Abstract
Luteinizing hormone (LH), produced in the anterior pituitary, has been detected in cadaver eyes and LH receptors (LHRs) have been identified in the retina, with the highest density in cone photoreceptors. Our aim was to confirm the presence of LH in the living, human eye as well as to examine the potential impact of a reduction in LHR signaling on visual processing. Vitreous samples were collected from 40 patients (23 diabetics, 17 non-diabetics) who were undergoing vitrectomies for various indications. LH concentration was quantified in each sample via an electro-chemiluminescence immunoassay and Meso Scale Discovery platform and normalized to total protein. In addition, full field electroretinography (ERG) was performed on 11 adult LHR knockout heterozygous mice (B6;129X1-Lhcgrtm1Zmlei/J) and 11 wildtypes using the Celeris - Diagnosys system. The median LH values (pg/mg total protein) for non-diabetics, diabetics without proliferative diabetic retinopathy (PDR) and diabetics with PDR were 40.7, 41.9 and 167.8 respectively. LH levels were significantly higher in diabetics with PDR. In our ERG investigation, heterozygous LHRKOs were found to have significantly reduced amplitudes of a-wave and b-waves at high stimulus intensities with no significant change in a-wave or b-wave amplitudes at lower intensities; this is consistent with a selective impairment of cone-mediated responses. Our findings confirm LH is present in the adult human eye. Our findings also suggest that a reduction in LH receptor signaling negatively impacts visual processing of the cone photoreceptors. Overall, our study results support the theory that LH likely plays a physiologic role in the eye.
Keywords: photoreceptors, ERG, electroretinography, visual processing, cone cells, luteinizing hormone
Introduction
Luteinizing hormone (LH) belongs to the glycoprotein hormone family which includes follicle stimulating hormone, thyroid stimulating hormone and human chorionic gonadotropin (hCG).(Rao and Lei, 2002) Within this hormone family, hCG, produced by the human placenta during pregnancy, is the most closely related to LH; LH and hCG share a common G-protein associated receptor.(Cole, 2010) From fetal life through adulthood, LH is produced in the anterior pituitary gland of both genders and plays a vital role in the functioning of the gonads. (Melmed, 2011) The ovary and testes are classical targets of LH action; LH stimulates the testes to produce testosterone and the ovaries to produce estrogen and progesterone. (Pierce and Parsons, 1981) Over the years, potential roles for LH outside of the gonadal systems have been suggested but have not been well delineated.(Pakarainen et al., 2005)
In 1986, LH was detected by radioimmunoassay in 11 out of 51 vitreous fluid samples extracted from cadaver eyes before autopsy.(Chong and Aw, 1986) However, it is not known whether the presence of intraocular LH in these cadaver eyes represents a postmortem artifact. Furthermore, the vitreous undergoes substantial biochemical changes and glycoprotein degradation following death.(Kokavec et al., 2016, Monteiro et al., 2015, Ortmann et al., 2016) Therefore, quantitation of LH in cadaver eyes cannot be assumed to reflect physiologic levels in the living eye.
A little over a decade later, in 1998, researchers identified luteinizing hormone receptor (LHR) gene expression in the neural retina.(Thompson et al., 1998) (Dukic-Stefanovic et al., 2012) By this point in time, LHRs had already been identified in numerous extragonadal organs such as the brain, placenta, skin and kidney but the extragonadal function of the LHRs remained elusive.(Huhtaniemi et al., 2002, Pakarainen et al., 2005, Ziecik et al., 1986) LHR transcript levels in the retina were found to be approximately equal to levels present in the cerebral cortex, but at least 100 fold lower than the levels in testis. The density of LHR receptor transcripts, and LHR protein were highest in cone photoreceptor cells, and then decreased throughout the inner retina. (Dukic-Stefanovic et al., 2012, Thompson et al., 1998) These findings raised the possibility that cone photoreceptor cells have the ability to mount cellular responses to LH; thus, the suggestion that intraocular LH may impact visual processing was raised but not fully explored.
The aim of the current study was two-fold. The first aim was to confirm the presence of LH in the living eye. To accomplish this, we assessed LH levels (via an electrochemiluminescence immunoassay developed specifically for this study) in vitreous fluid extracted from 40 live study participants who were undergoing a scheduled vitrectomy for a medical indication; we then compared vitreous LH levels with patient’s age, gender and diabetic status to assess potential correlations. The second aim of the study was to examine the potential physiologic impact of LHR signaling on visual function by comparing electroretinogram (ERG) results from adult heterozygous LHR knockout mice (Lhrko+/−) to their gender-and-age-matched wild type (WT) littermates.
Experimental Procedures:
In this study, the research involving human subjects adhered to the tenets of the Declaration of Helsinski; all procedures were carried out with the adequate understanding and written consent of the subjects. Approval to conduct the human subject study was obtained by the Western Institutional Review Board. The research involving experimental animals was carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Approval to conduct the animal experiments was obtained by the Institutional Animal Care and Use Committee at the University of Michigan. All efforts were made to minimize the number of animals used and their suffering.
Methods for Investigation #1: Identification and Analysis of LH in Human Vitreous Fluid
Vitreous Fluid Collection:
After study approval by Western Institutional Review Board, vitreous samples were collected from 40 adults who were undergoing scheduled vitrectomy surgery (23 diabetics, 17 non-diabetics) by a single retinal surgeon (initials: MDO) at Straith Hospital, Southfield, Ml. All patients had given informed consent to have a sample of their vitreous fluid used for this study. The study intake form included patient’s age, race, gender, type of diabetes and indication for vitrectomy. The de-identified clinical records with pre-surgical retinal exam were also made available. Exclusion criteria included a history of prior vitrectomy or previous ophthalmic surgery or laser therapy within the previous 3 months. Inclusion criteria included adult patients with diagnoses of either proliferative diabetic retinopathy (PDR), rhegmatogenous retinal detachment, visually significant floaters, dislocated intraocular lens, macular hole,or epiretinal membrane (macula pucker) and thus scheduled for a medically-indicated vitrectomy.
