Abstract
Human milk is considered to be the ideal food for infants. Accurate, representative, and up-to-date nutrient composition data of human milk are crucial for the management of infant feeding, assessment of infant and maternal nutritional needs, and as a guide for developing infant formula. Currently in the United States, the nutrient profiles of human milk can be found in the USDA National Nutrient Database for Standard Reference, and in books or review articles. Nonetheless, these resources all suffer major drawbacks, such as being outdated, incomplete profiles, limited sources of data, and uncertain data quality. Furthermore, no nutrient profile was developed specifically for the US population. The purposes of this review were to summarize the current knowledge of human milk nutrient composition from studies conducted in the United States and Canada, and to identify the knowledge gaps and research needs. The literature review was conducted to cover the years 1980–2017, and 28 research papers were found containing original data on macronutrients and micronutrients. Most of these 28 studies were published before 1990 and mainly examined samples from small groups of generally healthy lactating women. The experimental designs, including sampling, storage, and analytic methods, varied substantially between the different studies. Data of several components from these 28 studies showed some consistency for 1–6 mo postpartum, especially for protein, fat, lactose, energy, and certain minerals (e.g., calcium). The data for 7–12 mo postpartum and for other nutrients are very scarce. Comprehensive studies are required to provide current and complete nutrient information on human milk in the United States.
Keywords: human milk, composition, nutrient, database, USDA Food Data System
Introduction
Human milk is considered to be the ideal source of nutrition for infants. Given the documented short- and long-term medical and neurodevelopmental advantages of breastfeeding, the American Academy of Pediatrics affirmed its recommendation: “exclusive breastfeeding for about 6 months, followed by continued breastfeeding as complementary foods are introduced, with continuation of breastfeeding for 1 year or longer as mutually desired by mother and infant” (1). This recommendation was in agreement with the US Surgeon General's Call to Action, the Centers for Disease Control and Prevention, and The Joint Commission in the US (1), and was also supported by the WHO (2).
Breastfeeding confers unique nutritional and nonnutritional benefits to both the infant and the lactating mother (3). In addition to supporting normal growth and development, human milk offers numerous physiologic advantages through various functional components (4). Breastfeeding has also been associated with higher scores on tests of neurodevelopment and cognition in later life (5, 6).
Recently, the US Department of Health and Human Services Office of Disease Prevention and Health Promotion and the USDA Center for Nutrition Policy and Promotion initiated a project entitled “Evaluating the evidence base to support the inclusion of infants and children from birth to 24 months of age in the Dietary Guidelines for Americans—the B-24 Project.”
This project intended to respond to the challenge of developing and implementing US-driven, evidence-based health and nutrition promotion programs for this age group (7). Of the research priorities identified by the B-24 research committee, the first one is “Human milk composition: need for up-to-date analyses of human milk across populations including nutrients and bioactive components of human milk, and the factors influencing human milk composition” (7).
From a nutritional perspective, infancy is a critical and vulnerable period. Because of immaturity in tissues and organs involved in nutrient metabolism, infants display a narrow tolerance to deviations in nutrient intakes (8). Unlike commercial infant formula, which is standardized and tightly regulated, human milk is a very dynamic, constantly changing biological fluid. Therefore, accurate and up-to-date nutrient composition data and the factors that influence them are especially critical for the management of infant feeding and assessment of infant nutritional needs.
The composition of human milk changes constantly throughout the entire lactation period (2, 9). The colostrum and transitional milk in early lactation change rapidly and are distinct in many ways from mature milk. Mature milk remains relatively similar in composition with subtle changes over the course of lactation. The nutrient composition is also different between term and preterm human milk (10). Ethnicity, diet, and environment are known to be important factors influencing human milk composition. Some micronutrients can vary with nutritional status; and environmental toxins would differ based on the exposure of region-specific environmental chemicals. Therefore, understanding the human milk composition in one country or region, rather than combining international data, would be more relevant and provide useful information in assessing the infant and maternal nutritional need and supporting policy making of that specific country or region. For this current review, the efforts were restricted to term mature milk. The purposes of this review were to summarize the current knowledge of human milk nutrient composition studies conducted in the United States and Canada and to support an update to the USDA Food Data System for human milk with accurate, up-to-date, representative, and complete data gathered via valid analytic techniques. Although the actual data were not included, the studies on non-US/Canadian populations provided valuable information when discussing the influential factors and analytic methodology. This review is also intended to identify the research questions and provide information for developing a robust experimental design for the proposed sampling and analysis of human milk.
Current status
Currently in the United States, the nutrient profile of mature human milk can be found in the USDA National Nutrient Database for Standard Reference (SR) (11), and in the future, the USDA Food Data System, as well as in books and review articles (12–16). All these profiles share some major drawbacks, the first of which is their outdated sources. For instance, the nutrient profile in USDA SR was created in the 1970s, without being substantially updated since. Yet, it has still been widely used for various purposes and cited even in the most recently published books (17). In 1 book, the nutrient profiles of human milk were generated from data published between 1946 and 1980 (12). Second, the available data used to create the nutrient profiles came from very limited sources, or the sources were not clearly described (13). In some cases, values of certain nutrients came from only one or very few references (12). Third, there was no data quality evaluation. The data used to develop the aforementioned nutrient profiles were generated by different researchers, over a long period of time, for different populations and with different experimental designs and analytic methods. All of these factors would undoubtedly affect the quality of the data. Fourth, no statistical analyses of variability (e.g., mean, variance, and SD) were presented in any of the 5 profiles. Therefore, the sample variability could not be determined. Finally, none of the profiles were developed specifically for the US population. To create a US-specific human-milk nutrient profile, the data must be carefully examined to ensure they represent the US population.
Several excellent reviews have addressed human milk composition over the last 2 decades (3, 8–10, 13, 18–20). Nonetheless, very few of them summarized the traditional nutrient composition data and discussed the quality of these data. More recent data have focused mainly on so-called “bioactive components” and their potential health benefits.
Factors affecting data quality
Many factors need to be considered when discussing the quality of food composition databases. The USDA has maintained tables of food composition for >124 y (21). To ensure data quality, the USDA Nutrient Data Laboratory (NDL) has developed a comprehensive data quality evaluation system (22, 23). Five categories (sampling plan, number of samples, sample handling, analytic method, and analytic quality control) must be carefully assessed to ensure data quality for the food composition database development. Although these data quality criteria were initially developed for foods and food products, the same principles can be applied to determine the quality of human milk nutrient data. However, owing to the uniqueness of human milk, the 5 major categories are examined next specifically.
Sampling plan
One of the most important characteristics of a good food composition database is that the data effectively represent the foods consumed by the population that the database intends to cover. The sampling plan should be designed to fulfill this purpose. For designing a good sampling plan, the key is to understand the influential factors affecting the nutrient composition of a given food/food product. The composition of human milk is influenced by many factors. The main factors are discussed here to provide a basis for evaluating the existing data and designing appropriate sampling plans for future studies.
Maternal factors
Many maternal factors are known to influence human milk nutrient composition including stage of lactation, genetic background of the mother, parity, age, and health status.
Stage of lactation
Human lactation stage can be divided into 3 major phases: colostrum, transitional milk, and mature milk. Colostrum is rich in immunologic components and contains relatively low concentrations of lactose but higher protein content, suggesting its important functions to be immunologic and trophic besides nutritional (24). Transitional milk shares some of the characteristics of colostrum but represents a period of accelerated milk production to support the infant's nutritional and developmental needs for rapid growth. Mature milk is relatively similar to transitional milk, but the changes are not as remarkable as in the early weeks (9, 13). The influence of lactation stage differs for different nutrients. For example, total protein and lipids show a gradual decrease during the first 6 mo of lactation. Whereas the lactose is initially low in colostrum and transitional milk, it then increases in mature milk and remains at the same levels for up to 6 mo (25). In addition to the alterations of total protein, protein composition also changes. Milks mainly contain 2 types of protein: caseins and whey proteins. The ratio of whey to casein in human milk can vary from ∼80:20 in early lactation to ∼50:50 in late lactation (26). Because the amino acid compositions differ between caseins and whey proteins, the type and content of the amino acids of human milk in turn vary during lactation.
Maternal factors and infant status
Genetic factors of lactating women have been shown to influence human milk composition, which at least partly explains the considerable interindividual variations in milk composition. All physiologic and biochemical events that influence the composition of plasma may potentially affect the composition of milk. Hormones or other factors that are capable of influencing biosynthetic processes in the mammary gland can also modify the milk composition (14). For example, genetic variants of fatty acid desaturase were associated with variability of 20:4n–6, 20:5n–3, and DHA in human milk (27). Another good example is the array of human milk oligosaccharides (HMOs), which are believed to be genetically determined. Different profiles of HMOs occur as a result of specific transferase enzymes expressed in the mammary epithelial cells (18). For instance, human milk fucosylated oligosaccharide synthesis is controlled by the same fucosyltransferase genes (FUT2 and FUT3) that control secretor and Lewis blood group types (28). As few as 23 and as many as 130 different oligosaccharides were found in the milk from randomly selected mothers (29).
Infant need could be a driving force of the quantity and quality of human milk, as its composition changes in response to the infant's age and other characteristics. Human milk from women delivering prematurely supplies more protein and higher levels of many bioactive molecules compared with milk from women delivering at term (30, 31). Another important factor is the infant's gender. In a recent study, Fujita et al. (32) found that infant's gender and socioeconomic status interacted in their relation with milk fat concentration. In Northern Kenya, the economically sufficient mothers produced more milk fat for sons than for daughters (2.8 compared with 0.6 g/dL), whereas poor mothers produced more milk fat for daughters than for sons (2.6 compared with 2.3 g/dL). This finding was later confirmed by a study in other mammals, i.e., dairy cows (33). These studies may indicate a possible “programming” of mammary function by offspring in utero. However, how the programming is regulated, and how it affects human milk nutrient composition, are yet to be determined.
Milk volume
A key element defining lactation performance is the total amount of milk produced. The volume of milk transferred to the infant affects the infant's nutrient intake and the mother's nutrient requirements (34). The milk volume could also affect the milk composition. For example, milk protein concentration was negatively related to milk volume at 6 and 9 mo postpartum, whereas milk lactose concentration was positively related to milk volume at 6 and 9 mo postpartum (35). In another study, an association between weaning and significant changes in milk composition was observed. When milk volume fell below 300 mL/d, the protein and sodium increased whereas lactose, calcium, and zinc decreased (36).
Parity, age, and other characteristics
Human milk composition may be influenced by the parity and age of lactating women. Protein concentration was found to be the highest in the milk of mothers 20–30 y old (13). In a study to investigate the influence of diet and maternal parity on the fatty acid composition of mature milk in rural Gambian mothers, the authors found that the proportion of endogenous fatty acids was markedly reduced in the milk of mothers of very high parity. It was hypothesized that this represented an impairment of the ability to synthesize milk fatty acids de novo in these mothers (37). A more recent study investigated the effects of smoking, mother's age, BMI, and parity on the lipids and proteins of human milk. The study found that smoking was associated with lower milk lipid and protein concentrations, the increase of parity number led to an increase in lipid concentration, and the overweight mothers showed lower milk protein content (38). Carbohydrate content in mature milk was significantly higher in the older mothers group, and carbohydrates in mature milk correlated positively with maternal age (39).
Health conditions
The mother's health may also affect human milk composition, but this factor has not been well studied. One of the most common health problems for Americans is the prevalence of obesity (40). The prepregnancy obesity rate increased by an average of 0.5%/y, from 17.6% to 20.5%, in 2003–2009 (41). A recent study compared many nutritional components of human milk between obese and lean lactating women. The results showed that the composition of human milk from obese women differed in fatty acids, certain vitamins, and carotenoid composition from that of the lean women. Specifically, human milk from obese mothers contained lower DHA, vitamin D, and lutein + zeaxanthin content (42). Differences in the lipid composition of human milk have long been described in maternal diseases known to affect fat metabolism such as diabetes, cystic fibrosis, hypobetalipoproteinemia, and type I hyperlipoproteinemia (43). Other diseases, such as allergic disease, also alter fatty acid profiles and eicosanoids (44). Changes of fatty acid composition in human milk have also been observed in lactating mothers with cold-like syndrome (45). Cholesterol content is generally tightly regulated and is not affected by maternal diet. However, a 16-fold elevation was noted in milk of women with familial hypercholesterolemia (46).
