity. If so, we expect daf-3(rf) and daf-5(rf) mutants to display wild-type SIS and for this phenotype to be epistatic to the sleep defect of daf-7 mutants. We examined daf-5 and daf-3 mutants for SIS and found that they sleep similarly to wild type (Figure 3, B and C). We then examined daf-3;daf-7 double mutants and found that they sleep similarly to daf-3 single mutants (Figure 3F), consistent with DAF-7 promoting SIS by antagonizing DAF-3 activity. As DAF-7-dependent regulation of several processes relies on repression of DAF-3 within the RIM/RIC interneurons (Greer et al. 2008), and signaling from these neurons appears to be activated by starvation (Alkema et al. 2005; Suo et al. 2006; Greer et al. 2008), we reasoned that SIS may require inactivation of RIM/RIC by TGF-β signaling. To test this hypothesis, we performed a site-of-action analysis of the TGF-β type-1 coreceptor DAF-1. We used multiple transgenic lines of daf-1(rf) to target expression of DAF-1 to all neurons or specifically to the RIM/RIC interneurons (Alkema et al. 2005; Greer et al. 2008). We found that DAF-1 expression exclusively in RIM/RIC is sufficient to rescue the daf-1 SIS defect (Figure 3G), indicating that DAF-1 is required in the RIM/RIC interneurons for SIS.
Interestingly, it has been found that tyraminergic and octopaminergic transmissions originating in RIM and RIC mediate the effects of TGF-β signaling on feeding rate (Greer et al. 2008), and octopamine has been shown to promote wakefulness in Drosophila (Crocker and Sehgal 2008). Synthesis of tyramine from tyrosine requires tyrosine decarboxylase (TDC-1), which is expressed in both RIM and RIC, and subsequent conversion of tyramine to octopamine requires tyramine β-hydroxylase (TBH-1), expressed exclusively in RIC (Alkema et al. 2005). To determine if tyraminergic and/or octopaminergic neurotransmission are required for the SIS defect observed in daf-7(rf) mutants, we examined daf-7(rf) animals lacking either TDC-1 or TBH-1 for SIS following exposure to UV light. We found that neither mutation could rescue the daf-7 SIS defect (Figure S4F), suggesting that other signaling molecules are released from RIM and RIC to inhibit competence to engage in SIS.
The inhibitory effects of FD and population density on SIS are not mediated by DAF-3/co-SMAD
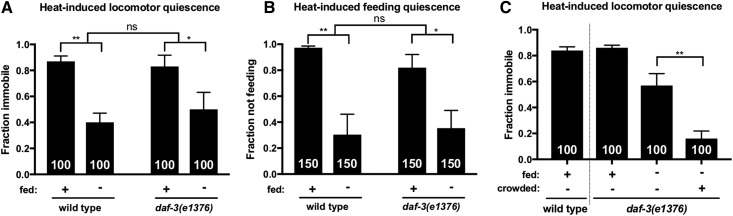
We have shown that TGF-β signaling in the RIM/RIC interneurons promotes SIS by antagonizing DAF-3 activity. If the sleep-suppressing effect of FD and crowding are mediated solely by modulation of TGF-β signaling, we expect daf-3(rf) mutants to be competent to sleep even under these conditions. We found, however, that FD suppresses sleep in daf-3(rf) animals as it does in wild-type animals (Figure 4, A and B), indicating that the sleep-suppressing effect of FD is not attributable to DAF-3 activation. To test whether DAF-3 activity is responsible for the enhancement of sleep suppression observed among food-deprived animals grown at high population density, we examined SIS in crowded and food-deprived daf-3(rf) mutants. We found that, similar to wild type (Figure 2A), crowding further suppressed sleep among food-deprived daf-3(rf) mutants (Figure 4C), indicating that DAF-3 activation is not responsible for the effect of crowding on SIS. To rule out the possibility that these results might be confounded by abnormal baseline locomotor activity, the rate of body bending was measured in daf-3(rf) animals in the absence of SIS. We found that daf-3(rf) mutants exhibit similar locomotor activity to wild-type animals in both the fed and food-deprived state (Figure S5). Together, these data indicate that the sleep-suppressing effects of FD and crowding are not mediated by DAF-3 activation. It is possible, however, that DAF-3 plays a role that is redundant with another SIS-suppressing pathway. In sum, while our data indicate that TGF-β signaling is required for SIS under well-fed conditions, our phenotypic observations and genetic analyses indicate that TGF-β signaling cannot be the only pathway linking food availability to sleep. We wished to examine other mechanisms that may inhibit SIS in response to FD.