A core vitrectomy was performed with a goal of collecting the first 1 cc of undiluted vitreous contents aspirated into a 10 mL syringe. Minimal vitreous cutting was applied at 100 cuts/minute from the central mid or anterior vitreous. The aspirated vitreous was immediately transferred to a polypropylene microcentrifuge tube, before being sealed by an airtight lid. All vitreous samples were frozen at −20°C immediately after extraction and then transferred on ice to the laboratory where they were frozen at −80°C until further analysis.
LH Assay Development
Given the anticipated low concentration of analyte in the vitreous fluid (in the low picogram range) and the small vitreous volume per sample, reliable measurements of LH utilizing commercially available immunoassays in the traditional ELISA format which uses color (Absorbance at 450nm) as the readout would be unlikely. Commercially-available LH ELISA assays have lower limits of quantitation in the range of 300–500 pg/ml. Instead, it was determined that a more sensitive immunoassay using electrochemiluminescence (ECL) technology and the Meso Scale Discovery (MSD) platform (Chaturvedi et al., 2013, Lee et al., 2006, Zhao et al., 2015) would be a more reasonable approach for LH quantification in human vitreous; thus, this approach was chosen for this investigation.(Chaturvedi et al., 2013, Lee et al., 2006, Zhao et al., 2015)
For the LH immunoassay development, we followed the methodology for ECL assay development on the MSD platform as previously described,(Chaturvedi et al., 2013, Lee et al., 2006, Zhao et al., 2015) with details related to capture antibody and detection antibody optimization provided below. The list of antibodies and proteins used for our final LH immunoassay are listed in Table 1. Of important note, LH is a heterodimer consisting of an alpha chain and a beta chain. The alpha chain amino acid sequence of LH is identical to that of human chorionic gonadotropin (hCG) but the beta chains of LH and hCG are unique to each of these two molecules. Thus, our LH immunoassay was designed to differentiate between LH and hCG by using capture antibody specific to the beta chain of the LH heterodimer. Captured LH was detected using an anti-alpha chain antibody.
Table 1:
Antibodies and hormone used in LH immunoassay
| Assay Component |
Antibody/ Hormone |
Clonality | Manufacturer | Product # |
|---|---|---|---|---|
| Capture | Anti-Luteinizing Hormone beta antibody [L1] | Monoclonal | Abcam | ab47112 |
| Calibrator | Luteinizing Hormone-Human Pituitary Glands | NA | Lee Biosolutions | 996–31 |
| 1° Detection Antibody | Affinity Purified Rabbit Anti-αHCG Antibody | Polyclonal | R&D Systems | R-114-C |
| 2° Detection Antibody | SULFO-TAG™ Goat Anti-Rabbit Antibody | Polyclonal | Meso Scale Discovery | R32AB-1 |
Capture antibody coating optimization:
Capture antibody solution coating concentration was optimized using 5 plating concentrations including 4 μg/mL, 2 μg/mL, 1 μg/mL, 0.5 μg/mL and 0.25 μg/mL. Assay conditions used dose response curves for LH starting with a high calibrator concentration of 10 ng/mL with 4 fold serial dilutions through 2.44 pg/mL. Polyclonal rabbit anti-alpha chain detection antibody was used at a concentration of 1 μg/mL. Signal was generated using Sulfo-Tag-goat anti-rabbit antibody at a final concentration of 0.5 μg/mL.
Detection antibody optimization:
Polyclonal rabbit anti-alpha chain detection antibody was evaluated at 3 concentrations including 2 μg/mL, 1 μg/mL and 0.5 μg/mL. Detection antibody concentrations were evaluated in dose response curves using optimal capture antibody plating concentration. Optimal capture and detection antibody concentrations were then used to determine assay’s range and lower limit of quantitation. The assay developed for this study was found to have a lower limit of quantitation of 2.44 pg/mL. In additional assay preparatory work, human vitreous cadaver samples spiked with various concentrations of LH revealed over 97% LH recovery.
LH Quantification in Human Vitreous Samples
LH levels of 40 human vitreous samples were quantified utilizing the LH assay described above. All vitreous samples were thawed on wet ice, vortexed and briefly centrifuged to consolidate the entire sample volume in the bottom of the tube. Each sample was diluted 2 fold into assay buffer prior to being assayed in duplicate. Assay buffer, referred to as diluent in protocol below, was composed of 1% bovine serum albumin (BSA) and 0.05% Tween-20 in 1 X phosphate buffered saline (PBS).
Capture Antibody Solution Coating:
1 standard MSD Plate was pre-wet with 100 μL/well 1X PBS. The capture antibody (Table 1) was diluted to 1 μg/mL in 1X PBS. After PBS removal, 30 μL/well capture antibody solution was added, sealed and incubated overnight at 4° C.
Plate Blocking:
Remaining capture antibody was decanted and 100 μL/well 5% blocker A (5% BSA and 0.05% Tween-20 in 1X PBS) was added, and then sealed and incubated at room temperature with shaking at 650 rpm for 30 min.
Calibrator Preparation:
The human LH (Lee Biosolutions, Product 996–31) was diluted to 10,000 pg/mL in diluent = High Standard. The High standard was serially diluted down 4-fold into diluent (50 μL [previous]: 150 μL diluent) 6 times with the final standard being diluent only.
Human Vitreous Preparation:
The vitreous samples were diluted 2-fold with diluent in 1X PBS (60 μL vitreous: 60 μL diluent), followed by 30-min. incubation with the blocker, and washed 3 times with 200 μL/well 1X Tris buffered saline-Tween-20 (TBST). Then 25 μL/well of appropriate sample/standard was added, sealed and incubated 2 hours at room temperature on a shaker at 650 rpm.
1° Detection Antibody Preparation:
Rabbit anti-αhCG affinity purified antibody (Table 1) was diluted to 0.5 μg/mL in diluent. Twenty-five μL/well of 1° detection antibody was added, sealed and incubated 1 hour at room temperature on a shaker at 650 rpm.
