Abstract
Rheumatoid nodules are the most common pulmonary manifestations of rheumatoid arthritis (RA) and are usually asymptomatic. In rare cases, they progress cavitary formation and cause severe clinical symptoms because of disease activity, infectious diseases, and other etiologies. The determination of clinical and histopathological features may be helpful for differential diagnosis in patients with RA with cavitary pulmonary nodules. Herein, we report two female patients with RA with cavitary pulmonary nodules and aim to obtain more detail data by means of reviewing previously reported cases.
Keywords: Cavitary pulmonary nodules, rheumatoid arthritis, leflunomide
Introduction
Rheumatoid arthritis (RA), primarily affecting joints, is a systemic chronic inflammatory disorder. Respiratory complications with the involvement of pleura, airway, pulmonary vascular system, and parenchymal lung might be seen in RA (1). Drug toxicity and opportunistic infection also might cause pulmonary system symptoms in patients with RA. Pulmonary rheumatoid nodules have been reported in up to 32% of individuals with RA and require a more detailed evaluation to exclude neoplasms, tuberculosis (TB), and fungal infection (2). They usually tend to be asymptomatic and 1–3 cm in size. In rare cases, they can progress to cavitary lesions, potentially causing difficulty in differential diagnosis and more severe pulmonary symptoms. We report two patients with RA with large cavitary pulmonary nodules and provide a review of the literature based on published cases.
Case Presentations
Case 1
A 55-year-old woman with RA, diagnosed 20 years ago, was initially treated with methotrexate (MTX) 15 mg/week, hydroxychloroquine (HCO) 200 mg/day, and prednisolone (P) 5 mg/days. After 5 years of treatment, MTX and HCO were withdrawn due to vomiting and retinal toxicity and then leflunomide (LEF) 20 mg/day was started as a monotherapy. There was no other significant disease in her and her family’s history.
She was admitted to our outpatient clinic complaining of fatigue, weight loss, cough, and chest pain for 2 months. In her physical examination, the tender and the swollen joint counts were 2 and 1, respectively. The 28-joint disease activity score (DAS28) was calculated to be 4.8. Serum biochemical test was normal, except for the increase in acute-phase reactants [erythrocyte sedimentation rate (ESR): 71 mm/h; C-reactive protein: 4 mg/dL; normal range <0.5]. Rheumatoid factor (RF) was found positive (143 IU/mL; normal value: <20), whereas anti-CCP, ANA, and ANCA were negative. Radiography of extremities showed erosions of the hands. Computed tomography (CT) of the chest revealed bilateral multiple nodular lesions with extensive cavitary areas in especially lower zones (Figure 1). The three negative sputum tests for acid-fast bacillus were obtained and culture examination of bronchoalveolar lavage for TB was also negative. Histopathological evaluation of pulmonary tissue obtained with transbronchial needle biopsy revealed central necrosis surrounded by granulomatous inflammation and perivascular lymphoplasmacytic infiltration with the destruction of medium-sized vessels (Figure 2a, b). Anti-TB therapy with isoniazid (5 mg/kg), rifampin (10 mg/kg), ethambutol (15 mg/kg), and pyrazinamide (20 mg/kg) was given as an empirical treatment after LEF was stopped. Anti-TB therapy was interrupted in the second month of treatment because the progression of pulmonary lesions was detected with control; the chest CT and polyarthritis worsened. It was considered as a disease activity, and prednisolone 500 mg pulse for 3 days was started followed by 1 mg/kg/day prednisolone along with rituximab treatment 1 gm intravenously on days 1 and 15. Low-disease activity of RA and considerable regression in pulmonary cavitary lesions were detected at the end of the third month of treatment.
Figure 1.