Environmental factors
Environmental factors, especially maternal diet, play important roles in human milk nutrient composition. Other factors such as season, region, and socioeconomic status are suggested to be more or less related to maternal diet.
Maternal diet
Maternal diet has a profound influence on the composition of human milk for some nutrients, depending on the nutrition status of the lactating women (27, 47, 48). Maternal diet has been shown to have little effect on total protein, carbohydrates, and certain minerals, but affects fatty acids, certain vitamins, zinc, calcium, selenium, iodine, and fluorine (13, 48, 49). The maternal nutrition could have an impact on human milk composition through 3 aspects: current dietary intake, nutrient stores, and alterations in nutrient utilization. For instance, the nature of the fat consumed by the mother influences the fatty acid composition of milk (14, 50). The vitamin content of human milk is influenced by the mother's vitamin status. It was suggested that when maternal intakes of a vitamin are chronically low, its levels in human milk are also low (14). Some major minerals such as calcium, phosphorus, and magnesium are shown to be tightly regulated in maternal serum. However, the concentrations of minor minerals (e.g., iron and copper) in human milk may be influenced largely by maternal nutrition (51).
Nutritional requirements to support lactation are among the highest priorities in human development. According to the theory of systems biology, robustness is a ubiquitously observed property of biological systems and is considered to be a fundamental feature of complex evolvable systems. Robustness enables the system to maintain its functionalities against external and internal swings in concentration (52). The more important the function, the more robustly the system will ensure self-regulation. Genetic buffering is one of the fundamental mechanisms that provides robustness. The likely reason why many studies did not find maternal diet to influence human milk nutrient composition is probably this buffering mechanism. Although maternal intake influences the provision of nutrients to the nursing infant, maternal hormonal adjustments and nutritional stores serve to buffer daily fluctuations, so that excesses or moderate dietary deficiencies do not always alter nutrient transfer to the infant (46). When maternal nutrition is continuously compromised but the nutrient content and the milk volume remain unchanged, the nutrients could be synthesized from the maternal stores or body tissues (14). However, any buffer system has its own capacity and range. Beyond its capacity or range, the buffer system will lose its function to absorb perturbations. If severe malnutrition has persisted for some time, the ability of the lactating mother to maintain the macronutrient in the human milk will reduce or even collapse. Two possible reasons may lead to a buffering function reduction: 1) low dietary intake and depletion of nutrient stores, because the nutrients in human milk ultimately come from the mother's diet; and 2) malnutrition or disease conditions causing the mother's body to be less capable of managing the buffer system. More research is needed to fully understand the buffer system, how it is regulated, and, more importantly, to understand its capacity and range. There are also no studies specifically designed to evaluate the consequences when prenatal stores become depleted (2).
Another important aspect is the use of dietary supplements. Because nutrient requirements increase during periods of lactation, many clinicians recommend dietary supplements during these important periods of the life cycle (53). Data suggest that in the United States, most (73%) lactating women take a multivitamin preparation, with fewer reporting use of specific supplements of calcium (11%), folic acid (7%), or iron (4%) (54). Some supplements, such as DHA, showed a strong and dose-dependent increase in human milk (55). However, not all nutrients found in human milk respond positively to supplementation. For instance, a dietary folate intake of ∼380 µg/g did not appear to prevent mobilization of maternal folate stores during lactation (56).
Region, season, and other environmental factors
Some distinct regional differences are evident, particularly in concentrations of certain protein components, minerals, vitamins, and trace elements. Season could be an influential factor for human milk nutrient composition. The influence may be more or less related to maternal diet, breastfeeding behavior, and sun exposure (13). In a recent study also conducted in China, significant differences among 3 distinct regions were observed in regard to total MUFAs and PUFAs in human milk. Different dietary habits were indicated as the main drivers behind the different fatty acid profiles between the 3 regions (57). HMO concentrations and profiles have also been shown to vary geographically in healthy women, which may be determined by a combination of genetic, sociocultural, behavioral, and environmental factors (58).
Sample size
Assessment of the number of samples analyzed is critical in developing the nutrient composition database of a given food. The sample size should be large enough to cover major variations, such as geographic and demographic factors, to allow valid statistical analyses. The analysis of an inadequate number of independent samples limits the ability to estimate the mean and variability. On the other hand, analyses of too many samples (either individual or composite samples) will add substantial unnecessary cost. USDA NDL has extensive experience in handling mainstream food products (22, 59). However, human milk is very different from other foods, and very few studies have discussed appropriate sampling quantities. In a recent study to develop an online database for human milk composition in China, the number of samples was estimated based on the variation and SD of the studied nutrients (60).
Sample collection and handling
Human milk composition can vary during the day and from the beginning to the end of a feeding. This variation is most pronounced for zinc, fat, and fat-soluble components such as vitamin A (61, 62). Human milk nutrient concentrations are also influenced by handling and storage conditions, including also the freeze-thaw cycles and whether or not pasteurization is used (9). Therefore, to obtain high-quality data, sample handling methods must be carefully examined, validated, and standardized.
Sample collection methods
Certain milk nutrients are known to be altered by diurnal variation. A study of milk from 71 mothers over a 24-h period found that the milk fat content was significantly lower in night and morning feedings compared with afternoon or evening feedings (63). In another study, the energy concentration of human milk was measured for samples collected at 4 different time periods: 1) 0000–0600; 2) 0600–1200; 3) 1200–1800; and 4) 1800–0000. The variability of the energy concentration of milk throughout the day was estimated to be 14% at month 1 and 22% at month 4 (64). The stage of the nursing process is probably responsible for some of the largest variabilities in milk composition. For example, the fat content gradually increases from the beginning to the end of a feed, but there were no significant changes for either lactose or protein (65). The gold standard of milk collection involves multiple collections from the same individuals over a 24-h period (9). Alternatively, the milk can be collected at a specific time of day (e.g., morning) by emptying the entire breast. But collection should be avoided from a breast that was used for nursing within the past 2–3 h (9).
Sample handling and storage
In most published studies, especially those with large sample numbers or with samples collected far from the analytic facilities, the milk samples were stored, shipped, and in many cases involved in multiple freeze-thaw cycles for aliquots to analyze different nutrients. The samples could be taken by the collectors or lactating women themselves, and were temporarily stored under different conditions. If not handled properly, varying degrees of nutrient loss occur, depending on the nutrient and the storage methods. Products of lipolysis, including diacylglycerols, monoacylglycerols, and FFAs, were found in human milk unless new milk was extracted and analyzed immediately after sample collection (50). Storage temperature at –20°C is not low enough to preserve the integrity of the milk lipid, for it results in hydrolysis of TGs and the appearance of FFAs (66). The amounts of hydrolytic substances increase as storage lengthens at all temperatures except −70°C (67); lipases in milk are inactive at this temperature (50). Freeze-thaw cycles should be minimized because thawing and warming change the integrity of previously frozen human milk (68).
Many published studies on human milk composition used samples from a milk bank. The milk bank receives milk from the milk donors. After being collected, donated milk is pooled and pasteurized to prevent the risk of transmitting pathogens. A variety of protocols have been developed for pasteurization of donor milk (69). Heat treatment has been shown to reduce the concentration and functionality of certain nutrients, particularly functional proteins (70). Moreover, the variability is lost by pooling, and the process method may significantly alter the nutrient profile and their concentrations. Therefore, donor milk from a milk bank is not appropriate to be used to generate data for a human milk composition database.
Analytic methods
Valid and meticulously applied analytic methodology is critical for obtaining accurate nutrient data. In some cases, it is analytic inaccuracies, rather than true biological variances, that result in the large variations of many milk constituents. Therefore, the entire analytic procedure, from pretreatment to extraction, separation, analysis, and quantification, must be carefully validated for optimal results. The methods to be used should be determined by the chemo-physical properties of nutrients, how they present in human milk, and their concentrations.
Sample preparation and extraction
Before the nutrients can be analyzed, they must be extracted from the milk. Human milk is a heterogeneous mixture, which consists of true solutions, colloids, membranes, membrane-bound globules, live cells, etc. (46). For analysis of some nutrients, pretreatment is necessary to either separate them from similar components or release them from binding to other compounds. For instance, the Kjeldahl method is the most commonly used method to determine total nitrogen, which can then be used to calculate total protein by a conversion factor. However, in human milk, about 20–25% of total nitrogen is nonprotein nitrogen (NPN). For this reason, the classic Kjeldahl method will considerably overestimate milk protein. A more accurate approach known as “corrected Kjeldahl” is to first measure the total nitrogen, and then NPN after precipitating protein by trichloroacetic acid. The total protein nitrogen is obtained by subtracting the NPN value from the total nitrogen (71).
Another seldom-mentioned but important aspect is the sample aliquot. Jensen reported that sampling milk and obtaining an aliquot that contains the true amount of lipid is critical for measuring total lipid. The heterogeneously sized lipid globules, with a density of 0.9, rise at different rates and must be shaken at ≥38°C to achieve a random distribution (50).
Nutrient analysis
In general, more than one method can be used to analyze a given nutrient, and each has its own advantages and disadvantages. For developing values for a database, the method that provides the most accurate measurement of the nutrient in its original form in the milk is the most desirable. In addition, analytic methods evolve with the development of new analytic technology and instrumentation. For instance, methods used in the past for analysis of B-vitamins were microbiological, but in recent years, spectroscopic and chromatographic methods coupled with UV, fluorometric, or MS detection have been developed (72). The most recently developed method, which is ultra-performance liquid chromatography—tandem MS (UPLC-MS/MS), has offered improved resolution, speed, and sensitivity for analytic determinations of B-vitamins (73, 74). Another issue is that the analytic methods designed to study constituents in bovine milk or other biological fluids have been directly applied in the analysis of human milk, thereby providing inaccurate information (14). To obtain accurate results, the methods must be validated or modified for human milk (e.g., through the use of standards from human milk rather than from bovine milk). Analytic methods will be discussed in detail in the following sections.
Analytic quality control
Analytic quality control ensures accuracy and precision in the day-to-day performance of the analytic method. It also serves as the foundation to compare analytic data that were conducted at different dates, in different laboratories, or by different instruments. A certified reference material, such as the standard material from the National Institute of Standards and Technology (NIST), is preferred, when possible. Consensus or in-house material, when carefully characterized, can also be used (23).
Methods
Literature search
PubMed and Google Scholar were selected as primary search engines for the literature review. The following keywords were used in different combinations: breast milk, human milk, composition, nutrient, protein, amino acid, lipid, fatty acid, carbohydrate, lactose, oligosaccharide, vitamin, and mineral/element/electrolyte. The search was conducted for the period from 1 January 1980 to 31 December 2017. Reference lists of the retrieved research and review articles were reviewed to identify references not found using electronic search engines. Only data in original research articles were included; secondary data in review articles or books were excluded. The detailed inclusive criteria are listed as follows:
The subjects were stated as living in the United States or Canada, or there was clear indication that the subjects were in the United States or Canada (e.g., the protocol was approved by a US institutional review board). Data from Canada were included because of the similarity in terms of ethnicity, diet, and environment between the United States and Canada.
Information on lactation stage, sample collection, and storage was provided. Therefore, the data from pooled samples or milk banks could not be included.
Only the data on traditional nutrients were included. In addition, considering the current interest in oligosaccharides and their quantities in human milk, total HMO was also included. The nutrient list is presented in the USDA SR (11).
Only the data of milk for full-term infants were included. Full-term was determined by gestation (>37 wk) (10, 75) or specified in the references.
Only the data for mature milk in the first year of lactation were included. There is no consensus on defining milk as mature milk. In this review, the definition of the Institute of Medicine was adopted, in which milk after 3 wk or 21 d lactation is considered mature milk (14). Therefore, the data from the lactation stage of 21–365 d were included.
From the papers that aimed to study the factors or influential effects of certain treatments/supplements, only the data from control or placebo groups were included.