2° Detection Antibody Preparation:
SulfoTag® goat anti rabbit IgG (Table 1) was diluted to 1 μg/mL in diluent. After 1-hour 1° detection antibody incubation, plates were washed 3 times with 200 μL/well 1X TBST. Twenty-five μL/well 2° detection antibody (Table 1) added, sealed and incubated 30 min at room temperature on a shaker at 650 rpm.
1X Read Buffer T Preparation:
Five ml 4X read buffer T (MSD #R92TC-3) was added to 15 mL water (4-Fold dilution). After incubation with 2° detection antibody (Table 1) for 30 min, plates were washed 3 times with 200 μL/well 1X TBST. One hundred fifty μL/well 1X Read Buffer T was added and plates were read on MSD Sector 6000 Imager.Total protein of each vitreous sample was measured by using Micro BSA protein assay kit (Thermo Fisher, Catalog # 23235) according to the manufacture suggested procedure. LH levels were normalized to total protein levels.
Statistics:
Statistical Analytic Software (SAS Institute Inc, Cary NC) was utilized for the statistical analyses. For each vitreous sample group, the 25th percentile, 50th percentile (median) and 75th percentiles of LH values (pg/total mg protein) are reported. Statistical comparisons between groups were performed with the non-parametric, Mann-Whitney U test, with p<0.05 indicating significance.
Methods for Investigation #2: Murine Electroretinography
After animal protocol approval by the Institutional Animal Care and Use Committee at the University of Michigan, 11 heterozygous LHR knockout (Lhrko+/−) mice (B6;129X1-Lhcgrtm1Zmlei/J) and 11 WT littermates were procured from Jackson Laboratory. All 22 JAX mice were 6 weeks old at time of arrival with an equal mix of genders in each group. At the time of this study, homozygous LHRKO mice, devoid of all LHR expression, were not available. Upon arrival, all animals were group housed at Unit of Laboratory Animal Medicine, University of Michigan for 4 weeks, provided ad libitum access to food and water, and maintained on a 12-hour light: 12-hour dark cycle. At ten weeks old, the mice were transferred to the Kellogg Eye Center, University of Michigan for electroretinography (ERG) measurements as a terminal procedure.
Corneal ERGs were recorded from 11 Lhrko+/− mice and 11 WT littermates using the Celeris-Diagnosys ° C platform of the Celeris-Diagnosys system. Under dim red light provided by the overhead lamp of the Celeris-Diagnosys system, tropicamide (1%) and phenylephrine (2.5%) were topically applied to dilate the pupils of both eyes, and a thin layer of hypromellose (2.5%) was applied to keep the eyes moist. After the light guide electrodes had been placed on the hypromellose, the overhead lamp was turned off and recording began 10 min afterward.
The light guide electrode on the right eye was used to present 4-ms full-field light flashes and record ERG responses, whereas the light guide electrode on the left eye served as the reference electrode. For each animal, 635 nm red flashes (11.3 log - 13.4 log photons cm−2 s−1) were first presented to evoke near-threshold ERGs, and 465 nm blue flashes (11.8 log - 15.9 log photons cm−2 s−1) were subsequently delivered to evoke larger responses. Rods (λmax = 498 nm) are 2.8 log units less sensitive to 635 nm than to 465 nm, whereas mid-wavelength cones (λmax = 508 nm) are 2.3 log units less sensitive to 635 nm than to 465 nm. (Jacobs et al., 1991) Voltage signals were sampled at 1 Hz and the recorded responses were either single responses or averages of 2–10 responses depending on signal-to-noise ratios. Inter-stimulus intervals ranged from 10 sec to 3 min depending on stimulus intensity.
The amplitude of the a-wave was measured as the difference between the pre-stimulus baseline and the trough of the a-wave, and a-wave latency was measured as the timing of the trough of the a-wave relative to stimulus onset. For the b-wave, its amplitude was measured from the trough of the a-wave to the peak of the b-wave, and its latency was measured from stimulus onset to the peak of the b-wave. Statistical comparisons used the Mann-Whitney U test, with p<0.05 indicating significance.
Results:
LH analyses in the human vitreous samples
Table 2 displays the demographics of the 40 study participants, of which 65% (26/40) were male, 70% (28/40) were white and 57.5% (23/40) were diabetics. Amongst the diabetic participants, 52.2 % (12/23) had a diagnosis of proliferative diabetic retinopathy (PDR). Amongst the diabetics with PDR, 66.6% (8/12) also had vitreous hemorrhage. Amongst participants with rhegmatogenous retinal detachments, 28.6% (2/7) also had vitreous hemorrhage. LH was successfully detected and quantified in all vitreous samples. Table 3 presents the 25th percentile, 50th percentile (median) and 75th percentile of LH concentrations by diabetic status (grouped by non-diabetics, diabetics without PDR and diabetics with PDR), by gender (subdivided into diabetic status