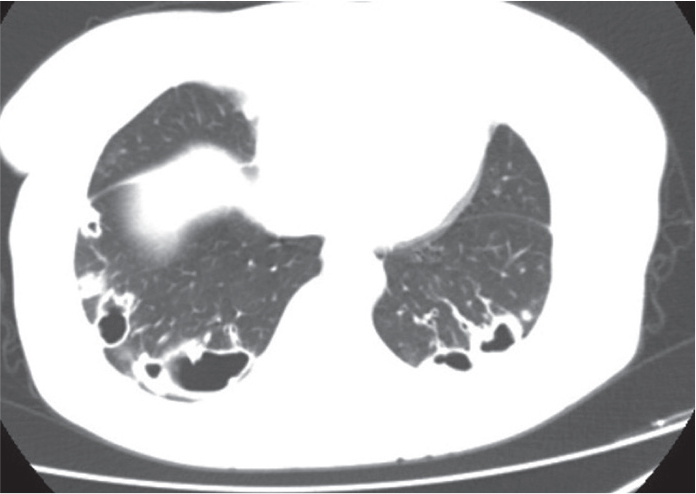
Thorax CT shows irregular nodules with large cavities located in the peripheral zone of bilateral lower lobes
Figure 2. a, b.
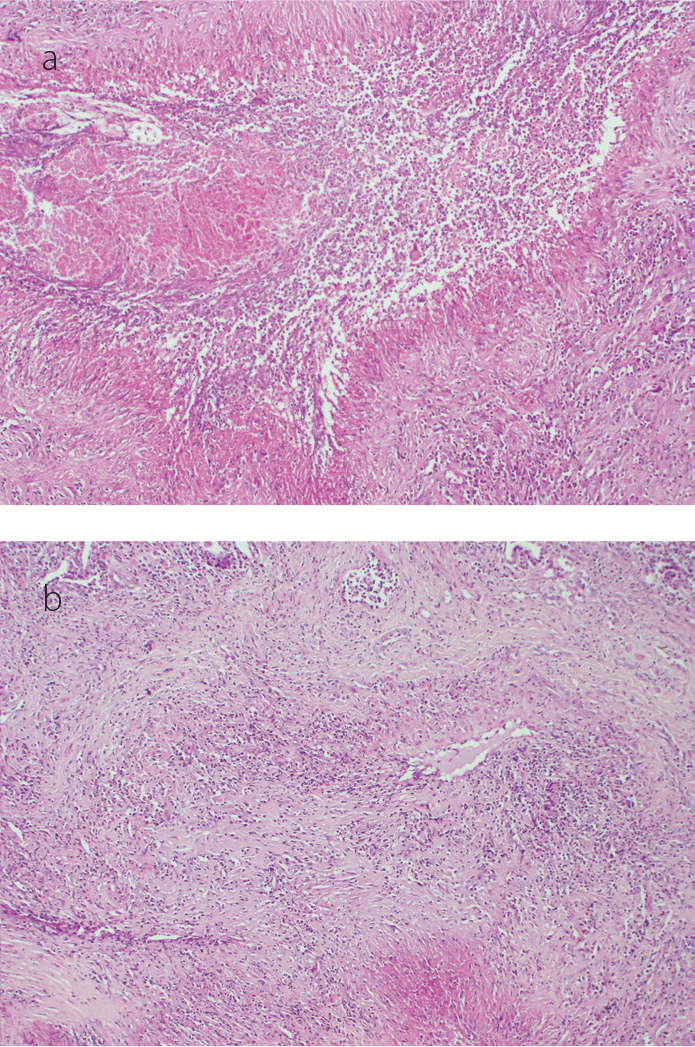
Histopathologic specimens show fibrinoid necrosis with histiocytes and giant cells (a). There are lymphohistiocytic infiltrates which disturb the integrity of medium-sized vessels (b) [hematoxylin and eosin stain (H&E), original magnification ×100, ×200, respectively]
Case 2
A 61-year-old female patient was admitted to our clinic with complaints of cough and pain and swelling of the wrists and small joints of the hands, shoulders, and knees. She also showed fatigue, weight loss, and loss of appetite. Joint symptoms started 2 years ago, and she was treated with LEF 20 mg/day and HCO 200 mg/day for 10 months with the diagnosis of RA. There was no comorbidity. Physical examination showed severe polyarthritis (DAS28 score was 7.2) and necrotic skin lesions in the breast (Figure 3). Laboratory examination revealed an increase of the acute-phase reactant (ESR: 88 mm/h; C-reactive protein: 17 mg/dL; normal range <0.5). The other serum biochemical test was in normal range. RF, anti-CCP, and anti-MPO were found positive [569 IU/mL (<20), 201 IU/mL (<12), and 93 IU/mL (<18), respectively]. Periarticular osteopenia and few lytic lesions were detected in X-ray of the