Abstract
Background
The HALT PKD trial in early autosomal dominant polycystic kidney disease (ADPKD) showed that intensive control of systolic blood pressure to 95-110 mmHg was associated with a 14% slower rate of kidney volume growth compared to standard control. It is unclear whether this result was due to greater blockade of the renin-angiotensin-aldosterone system (RAAS) by allowing the use of higher drug doses in the low blood pressure arm, or due to the lower blood pressure per se.
Methods
In this secondary analysis of HALT PKD Study A, we categorized participants into high and low dose groups based on the median daily equivalent dose of RAAS blocking drugs used after the initial dose titration period. Using linear mixed models, we compared the percent change in total kidney volume and the slope of estimated glomerular filtration rate (eGFR) between the 2 groups. We also assessed the effects of time-varying dose and time-varying blood pressure parameters on these outcomes.
Results
Subjects in the high dose group (n=252) did not experience a slower increase in total kidney volume than those in the low-dose (n=225) group, after adjustment for age, sex, genotype, and BP arm. The chronic slope of eGFR decline was similar in the 2 groups. Higher time-varying systolic blood pressure was associated with a steeper decline in eGFR.
Conclusion
ADPKD progression (as detected by eGFR decline and TKV increase) was ameliorated by intense blood pressure control as opposed to pharmacologic intensity of RAAS blockade.
Keywords: Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, autosomal dominant polycystic kidney disease, estimated glomerular filtration rate, HALT PKD trials, total kidney volume
1. Introduction
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is the most common potentially fatal monogenic disease, estimated to affect 1:500 to 1:1000 people in the United States [1, 2], and is the cause of 4-10% of all End-Stage Renal Disease (ESRD) cases worldwide [3]. Hypertension is an almost universal clinical manifestation, often developing in the second or third decade of life [4-6]. The early onset of hypertension has been attributed, among other factors, to activation of the Renin-Angiotensin-Aldosterone System (RAAS) by the enlarging cysts [7-10]. Treatment with RAAS blocking drugs reduced the progression of renal disease in animal models of ADPKD [11-13]. Angiotensin-Converting Enzyme Inhibitors (ACEI) and Angiotensin Receptor Blockers (ARB) have become first-line drugs to treat hypertension in ADPKD, based on experimental data and their benefits for cardiovascular disease [14-17].
The HALT PKD trials were designed to test the hypothesis that increased intensity of Blood Pressure (BP) control using primarily RAAS blocking drugs would decrease renal cystic growth and delay or slow the decline in renal function [18-20]. Study A (Early ADPKD) included young to middle-aged adults with preserved renal function and showed that randomization to a very low BP goal (95/60-110/75 mmHg) was associated with a statistically significant 14.2% slower annual increase in Total Kidney Volume (TKV) than randomization to the standard BP goal of 120/70-130/80 mmHg [19]. Because subjects in the low BP arm received higher doses of RAAS blocking drugs, it remains unclear whether the observed difference in renal enlargement was due to the lower BP per se, or due to more intensive blockade of the RAAS by allowing the use of higher drug doses.
We undertook this secondary analysis of HALT PKD Study A to determine whether the dosage of RAAS blocking drugs was associated with the rate of TKV growth or decline in estimated glomerular filtration rate (eGFR) independent of assignment to the low or standard BP arm. We also tested whether lower achieved time-varying BP was correlated with percent change in TKV or with the slope of eGFR decline, independent of the amount of RAAS blocking medications used.
2. Methods
The design of the HALT PKD trials (ClinicalTrials.gov NCT00283686 and NCT01885559) and primary results have been published [18-20]. The trials adhered to the Declaration of Helsinki and were approved by the Institutional Review Boards of each center. Briefly, HALT PKD Study A recruited 558 subjects with early ADPKD, i.e. age 15-49 years and preserved renal function (eGFR > 60 ml/min/1.73m2) [19]. With a 2x2 factorial design, subjects were randomized to a low BP goal (95/60-110/75 mmHg) or to the standard BP goal (120/70-130/80 mmHg) and to either lisinopril (2.5 to 40 mg daily) and placebo (40 or 80 mg daily) or lisinopril (2.5 to 40 mg daily) and telmisartan (40 or 80 mg daily), with other medications added as needed to achieve the assigned BP goal as defined by the mean of several standardized home BP recordings (3 sets of sitting BPs twice a day for at least 5 days every 3 months). The protocol specified the use of hydrochlorothiazide at 12.5 to 25 mg daily as the first add-on medication, followed by metoprolol, followed by calcium channel blockers or other vasodilators if needed. Follow-up was 5-8 years.