Figure 4.
The sleep-suppressing effects of FD and population density are not attributable to DAF-3. Animals were heat shocked at 37° for 11 min and examined for SIS. FD inhibits heat-induced (A) locomotor and (B) feeding quiescence in daf-3(rf) mutants. (C) Crowding enhances the sleep-suppressing effect of FD in daf-3(rf) mutants, similar to wild type (Figure 2A). The total number of animals examined is indicated at the bottom of each bar. Error bars represent SEM (25 animals per trial). Two-way ANOVA with Sidak’s (A and B) or one-way ANOVA with Tukey’s (C) multiple comparisons test. (See also Figure S5.) * P < 0.05, ** P ≤ 0.01. ns, not significant vs. control (unless otherwise indicated by connecting bars on graph).
TOR signaling promotes SIS in the fed state
TOR signaling is active during periods of ample nutrient availability and has myriad effects on cell growth, metabolism, and stress-responsive transcription (Wullschleger et al. 2006). We speculated that TOR signaling may promote competence to engage in SIS under well-fed conditions. As mutations in the C. elegans TOR homolog let-363 lead to developmentally arrested animals (Long et al. 2002), RNAi was used to inactivate let-363. We found that let-363(RNAi) impaired SIS in the fed state (Figure 5A). This raises the possibility that repression of TOR signaling during periods of FD may play a role in sleep suppression. To further investigate the role of TOR signaling in SIS, we examined the effect of exposure to the TOR inhibitor rapamycin. Rapamycin has been shown to phenocopy the life-span extension (Robida-Stubbs et al. 2012) but not the developmental or intestinal phenotypes (Long et al. 2002) associated with loss of C. elegans TOR signaling. We found that acute exposure to rapamycin does not induce hyperactivity (Figure S6) but does partially phenocopy the sleep-suppressing effect of TOR-RNAi (Figure 5B). Together, these data support a role for TOR in promoting competence to engage in SIS.
Figure 5.
TOR signaling promotes SIS. Animals were heat shocked at 37° for 11 min and examined for locomotor and feeding quiescence (as indicated in each panel) as measures of SIS. (A) LET-363/TOR signaling promotes SIS under well-fed conditions. RNAi-sensitized animals were fed bacteria expressing let-363 dsRNA or an empty vector. (B) Exposure to 200 μM rapamycin for 6 hr impairs feeding quiescence. (C and D) FD suppresses SIS in daf-16 null mutants. (E and F) Animals carrying a hypomorphic mutation in skn-1 show wild-type suppression of sleep in response to FD. (G–J) PHA-4/FOXA is required for starvation-induced sleep suppression. (G and H) Starvation does not significantly suppress heat-induced (G) locomotor or (H) feeding quiescence in pha-4(RNAi) animals, although in the case of feeding quiescence this outcome is not significantly different from vector control (interaction P = 0.16). RNAi-sensitized animals were fed pha-4 dsRNA or an empty vector. (I and J) FD has a significantly reduced effect on heat-induced (I) locomotor and (J) feeding quiescence in pha-4/FOXA mutants. The total number of animals examined is indicated at the bottom of each bar. Error bars represent SEM [25 animals per trial with the exception of (G and H)]. Two-way ANOVA with Sidak’s multiple comparisons test. (See also Figures S6–S8.) *P<0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001. ns, not significant.