groups) and by age (subdivided into gender groups). The median LH values (pg/mg total protein) for non-diabetics, diabetics without PDR and diabetics with PDR were 40.7, 41.9 and 167.8 respectively. Table 3 also summarizes the group comparison results from Mann-Whitney U testing. Whereas there was no significant difference in intraocular LH levels between diabetics without PDR and non-diabetics [U(11,17)=93], there was significantly higher LH levels in diabetics with PDR compared to non-diabetics [U(12,17)=56]. Within the PDR collection of vitreous samples, female eyes had significantly higher LH values than male eyes. [U(7,5)=2]. Table 4 presents the 25th percentile, 50th percentile (median) and 75th percentile and interquartile range of LH levels by 4 diagnosis types (macular hole, rhegmatogenous retinal detachment, epiretinal membrane, PDR)
Table 2:
Demographics of study participants (N=40)
| Gender | Males: 26 (65%) Females: 14 (35%) |
|
| Race | White: 28 (70%) Black: 12(30%) |
|
| Mean Age (yrs) | 64.2 (SD 10.7) | |
| Diabetic Status | Non-diabetics: 17 (42.5%) DM Type 2: 22(55.0%) DM Type1: 1(2.5%) |
|
| Indication for vitrectomy | Total Proliferative Diabetic Retinopathy (PDR) | 12 (30.0%) |
| • PDR with vitreous heme: 8/12 | ||
| Macular Hole: | 9 (22.5%) | |
| Epiretinal Membrane: | 8 (20.0%) | |
| Rhegmatogenous Retinal Detachment (RRD) | 7 (17.5%) | |
| • RRD with vitreous heme: 2/7 | ||
| Dislocated Intraocular Lens: | 2 (5.0%) | |
| Visually Significant Floaters: | 2 (5.0%) | |
Table 3:
Statistical comparison of vitreous levels by diabetic status, gender and age
| LH: Group 1 Units: pg/mg total protein |
LH: Group 2 Units: pg/mg total protein |
Mann-Whitney U (N1,N2)* |
Significance p<0.05 |
|---|---|---|---|
|
Proliferative diabetic retinopathy (PDR) 25th percentile: 41.6 LH Median: 167.8 75th percentile: 235.4 |
Non-diabetics 25th percentile: 30.2 LH Median: 40.7 75th percentile: 94.5 |
U (12,17) = 56 | YES |
|
Diabetics without PDR** 25th percentile: 28.6 LH Median: 41.9 75th percentile: 60.81 |
Non-diabetics 25th percentile: 30.2 LH Median: 40.7 75th percentile: 94.5 |
U (11, 17) = 93 | NO |
|
Males with PDR 25th percentile: 25.2 LH Median: 51.8 75th percentile: 163.4 |
Females with PDR 25th percentile: 185.5 LH Median: 238.4 75th percentile: 394.5 |
U (7, 5) = 2 | YES |
|
Males without diabetes 25th percentile: 29.7 LH Median: 36.5 75th percentile: 52.79 |
Females without diabetes 25th percentile: 30.8 LH Median: 70.4 75th percentile: 127.4 |
U (10, 7) = 24 | NO |
|
All females below mean female age (<67.8 years) 25th percentile: 59.7 LH Median: 118.7 75th percentile: 278.4 |
All females above mean female age (>67.8 yrs) 25th percentile: 70.4 LH Median: 185.5 75th percentile: 238.4 |
U (8,6)=22 | NO |
|
All males below mean male age (<61.4 yrs) 25th percentile: 28.6 LH Median: 38.3 75th percentile: 58.6 |
All males above mean male age (>61.4 yrs) 25th percentile: 27.0 LH Median: 39.9 75th percentile: 53.3 |
U (10,16) = 65 | NO |
N1= # samples in Group 1; N2= # samples in Group 2
Diabetics without PDR ranged from no retinopathy to non-proliferative retinopathy
Table 4:
25th percentile, 50th percentile (Median) and 75th percentile values of vitreous LH by diagnosis
| LH 25th Percentile |
LH Median |
LH 75th Percentile |
Interquartile Range |
|
|---|---|---|---|---|
| Units: pg/mg total protein | Units: pg/mg total protein | Units: pg/mg total protein | ||
| Macular Holes | 23.4 | 36.0 | 60.8 | 37.4 |
|
Rhegmatous Retinal Detachments |
35.2 | 49.5 | 136.4 | 101.2 |
|
Epiretinal Membranes |
34.0 | 39.9 | 79.1 | 45.1 |
|
Proliferative Diabetic Retinopathy |
41.6 | 167.8 | 235.4 | 193.8 |
Electrophysiological characterization of the Lhrko+/− and WT mice
To examine whether a disruption in LH signaling impacts retinal function, we recorded full-field ERGs from Lhrko+/− mice and their WT siblings, and used the a- and b-waves to assess light-evoked responses of the photoreceptors and ON bipolar cells, respectively. [Of note, ON bipolar cells are defined as bipolar neurons which depolarize in response to light stimulation of their presynaptic photoreceptors.] All recordings were made under dark-adapted conditions to preserve both rod- and cone-mediated responses. Example recordings are shown in Fig. 1 A. Population averages show that a-wave and b-wave latencies at all stimulus intensities were similar between Lhrko+/− and wild-type control mice (Fig. 1 B, D), and the amplitudes of these ERG components were also statistically indistinguishable between the two mouse groups at near-threshold and intermediate intensities (Fig. 1 C, E). But at the three highest intensities tested, both a- and b-waves were ~25% smaller in Lhrko+/− mice than in the controls, and this difference was statistically significant (Fig. 1 C, E), with p-values <0.05 and U11,11 values ranging from 21 to 29.
Fig. 1. Photoresponse deficits in Lhrko+/−mice.