The primary outcome for Study A was percent annual change in TKV measured by Magnetic Resonance Imaging (MRI), which was performed at baseline and after 2, 4 and 5 years using methods established by the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) [21]. Secondary outcomes were the rate of change in eGFR, renal blood flow as determined by MRI [21], Left Ventricular Mass Index (LVMI) measured by cardiac MRI, change in albuminuria and change in 24-hour urinary excretion of aldosterone. Serum creatinine was determined by Isotope Dilution Mass Spectrometry (IDMS) at the central laboratory of the Cleveland Clinic at baseline, at 4 and 12 months, then every 6 months for up to 8 years, and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD EPI) equation [22]. Mutation analysis was performed in all consenting subjects as described by Heyer et al. [23].
To determine the intensity of drug dosing, we used an approach similar to the Defined Daily Dose (DDD) categorization adopted by the World Health Organization for drug utilization studies [24]. In its last update from December 20, 2017, the DDD for lisinopril is 10 mg and for telmisartan 40 mg, reflecting the most commonly used doses, not necessarily therapeutically equivalent doses. Because we considered half maximum doses of each drug to be equivalent in terms of RAAS blockade, we defined 20 mg lisinopril as one DED (daily equivalent dose) and 40 mg telmisartan as one DED. Therefore a subject prescribed 40 mg lisinopril plus 80 mg telmisartan would be taking 4 DED, whereas 40 mg lisinopril plus 80 mg placebo would count as 2 DED (because placebo is 0 DED), and 10 mg lisinopril plus 40 mg telmisartan would be 1.5 DED.
We then divided Study A participants into 2 groups based on the DED they were taking at month 4, i.e. after the initial dose titration period. Subjects taking the median DED or more constituted the high dosage group, those taking less than the median DED comprised the low dosage group. Dose information at month 4 was missing for 81 subjects, therefore this analysis was performed on 477 study participants.
2.1. Statistical Analysis
Based on the median DED of 1.25 at 4 months, participants were grouped into high (> 1.25 DED; n = 252) and low (< 1.25 DED; n = 225) dosage groups for the main analysis. We compared the baseline demographic and clinical characteristics of participants between the dosage groups using two-sample t-test and Chi Square tests of significance, or their nonparametric counterparts when necessary. Several variables such as TKV, urine aldosterone and urine albumin were log-transformed in order to normalize. All statistical analyses were performed using SAS 9.4.
Linear mixed models were used to assess whether dosage group was predictive of outcomes (LnTKV and post 4-month eGFR slope). Predictors included month, month-by-BP arm, DED group, month-by-DED group, age, gender, and genotype. The latter 3 covariates were included because they predict TKV growth in ADPKD. For eGFR, only post-baseline measures were analyzed. For the LnTKV model, we also included the main effect of BP arm as a covariate due to baseline imbalances. Of interest was whether the interaction between month and DED group was significant.
Additionally, we assessed whether gender moderated the effect of DED group on the outcomes of TKV and eGFR. The same linear mixed models above were augmented with an interaction term between gender and DED group as well as the three-way interaction between gender, DED group, and month.
For sensitivity analyses, we ran the same models for TKV and eGFR using quartiles of DED and comparing participants in the lowest DED quartile with those in the highest. Similarly, we compared TKV and eGFR slopes between individuals taking < 1 DED and those taking > 3 DED.
We also determined whether time-varying DED (i.e. using DED measurements across time points) or time-varying
achieved home blood pressures (systolic, diastolic, mean) were correlated with annual percent increase in TKV or post 4-month eGFR slope. Similar linear mixed models as above were run with DED and home blood pressures as time-varying covariates instead of as cross-sectional covariates.