In mammals and C. elegans, the single TOR protein functions in two complexes, TORC1 and TORC2, defined by distinct proteins that make up each multimer (Wullschleger et al. 2006). C. elegans TORC1 is associated with DAF-15/Raptor while TORC2 is associated with RICT-1/Rictor, and the two protein complexes have distinct but overlapping functions (Lapierre and Hansen 2012; Robida-Stubbs et al. 2012). We examined the requirement for the TORC1 component DAF-15 by RNAi and found that interference with DAF-15 has no effect on SIS in the fed state (Figure S7A). We also examined RSKS-1/S6 kinase, a ribosomal protein known to be activated by TORC1 in mammals (Laplante and Sabatini 2012), and found that rsks-1(rf) mutants show no impairment for SIS (Figure S7, B and C). To investigate the role of TORC2 in promoting SIS, we examined animals with an rf mutation in the TORC2 component RICT-1 and found that, while they show wild-type locomotor quiescence (Figure S7B), they exhibit a mild but significant defect in heat-induced feeding quiescence (Figure S7C). Taken together with previous work indicating that TORC1 and TORC2 have overlapping functions in C. elegans (Robida-Stubbs et al. 2012), these data suggest that TORC1 and TORC2 play largely redundant roles in promoting SIS.
Under well-fed conditions, TOR signaling acts to inhibit the activity of several transcription factors including C. elegans DAF-16/FOXO, SKN-1/Nrf, and PHA-4/FOXA (Lapierre and Hansen 2012). We reasoned that one or more of these factors mediates starvation-induced sleep suppression. We found that FD suppresses SIS in both daf-16 null (Figure 5, C and D) and skn-1(rf) mutants (Figure 5, E and F), indicating that neither DAF-16 nor SKN-1 are required for starvation-induced sleep suppression. As complete loss of PHA-4 function results in embryonic arrest, we performed RNAi against pha-4 and found that pha-4(RNAi) reduces the sleep-suppressing effect of FD (Figure 5, G and H), an effect that is not attributable to decreased locomotor activity in food-deprived pha-4(RNAi) animals (Figure S8). To further examine the role of PHA-4, we examined smg-1;pha-4 animals, in which PHA-4 function is partially reduced (Gaudet and Mango 2002), and we observed a partial reduction in the inhibitory effect of FD on SIS (Figure 5, I and J). Interestingly, we noted that well-fed smg-1;pha-4 mutants exhibit reduced locomotor quiescence compared to wild type (P < 0.0001; Figure 5I), a phenotype not observed in pha-4(RNAi) animals (Figure 5G), opening the possibility that SMG-1 influences SIS. Together, our data suggest that TOR-mediated repression of FOXA activity plays a role in starvation-induced sleep suppression.
FD protects against the lethal effects of sleep loss
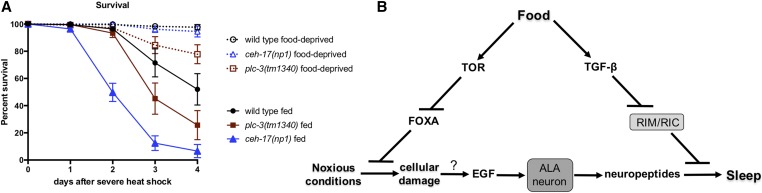
We wished to investigate the possibility that FD may inhibit SIS by reducing the need for it. As sleep need can be assessed by examining the lethal effects of sleep loss following severe damage (Hill et al. 2014), we examined whether FD could suppress the heat-induced lethality observed in SIS-defective mutants. To this end, we examined the effect of FD on the survival of ALA neuron-defective ceh-17 mutants as well as plc-3 mutants lacking an EGFR effector required for SIS (Hill et al. 2014). We found that FD significantly reduces the lethality associated with sleeplessness in both ceh-17 and plc-3 mutants (Figure 6A), suggesting that FD suppresses SIS at least in part by reducing the need for it. Further, the partial lethality seen in wild-type animals following heat shock is also suppressed by FD (Figure 6A), an observation consistent with previous studies showing that FD of C. elegans adults confers resistance to various stressors (Kaeberlein et al. 2006; Lee et al. 2006). These data indicate that FD suppresses SIS in part by reducing sleep need, possibly by mitigating the accrual of cellular damage (Figure 6B).