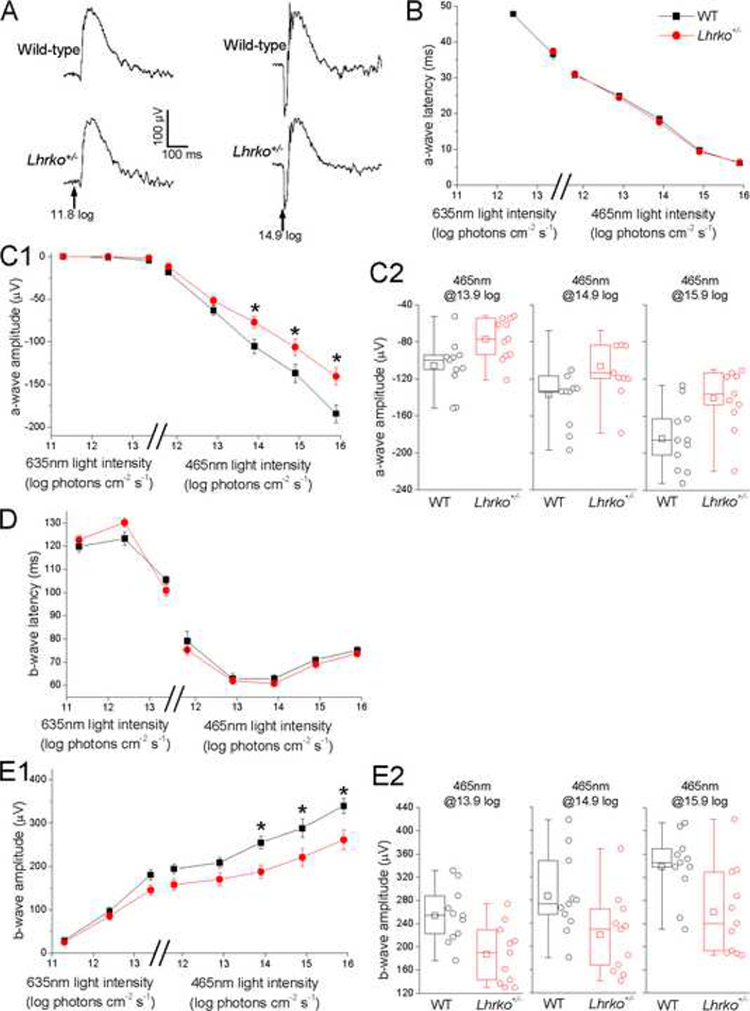
A) Examples of wild-type and Lhrko+/− electroretinogram responses to the 465 nm stimulus at 11.8 log (left) and 14.9 log photons cm−2 s−1 (right). Arrows indicate stimulus timing. B-E) Wild-type vs. Lhrko+/− comparisons in a-wave latency (panel B), a-wave amplitude (panel C), b-wave latency (panel D) and b-wave amplitude (panel E). In the plots in panels B, C1, D and E1, the first three stimuli are 635 nm red light while the rest are 465 nm blue light. The error bars represent SEM. *, p<0.05. Panels C2 and E2 show box-and-whisker plots for the three light intensities at which significant wild-type vs. Lhrko+/− differences were found. The lower and upper limits of each box depict the 25th and 75th percentiles. The horizontal line and square indicate the median and the mean, respectively. The ends of the whiskers represent the minimum and maximum of the data. The open circles depict all data points.
Discussion:
We show for the first time that the pituitary hormone LH is reliably present in the living human eye; we detected and quantified LH in 100% of our vitreous samples. To the best of our knowledge, LH has not been previously catalogued in libraries of the human vitreous proteome. (Monteiro et al., 2015, Murthy et al., 2014, Skeie et al., 2015, Zou et al., 2018) The most likely explanation is that our targeted MSD platform approach enabled much greater sensitivity of detection (in the picogram/mg total protein range) than the protein identification platforms (such as mass spectrometry) used in proteomic studies. For example, when utilizing mass spectrometry, it is imperative to consider not only the starting concentration of the originating sample but also the total amount of analyte that can be introduced into the mass spectrometer. Herein lies the challenge of measuring analytes of extremely low concentration by mass spectrometry in small samples volumes. Furthermore, protein detection in vitreous proteomic studies is first optimized by depletion of high abundant proteins (such as albumin). However, some low abundant proteins are sometimes unintentionally depleted along with the high abundant proteins. (Monteiro et al., 2015, Murthy et al., 2014, Skeie et al., 2015, Zou et al., 2018) It is possible that this may apply to vitreous LH.
Our findings suggest that despite its large molecular size and limited lipid solubility,(Stamatiades and Kaiser, 2017) LH most likely defies the blood-retinal barrier to enter the eye from the peripheral circulation. It has been previously established that (a) LH exerts its effect through binding to its receptor (Liu et al., 1993) and that (b) LHRs are present in the retinas of numerous species ranging from rats to rabbits to bovines to humans.(Dukic-Stefanovic et al., 2012, Thompson et al., 1998) We postulate that once LH enters the posterior segment of the eye, it activates retinal LHRs to elicit a biochemical and/or electrical response. In our second investigation, we show that genetically-altered mice with reduced numbers of LHRs have abnormal ERGs, suggesting that subnormal LHR signaling yields subpar visual processing. (Of note, heterozygous Lhrko mice and littermate WTs do not have significantly different levels of circulating serum LH.(Lei et al., 2001)) Taken together, these observations provide supporting evidence that LH likely plays a physiologic role in the visual system.
For obvious ethical considerations, vitreous samples from live individuals were drawn from patients otherwise undergoing a vitrectomy for retinal pathology. Most studies of the living vitreous proteome restrict their non-diabetic eyes to those with macular holes or macular puckers (also called extra-retinal membranes); the general consensus of the scientific community is that the vitreous proteome of these types of eyes most closely resemble that of the healthy, unadulterated eye.(Monteiro et al., 2015, Murthy et al., 2014, Yamane et al., 2003) However, in addition to macular holes and macular puckers, we intentionally included samples from vitrectomies performed for a variety of indications such as rhegmatogenous retinal detachments, and retained intraocular lenses. The reason for this is that retinal LH receptors are primarily expressed by cone photoreceptors near the macula.(Dukic-Stefanovic et al., 2012, Thompson et al., 1998) Thus, macular holes and macular puckers may potentially impact retinal LHR expression, which in turn, may or may not impact vitreous LH concentration. Furthermore, in contrast to normal eyes, all macular puckers and some macular holes involve the migration and proliferation of a wide variety of cell types from ocular tissues. (Bringmann and Wiedemann, 2009) Therefore, we included eyes with a variety of pathologies to diversify the representation of vitreous samples.