hands. Chest CT indicated cavitary pulmonary nodules (Figure 4). Skin biopsy revealed dermal lymphocyte and neutrophil infiltration. A percutaneous transthoracic needle biopsy was performed, and the subcutaneous nodule disclosed a necrotizing area surrounded by a palisade of histiocytes and chronic inflammatory cells and lymphoplasmacytic inflammation in all layers of vessels (Figure 5a, b). There were no laboratories or histopathological findings to support infectious diseases, including fungal diseases and TB. It was not possible to make an exact diagnosis between ANCA-associated vasculitis (AAV) and RA vasculitis (RV) because the findings of the former coincide with the latter. Since corticosteroid and immunosuppressive treatment could have been effective in both conditions, we started pulse methylprednisolone (1 gm’3 days) and cyclophosphamide (15 mg/kg/day) intravenously after excluding infective etiologies. Pneumothorax was observed in the second week of treatment. In follow-up, all symptoms resolved. Methylprednisolone dose was continued 1 mg/kg/day and diminished 10% daily dose per week.
Figure 3.
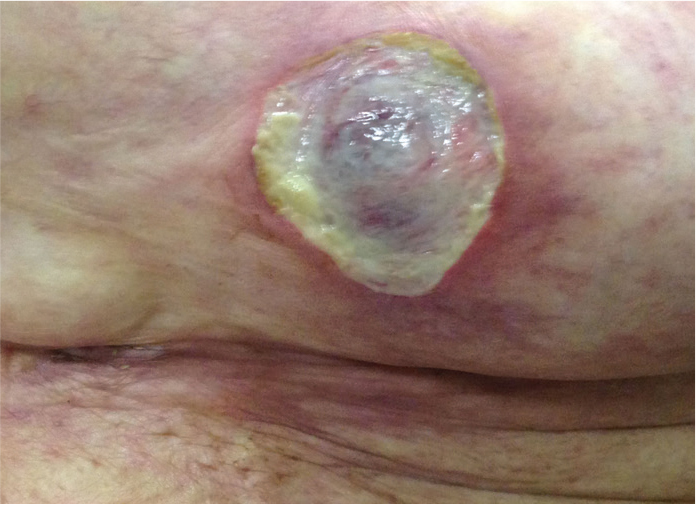
Livedoid view and a necrotic skin lesion in the breast
Figure 4.
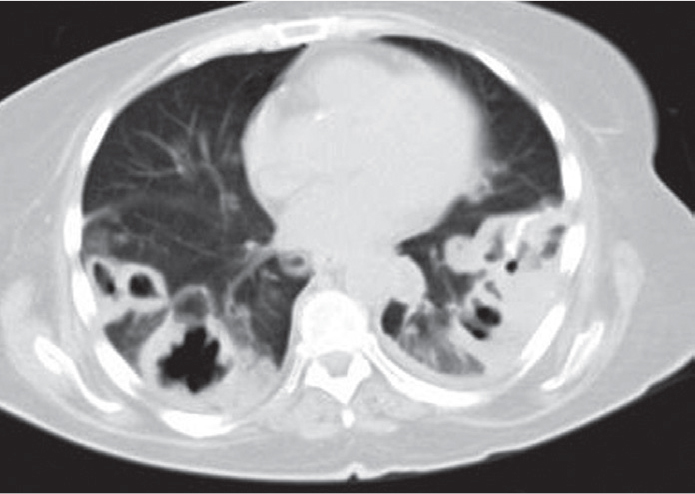
Thorax CT shows numerous nodules near pleural spaces, sizes ranging from 6 to 58 mm with central necrosis
Figure 5. a, b.