Figure 6.
FD reduces the need to sleep. (A) FD reduces the lethality associated with the SIS-defective ceh-17(np1) and plc-3(tm1340) mutations. Consistent with previous results (Hill et al. 2014), wild-type animals show a marked survival advantage over SIS-defective mutants (P < 0.0001, log-rank test) and here we find that food-deprived ceh-17 and plc-3 mutant animals show significantly greater survival than their fed counterparts (P < 0.0001, log-rank test). Percentage of animals surviving at daily intervals after a severe heat shock (40° for 20 min) are shown. Mean and ± SEM of a minimum of four trials, each containing at least 50 animals each, are shown. (B) Proposed model of starvation-induced plasticity in sleep drive. Under fed conditions, SIS requires DAF-7/TGF-β. In response to food scarcity, reduced DAF-7/TGF-β signaling allows activation of the RIM/RIC interneurons, potentially decreasing ALA target tissue competence to respond to sleep-promoting neuropeptides. Reduced nutrient availability and concomitant reduction of LET-363/TOR activity results in derepression of the PHA-4/FOXA transcription factor, leading to engagement of stress responses that potentially reduce the need to sleep. At this time the mechanism linking accrual of cellular damage to activation of EGF signaling in the ALA neuron is not known.
Discussion
Sleep occurs across the animal kingdom and is universally acknowledged to be beneficial, yet it interferes with essential behaviors such as foraging and reproduction, necessitating mechanisms that integrate and prioritize these competing behavioral drives. Indeed, FD has been observed to have a sleep-suppressing effect across phyla (Jacobs and McGinty 1971; MacFadyen et al. 1973; Rashotte et al. 1998; Roky et al. 2003; Keene et al. 2010). The nematode C. elegans has been found to enter a sleep state following exposure to noxious conditions (Hill et al. 2014; Nelson et al. 2014; Iannacone et al. 2017). This SIS is mediated by EGF signaling within the sleep-inducing ALA neuron (Hill et al. 2014), which in turn releases neuropeptides with distinct but overlapping effects on quiescent behavior (Nath et al. 2016). Using C. elegans SIS as a model, we investigated the effect of FD on sleep in young adult animals. We found that 24 hr of FD potently suppresses SIS and that the transcriptional regulator PHA-4/FOXA is required for this effect, demonstrating a mechanistic link between FD and sleep suppression.
The conserved nutrient sensing TOR pathway is a key regulator of growth and metabolism, with outputs including the stimulation of messenger RNA translation and the repression of transcriptional regulators including SKN-1/Nrf and the Forkhead box proteins DAF-16/FOXO and PHA-4/FOXA. While mutation of any of these factors interferes with the life-span extension normally associated with dietary restriction (Henderson et al. 2006; Bishop and Guarente 2007; Zhong et al. 2010), reduction of PHA-4 activity alone interferes with the suppression of SIS by FD. As DAF-16 and SKN-1 are also targets of insulin/IGF-1 signaling (IIS), our finding that these factors are not required for starvation-induced sleep suppression is consistent with our observation that a reduction in IIS has no detectable effect on SIS. The specific requirement for PHA-4 in this process suggests that sleep suppression is mediated by a subbattery of starvation-responsive transcription, affecting the accrual of cellular damage as well as the capacity to execute the SIS response. Genome-wide identification of starvation-enriched PHA-4 binding sites (Zhong et al. 2010) revealed components of autophagy and metabolism as well as signaling molecules such as nuclear hormone receptors and components of TGF-β and acetylcholine signaling. Our findings together with recent evidence that TOR affects C. elegans taste-associative learning (Sakai et al. 2017) point to a broad role for TOR signaling in food-dependent behavioral plasticity.