Data extraction
Data extracted from studies were converted to units of gram, milligram, or microgram per 100 g basis. The human milk specific gravity of 1.031 g/mL was used for the conversion (76). For protein content analysis using the Kjeldahl method, protein nitrogen was converted to total protein with 6.38 as the conversion factor (77, 78). When other conversion factors (i.e., generic Jones factor 6.25) were used to estimate the true protein content, the data were recalculated with 6.38 as the conversion factor. Fatty acids, presented as percentage of total lipid, were calculated to grams per 100 g human milk based on the value of the total lipid.
As mentioned earlier, human milk composition constantly changes over the entire lactation. Currently, only 1 nutrient profile for mature human milk is present in the USDA food composition databases and other sources (11–16). It does not reflect the variation of human milk composition during the entire lactation. On the other hand, it is not necessary to present the values for every week or month of postpartum. To comply with the recommendation of the American Academy of Pediatrics which states “exclusive breastfeeding for about 6 months, followed by continued breastfeeding as complementary foods are introduced, with continuation of breastfeeding for 1 year or longer,” it is reasonable to us that at least 2 nutrient profiles should be developed, representing 1–6 mo and 7–12 mo postpartum, respectively. Considering the recommendation “exclusive breastfeeding for about 6 months,” it is more critical to understand the nutrient profile of this period. The nutrient profile for 7–12 mo postpartum may be less critical because supplemental foods are typically added to the infant's diet during that time, although data for the nutrient profile of human milk for 7–12 mo postpartum are important for guiding the type and quantity of supplemental foods to be added.
For individual nutrients analyzed for milk from these 2 time periods, the data were pooled from different time points. Each nutrient value was calculated as a mean and the SD was calculated as a pooled SD via the following equation:
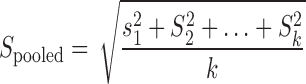 |
(1) |
A t test was run in Excel (Excel 2013, Microsoft) to calculate the P values of the 2 paired sample groups.
Results
Overview of the publications
After several sequential stages of screening, 52 papers were found that met all of the aforementioned criteria. Among them, data from 28 papers were extracted for this review (Table 1). The data in the other 24 papers were excluded after assessing the subjects and data quality, if ≥1 of the following issues were present:
The data were presented only in graph format with no numerical data included (79–82). Unless the raw data were obtained through personal communication, the data could not be extracted.
Data represented only a portion or were not complete for a given nutrient. For instance, only phospholipid and fatty acids of phospholipids were presented (83) and because they are only a part of total fat and total fatty acids, the data were not included. For protein, if only total nitrogen was measured, the data were not included because of the considerable amount of nonprotein nitrogen in human milk (84).
Subjects were following a special diet. For example, in a study to examine the effect of folate supplements on human milk folate, in addition to the treatment group receiving a folic acid supplement, all participants received a folate-free daily multivitamin and mineral supplement (85). However, those studies that include the special diet or dietary supplements into experimental designs were still included.
TABLE 1.
Summary of the studies that contained the original nutrient composition data for populations in the United States and Canada (1980–2017)1
| Protein | Fat | Carbohydrates | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Year | Subjects | Location | Lactation stage, d | Subject number | Sample collection | Storage | Total protein | Amino acids | Total fat | Fatty acids | Others | Lactose | HMO | Others | Vitamin | Mineral | Energy |
| Gross et al. (92) | 1980 | NA | USA | 21–28 | 18 | Morning | Lyophilized and stored –20°C | X | X | X | X | X | ||||||
| Clark et al. (88) | 1982 | NA | CT, MA | 42–112 | 10 | Morning and afternoon | Transport on dry ice, stored –70°C | X | X | X | ||||||||
| Garza et al. (125) | 1983 | NA | USA | 180 | 6 | Between 0800 and 1200 | Transport at 4°C, stored –20°C | X | X | X | X | |||||||
| Clark et al. (126) | 1983 | NA | USA | 42–112 | 10 | Morning and afternoon | Transport on dry ice, stored –70°C | X | X | |||||||||
| Feeley et al. (94) | 1983 | Caucasian, black, oriental | Athens, GA | 30–45 | 105 | Morning and afternoon | Stored –20°C | X | ||||||||||
| Feeley et al. (127) | 1983 | Caucasian, black, oriental | Athens, GA | 30–45 | 105 | Morning and afternoon | Stored –20°C | X | ||||||||||
| Dewey et al. (36) | 1984 | NA | Davis, CA and northern CA | 120–330 | 12 | 2nd feeding, morning | Stored –20°C | X | X | X | X | |||||||
| Butte et al. (90) | 1984 | NA | Houston, TX | 28–84 | 13 | Between 0800 and 1200 | Transport at 4°C, stored –20°C | X | X | X | X | |||||||
| Butte et al. (128) | 1984 | NA | Houston, TX | 30–120 | 41 | 24 h | Transport on ice, stored temp NA | X | X | X | X | |||||||
| Casey et al. (93) | 1985 | White | USA | 28 | 9 | Midfeed, midmorning | Stored –70°C | X | ||||||||||
| Janas and Picciano (129) | 1986 | NA | IL, USA | 28–56 | 10 | Foremilk and hind milk, morning | Stored –20°C | X | ||||||||||
| Garza et al. (64) | 1986 | NA | USA | 30–120 | 20 | 24 h | Transport on ice, stored temp NA | X | ||||||||||
| Janas et al. (89) | 1987 | NA | Urbana-Champaign, IL | 28–56 | 40 | Foremilk and hind milk, time NA | Freezing, temp NA | X | ||||||||||
| Ferris et al. (130) | 1988 | NA | USA | 42–112 | 12 | Morning and afternoon | Transport on dry ice, stored –70°C | X | X | X | ||||||||
| Dewey and Lonnerdal (91) | 1983 | NA | Davis, CA | 30–180 | 20 | 2nd feeding, morning | Freezing, temp NA | X | X | X | X | X | ||||||
| Casey et al. (87) | 1989 | Caucasian, 1 Native American, 1 Hispanic | Denver, CO | 4–360 | 22 | Midfeed, midmorning | Stored –70°C | X | ||||||||||
| Stuff and Nichols (131) | 1989 | NA | Houston, TX | 112–252 | 58 | 24 h | NA | X | X | |||||||||
| Butte et al. (111) | 1990 | NA | TX, USA | 30–120 | 20 | 24 h | Freezing, temp NA | X | X | X | X | |||||||
| Allen et al. (79) | 1991 | Caucasian | Denver, CO | 21–180 | 13 | Midmorning | Transport on ice, stored –70°C | X | X | |||||||||
| Nommsen et al. (35) | 1991 | NA | CA, USA | 90–360 | 21–58 | 24 h | Freezing, temp NA | X | X | X | X | |||||||
| Wack et al. (132) | 1997 | NA | USA | 0–360 | 30 | Between 1000 and 1400 | Stored –35°C | X | X | |||||||||
| Friel et al. (95) | 1999 | NA | Newfoundland, Canada | 7–84 | 17 | Between 1000 and 1400 | Transport in cooler, stored –20°C | X | ||||||||||
| Glew et al. (96) | 2008 | NA | NM, USA | 30–180 | 29 | Between 0700 and 1000 | Stored –70°C | X | X | |||||||||
| Hannan et al. (97) | 2009 | Mexican-American | Rio Grande Valley, south TX | 30–90 | 17 | NA | Stored –20°C | X | ||||||||||
| Tijerina-Saenz et al. (123) | 2009 | Caucasian | Vancouver, Canada | 30 | 60 | Hindmilk | Transport frozen, stored –80°C | X | X | |||||||||
| Glew et al. (122) | 2011 | Hispanic and non-Hispanic white | NMn, USA | 30–180 | 19 | Between 0830 and 1030 | Stored –70°C | X | X | |||||||||
| Alderete et al. (133) | 2015 | NA | OK, USA | 30–180 | 25 | Morning, 0800–1000 | Stored –80°C | X | ||||||||||
| Perrin et al. (98) | 2016 | NA | Raleigh, NC | 330–360 | 19 | 1st or 2nd feeding, morning | In subject's freezer until study completed, then –80°C | X | X | X | X | X | ||||||
1NA, not available; Ref, reference.
Overall, the number of studies on human milk has steadily increased. Nonetheless, the most recent studies on human milk composition have focused on nonnutritional bioactive components, including cells, antimicrobial factors, cytokines and anti-inflammatory agents, hormones, growth factors, prebiotics, and digestive enzymes (13, 86). Of the 28 studies whose data we included in this review, most were published in the 1980s or early 1990s, possibly due to the assumption that not much new research was needed for traditional nutrients (Figure 1). Even for these 28 studies, not 1 was designed to develop a dedicated nutrient database for human milk. These studies were primarily designed for various other purposes, such as the influential factors on human milk composition, comparison between term and preterm milk, impact of certain diet/supplements on milk nutrients, human milk and infant growth, or sampling/analytic method development. Therefore, the experimental design generally did not take into full consideration the factors discussed earlier, so some key information was missing or not presented in the papers.
FIGURE 1.
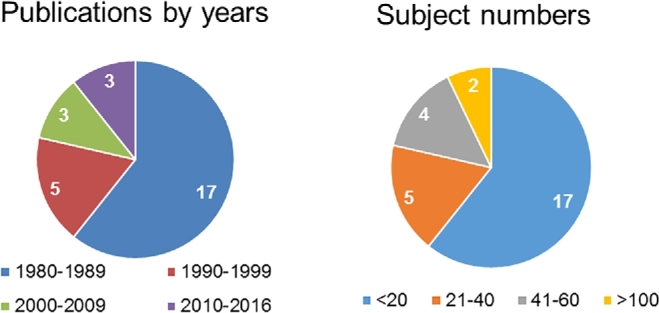
Summary of publication years and sample sizes of studies on human milk nutrient composition.
The number of the subjects in these 28 studies was generally small, with most of them <40. Only 2 papers (apparently from the same study) included >100 subjects (Figure 1). The ethnicity of the subjects was typically not mentioned. For those that were mentioned, the subjects were mostly Caucasian and generally healthy, hence the data from these 28 studies may not well represent the US population. However, in terms of geographic variation, the subjects came from diverse regions of the United States and Canada.
Discussion
Influential factors
Lactation stage is a key factor influencing human milk composition. Most of the data from these 28 studies reflected 4 mo postpartum (<120 d). Only 5 studies provided data for lactation at 7–12 mo (181–360 d).
Diurnal variation has been shown to dramatically influence human milk composition. In a recent study, circadian variation in concentration was significant for all vitamins, with riboflavin showing the greatest range (62–220%) from the daily median (73). Among these 28 studies, only 5 employed 24-h collections. The rest of the studies collected samples using different protocols: 1) sampling at the midmorning, midfeed from both breasts (87); 2) sampling at morning and afternoon from 1 breast (88); 3) sampling foremilk and/or hindmilk (89); 4) second feeding in the morning from 1 breast (36); and 5) sampling over a 2–4 h period ≥2 h after a feeding and emptying 1 breast (90). There are limited studies that indicate the methods which represent a 24-h mean or minimize the influence of diurnal variation, such as midmorning, midfeed from both breasts (81), and second feeding in the morning from 1 breast (91). However, these studies only analyzed and compared certain nutrients, not the complete nutrient profile. Again, the impact of diurnal variation on specific nutrients may be quite different. For instance, the highest correlation for energy between single samples and the 24-h value was from the samples collected between 0000 and 0600 (64). More studies are required to evaluate the influences of sampling time and to determine the best alternative sampling procedure should 24-h collections be unsuitable or not feasible.
Sample handling and storage is another important factor that may influence certain unstable or heat-sensitive nutrients but not others (e.g., minerals). In many studies, milk samples were collected by lactating women at their own homes; therefore, optimized transportation conditions of the sample shipment to the laboratory should be considered. Different transportation procedures were described in these 28 studies, including keeping the milk in ice, dry ice, a cooler, or at 4°C without further detail. Depending on the ways that the samples were transported, these different methods could lead to deterioration of certain nutrients. In terms of storage, −70°C storage is preferable, especially for the long term. However, milk samples were stored at −20°C in over half of the 28 studies. Interestingly, in 1 study, samples were lyophilized and stored at −20°C (92). There was no evaluation of how this method affected the nutrient composition of the human milk.
Method validation and quality control are keys to ensuring data accuracy. However, of the 28 studies, only 8 studies applied some kind of method validation or quality control procedures (79, 87, 93–98). Of these, 6 used standard reference materials (87, 93, 94, 96–98).