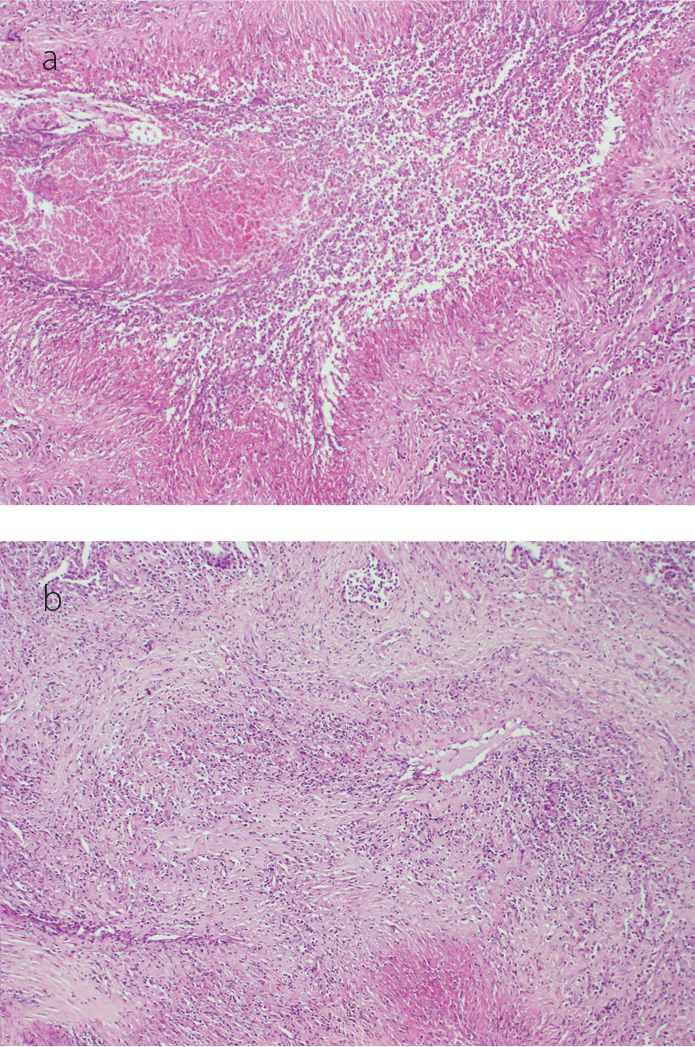
Large number of macrophages, multinucleated giant cells, and epithelioid histiocytes surrounded by fibrinoid necrosis (a) and dense lymphoplasmacytic infiltrates which cause vascular wall damage (b) (H&E, original magnification ×100, ×200 respectively)
Discussion
Rheumatoid pulmonary nodules contained central necrosis surrounded by epithelioid histiocytes, lymphocytes, plasma cells, and proliferating fibroblasts. They are observed more frequently in smokers, males, and patients with advanced seropositive RA (3). The necrotic central zone may develop into cavitation as a result of the resolution of necrosis and consequent inflation due to airflow (4). In this case, more severe symptoms could be seen, and the differentiation of RV, AAV, and/or pulmonary TB in patients with RA may be difficult with regards to similar histopathological findings. The detection of caseification necrosis or mycobacteria with specific microbiological tests is diagnostic for TB. In our first case, we started anti-TB therapy empirically because TB infection was not clinically excluded. We thought that pulmonary lesions may be associated with RA due to the progression of pulmonary lesions observed under the anti-TB therapy. The differential diagnosis was also difficult for the second patient with RA, with vasculitic findings in histopathological evaluation and anti-MPO positivity. According to RA cohorts, the rate of ANCA positivity in ELISA is up to 32% (5). Other studies reported that ANCA and anti-CCP double-positive patients with RA may more frequently present with pulmonary nodules (6, 7). Histopathological findings of pulmonary nodules may not be helpful enough at differentiating RV from AAV in RA. Necrotizing granulomatosis inflammation and perivascular lymphocyte infiltration might be seen in both diagnoses. Therefore, we could not be sure that the diagnosis was RV or AAV in our second patient.