Previous studies demonstrating a connection between sleep and feeding have raised interesting questions about whether suppressing the drive to sleep also suppresses the need to sleep. For example, findings in Drosophila indicate that sleep loss due to fasting does not lead to the learning impairments that are normally associated with prolonged wakefulness (Thimgan et al. 2010), suggesting that fasting reduces sleep need. In this study, we provide evidence that fasting reduces the need for sleep that normally follows exposure to damaging conditions in C. elegans. We show that the loss of sleep in food-deprived animals is not compensated for upon refeeding and that FD eliminates the lethality seen among SIS-defective mutants following noxious heat exposure. We show that FD likely affects the SIS pathway upstream of ALA neuron activation, as would be expected for the modulation of sleep need. Given previous studies showing that FD of adult C. elegans confers stress resistance (Kaeberlein et al. 2006; Lee et al. 2006), it seems plausible that this reduced sleep need may be conferred by heightened cellular repair conferred by inactivation of TOR. An observation that appears to counter this notion is our finding that daf-2/InsR mutants show robust SIS yet are known to be thermotolerant (Lithgow et al. 1995) and UV resistant (Murakami and Johnson 1996). However, the thermotolerance conferred by adult FD has been shown to be greater than, and capable of enhancing, that of daf-2 mutants (Kaeberlein et al. 2006). Nonetheless, these data are consistent with the possibility that factors in addition to stress resistance contribute to the sleep-suppressing effect of FD. For example, certain PHA-4 targets may affect the capacity to execute the SIS response. In Drosophila, one such factor may be the DNA/RNA binding protein TRANSLIN, which was recently shown to be required for starvation-induced sleep suppression without concurrently altering stress responses or metabolic functions (Murakami et al. 2016).
Under conditions of limiting food and high population density, the RIM and RIC interneurons repress myriad processes including reproductive growth, feeding rate (Greer et al. 2008), and competence to engage in a satiety response (Gallagher et al. 2013). Under favorable conditions, DAF-7 secretion from the ASI sensory neurons is known to antagonize the activity of the RIM/RIC, thereby derepressing these potentially costly activities. Our data indicate that competence to engage in SIS falls under RIM/RIC repression, but that this does not account for all of the effects of FD. This suggests that either the role of TGF-β signaling in SIS is unrelated to food status, or, more likely, that FD affects SIS through both TGF-β and TOR/FOXA pathways. Our genetic analysis indicates that TGF-β signaling affects SIS downstream of ALA neuron activation, consistent with its differential impact on feeding and locomotor quiescence, subbehaviors regulated by distinct but overlapping sets of ALA neuropeptides (Nath et al. 2016). We propose that under adverse conditions, RIM and RIC activation limits the ability of target tissues to respond to sleep-inducing signals from the ALA neuron. This effect appears to be exacerbated by crowding, in particular at the level of locomotor activity. This suggests that crowding can affect a specific subbehavior of sleep and may promote foraging at greater distances when local population density is high. As population density is sensed via constitutively secreted pheromones in C. elegans (Golden and Riddle 1984a), this raises the possibility that pheromones may affect SIS.
Together our data reveal multilevel modulation of the SIS response by FD and provide insight into mechanistic links between sleep and feeding. While C. elegans SIS is not under circadian control, it does share molecular conservation with sleep in mammals, including the involvement of EGF and neuropeptide signaling. We can then conjecture that the accrual of sleep debt in higher organisms may be partially attributable to various cellular insults incurred in the waking state, and that the mechanisms underlying plasticity in sleep drive will also be deeply conserved.