3. Results
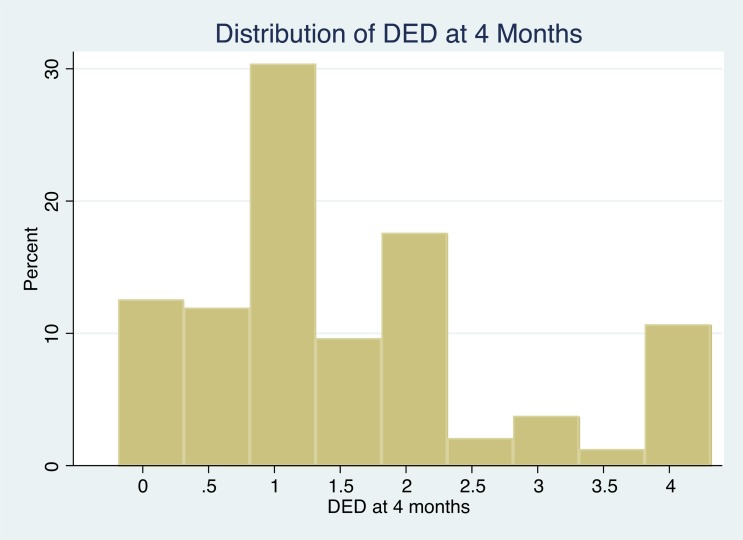
The median DED at the end of the 4-month medication titration period was 1.25, and only 10.5% of HALT A participants were taking the full doses of both lisinopril and telmisartan (Fig. 1). Mean DED in the low dose group was 0.7 versus 2.3 in the high dose group, a good separation. Baseline characteristics of subjects in the low and high DED groups are shown in Table 1. The high dose group had more men than the low dose group (65 vs 42%), and as expected, two thirds of subjects in the high DED group had been randomized to the low BP goal compared to only 35% of those in the low DED group. Participants in the high DED group had a higher body mass index and higher systolic and diastolic home BP at baseline, before randomization. They also had higher left ventricular mass index, higher renal blood flow, and lower urinary aldosterone excretion.
Fig. (1).
Histogram of DED (daily equivalent dose of RAAS-blocking medication) distribution at month 4 (i.e. after dose titration) among 477 participants in HALT PKD Study A. Shown on the X-axis are the dose increments from 0 to the maximum of 4, and on the Y-axis the percentage of participants taking a particular DED.
Table 1.
Baseline characteristics of Study A participants by dosage group.
| - | - |
Below Median
DED (<1.25) at month 4 (n=225) Mean DED 0.68 |
At or Above Median DED (≥1.25) at month 4 (n=252) Mean DED 2.33 | |
|---|---|---|---|---|
| Measure | Category | n (%) | n (%) | P Chisq |
| Sex | Male Female |
95 (42.2%) 130 (57.8%) |
164 (65.1%) 88 (34.9%) |
< 0.001 |
| PKD genotype – 4 levels | NMD | 22 (10.2%) | 17 (6.9%) | 0.27 |
| PKD1-NT | 56 (25.9%) | 75 (30.4%) | ||
| PKD1-T | 107 (49.5%) | 110 (44.5%) | ||
| PKD2 | 31 (14.4%) | 45 (18.2%) | ||
| Previous use of any ARB | Yes | 38 (18.4%) | 46 (19.2%) | 0.83 |
| Previous use of any ACE-Inhibitor | Yes | 96 (46.6%) | 126 (52.7%) | 0.20 |
| Treatment group | Lis + Tel Lis + Placebo |
83 (36.9%) 142 (63.1%) |
158 (62.7%) 94 (37.3%) |
< 0.001 |
| Blood pressure group | Low BP Standard BP |
79 (35.1%) 146 (64.9%) |
166 (65.9%) 86 (34.1%) |
< 0.001 |
| MRI Class ǂ | 1A + 2A | 32 (14.3%) | 20 (8.0%) | 0.09 |