Nutrients
Protein
The protein content of mature human milk is ∼8–10 g/L (2). Several methods have been developed over the years to determine the total protein content of human milk and each may yield different results. Among them, amino acid analysis is the most accurate direct method for determining true protein content. However, this method is rather time consuming and costly, so it has not been routinely used to quantify total protein. In future, because the amino acid profile is an important part of the food nutrient profile, amino acid analysis is recommended as the standard method for quantifying total protein in human milk. Another direct method of analysis is the classic Kjeldahl method. This method measures total nitrogen and the total protein is further calculated by a conversion factor (77). Total protein can also be measured by indirect colorimetric assays, based on the proteins’ functional groups, such as the Biuret method (peptide bond), the bicinchoninic acid (BCA) method (dye-binding sites), and the Lowry method (tyrosine and phenylalanine content) (2). However, overestimation of ∼25–40% is possible if the human milk protein content is measured by colorimetry (99). The accuracy of indirect measurements depends on the standard, calibration of the individual machines, and the technique. The protein content of mature human milk was reported as ∼9 g/L by the Kjeldahl method, and 12–14 g/L by the Lowry method. In the Lowry method, bovine serum albumin is commonly used as the standard. However, BSA has fewer aromatic amino acids than human milk, which explains the 25% higher values (2).
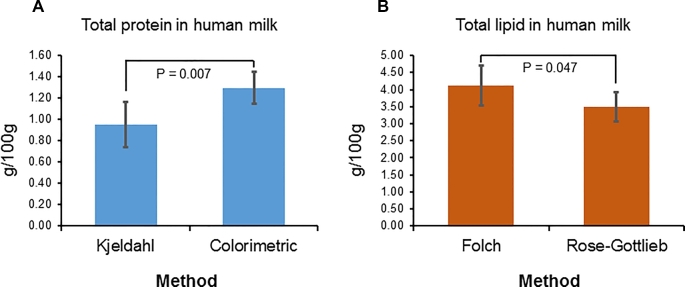
Of the 28 studies, 10 reported total protein or total protein nitrogen (Table 2). The total protein nitrogen was converted to total protein by the Jones factor of 6.38 for milk (77). Among these 10 studies, no study reported total protein by summing up total amino acids, 6 used the Kjeldahl method to calculate total protein nitrogen, and the other 4 studies used colorimetric assays. The protein contents from the Kjeldahl method, except for the data from 1 study (92), were generally lower than from colorimetric assays. Statistical analysis of the values from these 2 types of method across all studies indicated that the protein contents calculated by the Kjeldahl method were significantly lower (P = 0.007) (Figure 2A). These findings are in accordance with a previous report (14). Considering the potential drawbacks of colorimetric assays, and that the evidence showing the true protein contents determined by the “corrected Kjeldahl” method and by amino acid analysis has agreed closely (26), the “corrected Kjeldahl” method is accepted for database development should total amino acids analysis not be available.
TABLE 2.
Total protein contents in human milk for populations in the United States and Canada (1980–2017)1
| Protein | |||||
|---|---|---|---|---|---|
| 1–6 mo | 7–12 mo | ||||
| Ref | Mean | SD | Mean | SD | Method |
| Perrin et al. (98) | — | — | 1.55 | 0.19 | BCA |
| Dewey et al. (36) | 1.29 | 0.37 | 1.35 | 0.37 | Lowry assay |
| Nommsen et al. (35) | 1.14 | 0.15 | 1.16 | 0.16 | Lowry assay |
| Butte et al. (111) | 0.92 | 0.21 | — | — | Kjeldahl method for protein N |
| Stuff and Nichols (131) | 0.79 | 0.13 | 0.78 | 0.13 | Kjeldahl method for protein N |
| Gross et al. (92) | 1.40 | 0.18 | — | — | Kjeldahl method for protein N |
| Butte et al. (90) | 0.95 | 0.15 | — | — | Kjeldahl method for protein N |
| Dewey and Lonnerdal (91) | 1.28 | 0.22 | — | — | Dye-binding assay |
| Butte et al. (128) | 0.89 | 0.12 | — | — | Kjeldahl method for protein N |
| Garza et al. (125) | 0.82 | 0.04 | — | — | Kjeldahl method for protein N |
1BCA, bicinchoninic acid assay; Ref, reference.
FIGURE 2.
Comparisons between the methods for total protein (A) and total lipid (B) (data were expressed as means ± SDs).
Lipids
Lipids are the main source of energy in human milk, contributing 40–55% of the total energy depending on state of lactation. In addition, they provide essential nutrients such as PUFAs and lipid-soluble vitamins. Lipids are present as an emulsion in human milk. The vast majority of lipids are TGs, contributing about 98% of the lipid fraction, followed by phospholipids (0.8%), cholesterol (0.5%), and others. Lipid components are packaged into milk fat lipid globules, with the phospholipids forming the bulk of the membrane and the triacylglycerols found in the core (18, 25, 50).
The method most widely used is solvent extraction followed by gravimetry. Common methods for determining total lipid concentration include the Folch and Roese-Gottlieb methods, which both use solvent extraction followed by gravimetric determination. Folch originally employed 2:1 chloroform:methanol but later modified by substituting chloroform with dichloromethane for lower toxicity (50). The Roese-Gottlieb and its modified methods (e.g., Mojonnier method) use a hexane-ethyl ether-petroleum ether-ethyl alcohol-ammonia solvent system (100, 101). In general, solvent in the Folch method is more polar than that in the Roese-Gottlieb method, therefore total lipid measured by the Folch method tends to be higher because more polar lipids such as phospholipids can be recovered. The Creamatocrit procedure is a simple and quick method to measure lipid content of human milk (102). It was widely used for samples from field studies but is not accurate for database development. In addition, other methods such as turbidimetry, colorimetry, densitometry, and infrared spectrophotometry have also been used to measure total lipids. Nonetheless, owing to their inherent drawbacks, they are not commonly used at present (50, 101, 103). Finally, total lipid can be estimated by summing up individual fatty acids analyzed by GC or GC-MS, based on the fact that over 98% of milk fat is TGs. Again, this method is very time consuming, costly, and requires good chromatographic separation and standards to identify and quantify individual fatty acids. For this method to be most accurate, theoretically, a conversion factor needs to be determined in order to include the glycerol parts, which provides the added advantage of obtaining a fatty acid profile at the same time.
Fifteen studies contained total lipid data, of which Folch or Roese-Gottlieb–based methods were found to be the predominant methods, with each being used in 6 studies (Table 3). Surprisingly, the degree of fluctuation of total lipid was quite consistent across different studies, methods, and lactation stages. The CV was 15.2% for lactation 1–6 mo and 5.9% for 7–12 mo. In accordance with the previous discussion, total lipids measured by the Folch method usually showed significantly higher values than the Roese-Gottlieb methods (P = 0.047) (Figure 2B). However, in some cases, the Folch method gave low values (3.27 ± 2.27 g/100 g) while the Roese-Gottlieb method yielded higher values (4.26 ± 1.14 g/100 g). In addition to the possible variations due to differences in samples and solvents, detailed experimental procedures, such as solvent to sample ratio, extraction times, and separation between lipid phase and aqueous phase, could also contribute to the difference. Based on the foregoing discussion, the Folch or Roese-Gottlieb–based methods are easy to conduct and could generate agreeable data. However, in the future, analyzing fatty acids could provide a good estimate of total lipids. Whereas total lipids remain stable, the fatty acid profile of human milk may vary in relation to maternal diet and health conditions. For instance, with long-chain PUFA intake in the Western world shifting toward ω-6 fatty acids, the DHA composition of human milk is particularly low in North American populations (9). The human milk inflammatory factors and fatty acid composition might be related. A possible association was observed between TGF-β2 and the fatty acids in human milk (44).
TABLE 3.
Total lipid contents in human milk for populations in the United States and Canada (1980–2017)
| Lipid | |||||
|---|---|---|---|---|---|
| 1–6 mo | 7–12 mo | ||||
| Ref1 | Mean | SD | Mean | SD | Method |
| Clark et al. (88) | 4.49 | 0.53 | — | — | Folch procedure |
| Perrin et al. (98) | — | — | 3.83 | 1.94 | Moisture/fat analyzer |
| Dewey et al. (36) | 4.09 | 2.02 | 3.63 | 2.21 | Colorimetric |
| Nommsen et al. (35) | 3.58 | 0.82 | 3.65 | 0.95 | Folch procedure |
| Butte et al. (111) | 3.08 | 0.81 | — | — | Roese-Gottlieb mixed ethers |
| Stuff and Nichols (131) | 3.04 | 0.83 | 3.32 | 1.15 | Roese-Gottlieb mixed ethers |
| Gross et al. (92) | 3.83 | 1.37 | — | — | Roese-Gottlieb mixed ethers |
| Butte et al. (90) | 4.26 | 1.14 | — | — | Roese-Gottlieb mixed ethers |
| Ferris et al. (130) | 4.78 | 1.11 | — | — | Folch procedure |
| Dewey and Lonnerdal (91) | 4.42 | 1.53 | — | — | Colorimetric |
| Butte et al. (128) | 3.44 | 0.84 | — | — | Roese-Gottlieb mixed ethers |
| Garza et al. (125) | 3.49 | 0.78 | — | — | Roese-Gottlieb mixed ethers |
| Clark et al. (126) | 4.49 | 0.55 | — | — | Folch procedure |
| Glew et al. (96) | 3.27 | 2.27 | — | — | Folch procedure |
| Glew et al. (122) | 4.53 | 1.84 | — | — | Folch procedure |
1Ref, reference.
Carbohydrates
The principal carbohydrate of human milk is lactose, a β-disaccharide consisting of glucose and galactose. Of all mammals, lactose is present in the highest concentration in the milk of humans, corresponding to the high energy demands of the human brain (18). In addition, human milk is comprised of a complex mixture of HMOs that differ in size, charge, and abundance. In mature milk, the concentration of HMOs was found to be 5–20 g/L (104). HMOs are composed of both neutral and anionic species with building blocks of 5 monosaccharides: D-glucose, D-galactose, N-acetylglucosamine, L-fucose, and N-acetylneuraminic acid. The basic structure of HMOs includes a lactose core at the reducing end and they are elongated by N-acetyllactosamine units, with greater structural diversity provided by extensive fucosylation and/or sialylation wherein fucose and sialic acid residues are added at the terminal positions. Milks from randomly selected mothers contain 23–130 different HMOs (29). Although research on HMOs started in the early 1900s on the function of HMOs for breastfed neonates related to their high quantity and diversity, their indigestibility has only been revealed since the 1980s (29, 105). HMOs have now been widely recognized as prebiotics—indigestible carbohydrates that stimulate the colonization of beneficial gut microflora (86). Most recent studies have suggested that their functions may go beyond prebiotic effects, including acting as signaling molecules, immune-modulators, and possible brain function developers (106).
As for other macronutrients, various methods based on different analytic principles were developed to analyze lactose in milk or milk products (107). Among them, the most commonly used one is based on the enzyme hydrolysis of lactose. The lactose is hydrolyzed by β-galactosidase or acid/base into glucose and galactose, then the glucose or galactose is quantified colorimetrically by reacting with an enzyme (e.g., glucose oxidase) and a color reagent (108–110). The assay can be performed manually or by a more automated biochemistry analyzer (111). However, chromatography-based methods, especially HPLC and LC-MS, are superior to enzymatic methods in many different ways. First, no reactions are involved and thus possible artificial products are not produced. Second, they are direct methods that can analyze different mono- and disaccharides and quantify them with a single run. Third, a much smaller sample size is required for analysis, because usually a milliliter-level quantity is enough. In recent years, mid-infrared spectroscopy has been used to analyze macronutrients in human milk (112–114). Although shown to be a quick alternative technology with acceptable accuracy in field studies (115), it behaved poorly for quantifying lactose because measurement is confounded by the presence of oligosaccharides (113).
Tremendous efforts have been made in recent years to understand the compositional profile of HMOs in human milk (116). However, in regard to quantifying total HMOs, there is still no consensus method available. Accurate quantification of individual and total HMOs remains a challenge (117). Traditionally, quantification of HMOs has involved multiple steps. The oligosaccharide fraction of milk is usually obtained by precipitating protein and then the HMOs are separated from the lactose. The weight of the oligosaccharide fraction could be used as a rough estimate of total HMOs. Analysis and quantification of individual HMOs are accomplished by chromatographic technology such as HPLC and high pH anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and, more recently, mass spectrometric technology (118).