We searched case reports and case series published in the last 30 years with keywords including “rheumatoid arthritis,” “cavitary pulmonary nodules,” and “vasculitis” in the PubMed database (8–15). Case reports diagnosed with diseases other than RA and suspicions of infectious disease which can cause cavitary pulmonary nodules were excluded. Consequently, clinical and histopathological features of 11 patients with RA (7 female) and two female patients with RA with cavitary pulmonary nodules are seen in Table 1. The disease duration ranged 2–20 years, and seven of the patients were older than 60 years. Skin ulcers were detected in two patients. Five cases were complicated with pneumothorax. Eleven of 12 patients with RA were seropositive. ANCA was positive in one of nine patients. There was usually granulomatous inflammation with necrosis in pathological specimens. Favorable outcomes were reported with corticosteroids and/or other immunosuppressive therapy except for 2 cases who died of sepsis and interstitial pneumonia.
Table 1.
Clinical features of patients with RA reported since 1996 and our patients with cavitary pulmonary nodules
| Ref. no. | Age, y | Sex | Disease Duration, y | RF | Anti-CCP | ANCA | Therapy | Duration of LEF therapy | Pulmonary radiological findings | Pulmonary histopathological findings | Treatment and disease course |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 51 | F | 10 years | Seropositive* | NA | PRD, LEF | 10 years | Multiple nodules, pleural effusion, PX | NA | Died from sepsis | |
| 7 | 46 | F | 2 years | Seropositive | NA | PRD, LEF, HCO | 2 years | A large right PX, multiple bilateral nodules | Necrosis, granuloma, epithelioid mononuclear inflammation withgiant cells | Favorable LEF was stopped | |
| 8 | 77 | M | 18 years | + | NA | - | LEF | 13 months | Cherry-like nodes partially with cavitation | Epithelioid and giant cell inflammation with necrosis | Favorable LEF was stopped |
| 66 | M | 22 years | + | NA | - | PRD, LEF, AZA | NA | Small nodules in right side with cavitation, PX | Histiocytes, multinucleated giant cells surrounding focus of necrosis | Favorable LEF was stopped | |
| 9 | 66 | F | 7 years | NA | NA | - | MPRD, HCO, MTX | - | Multiple cavitary consolidations | NA | Favorable |
| 10 | 67 | F | 14 years | + | NA | NA | Only NSAID | - | Small nodules with cavities | Fibrinoid necrosis, histiocytes, lymphocytes, plasma cells, and multinucleated giant cells | Died from severe interstitial pneumonia |
| 11 | 60 | F | 10 years | + | NA | - | LEF, Deflazacort | 8 years | Multiple cavitary pulmonary nodules | Acute suppurative inflammation with necrosis | Favorable with abatacept. LEF was stopped |
| 12 | 64 | M | >3 years | + | NA | NA | MPRD, MTX | - | Bilateral multiple nodules and a large cavitation, PX | Interstitial lymphoid hyperplasia with giant cells | Favorable with CS MTX was stopped |
| 13 | 42 | F | 20 years | + | NA | - | MPRD, HCO, LEF | 13 months | Pleural effusion and bilateral cavitary nodules | NA | NA |
| 44 | F | 9 years | + | NA | - | MTX, SSZ, LEF | 15 months | Pleural effusions and four cavitary lesions | NA | NA | |
| 65 | M | 14 years | + | NA | - | MTX, HCO | - | A cavitary nodule Bilateral multiple nodular lesions with extensive cavitary areas | Epithelioid histiocytes Central necrosis, granulomatous inflammation, and perivascular lymphoplasmacytic infiltration | NA Favorable with CS and rituximab. LEF was stopped | |
| Our case 1 | 55 | F | 20 years | + | + | - | LEF | 15 years | |||
| Our case 2 | 61 | F | 2 years | + | + | + | LEF, HCO | 10 months | Bilateral massive cavitary pulmonary nodules, PX | Giant cell inflammation with necrosis and lymphoplasmacytic inflammation at vessels wall | Favorable with CS and cyclophosphamide. LEF was stopped |
F: female; M: male; PRD: prednisolon; MPRD: metyprednisolon; CS: cortiocteoid; LEF: leflunomide; HCO: hydroxichlorocine; MTX: methotrexate; AZA: azathioprin; NSAID: nonsteroid anti-inflammatory drugs; SSZ: sulphasalazine; NA: not available; PX: pneumothorax
Only seropositivity was reported, RF or anti-CCP results were not obtained.