| 1B + 1C | 118 (52.9%) | 142 (56.8%) | ||
| 1D + 1E | 73 (32.7%) | 88 (35.2%) | ||
| Measure (continuous) at baseline | Mean ± SD | Mean ± SD | ProbF | |
| Age (years) | 37.0 ± 8.1 | 36.8 ± 8.2 | 0.77 | |
| BMI (kg/m2) | 26.8 ± 5.1 | 28.0 ± 5.1 | 0.01 | |
| Average home systolic BP (mmHg) | 121.1 ± 7.2 | 127.6 ± 9.7 | < 0.001 | |
| Average home diastolic BP (mmHg) | 81.8 ± 7.0 | 84.1 ± 7.8 | < 0.01 | |
| CKD EPI eGFR (mL/min/1.73 m2) | 92.3 ± 17.0 | 89.4 ± 17.3 | 0.06 | |
| Urine aldosterone (µg/24 hrs) | 13.1 ± 10.2 | 11.1 ± 9.4 | 0.01* | |
| Urine sodium (mEq/24 hrs) | 177.8 ± 82.9 | 183.2 ± 79.4 | 0.48 | |
| Urine albumin (mg/24 hrs): Median (p25, p75) | 19.6 (12.2, 32.4) | 17.7 (11.8, 33.3) | 0.55* | |
| Total kidney volume (mL) | 1186.3 ± 737.1 | 1304.1 ± 741.3 | 0.02* | |
| Height-adjusted TKV (mL/m) | 682.1 ± 409.6 | 736.1 ± 409.1 | 0.05* | |
| Renal blood flow (mL/min/1.73 m2) | 576.5 ± 197.9 | 625.8 ± 210.9 | 0.03 | |
| Left ventricular mass index (g/m2) | 61.4 ± 12.2 | 67.1 ± 12.0 | < 0.001 | |
*p value from log-transformed variable.
Baseline age, height-adjusted TKV (htTKV), urinary albumin excretion, eGFR, and distribution of genotypes and MRI classes (according to the Irazabal [25] imaging classification) were not significantly different between the high and low DED groups (Table 1).
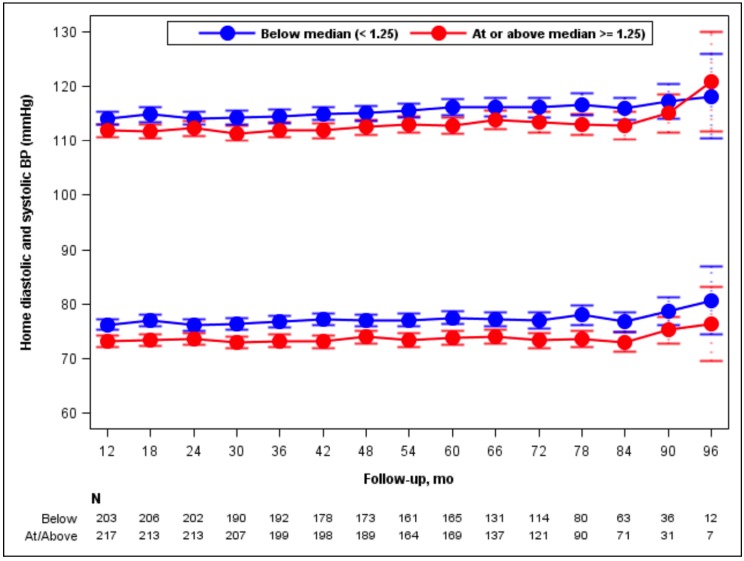
Averaged home BPs during the trial were slightly higher in the low compared to the high DED group (115 ± 8 vs 113 ± 8.5 mmHg for systolic [p = 0.002] and 77 ± 6.5 vs 74 ± 7 mmHg for diastolic BP [p < 0.001]; Fig. 2), but the changes of systolic and diastolic BP over time were not different between the groups. Urine aldosterone levels declined similarly between baseline and month 4 in the 2 DED groups (Table 2). Diuretic use during the trial was more common in the high than the low DED group (Table 2).
Fig. (2).
Mean systolic and diastolic blood pressures over time (month 12-96) among participants classified at 4 months as High DED or Low DED. Bars indicate 95% confidence intervals. Numbers of participants in the 2 groups who reported their home blood pressures at the indicated time points are stated at the bottom of the figure.
Table 2.