Eight studies provided data for lactose, of which 7 were for the lactation stage 1–6 mo (Table 4). Except for 1 recent study using HPLC-MS (98), all other papers used enzymatic methods. The data from 7 studies for the lactation stage 1–6 mo yielded quite consistent data, with the CV only 5.6%. This is in accordance with the previous finding that the concentration of lactose in human milk is the least variable of the macronutrients (35). The reason is probably due to the critical role lactose plays in addition to being a major source of energy. Lactose exerts 60–70% of the total osmotic pressure of human milk. Compared with glucose, lactose provides nearly twice the energy value per molecule (14). Notably, the lactose analyzed by LC-MS yielded a much lower value compared with enzymatic methods. However, because the result is from only 1 study, no statistical analysis could be done, nor do we know the cause for the variability. More studies are needed to compare and evaluate the 2 types of methods. Despite the extensive research on HMOs in recent years, only 2 studies reported total HMOs for the US population.
TABLE 4.
Lactose contents in human milk for populations in the United States and Canada (1980–2017)
| Lactose | |||||
|---|---|---|---|---|---|
| 1–6 mo | 7–12 mo | ||||
| Ref1 | Mean | SD | Mean | SD | Method |
| Perrin et al. (98) | — | — | 5.67 | 0.73 | LC-MS |
| Dewey et al. (36) | 7.56 | 0.29 | 7.49 | 0.51 | Enzymatic colorimetric (Dahlqvist) |
| Nommsen et al. (35) | 7.22 | 0.17 | 7.15 | 0.27 | Enzymatic colorimetric (Dahlqvist) |
| Butte et al. (111) | 6.51 | 0.20 | — | — | Colorimetric (Biochemistry analyzer) |
| Gross et al. (92) | 6.97 | 0.61 | — | — | Enzymatic colorimetric |
| Ferris et al. (130) | 6.67 | 0.67 | — | — | Enzymatic colorimetric |
| Dewey and Lonnerdal (91) | 7.17 | 0.51 | — | — | Enzymatic colorimetric |
| Butte et al. (128) | 6.59 | 0.24 | — | — | Colorimetric (Biochemistry analyzer) |
1Ref, reference.
Energy
Energy in human milk can either be measured by bomb calorimetry, or be calculated from proximate composition (protein, fat, and carbohydrate) by applying energy conversion factors (119). Bomb calorimetry directly measures energy and thus is considered an accurate method. However, a lot of researchers still use calculated methods owing to their simplicity and generally satisfactory results. The general energy conversion factor was originally developed by WO Atwater and his colleagues at USDA (120), and it is recognized as the Atwater general factor. It uses a single factor for each of the energy-yielding substances, with the values 4, 9, and 4 kcal/g for protein, fat, and carbohydrate, respectively (120). After the Atwater general factor was developed, a more extensive general factor system was developed for specific foods. Over the years, different researchers have used slightly different energy conversion factors to calculate energy in human milk, which obviously affected the values obtained. They are superior to the original Atwater factors because they take into consideration the differences of different energy sources (77), although there is still no consensus on which energy conversion factors should be used. The UN’s FAO published energy conversion factors for milk and milk products as 4.27, 8.79, and 3.87 kcal/g for protein, fat, and carbohydrate, respectively (77). A comparison between measured and calculated energy contents of milk was conducted in a recent review, which showed measured values were –6 to 10 kcal/dL (–9 to 13%) greater than the calculated values (10).
Nine studies provided data on energy of human milk (Table 5). Of them, 5 used bomb calorimetry and 4 used the calculated method. Interestingly, 4 different energy conversion factors were used in the calculations, including general Atwater factors and 3 food-specific modified Atwater factors. Despite this difference, the values were actually quite consistent, with the CV only 8.6%.
TABLE 5.
Energy in human milk for populations in the United States and Canada (1980–2017)
| Energy | |||||
|---|---|---|---|---|---|
| 1–6 mo | 7–12 mo | ||||
| Ref1 | Mean | SD | Mean | SD | Method |
| Nommsen et al. (35) | 68.09 | 7.81 | 68.62 | 9.09 | Calculated 5.65, 3.95, 9.25 kcal/g |
| Butte et al. (111) | 64.00 | 7.07 | — | — | Bomb calorimeter |
| Gross et al. (92) | 67.07 | 11.74 | — | — | Calculated 4.27, 3.87, 8.87 kcal/g |
| Butte et al. (90) | 65.62 | 9.89 | — | — | Bomb calorimeter |
| Ferris et al. (130) | 75.43 | 10.71 | — | — | Calculated 4.27, 4.27, 8.87 kcal/g |
| Garza and Butte (64)2 | 70.47 | 9.35 | — | — | Bomb calorimeter |
| Dewey and Lonnerdal (91) | 73.59 | 14.04 | — | — | Calculated 4, 4, 9 kcal/g |
| Butte et al. (128) | 64.50 | 8.79 | — | — | Bomb calorimeter |
| Garza et al. (125) | 55.87 | 3.98 | — | — | Bomb calorimeter |
1Ref, reference.
224-h collection.
Minerals
Minerals in human milk can be divided into 3 major categories: 1) major minerals, such as calcium, phosphorus, and magnesium, which are tightly regulated in maternal serum; 2) electrolytes, including sodium, potassium, and chloride, which in milk are determined by an electrical potential gradient in the secretory cell; and 3) trace elements, such as iron, copper, and zinc, which exist at very low concentrations in human milk and may be influenced to a large extent by maternal diet (14).
When analyzing minerals, electrolytes, and trace elements in human milk, it is important to remember that the samples must be collected in acid-washed, mineral-free containers to eliminate contamination. Whole milk (not defatted or deproteinized milk) should be used because considerable amounts of certain minerals may be present in these removed fractions (109). Analytic methods for measuring minerals in milk and milk products were reviewed in a recent paper (121). Historically, a number of techniques were used to determine minerals and elements in milk, including colorimetry, titrimetry, and ion-selective electrodes. However, they have been largely replaced since the 1980s with atomic absorption spectrometry (AAS) and atomic emission spectrometry (AES) with proper digestion procedures. AAS was firstly introduced with a flame atomization source (FAAS) and later a graphite atomizer. The AES technique can be used either as flame emission spectrometry or as inductively coupled plasma-atomic emission spectrometry (ICP). ICP coupled with a mass spectrometer (ICP-MS) after high-pressure digestion is currently the best technology for mineral analysis (121).
Data from 14 studies are summarized in Tables 6–8. Again, most of the data were generated for 1–6 mo postpartum. The majority of papers used either AAS or AES, and only 3 early papers used colorimetry or ion-selective electrodes. Calcium, magnesium, sodium, potassium, zinc, copper, and iron are among the most measured minerals; each was presented in >3 studies. In general, the levels for major minerals from different times, researchers, and by different analytic methods are fairly consistent. For instance, the CV of calcium for 1–6 mo is 9.1%, and for 7–12 mo, 8.8%.
TABLE 6.
Major minerals in human milk for populations in the United States and Canada (1980–2017)1
| Calcium | Magnesium | Phosphorus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–6 mo | 7–12 mo | 1–6 mo | 7–12 mo | 1–6 mo | |||||||
| Ref | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Method |
| Allen et al. (79) | 28.07 | 2.01 | 22.95 | 3.19 | 3.87 | 0.24 | 3.98 | 0.48 | — | — | Ion-selective electrodes and colorimetry |
| Perrin et al. (98) | — | — | 19.40 | 2.63 | — | — | — | — | — | — | ICP-MS |
| Dewey et al. (36) | 22.94 | 4.01 | 22.36 | 4.27 | 3.12 | 0.56 | 3.06 | 0.53 | — | — | AAS and AES |
| Gross et al. (92) | 24.98 | 5.98 | — | — | 2.62 | 0.70 | — | — | 15.08 | 3.71 | AAS and colorimetric |
| Butte et al. (90) | 25.38 | 3.16 | — | — | 3.46 | 0.83 | — | — | 14.44 | 2.75 | AAS and colorimetric |
| Dewey and Lonnerdal (91) | 25.30 | 4.54 | — | — | 3.17 | 0.55 | — | — | — | — | AAS |
| Garza et al. (125) | 21.34 | 1.16 | — | — | — | — | — | — | — | — | AAS |
| Friel et al. (95) | 27.96 | 3.69 | — | — | 2.91 | 0.51 | — | — | — | — | ICP-MS |
| Feeley et al. (127) | 26.20 | 6.14 | — | — | 5.00 | 1.26 | — | — | 13.30 | 3.76 | ICP |
1AAS, atomic absorption spectrometry; AES, atomic emission spectrometry; ICP, inductively coupled plasma-atomic emission spectrometry; Ref, reference.
TABLE 8.
Trace minerals in human milk for populations in the United States and Canada (1980–2017)1
| Zinc | Copper | Iron | Selenium μg/100 g | Iodine μg/100 g | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–6 mo | 7–12 mo | 1–6 mo | 7–12 mo | 1–6 mo | 7–12 mo | 1–6 mo | 1–6 mo | ||||||||||
| Ref | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Method |
| Casey et al. (87) | 0.20 | 0.08 | 0.07 | 0.01 | 0.030 | 0.008 | 0. 017 | 0.001 | — | — | — | — | — | — | — | — | AAS |
| Hannan et al. (97) | 0.20 | 0.10 | — | — | — | — | — | — | 0.04 | 0.06 | — | — | 1. 53 | 0.31 | 4.37 | 1.01 | AAS |
| Perrin et al. (98) | — | — | 0.06 | 0.03 | — | — | — | — | — | — | 0.02 | 0.01 | — | — | — | — | ICP-MS |
| Dewey et al. (36) | 0.09 | 0.05 | 0.07 | 0.04 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | — | — | — | — | AAS and AES |
| Butte et al. (90) | 0.20 | 0.08 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | AAS and colorimetry |
| Dewey and Lonnerdal (91) | 0.13 | 0.04 | — | — | 0.03 | 0.01 | — | — | 0.02 | 0.01 | — | — | — | — | — | — | AAS |
| Garza et al. (125) | 0.12 | 0.02 | — | — | — | — | — | — | 0.003 | 0.001 | — | — | — | — | — | — | AAS |
| Friel et al. (95) | 0.23 | 0.11 | — | — | 0.04 | 0.03 | — | — | — | — | — | — | — | — | — | — | ICP-MS |
| Casey et al. (93) | 0.29 | 0.32 | — | — | 0.04 | 0.02 | — | — | — | — | — | — | — | — | — | — | FES-AES |
| Feeley et al. (94) | 0.29 | 0.12 | — | — | 0.08 | 0.05 | — | — | 0.08 | 0.05 | — | — | — | — | — | — | ICP |
1AAS, atomic absorption spectrometry; AES, atomic emission spectrometry; FES, flame emission spectrometry; ICP, inductively coupled plasma-atomic emission spectrometry; Ref, reference.
TABLE 7.
Electrolytes in human milk for populations in the United States and Canada (1980–2017)1
| Sodium | Potassium | Chloride | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–6 mo | 7–12 mo | 1–6 mo | 7–12 mo | 1–6 mo | 7–12 mo | ||||||||
| Ref | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Method |
| Allen et al. (79) | 15.48 | 2.85 | 16.15 | 4.61 | 54.29 | 4.82 | 50.04 | 4.70 | 48.15 | 7.35 | 50.40 | 12.74 | Ion-selective electrodes and colorimetry |
| Perrin et al. (98) | — | — | 6.79 | 2.10 | — | — | 36.37 | 5.88 | — | — | — | — | ICP-MS |
| Dewey et al. (36) | 9.46 | 5.14 | 8.05 | 3.91 | 39.77 | 7.10 | 37.15 | 6.17 | — | — | — | — | AAS and AES |
| Gross et al. (92) | 21.53 | 13.16 | — | — | 58.25 | 8.54 | — | — | 48.72 | 25.16 | — | — | AAS and colorimetric |
| Butte et al. (90) | 15.49 | 5.11 | — | — | — | — | — | — | — | — | — | — | AAS and colorimetric |
| Dewey and Lonnerdal (91) | 18.59 | 14.50 | — | — | 45.72 | 7.60 | — | — | — | — | — | — | AAS |
| Garza et al. (125) | 13.19 | 1.55 | — | — | — | — | — | — | — | — | — | — | AAS |
| Wack et al. (132) | 14.45 | 7.17 | 12.45 | 11.13 | 50.44 | 9.19 | 44.88 | 6.12 | 38.80 | 12.61 | 40.87 | 18.60 | ICP |
1AAS, atomic absorption spectrometry; AES, atomic emission spectrometry; ICP, inductively coupled plasma-atomic emission spectrometry; Ref, reference.