Leflunomide was a remarkable anti-rheumatic drug in the treatment of 10 patients. LEF inhibits the mitochondrial enzyme dihydroorotate dehydrogenase (DHODH), which plays a key role in the de novo synthesis of pyridine-containing ribonucleotides (rUMP). This agent targets the activated T cells and suppresses inflammatory reaction on synovium in RA (16). Although LEF therapy was reportedly less than other anti-rheumatic drugs such as MTX, TNF-alpha inhibitors in patients with RA with pulmonary non-cavitary nodules and fatal interstitial lung disease in this review, the high frequency of LEF therapy in patients with RA with cavitary pulmonary nodules should be emphasized (17–19). However, the underlying mechanism for the association between LEF therapy and the pulmonary cavitary lesion is not known at present.
In conclusion, patients with RA with cavitary pulmonary nodules tend to present with seropositive, long disease duration, and more severe pulmonary findings compared with non-cavitary lesions. Anti-rheumatic drugs, especially LEF, may play a role in the development of this clinical presentation. On the other hand, the vasculitic course in RA might be responsible for the development of cavitary pulmonary lesions. In literature, there is insufficient data about evaluations of vasculitis in such patients. We need to collect more information from new studies designed with descriptive clinical, laboratory, and histopathological perspective to manage these challenging patients. Written informed consent was obtained from the patients.
Footnotes
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - N.A.K., S.Ç.; Design - N.A.K., S.Y.Ö.; Supervision - E.Ç., C.B.; Resources - H.N.Ü., N.A.K.; Materials - H.N.Ü., N.A.K.; Data Collection and/or Processing - N.A.K., S.Ç.; Analysis and/or Interpretation - N.A.K., S.Ç.; Literature Search - N.A.K., S.Ç.; Writing Manuscript - N.A.K., S.Ç.; Critical Review - C.B., E.Ç.; Other - S.Y.Ö., E.Ç.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;15:443–8. doi: 10.1513/pats.200703-045MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez Herrero H, ArraizaSarasa M, Rubio Marco I, Garciade Eulate Martin-Moro I. Pulmonary rheumatoid nodules: presentation, methods, diagnosis and progression in reference to 5 cases. Reumatol Clin. 2012;8:212–5. doi: 10.1016/j.reumae.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Ziff M. Rheumatoid nodule. Arthritis Rheum. 1990;33:761–7. doi: 10.1002/art.1780330601. [DOI] [PubMed] [Google Scholar]
- 4.Cortet B, Flipo RM, Rémy-Jardin M, Coquerelle P, Duquesnoy B, Rêmy J, et al. Use of high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:815–9. doi: 10.1136/ard.54.10.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagnoux C, Seror R, Berezne A, Rouabhia S, Goulvestre C, Guillevin L. Remittent non-destructive polysynovitis in p-ANCA-positive vasculitis patients with anti-CCP antibodies. Joint Bone Spine. 2010;77:604–7. doi: 10.1016/j.jbspin.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Chinoy H, Mc Kenna F. Wegener’s granulomatosis in patients with rheumatoid arthritis. Rheumatology (Oxford) 2002;41:588–9. doi: 10.1093/rheumatology/41.5.588. [DOI] [PubMed] [Google Scholar]