Characteristics of Study A participants at month 4 by dosage group.
|
Below Median
DED (<1.25) (n=225) |
At or Above Median
DED (≥1.25) (n=252) |
||||
|---|---|---|---|---|---|
| Measure at month 4 | n | Mean ± SD | n | Mean ± SD | ProbF |
| Average home systolic BP (mmHg) | 221 | 114.0 ± 8.2 | 242 | 113.1 ± 9.0 | 0.29 |
| Average home diastolic BP (mmHg) | 221 | 76.7 ± 7.1 | 242 | 74.2 ± 7.5 | < 0.001 |
| CKD EPI eGFR (mL/min/1.73 m2) | 225 | 91.4 ± 17.5 | 251 | 86.3 ± 19.1 | < 0.01 |
| Lisinopril dose (mg/day) | 175 | 8.9 ± 6.6 | 248 | 27.9 ± 14.2 | < 0.001 |
| Telmisartan dose (mg/day | 217 | 45.4 ± 15.5 | 251 | 68.6 ± 18.0 | < 0.001 |
| Urine aldosterone (µg/24 hrs) | 214 | 10.1 ± 8.0 | 231 | 8.4 ± 10.9 | 0.06 |
| Urine sodium (mEq/24 hrs) | 214 | 179.3 ± 72.6 | 237 | 193.0 ± 74.3 | 0.05 |
| Urine albumin (mg/24 hrs): Median (p25, p75) | 213 | 16.3 (10.2, 29.3) | 236 | 16.5 (10.6, 27.8) | 0.28 |
| Measure during the trial | n | % | n | % | Chi-sq |
| Any diuretic reported at any study visit (yes) | 44 | 19.6 | 145 | 57.5 | < 0.001 |
BP: Blood Pressure; CKD EPI eGFR: estimated Glomerular Filtration Rate using the Chronic Kidney Disease Epidemiology Collaboration equation; DED: Daily Equivalent Dose (see text, Methods section); p25: 25th percentile; p75: 75th percentile; SD: Standard Deviation.
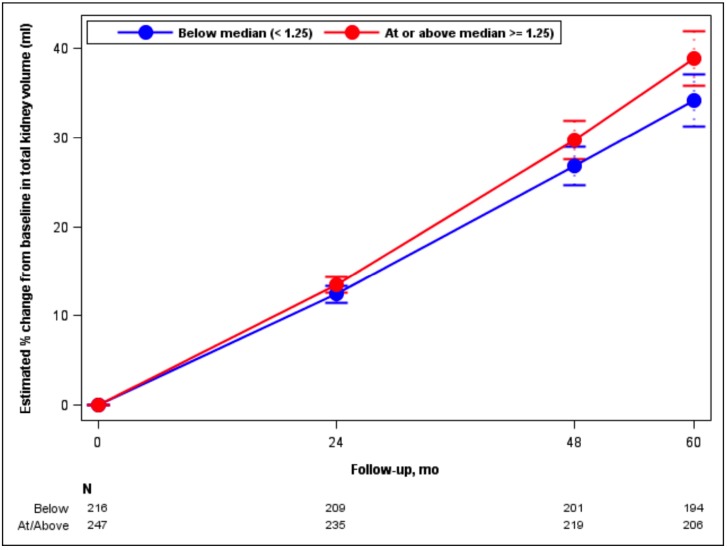
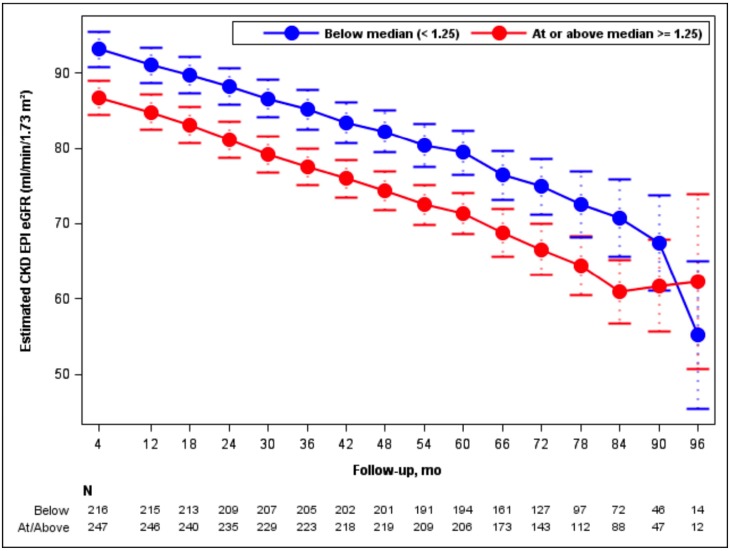
Adjusting for age, sex, genotype and BP arm, TKV increased by 6.7% per year in the high DED group, compared to 5.7% per year in the low DED group (p = 0.008; Fig. 3). The chronic eGFR slope (from month 4 until the end of the trial) was -2.9 and -3.1 ml/min/ 1.73 m2 per year for the low and high DED groups, respectively, not significantly different (p = 0.36; Fig. 4). There was no interaction between gender and DED on TKV slopes (p = 0.43) or on eGFR slopes (p = 0.96), meaning that the effect of DED on TKV and eGFR did not depend on gender.