Acknowledgments
The authors thank the laboratories of Kaveh Ashrafi, Dennis Kim, and Susan Mango for generously providing several of the strains used in this study. We are grateful to the students of the Fall 2015 BIOL447/L Full Immersion Research Experience laboratory for their investigation of sleep responses among dauer-defective mutants and to Andrew J. Hill for comments on the manuscript. This work was supported by an National Science Foundation Faculty Early Career Development Program (CAREER) award IOS#1553673 to C.V.B. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD-010440).
Author contributions: D.L.G. initiated the project, designed and performed experiments, performed statistical analyses, and prepared the manuscript. C.V.B. designed and performed experiments and edited the manuscript. R.S. performed experiments and contributed to manuscript editing.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6513068.
Communicating editor: M. Sundaram
Literature Cited
- Alkema M. J., Hunter-Ensor M., Ringstad N., Horvitz H. R., 2005. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46: 247–260. 10.1016/j.neuron.2005.02.024 [DOI] [PubMed] [Google Scholar]
- Allada R., Siegel J. M., 2008. Unearthing the phylogenetic roots of sleep. Curr. Biol. 18: R670–R679. 10.1016/j.cub.2008.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo G., Van Gilst M. R., 2009. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326: 954–958. 10.1126/science.1178343 [DOI] [PubMed] [Google Scholar]
- Bishop N. A., Guarente L., 2007. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447: 545–549. 10.1038/nature05904 [DOI] [PubMed] [Google Scholar]
- Campbell S. S., Tobler I., 1984. Animal sleep: a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8: 269–300. 10.1016/0149-7634(84)90054-X [DOI] [PubMed] [Google Scholar]
- Cho J. Y., Sternberg P. W., 2014. Multilevel modulation of a sensory motor circuit during C. elegans sleep and arousal. Cell 156: 249–260. 10.1016/j.cell.2013.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C., 2009. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat. Rev. Neurosci. 10: 549–560. 10.1038/nrn2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A., Sehgal A., 2008. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms. J. Neurosci. 28: 9377–9385. 10.1523/JNEUROSCI.3072-08a.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A., Sehgal A., 2010. Genetic analysis of sleep. Genes Dev. 24: 1220–1235. 10.1101/gad.1913110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Graca L. S., Zimmerman K. K., Mitchell M. C., Kozhan-Gorodetska M., Sekiewicz K., et al. , 2004. DAF-5 is a Ski oncoprotein homolog that functions in a neuronal TGF beta pathway to regulate C. elegans dauer development. Development 131: 435–446. 10.1242/dev.00922 [DOI] [PubMed] [Google Scholar]
- Fielenbach N., Antebi A., 2008. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 22: 2149–2165. 10.1101/gad.1701508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T., Kim J., Oldenbroek M., Kerr R., You Y.-J., 2013. ASI regulates satiety quiescence in C. elegans. J. Neurosci. 33: 9716–9724. 10.1523/JNEUROSCI.4493-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet J., Mango S. E., 2002. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science 295: 821–825. 10.1126/science.1065175 [DOI] [PubMed] [Google Scholar]
- Georgi L. L., Albert P. S., Riddle D. L., 1990. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell 61: 635–645. 10.1016/0092-8674(90)90475-T [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L., 1984a The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 102: 368–378. 10.1016/0012-1606(84)90201-X [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L., 1984b A pheromone-induced developmental switch in Caenorhabditis elegans: temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc. Natl. Acad. Sci. USA 81: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer E. R., Pérez C. L., Van Gilst M. R., Lee B. H., Ashrafi K., 2008. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 8: 118–131. 10.1016/j.cmet.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. T., Bonafè M., Johnson T. E., 2006. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J. Gerontol. A Biol. Sci. Med. Sci. 61: 444–460. [DOI] [PubMed] [Google Scholar]