Other nutrients
There are scattered data on other nutrients, including fatty acids, cholesterol, glucose, vitamin E, and retinol. Four studies provided data on fatty acids (Table 9). Although over 200 fatty acids have been found in human milk (18), about 20 fatty acids are major fatty acids with 7 of them (12:0, 14:0, 16:0, 18:0, 16:1, 18:1, and 18:2) in amounts >1% (100). Data from the 4 studies largely agreed with previous findings. Fatty acids must be converted to methyl esters prior to analysis by GC or GC-MS to increase volatility. Methylation of fatty acids is usually done by reactions with sodium hydroxide-methanol or boron trifluoride-methanol (100). There have been recommendations to usw the boron trifluoride method for human milk and the sodium methoxide method for formulas and bovine milk (50). Of the 4 studies, 1 used the sodium methoxide method (88) and 2 used the sodium methoxide/boron trifluoride method (96, 122). Acetyl chloride in methanol-benzene was used in the fourth paper (123), which may lead to incomplete methylation, thus partly explaining the low total fatty acids reported (Table 10).
TABLE 9.
Fatty acids in human milk for populations in the United States and Canada (1980–2017)
| Clark et al. (88) | Tijerina-Saenz et al. (123) | Glew et al. (96) | Glew et al. (122) | |||||
|---|---|---|---|---|---|---|---|---|
| Fatty acid | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 4:0 | — | — | — | — | — | — | 0.000 | 0.000 |
| 6:0 | — | — | — | — | 0.001 | 0.001 | 0.001 | 0.001 |
| 8:0 | — | — | — | — | 0.003 | 0.001 | 0.004 | 0.002 |
| 10:0 | 0.043 | 0.003 | 0.026 | 0.009 | 0.036 | 0.009 | 0.054 | 0.014 |
| 12:0 | 0.191 | 0.023 | 0.148 | 0.056 | 0.140 | 0.042 | 0.250 | 0.113 |
| 14:0 | 0.256 | 0.019 | 0.188 | 0.059 | 0.163 | 0.050 | 0.285 | 0.116 |
| 15:0 | — | — | — | — | 0.009 | 0.003 | 0.011 | 0.003 |
| 16:0 | 0.976 | 0.089 | 0.615 | 0.089 | 0.615 | 0.069 | 1.004 | 0.105 |
| 17:0 | — | — | — | — | 0.010 | 0.002 | 0.015 | 0.005 |
| 18:0 | 0.342 | 0.062 | 0.194 | 0.047 | 0.208 | 0.039 | 0.340 | 0.043 |
| 20:0 | 0.009 | 0.001 | — | — | 0.006 | 0.001 | 0.009 | 0.002 |
| 22:0 | 0.008 | 0.001 | — | — | 0.004 | 0.002 | 0.009 | 0.002 |
| 24:0 | — | — | — | — | 0.001 | 0.000 | 0.002 | 0.017 |
| 16:1 | 0.162 | 0.012 | 0.082 | 0.021 | 0.072 | 0.019 | — | — |
| 18:1 | 1.685 | 0.256 | 1.070 | 0.131 | 1.060 | 0.111 | 1.452 | 0.163 |
| 20:1 | — | — | — | — | 0.012 | 0.003 | — | — |
| 22:1 | — | — | — | — | 0.003 | 0.001 | 0.003 | 0.003 |
| 24:1 | — | — | — | — | 0.001 | 0.002 | 0.002 | 0.002 |
| 18:2 | 0.680 | 0.080 | 0.406 | 0.096 | 0.651 | 0.150 | 0.834 | 0.151 |
| 18:3 | 0.0681 | 0.009 | 0.045 | 0.016 | 0.044 | 0.001 | 0.061 | 0.024 |
| 20:2 | — | — | — | — | 0.020 | 0.005 | — | — |
| 20:3 | 0.014 | 0.001 | — | — | 0.012 | 0.003 | 0.019 | 0.016 |
| 20:4 | 0.019 | 0.001 | 0.013 | 0.002 | 0.014 | 0.003 | 0.014 | 0.004 |
| 20:5 | — | — | 0.003 | 0.002 | 0.003 | 0.001 | 0.002 | 0.004 |
| 22:2 | — | — | — | — | 0.001 | 0.000 | — | — |
| 22:4 | — | — | — | — | 0.003 | 0.001 | 0.004 | 0.002 |
| 22:5 | — | — | — | — | 0.005 | 0.000 | 0.003 | 0.001 |
| 22:6 trans | — | — | 0.009 | 0.005 | 0.004 | 0.001 | 0.005 | 0.002 |
| 16:1 trans | — | — | — | — | 0.005 | 0.001 | 0.019 | 0.004 |
| 18:1 trans | — | — | — | — | 0.133 | 0.018 | 0.222 | 0.002 |
| 18:2 trans | — | — | — | — | 0.022 | 0.001 | — | — |
| Total | 4.453 | — | 2.799 | — | 3.259 | — | 4.623 | — |
118:3 + 20:1.
TABLE 10.
Other nutrients in human milk for populations in the United States and Canada (1980–2017)1
| Cholesterol | Glucose | HMO | Vitamin E | Retinol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–6 mo | 1–6 mo | 7–12 mo | 1–6 mo | 7–12 mo | 1–6 mo | 1–6 mo | |||||||||
| Ref | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Method |
| Clark et al. (88) | 9.83 | 0.37 | — | — | — | — | — | — | — | — | — | — | — | — | GC-MS |
| Allen et al. (79) | — | — | 27.70 | 2.55 | 24.33 | 5.50 | — | — | — | — | — | — | — | — | Analyzer |
| Perrin et al. (98) | — | — | — | — | — | — | — | — | 0.70 | 0.19 | — | — | — | — | HPLC |
| Alderete et al. (133) | — | — | — | — | — | — | 1.05 | 0.21 | — | — | — | — | — | — | HPLC |
| Tijerina-Saenz et al. (123) | — | — | — | — | — | — | — | — | — | — | 280.31 | — | 7.76 | 7.51 | HPLC |
1HMO, human milk oligosaccharide; Ref, reference.
However, for other nutrients, because the numbers of studies are too few (≤2), no statistical analysis could be conducted and the data quality cannot be evaluated (Table 10).
Closing remarks and future directions
This review focused on summarizing the current knowledge and identifying knowledge gaps in human milk nutrient composition. Based on the foregoing discussions, several conclusions can be drawn with suggestions for future research directions:
Human milk nutrient composition is fundamental information for the management of infant breastfeeding, and serves as the gold standard for developing infant formulas. Despite the great need for more accurate, representative, and up-to-date data, the current review found that in the last 37 y, at least for the research published for the US and Canadian populations, the data are quite limited. Only 28 studies containing original data were found.
Of the 28 studies, most of them were published before 1990. Problems associated with these old data could be: 1) the analytic methods used are outdated, thus data quality could be questionable; and 2) the data may not reflect the current population. Rapid diet and demographic changes over the past few decades may affect human milk composition. For instance, there is a continual trend to replace saturated fats with polyunsaturated fats, which may in turn change the human milk fatty acid composition. In an Australian study, the linoleic acid content in human milk increased during 1981–2000, whereas DHA decreased at the same time (124).
In general, the study designs of these 28 studies were not comprehensive. The subject numbers in most of these studies were small (<50) and the subjects were largely healthy Caucasians, so the study populations did not represent the diversity of the current American population. Because these studies were published over a 37-y period, the experimental designs, including sampling, storage, and analytic methods, varied substantially between the different studies. All of these factors make data quality evaluation and comparison very difficult.
From the standpoint of updating the data in the USDA Food Data System, data for several nutrients from these 28 studies showed some consistency for 1–6 mo, especially for protein, fat, lactose, energy, and certain minerals (e.g., calcium). The data for 7–12 mo and for other nutrients are very scarce, and definitely need additional research.
Human milk is recommended as the sole source of nutrition for young infants, which contributes to normal physiologic and cognitive development during infancy and in later life. Country-specific human milk nutrient information is the foundation for the management of infant feeding. Nevertheless, based on this current review, knowledge on human milk nutrient composition in the United States is still very limited. More comprehensive studies or a set of studies on human milk nutrient composition, representative of the current US population, are necessary to provide accurate, representative, current data, and data variability. The factors that affect the nutrient content of milk and data quality must be considered in experimental design: stage of lactation, mother's diet and body nutritional needs, mother's genetic background and health condition, time of day, region, sample collection, sample storage methods, and nutrient analytic methods.
Acknowledgments
We thank Naomi Fukagawa and Janet Roseland for comments that greatly improved the manuscript, Margaret Neville for sharing the research data, and Ying Li and Amanda Moran for assistance with manuscript preparation. The authors’ responsibilities were as follows—PRP, XW, and RTJ: designed the study; XW and SAK: performed the literature review and analyzed the data; XW, PRP, JA, and RTJ: wrote the manuscript; and all authors; read and approved the manuscript.
Notes
Supported by the USDA.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Author disclosures: XW, RTJ, SAK, JA, and PRP, no conflicts of interest.
Abbreviations used:
- AAS
atomic absorption spectrometry
- AES
atomic emission spectrometry
- HMO
human milk oligosaccharide
- ICP
inductively coupled plasma-atomic emission spectrometry
- NPN
nonprotein nitrogen
- SR
USDA National Nutrient Database for Standard Reference.
Contributor Information
Xianli Wu, Email: xianli.wu@ars.usda.gov.
Pamela R Pehrsson, Email: pamela.pehrsson@ars.usda.gov.