Fig. (3).
Model-based estimates of percent change in Total Kidney Volume (TKV) from baseline over 60 months by DED group. Point estimates and 95% confidence intervals derived from linear mixed models including predictors for month, BP arm, month-by-BP arm, DED group, month-by-DED group, age, gender, and genotype. Numbers of participants in the 2 groups who had TKV measurements at the 4 time points are stated at the bottom of the figure.
Fig. (4).
Model-based estimates of post-baseline eGFR over 96 months by DED group. Point estimates and 95% confidence intervals derived from linear mixed models including predictors for month, month-by-BP arm, DED group, month-by-DED group, age, gender, and genotype. Numbers of participants in the 2 groups who had eGFR determinations at the indicated time points are stated at the bottom of the figure.
Similar trends were observed in the sensitivity analyses when comparing participants in the lowest DED quartile (n = 118; mean DED 0.4) with those in the highest (n = 169; mean DED 2.8): TKV increased by 6 and 6.7% per year (p = 0.17) and eGFR declined by 2.7 and 3 ml/min/1.73 m2 per year (p = 0.35). When using a third definition of low dose (DED < 1; n = 197; mean DED 0.6) and high dose (DED > 3; n = 75; mean DED 3.7), TKV increased by 5.9 and 6.7% per year in the low and high dose groups (p = 0.14) and eGFR declined by 2.8 and 3.2 ml/min/1.73 m2 per year (p = 0.33).
A within-person increase of one unit in DED was associated with a slightly steeper increase in TKV by 0.6% if adjusted for the low BP arm (p = 0.0006). A higher time-varying (within-person change) DED had no effect on the slope of chronic eGFR decline (data not shown).
We also examined the relationship between achieved systolic, diastolic, or mean arterial blood pressure (SBP, DBP, MAP) and the rate of TKV enlargement, but found no significant association between time-varying SBP and annual increase in TKV (0.12% per year increase for every 10 mmHg higher SBP; p = 0.35) after adjustment for age, gender, genotype and baseline eGFR. However, for every 10 mmHg higher SBP, there was a significantly steeper decline in eGFR (by 0.2 ml/min/1.73 m2 per year; p = 0.004) in the chronic phase. There were no relationships between diastolic or mean blood pressures and TKV growth or eGFR decline (data not shown).
4. Discussion
The HALT trial of early ADPKD (Study A) showed slower TKV growth in participants randomized to a very low BP goal, but the mechanism remained unresolved, particularly the question whether the observed benefit was due to allowing the use of higher doses of RAAS blocking drugs. In this post hoc analysis of HALT Study A we attempt to dissect the effects of the low BP goal from those of higher medication doses. RAAS blockade as a therapeutic strategy for ADPKD is supported by evidence from human and animal studies demonstrating that the RAAS is activated in ADPKD [7-13]. Increased angiotensin 2 promotes tubular cell proliferation, a prerequisite for cyst enlargement, as well as interstitial inflammation and fibrosis, by stimulating the release of transforming growth factor beta (TGF-β) and other cytokines [26-29]. Angiotensin 2 also decreases renal blood flow, an early finding in ADPKD that precedes the decline in GFR [30, 31]. Therefore, we hypothesized that HALT A participants who received more intense blockade of the RAAS, i.e. higher doses of lisinopril and telmisartan might experience slower TKV growth and eGFR decline than those who received lower doses, independently from BP level. However, the present analysis does not support that hypothesis.