- 7.Bosch X, Llena J, Collado A, Front J, Mirapeix E, Ingelmo M, et al. Occurrence of antineutrophil cytoplasmic and antineutrophil (peri)nuclear antibodies in rheumatoid arthritis. J Rheumatol. 1995;22:2038–45. [PubMed] [Google Scholar]
- 8.Corcoran JP, Ahmad M, Mukherjee R, Redmond KC. Pleuro-pulmonary complications of rheumatoid arthritis. Respir Care. 2014;59:55–9. doi: 10.4187/respcare.02597. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Yoo WH. Recurrent pneumothorax associated with pulmonary nodulesafter leflunomide therapy in rheumatoid arthritis: a case reportand review of the literature. Rheumatol Int. 2011;31:919–22. doi: 10.1007/s00296-009-1240-9. [DOI] [PubMed] [Google Scholar]
- 10.Rozin A, Yigla M, Guralnik L, Keidar Z, Vlodavsky E, Rozenbaum M, et al. Rheumatoid lung nodulosis and osteopathy associated with leflunomide therapy. Clin Rheumatol. 2006;25:384–8. doi: 10.1007/s10067-005-0024-1. [DOI] [PubMed] [Google Scholar]
- 11.Be M, Cha HJ, Park C, Park Y, Jung H, Lee Y, et al. Multiple pulmonary cavitary nodules with pyoderma gangrenosum in patient with rheumatoid arthritis. Ann Transl Med. 2016;4:39. doi: 10.3978/j.issn.2305-5839.2016.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi T, Satoh K, Ohkawa M, Satoh A. Multiple rheumatoid nodules with rapid thin-walled cavity formation producing pneumothorax. J Thorac Imaging. 2005;20:47–9. doi: 10.1097/01.rti.0000139387.80395.8a. [DOI] [PubMed] [Google Scholar]
- 13.Yoshikawa GT, Dias GA, Fujihara S, Silva LF, Cruz Lde B, Fuzii HT, et al. Formation of multiple pulmonary nodules during treatment with leflunomide. J Bras Pneumol. 2015;41:281–4. doi: 10.1590/S1806-37132015000004247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotsman I, Goral A, Nusair S. Secondary spontaneous pneumothorax in a patient with pulmonary rheumatoid nodules during treatment with methotrexate. Rheumatology (Oxford) 2001;40:350–1. doi: 10.1093/rheumatology/40.3.350. [DOI] [PubMed] [Google Scholar]
- 15.Ayse B, Veli Ç. Pulmonary nodulosis associated with leflunomide therapy in rheumatoid arthritis: report of four cases and review of the literature. J Clin Exp Invest. 2016;7:98–102. doi: 10.5799/jcei.328697. [DOI] [Google Scholar]
- 16.Martin K, Bentaberry F, Dumoulin C, Dehais J, Haramburu F, Be’gaud B, et al. Effectiveness and safety profile of leflunomide in rheumatoid arthritis: actualpractice compared with clinical trials. Clin Exp Rheumatol. 2015;223:80–84. [PubMed] [Google Scholar]
- 17.Sawada T, Inokuma S, Sato T, Otsuka T, Saeki Y, Takeuchi T, et al. Study committee forleflunomide-induced lung injury, Japan College of Rheumatology. Leflunomide-induced interstitial lung disease: prevalence and risk factors in Japanese patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1069–72. doi: 10.1093/rheumatology/kep052. [DOI] [PubMed] [Google Scholar]
- 18.Braun MG, Van Rhee R, Becker-Capeller D. Development and/or increase of rheumatoid nodules in RA patients following leflunomide therapy. Z Rheumatol. 2004;63:84–7. doi: 10.1007/s00393-004-0537-z. [DOI] [PubMed] [Google Scholar]
- 19.Horvath IF, Szanto A, Csiki Z, Szodoray P, Zeher M. Intrapulmonary rheumatoid nodules in a patient with long-standing rheumatoid arthritis treated with leflunomide. Pathol Oncol Res. 2008;14:101–4. doi: 10.1007/s12253-008-9003-6. [DOI] [PubMed] [Google Scholar]