References
- 1. Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129(3):e827–41. [DOI] [PubMed] [Google Scholar]
- 2. Butte N, Lopez-Alarcon M, Garza C. Nutrient adequacy of exclusive breastfeeding for the term infant during the first 6 months of life. Geneva: World Health Organization, 2001. [Google Scholar]
- 3. Mosca F, Gianni ML. Human milk: composition and health benefits. Pediatr Med Chir 2017;39(2):155. [DOI] [PubMed] [Google Scholar]
- 4. Shamir R. The benefits of breast feeding. Nestle Nutr Inst Workshop Ser 2016;86:67–76. [DOI] [PubMed] [Google Scholar]
- 5. Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res 2010;67(4):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. The association between duration of breastfeeding and adult intelligence. JAMA 2002;287(18):2365–71. [DOI] [PubMed] [Google Scholar]
- 7. Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. Executive summary: evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—“the B-24 Project”. Am J Clin Nutr 2014;99(3):663s–91s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Picciano MF. Nutrient composition of human milk. Pediatr Clin North Am 2001;48(1):53–67. [DOI] [PubMed] [Google Scholar]
- 9. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60(1):49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. USDA NDL Nutrient data laboratory. Version release 28 May 2016. [Internet]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl. [Google Scholar]
- 12. Casey CE, Hambidge KM. Nutritional aspects of human lactation. In: Neville MC,, Neifert MR, editors. Lactation: physiology, nutrition, and breast-feeding. New York: Plenum Press, 1983. p. 199–248. [Google Scholar]
- 13. Prentice A. Constituents of human milk. Food Nutr Bull 1996;17(4):305–12. [Google Scholar]
- 14. Institute of Medicine (US) Committee on Nutritional Status during Pregnancy and Lactation Milk composition. In: Nutrition during lactation. Washington (DC): National Academies Press, 1991. p. 113–52. [Google Scholar]
- 15. Akers S, Groh-Wargo S. Normal nutrition during infancy. In: Samour PQ,, King K, editors. Handbook of pediatric nutrition. Sudbury, MA: Jones and Bartlett Publishers, 2005. p. 75–106. [Google Scholar]
- 16. Picone TA, Benson JD, MacLean WC, Sauls HS. Amino acid metabolisms in human milk-fed and formula-fed term infants. Boca Rotan, FL: CRC Press, 1989. [Google Scholar]
- 17. Brown JE. Nutrition through the life cycle. 6th ed Boston, MA: Cengage Learning, 2016. [Google Scholar]
- 18. Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: a review on its composition and bioactivity. Early Hum Dev 2015;91(11):629–35. [DOI] [PubMed] [Google Scholar]
- 19. Hambraeus L. Composition of human milk: nutritional aspects. Bibl Nutr Dieta 1996;53:37–44. [PubMed] [Google Scholar]
- 20. Pereira PC. Milk nutritional composition and its role in human health. Nutrition 2014;30(6):619–27. [DOI] [PubMed] [Google Scholar]
- 21. Haytowitz DB, Pehrsson PR, Holden JM. The National Food and Nutrient Analysis program: a decade of progress. J Food Compos Anal 2008;21(S1):S94–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhagwat SA, Patterson KY, Holden JM. Validation study of the USDA's Data Quality Evaluation System. J Food Compos Anal 2009;22(5):366–72. [Google Scholar]
- 23. Holden JM, Bhagwat SA, Patterson KY. Development of a multi-nutrient Data Quality Evaluation System. J Food Compos Anal 2002;15(4):339–48. [Google Scholar]
- 24. Munblit D, Treneva M, Peroni DG, Colicino S, Chow LY, Dissanayeke S, Pampura A, Boner AL, Geddes DT, Boyle RJ, et al. Immune components in human milk are associated with early infant immunological health outcomes: a prospective three-country analysis. Nutrients 2017;9(6):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koletzko B. Human milk lipids. Ann Nutr Metab 2016;69(Suppl 2):28–40. [DOI] [PubMed] [Google Scholar]
- 26. Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr 2003;77(6):1537S–43S. [DOI] [PubMed] [Google Scholar]
- 27. Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr 2014;99(3):734S–41S. [DOI] [PubMed] [Google Scholar]
- 28. Davidson B, Meinzen-Derr JK, Wagner CL, Newburg DS, Morrow AL. Fucosylated oligosaccharides in human milk in relation to gestational age and stage of lactation. Adv Exp Med Biol 2004;554:427–30. [DOI] [PubMed] [Google Scholar]
- 29. German JB, Freeman SL, Lebrilla CB, Mills DA. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program 2008;62:205–18; discussion 218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tudehope DI. Human milk and the nutritional needs of preterm infants. J Pediatr 2013;162(3 Suppl):S17–25. [DOI] [PubMed] [Google Scholar]
- 31. Underwood MA. Human milk for the premature infant. Pediatr Clin North Am 2013;60(1):189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fujita M, Roth E, Lo YJ, Hurst C, Vollner J, Kendell A. In poor families, mothers’ milk is richer for daughters than sons: a test of Trivers-Willard hypothesis in agropastoral settlements in Northern Kenya. Am J Phys Anthropol 2012;149(1):52–9. [DOI] [PubMed] [Google Scholar]
- 33. Hinde K, Carpenter AJ, Clay JS, Bradford BJ. Holsteins favor heifers, not bulls: biased milk production programmed during pregnancy as a function of fetal sex. PloS One 2014;9(2):e86169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Insitute of Medicine Milk volume. In: Nutrition during lactation. Washington (DC): National Academies Press, 1991. p. 80–112. [Google Scholar]
- 35. Nommsen LA, Lovelady CA, Heinig MJ, Lönnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING study. Am J Clin Nutr 1991;53(2):457–65. [DOI] [PubMed] [Google Scholar]
- 36. Dewey KG, Finley DA, Lonnerdal B. Breast milk volume and composition during late lactation (7–20 months). J Pediatr Gastroenterol Nutr 1984;3(5):713–20. [DOI] [PubMed] [Google Scholar]
- 37. Prentice A, Jarjou LM, Drury PJ, Dewit O, Crawford MA. Breast-milk fatty acids of rural Gambian mothers: effects of diet and maternal parity. J Pediatr Gastroenterol Nutr 1989;8(4):486–90. [DOI] [PubMed] [Google Scholar]
- 38. Bachour P, Yafawi R, Jaber F, Choueiri E, Abdel-Razzak Z. Effects of smoking, mother's age, body mass index, and parity number on lipid, protein, and secretory immunoglobulin A concentrations of human milk. Breastfeed Med 2012;7(3):179–88. [DOI] [PubMed] [Google Scholar]
- 39. Lubetzky R, Sever O, Mimouni FB, Mandel D. Human milk macronutrients content: effect of advanced maternal age. Breastfeed Med 2015;10(9):433–6. [DOI] [PubMed] [Google Scholar]
- 40. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303(3):235–41. [DOI] [PubMed] [Google Scholar]
- 41. Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med 2013;56(6):372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Panagos PG, Vishwanathan R, Penfield-Cyr A, Matthan NR, Shivappa N, Wirth MD, Hebert JR, Sen S. Breastmilk from obese mothers has pro-inflammatory properties and decreased neuroprotective factors. J Perinatol 2016;36(4):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hamosh M, Bitman J. Human milk in disease: lipid composition. Lipids 1992;27(11):848–57. [DOI] [PubMed] [Google Scholar]
- 44. Laiho K, Lampi AM, Hamalainen M, Moilanen E, Piironen V, Arvola T, Syrjanen S, Isolauri E. Breast milk fatty acids, eicosanoids, and cytokines in mothers with and without allergic disease. Pediatr Res 2003;53(4):642–7. [DOI] [PubMed] [Google Scholar]
- 45. Gardner AS, Rahman IA, Lai CT, Hepworth A, Trengove N, Hartmann PE, Geddes DT. Changes in fatty acid composition of human milk in response to cold-like symptoms in the lactating mother and infant. Nutrients 2017;9(9):1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Picciano MF. Human milk: nutritional aspects of a dynamic food. Biol Neonate 1998;74(2):84–93. [DOI] [PubMed] [Google Scholar]
- 47. Lonnerdal B. Effects of maternal dietary intake on human milk composition. J Nutr 1986;116(4):499–513. [DOI] [PubMed] [Google Scholar]
- 48. Bravi F, Wiens F, Decarli A, Dal Pont A, Agostoni C, Ferraroni M. Impact of maternal nutrition on breast-milk composition: a systematic review. Am J Clin Nutr 2016;104(3):646–62. [DOI] [PubMed] [Google Scholar]
- 49. Keikha M, Bahreynian M, Saleki M, Kelishadi R. Macro- and micronutrients of human milk composition: are they related to maternal diet? A comprehensive systematic review. Breastfeed Med 2017;12(9):517–27. [DOI] [PubMed] [Google Scholar]
- 50. Jensen RG. Lipids in human milk. Lipids 1999;34(12):1243–71. [DOI] [PubMed] [Google Scholar]
- 51. Dorea JG. Iron and copper in human milk. Nutrition 2000;16(3):209–20. [DOI] [PubMed] [Google Scholar]
- 52. Kitano H. Biological robustness. Nat Rev Genet 2004;5(11):826–37. [DOI] [PubMed] [Google Scholar]
- 53. Picciano MF, McGuire MK. Use of dietary supplements by pregnant and lactating women in North America. Am J Clin Nutr 2009;89(2):663s–7s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stultz EE, Stokes JL, Shaffer ML, Paul IM, Berlin CM. Extent of medication use in breastfeeding women. Breastfeed Med 2007;2(3):145–51. [DOI] [PubMed] [Google Scholar]
- 55. Makrides M, Neumann MA, Gibson RA. Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. Eur J Clin Nutr 1996;50(6):352–7. [PubMed] [Google Scholar]
- 56. Mackey AD, Picciano MF. Maternal folate status during extended lactation and the effect of supplemental folic acid. Am J Clin Nutr 1999;69(2):285–92. [DOI] [PubMed] [Google Scholar]
- 57. Jiang J, Wu K, Yu Z, Ren Y, Zhao Y, Jiang Y, Xu X, Li W, Jin Y, Yuan J, et al. Changes in fatty acid composition of human milk over lactation stages and relationship with dietary intake in Chinese women. Food Func 2016;7(7):3154–62. [DOI] [PubMed] [Google Scholar]
- 58. McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, Moore SE, et al. What's normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr 2017;105(5):1086–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Trainer D, Pehrsson PR, Haytowitz DB, Holden JM, Phillips KM, Rasor AS, Conley NA. Development of sample handling procedures for foods under USDA's National Food and Nutrient Analysis Program. J Food Compos Anal 2010;23(8):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yin SA, Yang ZY. An on-line database for human milk composition in China. Asia Pac J Clin Nutr 2016;25(4):818–25. [DOI] [PubMed] [Google Scholar]
- 61. Silvestre MD, Lagarda MJ, Farre R, Martinez-Costa C, Brines J, Molina A, Clemente G. A study of factors that may influence the determination of copper, iron, and zinc in human milk during sampling and in sample individuals. Biol Trace Elem Res 2000;76(3):217–27. [DOI] [PubMed] [Google Scholar]
- 62. Miller EM, Aiello MO, Fujita M, Hinde K, Milligan L, Quinn EA. Field and laboratory methods in human milk research. Am J Hum Biol 2013;25(1):1–11. [DOI] [PubMed] [Google Scholar]
- 63. Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006;117(3):e387–95. [DOI] [PubMed] [Google Scholar]
- 64. Garza C, Butte NF. Energy concentration of human milk estimated from 24-h pools and various abbreviated sampling schemes. J Pediatr Gastroenterol Nutr 1986;5(6):943–8. [DOI] [PubMed] [Google Scholar]
- 65. Mitoulas LR, Kent JC, Cox DB, Owens RA, Sherriff JL, Hartmann PE. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br J Nutr 2002;88(1):29–37. [DOI] [PubMed] [Google Scholar]
- 66. Bitman J, Wood DL, Mehta NR, Hamosh P, Hamosh M. Lipolysis of triglycerides of human milk during storage at low temperatures: a note of caution. J Pediatr Gastroenterol Nutr 1983;2(3):521–4. [DOI] [PubMed] [Google Scholar]
- 67. Lavine M, Clark RM. Changing patterns of free fatty acids in breast milk during storage. J Pediatr Gastroenterol Nutr 1987;6(5):769–74. [DOI] [PubMed] [Google Scholar]
- 68. Handa D, Ahrabi AF, Codipilly CN, Shah S, Ruff S, Potak D, Williams JE, McGuire MA, Schanler RJ. Do thawing and warming affect the integrity of human milk? J Perinatol 2014;34(11):863–6. [DOI] [PubMed] [Google Scholar]
- 69. Morrow AL, Chantry CJ. Breastfeeding updates for the pediatrician. Oxford: Elsevier Health Sciences, 2011. [Google Scholar]
- 70. Peila C, Moro GE, Bertino E, Cavallarin L, Giribaldi M, Giuliani F, Cresi F, Coscia A. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: a review. Nutrients 2016;8(8):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Atkinson SA, Lönnerdal BO. Nonprotein nitrogen fractions of human milk. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press, 1995. p. 369–87. [Google Scholar]