The vitreous is a non-homogenous, hydrated matrix containing >1200 proteins; the concentration of vitreous proteins differs substantially from one individual to the next under both physiologic and pathologic circumstances. (Monteiro et al., 2015, Murthy et al., 2014, Simo-Servat et al., 2012, Yamane et al., 2003) For example, protein concentrations are substantially higher in eyes with PDR compared to non-diabetic eyes.(Simo-Servat et al., 2012) Perhaps, there are also higher protein concentrations in vitreous samples from eyes with retinal detachments than in eyes with macula holes. Consistent with methods employed by other vitreous proteomic researchers,(Simo-Servat et al., 2012) we corrected for this by normalizing the vitreous LH levels to total protein content. In recent years, normalization of analyte concentrations to total protein content is being performed in a number of non-homogenous matrices such as urine, whole blood spots and whole fetal eyes(Leviton et al., 2017, Ma et al., 2015). We found that after normalization to total protein content, the median LH levels (pg/mg total protein) for the diagnoses of macular holes, rhegmatogenous retinal detachments and epiretinal membranes were remarkably similar in magnitude (36.0, 49.5 and 39.9 respectively). Of these diagnoses, the interquartile range was highest for rhegmatogenous retinal detachments; this is expected given the greater variation in retinal detachment pathology (due to variety of location, extent, and chronicity of detachment) compared to epiretinal membranes and macular holes.
In this study, we show that PDR is significantly associated with higher levels of vitreous LH. This finding is particularly intriguing since both LH and its placental analog hCG (which binds to the same receptor) have been shown to promote angiogenesis in several tissue types with concomitant activation of vascular endothelial growth factor (VEGF) expression.(Babitha et al., 2014, Cole, 2010, Licht et al., 1998, Rasmussen et al., 2010, Trau et al., 2015, Tsampalas et al., 2010) Our lab has just published a study demonstrating a strong, significant correlation between vitreous levels of LH and VEGF in healthy bovine and porcine eyes. (Movsas et al., 2018) Elevated levels of intraocular VEGF are strongly implicated in the progression of PDR.(Aiello et al., 1994, Caldwell et al., 2003, Semeraro et al., 2014, Tarr et al., 2013) Increased expression of intraocular VEGF in PDR is mainly attributed to retinal ischemia.(Wirostko et al., 2008) However, overstimulation of the retinal LH receptors (presumably by supraphysiologic intraocular LH levels) may also potentially contribute to intraocular VEGF overexpression and thus, participate in retinopathy progression. Studies in our lab are being planned to investigate this.
We partially attribute the increased LH levels in PDR to be secondary to the deterioration of the blood-retina barrier; the compromised blood-retinal barrier in PDR most likely allows increased amounts of LH to enter the eye. Furthermore, in PDR accompanied by vitreous heme, additional LH probably escapes from hemorrhaging vessels to further increase intraocular levels. However, since our total number of PDR cases (N=12) was relatively small and vitreous heme complicated the majority of these cases (8/12), our study was not powered to examine the relative contribution from each of these etiologies.
By and large, the composition of human vitreous is described as largely similar in males and (non-pregnant) females.(Kokavec et al., 2016) Our study illustrates an exception to this; our findings show that LH levels are significantly elevated in female eyes with PDR compared to male eyes with PDR. Given that mean serum LH values are sex-related,(Melmed, 2011) this result is not especially surprising. Though we did not show a significant sex-related LH difference in non-diabetic eyes, the median LH level in female non-diabetic eyes (70.4 pg/mg protein) is almost twice that of male non-diabetic eyes (36.5 pg/mg protein). Perhaps, with a larger sample size, we would have been able to demonstrate a significant sex-related difference in non-diabetic eyes as well. Potential sex-related correlations between serum LH levels and intraocular LH levels and possible sex-related differences in density of intraocular LHRs will require future investigations.
In men, serum LH levels gradually and continually increase with age whereas in women, serum LH markedly increases after menopause and then levels off. (Melmed, 2011) We examined age in our study by calculating the mean age of male participants (61.4 years) and mean age of female participants (67.8 years). In gender-specific groups, we compared LH levels for those below gender-specific mean age to those above. We did not find that above mean age versus below mean age is a significant determinant of vitreous LH levels. That said, this study was not powered to detect subtle difference in vitreous LH levels that may possibly arise in the aging male. In addition, virtually all female participants in this study were already post-menopausal.
We utilized electroretinography, an effective tool in assessing in vivo retinal activity,(Bouskila et al., 2014, Marmor et al., 2009) to examine the impact of reduced LHR expression on visual processing. Although the ERG is a composite signal generated by the entire retina, the a- wave and b-wave of the ERG emerge from different levels of retinal processing; the a-wave is primarily generated by the photoreceptors, whereas the b-wave is mainly generated by retinal cells that are post-synaptic to the photoreceptors (such as the bipolar, amacrine and Muller cells).(Bouskila et al., 2014, Marmor et al., 2009) Thus, the ERG allows distinctions to be made between contributions from various retinal cell types. Lhrko heterozygous mice (with 50% reduction in global LHR expression) were found to have significantly reduced amplitudes of both a-wave and b-waves at high stimulus intensities (compared to their WT counterparts) with no significant change in either a-wave or b-wave amplitudes at lower intensities; this indicates a selective impairment of cone-mediated responses. At these intensities, both a- and b-waves were reduced by comparable amounts (~25%), suggesting that the photoresponse impairment was probably initiated within the cones and subsequently propagated to postsynaptic bipolar cells.
Overall, our ERG results suggest that LH signaling in normal retinas may potentially serve to enhance the gain of the phototransduction cascade in cones; these findings are consistent with previously published findings that the density of LHR receptor transcripts, and LHR protein are highest in cone photoreceptor cells.(Thompson et al., 1998) (Dukic-Stefanovic et al., 2012) Owing to the homology between retinal tissue and neural tissue, we may expect Lhrko heterozygous to have a similar reduction in neural activity in the hippocampus, the area of the brain with the highest density of LHRs.(Lei et al., 1993) In fact, a prior study did indeed show a similar reduction (approx. 23%, p<0.05) in hippocampally-associated cognitive performance (as measures by the Y-maze task(Lalonde, 2002)) in Lhrko heterogenous mice compared to WT littermates.(Casadesus et al., 2007)
Given that LH is known to induce VEGF expression (Babitha et al., 2014, Cole, 2010, Licht et al., 1998, Rasmussen et al., 2010, Trau et al., 2015, Tsampalas et al., 2010) and that LHRs are located on the cone photoreceptors (Dukic-Stefanovic et al., 2012, Thompson et al., 1998), we propose that LH may participate in maintenance of homeostatic VEGF levels necessary for healthful cone function. Targeted VEGF deletion has been shown to causes rapid dysfunction of cone photoreceptors.(Kurihara et al., 2012). Supporting this hypothesis, we have recently shown that vitreous levels of VEGF and LH are strongly correlated in healthy, mammalian eyes.(Movsas et al., 2018) Perhaps a neuroendocrine circuit exists in the retina which involves signaling from cone photoreceptors cells (site of LH receptors) to VEGF-producing cells (RPE, astrocytes, Muller cells, vascular endothelial cells, ganglion cells and ciliary epithelium.) Further studies will be needed to characterize this potential circuit.