- Hill A. J., Mansfield R., Lopez J. M. N. G., Raizen D. M., Van Buskirk C., 2014. Cellular stress induces a protective sleep-like state in C. elegans. Curr. Biol. 24: 2399–2405. 10.1016/j.cub.2014.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannacone M. J., Beets I., Lopes L. E., Churgin M. A., Fang-Yen C., et al. , 2017. The RFamide receptor DMSR-1 regulates stress-induced sleep in C. elegans. eLife 6: e19837 10.7554/eLife.19837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R. E., Li C., Leedale A. E., Shirras A. D., 2010. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc. Biol. Sci. 277: 65–70. 10.1098/rspb.2009.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B. L., McGinty D. J., 1971. Effects of food deprivation on sleep and wakefulness in the rat. Exp. Neurol. 30: 212–222. 10.1016/S0014-4886(71)80002-X [DOI] [PubMed] [Google Scholar]
- Kaeberlein T. L., Smith E. D., Tsuchiya M., Welton K. L., Thomas J. H., et al. , 2006. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 5: 487–494. 10.1111/j.1474-9726.2006.00238.x [DOI] [PubMed] [Google Scholar]
- Keene A. C., Duboue E. R., McDonald D. M., Dus M., Suh G. S. B., et al. , 2010. Clock and cycle limit starvation-induced sleep loss in drosophila. Curr. Biol. 20: 1209–1215. 10.1016/j.cub.2010.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G., 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Kramer A., Yang F. C., Snodgrass P., Li X., Scammell T. E., et al. , 2001. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294: 2511–2515. 10.1126/science.1067716 [DOI] [PubMed] [Google Scholar]
- Kushikata T., Fang J., Chen Z., Wang Y., Krueger J. M., 1998. Epidermal growth factor enhances spontaneous sleep in rabbits. Am. J. Physiol. 275: R509–R514. [DOI] [PubMed] [Google Scholar]
- Lapierre L. R., Hansen M., 2012. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol. Metab. 23: 637–644. 10.1016/j.tem.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D. M., 2012. mTOR signaling in growth control and disease. Cell 149: 274–293. 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. D., Wilson M. A., Zhu M., Wolkow C. A., De Cabo R., et al. , 2006. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell 5: 515–524. 10.1111/j.1474-9726.2006.00241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow G. J., White T. M., Melov S., Johnson T. E., 1995. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA 92: 7540–7544. 10.1073/pnas.92.16.7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X., Spycher C., Han Z. S., Rose A. M., Müller F., et al. , 2002. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr. Biol. 12: 1448–1461. 10.1016/S0960-9822(02)01091-6 [DOI] [PubMed] [Google Scholar]
- MacFadyen U. M., Oswald I., Lewis S. A., 1973. Starvation and human slow-wave sleep. J. Appl. Physiol. 35: 391–394. 10.1152/jappl.1973.35.3.391 [DOI] [PubMed] [Google Scholar]
- Murakami K., Yurgel M. E., Stahl B. A., Masek P., Mehta A., et al. , 2016. Translin is required for metabolic regulation of sleep. Curr. Biol. 26: 972–980. 10.1016/j.cub.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Johnson T. E., 1996. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics 143: 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R. D., Chow E. S., Wang H., Schwarz E. M., Sternberg P. W., 2016. C. elegans stress-induced sleep emerges from the collective action of multiple neuropeptides. Curr. Biol. 26: 2446–2455. 10.1016/j.cub.2016.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R. D., Bedbrook C. N., Abrams M. J., Basinger T., Bois J. S., et al. , 2017. The jellyfish Cassiopea exhibits a sleep-like state. Curr. Biol. 27: 2984–2990.e3. 10.1016/j.cub.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. D., Lee K. H., Churgin M. A., Hill A. J., Van Buskirk C., et al. , 2014. FMRFamide-like FLP-13 neuropeptides promote quiescence following heat stress in Caenorhabditis elegans. Curr. Biol. 24: 2406–2410. 