- 72. Hampel D, Allen LH. Analyzing B-vitamins in human milk: methodological approaches. Crit Rev Food Sci Nutr 2016;56(3):494–511. [DOI] [PubMed] [Google Scholar]
- 73. Hampel D, Shahab-Ferdows S, Islam MM, Peerson JM, Allen LH. Vitamin concentrations in human milk vary with time within feed, circadian rhythm, and single-dose supplementation. J Nutr 2017;147(4):603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hampel D, York ER, Allen LH. Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. J Chromatogr B 2012;903:7–13. [DOI] [PubMed] [Google Scholar]
- 75. Bokor S, Koletzko B, Decsi T. Systematic review of fatty acid composition of human milk from mothers of preterm compared to full-term infants. Ann Nutr Metab 2007;51(6):550–6. [DOI] [PubMed] [Google Scholar]
- 76. Neville MC, Jensen RG. The physical properties of human and bovine milks. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press, 1995. p. 81–5. [Google Scholar]
- 77. FAO Food energy – methods of analysis and conversion factors. In: United Nations , editor. Rome, Italy: FAO, 2002. [Google Scholar]
- 78. Maubois JL, Lorient D. Dairy proteins and soy proteins in infant foods nitrogen-to-protein conversion factors. Dairy Sci Technol 2016;96:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Allen JC, Keller RP, Archer P, Neville MC. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr 1991;54(1):69–80. [DOI] [PubMed] [Google Scholar]
- 80. Karra MV, Kirksey A, Galal O, Bassily NS, Harrison GG, Jerome NW. Zinc, calcium, and magnesium concentrations in milk from American and Egyptian women throughout the first 6 months of lactation. Am J Clin Nutr 1988;47(4):642–8. [DOI] [PubMed] [Google Scholar]
- 81. Neville MC, Keller RP, Seacat J, Casey CE, Allen JC, Archer P. Studies on human lactation. I. Within-feed and between-breast variation in selected components of human milk. Am J Clin Nutr 1984;40(3):635–46. [DOI] [PubMed] [Google Scholar]
- 82. Page R, Robichaud A, Arbuckle TE, Fraser WD, MacFarlane AJ. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am J Clin Nutr 2017;105(5):1101–9. [DOI] [PubMed] [Google Scholar]
- 83. Bitman J, Wood DL, Mehta NR, Hamosh P, Hamosh M. Comparison of the phospholipid composition of breast milk from mothers of term and preterm infants during lactation. Am J Clin Nutr 1984;40(5):1103–19. [DOI] [PubMed] [Google Scholar]
- 84. Jackson JG, Janszen DB, Lonnerdal B, Lien EL, Pramuk KP, Kuhlman CF. A multinational study of α-lactalbumin concentrations in human milk. J Nutr Biochem 2004;15(9):517–21. [DOI] [PubMed] [Google Scholar]
- 85. Houghton LA, Yang J, O'Connor DL. Unmetabolized folic acid and total folate concentrations in breast milk are unaffected by low-dose folate supplements. Am J Clin Nutr 2009;89(1):216–20. [DOI] [PubMed] [Google Scholar]
- 86. Barile D, Rastall RA. Human milk and related oligosaccharides as prebiotics. Curr Opin Biotechnol 2013;24(2):214–19. [DOI] [PubMed] [Google Scholar]
- 87. Casey CE, Neville MC, Hambidge KM. Studies in human lactation: secretion of zinc, copper, and manganese in human milk. Am J Clin Nutr 1989;49(5):773–85. [DOI] [PubMed] [Google Scholar]
- 88. Clark RM, Ferris AM, Fey M, Brown PB, Hundrieser KE, Jensen RG. Changes in the lipids of human milk from 2 to 16 weeks postpartum. J Pediatr Gastroenterol Nutr 1982;1(3):311–16. [DOI] [PubMed] [Google Scholar]
- 89. Janas LM, Picciano MF, Hatch TF. Indices of protein metabolism in term infants fed either human milk or formulas with reduced protein concentration and various whey/casein ratios. J Pediatr 1987;110(6):838–48. [DOI] [PubMed] [Google Scholar]
- 90. Butte NF, Garza C, Johnson CA, Smith EO, Nichols BL. Longitudinal changes in milk composition of mothers delivering preterm and term infants. Early Hum Dev 1984;9(2):153–62. [DOI] [PubMed] [Google Scholar]
- 91. Dewey KG, Lonnerdal B. Milk and nutrient intake of breast-fed infants from 1 to 6 months: relation to growth and fatness. J Pediatr Gastroenterol Nutr 1983;2(3):497–506. [DOI] [PubMed] [Google Scholar]
- 92. Gross SJ, David RJ, Bauman L, Tomarelli RM. Nutritional composition of milk produced by mothers delivering preterm. J Pediatr 1980;96(4):641–4. [DOI] [PubMed] [Google Scholar]
- 93. Casey CE, Hambidge KM, Neville MC. Studies in human lactation: zinc, copper, manganese and chromium in human milk in the first month of lactation. Am J Clin Nutr 1985;41(6):1193–200. [DOI] [PubMed] [Google Scholar]
- 94. Feeley RM, Eitenmiller RR, Jones JB Jr, Barnhart H. Copper, iron, and zinc contents of human milk at early stages of lactation. Am J Clin Nutr 1983;37(3):443–8. [DOI] [PubMed] [Google Scholar]
- 95. Friel JK, Andrews WL, Jackson SE, Longerich HP, Mercer C, McDonald A, Dawson B, Sutradhar B. Elemental composition of human milk from mothers of premature and full-term infants during the first 3 months of lactation. Biol Trace Elem Res 1999;67(3):225–47. [DOI] [PubMed] [Google Scholar]
- 96. Glew RH, Wold RS, Herbein JH, Wark WA, Martinez MA, Vanderjagt DJ. Low docosahexaenoic acid in the diet and milk of women in New Mexico. J Am Diet Assoc 2008;108(10):1693–9. [DOI] [PubMed] [Google Scholar]
- 97. Hannan MA, Faraji B, Tanguma J, Longoria N, Rodriguez RC. Maternal milk concentration of zinc, iron, selenium, and iodine and its relationship to dietary intakes. Biol Trace Elem Res 2009;127(1):6–15. [DOI] [PubMed] [Google Scholar]
- 98. Perrin MT, Fogleman AD, Newburg DS, Allen JC. A longitudinal study of human milk composition in the second year postpartum: implications for human milk banking. Matern Child Nutr 2016;13(1):e12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lonnerdal B, Woodhouse LR, Glazier C. Compartmentalization and quantitation of protein in human milk. J Nutr 1987;117(8):1385–95. [DOI] [PubMed] [Google Scholar]
- 100. Jensen RG, Bitman J, Carlson SE, Couch SC, Hamosh M, Newburg DS. Milk lipids: A. Human milk lipids. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press, 1995. p. 495–542. [Google Scholar]
- 101. Jensen RG, Clark RM. Methods of lipid analysis. J Pediatr Gastroenterol Nutr 1984;3(2):296–9. [DOI] [PubMed] [Google Scholar]
- 102. Du J, Gay MCL, Lai CT, Trengove RD, Hartmann PE, Geddes DT. Comparison of gravimetric, creamatocrit and esterified fatty acid methods for determination of total fat content in human milk. Food Chem 2017;217:505–10. [DOI] [PubMed] [Google Scholar]
- 103. Ferris AM, Jensen RG. Lipids in human milk: a review. 1: sampling, determination, and content. J Pediatr Gastroenterol Nutr 1984;3(1):108–22. [DOI] [PubMed] [Google Scholar]
- 104. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012;22(9):1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kunz C. Historical aspects of human milk oligosaccharides. Adv Nutr 2012;3(3):430S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jantscher-Krenn E, Bode L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr 2012;64(1):83–99. [PubMed] [Google Scholar]
- 107. Newburg DS, Neubauer SH. Carbohydrates in milks: analysis, quantities, and significance. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press, 1995. p. 273–349. [Google Scholar]
- 108. Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem 1964;7(1):18–25. [DOI] [PubMed] [Google Scholar]
- 109. Lonnerdal B, Smith C, Keen CL. Analysis of breast milk: current methodologies and future needs. J Pediatr Gastroenterol Nutr 1984;3(2):290–5. [DOI] [PubMed] [Google Scholar]
- 110. Nickerson TA, Vujicic IF, Lin AY. Colorimetric estimation of lactose and its hydrolytic products. J Dairy Sci 1975;59(3):386–90. [Google Scholar]
- 111. Butte NF, Wong WW, Ferlic L, Smith EO, Klein PD, Garza C. Energy expenditure and deposition of breast-fed and formula-fed infants during early infancy. Pediatr Res 1990;28(6):631–40. [DOI] [PubMed] [Google Scholar]
- 112. Casadio YS, Williams TM, Lai CT, Olsson SE, Hepworth AR, Hartmann PE. Evaluation of a mid-infrared analyzer for the determination of the macronutrient composition of human milk. J Hum Lact 2010;26(4):376–83. [DOI] [PubMed] [Google Scholar]
- 113. Groh-Wargo S, Valentic J, Khaira S, Super DM, Collin M. Human milk analysis using mid-infrared spectroscopy. Nutr Clin Pract 2016;31(2):266–72. [DOI] [PubMed] [Google Scholar]
- 114. Silvestre D, Fraga M, Gormaz M, Torres E, Vento M. Comparison of mid-infrared transmission spectroscopy with biochemical methods for the determination of macronutrients in human milk. Matern Child Nutr 2014;10(3):373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhu M, Yang Z, Ren Y, Duan Y, Gao H, Liu B, Ye W, Wang J, Yin S. Comparison of macronutrient contents in human milk measured using mid-infrared human milk analyser in a field study vs. chemical reference methods. Matern Child Nutr 2017;13(1):12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Thurl S, Munzert M, Boehm G, Matthews C, Stahl B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr Rev 2017;75(11):920–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kunz C, Kuntz S, Rudloff S. Bioactivity of human milk oligosaccharides. In: Javier Moreno F,, Luz Sanz M, editors. Food oligosaccharides: Production, Analysis and Bioactivity. Hoboken, NJ: John Wiley & Sons, Ltd, 2014. p. 1–20. [Google Scholar]
- 118. Mantovani V, Galeotti F, Maccari F, Volpi N. Recent advances on separation and characterization of human milk oligosaccharides. Electrophoresis 2016;37(11):1514–24. [DOI] [PubMed] [Google Scholar]
- 119. Neville MC. Volume and caloric density of human milk. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press, 1995. p. 99–113. [Google Scholar]
- 120. Nichols BL. Atwater and USDA nutrition research and service: a prologue of the past century. J Nutr 1994;124(9 Suppl):1718s–27s. [DOI] [PubMed] [Google Scholar]
- 121. Poitevin E. Official methods for the determination of minerals and trace elements in infant formula and milk products: a review. J AOAC Int 2016;99(1):42–52. [DOI] [PubMed] [Google Scholar]
- 122. Glew RH, Wold RS, Corl B, Calvin CD, Vanderjagt DJ. Low docosahexaenoic acid in the diet and milk of American Indian women in New Mexico. J Am Diet Assoc 2011;111(5):744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tijerina-Saenz A, Innis SM, Kitts DD. Antioxidant capacity of human milk and its association with vitamins A and E and fatty acid composition. Acta Paediatrica 2009;98(11):1793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gibson RA, Muhlhausler B, Makrides M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern Child Nutr 2011;7(Suppl 2):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Garza C, Johnson CA, Smith EO, Nichols BL. Changes in the nutrient composition of human milk during gradual weaning. Am J Clin Nutr 1983;37(1):61–5. [DOI] [PubMed] [Google Scholar]
- 126. Clark RM, Fey MB, Jensen RG, Hill DW. Desmosterol in human milk. Lipids 1983;18(3):264–6. [DOI] [PubMed] [Google Scholar]
- 127. Feeley RM, Eitenmiller RR, Jones JB Jr, Barnhart H. Calcium, phosphorus, and magnesium contents of human milk during early lactation. J Pediatr Gastroenterol Nutr 1983;2(2):262–7. [PubMed] [Google Scholar]
- 128. Butte NF, Garza C, Smith EO, Nichols BL. Human milk intake and growth in exclusively breast-fed infants. J Pediatr 1984;104(2):187–95. [DOI] [PubMed] [Google Scholar]
- 129. Janas LM, Picciano MF. Quantities of amino acids ingested by human milk-fed infants. J Pediatr 1986;109(5):802–7. [DOI] [PubMed] [Google Scholar]
- 130. Ferris AM, Dotts MA, Clark RM, Ezrin M, Jensen RG. Macronutrients in human milk at 2, 12, and 16 weeks postpartum. J Am Diet Assoc 1988;88(6):694–7. [PubMed] [Google Scholar]
- 131. Stuff JE, Nichols BL. Nutrient intake and growth performance of older infants fed human milk. J Pediatr 1989;115(6):959–68. [DOI] [PubMed] [Google Scholar]
- 132. Wack RP, Lien EL, Taft D, Roscelli JD. Electrolyte composition of human breast milk beyond the early postpartum period. Nutrition 1997;13(9):774–7. [DOI] [PubMed] [Google Scholar]
- 133. Alderete TL, Autran C, Brekke BE, Knight R, Bode L, Goran MI, Fields DA. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr 2015;102(6):1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]