In summary, we have successfully confirmed that LH is reliably present in the living, adult human eye and that proliferative diabetic retinopathy is significantly associated with increased intraocular LH concentration. When proliferative diabetic retinopathy is present, female gender is an additional significant risk factor for increased intraocular LH. Furthermore, we have shown that a deficiency in LH receptors negatively impacts visual processing, particularly of the cone photoreceptors. Our study supplies supporting evidence that LH potentially plays a role in ocular physiology, possibly via enhancement of the phototransduction cascade in cone photoreceptors. Further studies exploring the effect of LHR signaling on retinal function are indeed warranted.
Highlights:
Luteinizing hormone (LH) is reliably present in all living, adult human eyes.
Proliferative diabetic retinopathy (PDR) is associated with increased vitreous LH
In eyes with PDR, there are increased levels of vitreous LH in females
A deficiency in LH receptors negatively impacts visual processing.
LH possibly enhances the cone phototransduction cascade.
Acknowledgements:
We would like to acknowledge Rose Colton and Lea McTavish for their meticulous handling of the vitreous samples and Chrystal Hurst, Rachel Russell, Lindsey Allen and Angela Rosen for their expert care and handling of the animals. State of Michigan TCA/SCIP grant funds, NIH grant funds (NEI Grant # R43EY026281–01) and University of Michigan’s Vision Research Core grant, NIH P30 EY007003 funded the study. T. Movsas conceived and designed the study, participated in data analysis and wrote the manuscript. K. Wong performed and analyzed the ERGs and contributed to writing of the manuscript. M. Ober helped design and perform human vitreous investigation as well as edit the manuscript. R. Sigler helped with study design, data analysis and editing of the manuscript. Z. Lei, the inventor of the lhrko mice, contributed his expertise in this regard and critically edited the manuscript. A. Muthusamy performed the vitreous laboratory analyses and critically edited the manuscript.
Abbreviations
- BSA
bovine serum albumin
- ECL
electrochemiluminescence
- ERG
electroretinography
- hCG
human chorionic gonadotropin
- LH
luteinizing hormone
- LHR
luteinizing hormone receptor
- Lhrko +/−
Heterozygous luteinizing hormone receptor knock out
- LLOQ
lower limit of quantitation
- MSD
Meso Scale Discovery
- PBS
phosphate buffered saline
- PDR
proliferative diabetic retinopathy
- TBST
Tris buffered saline-Tween-20
- VEGF
Vascular endothelial growth factor
- WT
wild type
Footnotes
Potential Conflicts of Interest:
Dr. Tammy Z Movsas is the Director and Principal Investigator at Zietchick Research Institute (ZRI), a private research institute, and has a pending patent application for the use of gonadotropin antagonists for the treatment of ocular disorders. Dr. Arivalagan Muthusamy is a salaried employee at Zietchick Research Institute. The other co-authors are not employees of Zietchick Research Institute nor do they have any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, et al. (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487. [DOI] [PubMed] [Google Scholar]
- Babitha V, Yadav VP, Chouhan VS, Hyder I, Dangi SS, Gupta M, Khan FA, Taru Sharma G, et al. (2014) Luteinizing hormone, insulin like growth factor-1, and epidermal growth factor stimulate vascular endothelial growth factor production in cultured bubaline granulosa cells. Gen Comp Endocrinol 198:112. [DOI] [PubMed] [Google Scholar]
- Bouskila J, Javadi P, Palmour RM, Bouchard JF, Ptito M (2014) Standardized full-field electroretinography in the Green Monkey (Chlorocebus sabaeus). PLoS One 9:e111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Wiedemann P (2009) Involvement of Muller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol 247:865–883. [DOI] [PubMed] [Google Scholar]
- Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Caldwell RW (2003) Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 19:442–455. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, et al. (2007) Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol 269:107–111. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, Dell E, Siegel D, Brittingham G, Seetharam S (2013) Development of a rapid streptavidin capture-based assay for the tyrosine phosphorylated CSF-1R in peripheral blood mononuclear cells. Int J Biol Sci 9:1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong A, Aw S (1986) Postmortem endocrine levels in the vitreous humor. Ann Acad Med Singapore 15:606–609. [PubMed] [Google Scholar]
- Cole LA (2010) Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol 8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukic-Stefanovic S, Walther J, Wosch S, Zimmermann G, Wiedemann P, Alexander H, Claudepierre T (2012) Chorionic gonadotropin and its receptor are both expressed in human retina, possible implications in normal and pathological conditions. PLoS One 7:e52567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I, Zhang FP, Kero J, Hamalainen T, Poutanen M (2002) Transgenic and knockout mouse models for the study of luteinizing hormone and luteinizing hormone receptor function. Mol Cell Endocrinol 187:49–56. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J, Deegan JF 2nd, (1991) Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature 353:655–656. [DOI] [PubMed] [Google Scholar]
- Kokavec J, Min SH, Tan MH, Gilhotra JS, Newland HS, Durkin SR, Grigg J, Casson RJ (2016) Biochemical analysis of the living human vitreous. Clin Exp Ophthalmol 44:597–609. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M (2012) Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest 122:4213–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R (2002) The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26:91–104.. [DOI] [PubMed] [Google Scholar]
- Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, Keller S, Weinryb I, et al. (2006) Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res 23:312–328. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV (2001) Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol 15:184–200. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES (1993) Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology 132:2262–2270. [DOI] [PubMed] [Google Scholar]
- Leviton A, Ryan S, Allred EN, Fichorova RN, Michael O’Shea T, Kuban K, Dammann O, Investigators ES (2017) Antecedents and early correlates of high and low concentrations of angiogenic proteins in extremely preterm newborns. Clin Chim Acta 471:1–5. [DOI] [PubMed] [Google Scholar]
- Licht P, Losch A, Dittrich R, Neuwinger J, Siebzehnrubl E, Wildt L (1998) Novel insights into human endometrial paracrinology and embryo-maternal communication by intrauterine microdialysis. Hum Reprod Update 4:532–538. [DOI] [PubMed] [Google Scholar]
- Liu X, Davis D, Segaloff DL (1993) Disruption of potential sites for N-linked glycosylation does not impair hormone binding to the lutropin/choriogonadotropin receptor if Asn-173 is left intact. J Biol Chem 268:1513–1516. [PubMed] [Google Scholar]
- Ma IT, McConaghy S, Namachivayam K, Halloran BA, Kurundkar AR, MohanKumar K, Maheshwari A, Ohls RK (2015) VEGF mRNA and protein concentrations in the developing human eye. Pediatr Res 77:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M, International Society for Clinical Electrophysiology of V (2009) ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol 118:69–77. [DOI] [PubMed] [Google Scholar]
- Melmed SP, Kenneth S.; Larsen P. Reed; Kroneberg Henry M., Williams Textbook of Endocrinology, 12th Edition ed., Elsevier, Philadelphia, PA, 2011. [Google Scholar]
- Monteiro JP, Santos FM, Rocha AS, Castro-de-Sousa JP, Queiroz JA, Passarinha LA, Tomaz CT (2015) Vitreous humor in the pathologic scope: insights from proteomic approaches. Proteomics Clin Appl 9:187–202. [DOI] [PubMed] [Google Scholar]
- Movsas TZ, Sigler R, Muthusamy A (2018) Vitreous levels of luteinizing hormone and VEGF are strongly correlated in healthy mammalian eyes. Curr Eye Res DOI 10.1080/02713683.2018.1467932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KR, Goel R, Subbannayya Y, Jacob HK, Murthy PR, Manda SS, Patil AH, Sharma R, et al. (2014) Proteomic analysis of human vitreous humor. Clin Proteomics 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann J, Markwerth P, Madea B (2016) Precision of estimating the time since death by vitreous potassium-Comparison of 5 different equations. Forensic Sci Int 269:1–7. [DOI] [PubMed] [Google Scholar]
- Pakarainen T, Zhang FP, Poutanen M, Huhtaniemi I (2005) Fertility in luteinizing hormone receptor-knockout mice after wild-type ovary transplantation demonstrates redundancy of extragonadal luteinizing hormone action. J Clin Invest 115:1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF (1981) Glycoprotein hormones: structure and function. Annu Rev Biochem 50:465–495. [DOI] [PubMed] [Google Scholar]
- Rao CV, Lei ZM (2002) Consequences of targeted inactivation of LH receptors. Mol Cell Endocrinol 187:57–67. [DOI] [PubMed] [Google Scholar]
- Rasmussen KL, Laugesen CS, Ringholm L, Vestgaard M, Damm P, Mathiesen ER (2010) Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia 53:1076–1083. [DOI] [PubMed] [Google Scholar]
- Semeraro F, Cancarini A, Morescalchi F, Romano M, dellaEl Omo R, Ruggeri G, Agnifili L, Costagliola C (2014) Serum and intraocular concentrations of erythropoietin and vascular endothelial growth factor in patients with type 2 diabetes and proliferative retinopathy. Diabetes Metab 40:445–451. [DOI] [PubMed] [Google Scholar]
- Simo-Servat O, Hernandez C, Simo R (2012) Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediators Inflamm 2012:872978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeie JM, Roybal CN, Mahajan VB (2015) Proteomic insight into the molecular function of the vitreous. PLoS One 10:e0127567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatiades GA, Kaiser UB (2017) Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol Cell Endocrinol DOI 10.1016/j.mce.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R (2013) Pathophysiology of diabetic retinopathy. ISRN ophthalmology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Othman M, Lei Z, Li X, Huang Z-H, Eadie D, Rao CV (1998) Localization of receptors for luteinizing hormone/chorionic gonadotropin in neural retina. Life sciences 63:1057–1064. [DOI] [PubMed] [Google Scholar]
- Trau HA, Davis JS, Duffy DM (2015) Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol Reprod 92:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier d’Hauterive S (2010) Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod Immunol 85:93–98. [DOI] [PubMed] [Google Scholar]
- Wirostko B, Wong TY, Simó R (2008) Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res 27:608–621. [DOI] [PubMed] [Google Scholar]
- Yamane K, Minamoto A, Yamashita H, Takamura H, Miyamoto-Myoken Y, Yoshizato K, Nabetani T, Tsugita A, et al. (2003) Proteome analysis of human vitreous proteins. Mol Cell Proteomics 2:1177–1187. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ayanoglu G, Bilardello M, Fayadat-Dilman L, Zhang JH, Li HL, Bald L, McClanahan T, et al. (2015) A clinical biomarker assay to quantitate thymic stromal lymphopoietin in human plasma at sub-pg/ml level. Bioanalysis 7:573–582. [DOI] [PubMed] [Google Scholar]
- Ziecik AJ, Stanchev PD, Tilton JE (1986) Evidence for the presence of luteinizing hormone/human chorionic gonadotropin-binding sites in the porcine uterus. Endocrinology 119:1159–1163. [DOI] [PubMed] [Google Scholar]
- Zou C, Han C, Zhao M, Yu J, Bai L, Yao Y, Gao S, Cao H, et al. (2018) Change of ranibizumab-induced human vitreous protein profile in patients with proliferative diabetic retinopathy based on proteomics analysis. Clin Proteomics 15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]