10.1016/j.cub.2014.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson G. I., Koweek A., Wong A., Liu Y., Ruvkun G., 1997. The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev. 11: 2679–2690. 10.1101/gad.11.20.2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte M. E., Pastukhov I. F., Poliakov E. L., Henderson R. P., 1998. Vigilance states and body temperature during the circadian cycle in fed and fasted pigeons (Columba livia). Am. J. Physiol. Integr. Comp. Physiol. 275: R1690–R1702. 10.1152/ajpregu.1998.275.5.R1690 [DOI] [PubMed] [Google Scholar]
- Ren P., Lim C. S., Johnsen R., Albert P. S., Pilgrim D., et al. , 1996. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science 274: 1389–1391. 10.1126/science.274.5291.1389 [DOI] [PubMed] [Google Scholar]
- Robida-Stubbs S., Glover-Cutter K., Lamming D. W., Mizunuma M., Narasimhan S. D., et al. , 2012. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15: 713–724. 10.1016/j.cmet.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roky R., Chapotot F., Benchekroun M. T., Benaji B., Hakkou F., et al. , 2003. Daytime sleepiness during Ramadan intermittent fasting: polysomnographic and quantitative waking EEG study. J. Sleep Res. 12: 95–101. 10.1046/j.1365-2869.2003.00341.x [DOI] [PubMed] [Google Scholar]
- Sakai N., Ohno H., Tomioka M., Iino Y., 2017. The intestinal TORC2 signaling pathway contributes to associative learning in Caenorhabditis elegans. PLoS One 12: e0177900 10.1371/journal.pone.0177900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin E. R., Ranganathan R., Horvitz H. R., 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631. 10.1016/S0896-6273(00)81199-X [DOI] [PubMed] [Google Scholar]
- Schackwitz W. S., Inoue T., Thomas J. H., 1996. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17: 719–728. 10.1016/S0896-6273(00)80203-2 [DOI] [PubMed] [Google Scholar]
- Shukla C., Basheer R., 2016. Metabolic signals in sleep regulation: recent insights. Nat. Sci. Sleep 8: 9–20. 10.2147/NSS.S62365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo S., Kimura Y., Van Tol H. H. M., 2006. Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J. Neurosci. 26: 10082–10090. 10.1523/JNEUROSCI.0819-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimgan M. S., Suzuki Y., Seugnet L., Gottschalk L., Shaw P. J., 2010. The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 8: e1000466 10.1371/journal.pbio.1000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski N. F., Raizen D. M., 2016. Call it worm sleep. Trends Neurosci. 39: 54–62. 10.1016/j.tins.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk C., Sternberg P. W., 2007. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nat. Neurosci. 10: 1300–1307. 10.1038/nn1981 [DOI] [PubMed] [Google Scholar]
- Van Buskirk C., Sternberg P. W., 2010. Paired and LIM class homeodomain proteins coordinate differentiation of the C. elegans ALA neuron. Development 137: 2065–2074. 10.1242/dev.040881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M. N., 2006. TOR signaling in growth and metabolism. Cell 124: 471–484. 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- You Y. J., Kim J., Raizen D. M., Avery L., 2008. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 7: 249–257. 10.1016/j.cmet.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M., Niu W., Lu Z. J., Sarov M., Murray J. I., et al. , 2010. Genome-wide identification of binding sites defines distinct functions for Caenorhabditis elegans PHA-4/FOXA in development and environmental response. PLoS Genet. 6: e1000848 10.1371/journal.pgen.1000848 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for strains and reagents will be fulfilled by the lead contact, cheryl.vanbuskirk@csun.edu. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and supplemental material. Supplemental material includes Figures S1–S8 and the Reagent Table, available at Figshare: https://doi.org/10.25386/genetics.6513068.