ǂImaging classification by Irazabal et al.25, where MRI class 1A and 2A represent the lowest growth rates for TKV (< 3% per year) and the lowest risk for GFR decline, class 1B and 1C an intermediate risk, and class 1D and 1E the highest TKV-growth rate (> 6% per year) and highest risk for rapid decline in GFR.
ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; BMI: body mass index; BP: blood pressure; CKD EPI eGFR: estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation; DED: daily equivalent dose (see text, Methods section); HTN: hypertension; Lis: lisinopril; MRI: magnetic resonance imaging; NMD: no mutation detected; p25: 25th percentile; p75: 75th percentile; PKD1-NT: non-truncating PKD1 mutation; PKD1-T: truncating PKD1 mutation; SD: standard deviation; Tel: telmisartan; TKV: total kidney volume.
As is inherent in a post-hoc design, several baseline variables were different between the low and high DED groups. Participants in the high DED group had more difficult to control hypertension, as suggested by their higher body mass index and higher baseline home blood pressures before randomization. Their higher LVMI may have been due to the male predominance in that group, because healthy men have higher LVMI than women [32, 33]. The unexpectedly higher renal blood flow and lower aldosterone excretion in the high DED group may also reflect the male predominance in that group as shown by the baseline characteristics of all HALT Study A participants, where men had higher renal blood flow and lower urinary aldosterone excretion than women [34].
Although two thirds of participants in the high DED group were randomized to the low BP arm compared to approximately one third of the low DED subjects, BP levels during the trial were only slightly lower (by 2 mmHg for SBP and 3 mmHg for DBP) in the high DED group, indicating that high DED participants did not always achieve their BP goal despite higher RAAS-blocking drug doses and more frequent use of diuretics. In contrast to having more severe hypertension, high DED subjects did not have more severe kidney manifestations, as baseline height-adjusted TKV, MRI class and ADPKD genotype distribution, urinary albumin excretion and baseline eGFR were not significantly different between the high and low DED groups. Very likely the severity of hypertension in ADPKD is determined by multiple genetic (e.g. history of hypertension in the unaffected parent) and environmental (e.g. obesity, salt intake, physical activity) factors in addition to the PKD genotype.
The fact that almost two thirds of subjects in the high DED group were men, despite gender-balanced randomization to the low BP goal and to combination (ACEI + ARB) treatment [34], suggests that men with ADPKD have more severe hypertension than women of similar age, therefore requiring higher doses of BP medications to achieve the BP goal. Previous natural history studies of ADPKD also reported that hypertension was significantly more common in men than women of the same age (mean 32 years), and BP was significantly higher in men than women in both the hypertensive and normotensive groups, despite normal renal function [35]. A subsequent much larger study confirmed these findings, which were attributed to the greater male predisposition for hypertension in the general population [35, 36]. Experimental studies show that estrogens inhibit the ACE/angiotensin-2-receptor axis while upregulating the vasodilatory peptide angiotensin-(1-7), thus contributing to lower BP in females compared to males [37]. Therefore we examined whether gender modulated the effect of DED on the outcomes TKV and eGFR, but this was not the case. Interestingly, a pre-specified subgroup analysis of HALT Study A revealed that the benefit of the low BP goal for limiting kidney growth was significant only in men but not in women [19], possibly because men have higher cyst growth rates [25] and more severe hypertension. Faster TKV progression in men compared to women was also observed in the trial of tolvaptan for ADPKD, the TEMPO 3:4 trial [38]. Higher male TKV growth rates likely account for the unexpected finding of faster TKV enlargement in the high DED group which had significantly more men than women. Because HALT Study A recruited participants younger than 50 years, the vast majority of women were premenopausal.
How targeting a very low BP goal might lead to reduced cystic kidney growth remains unclear. It is possible that the polycystic kidney is exquisitely sensitive to hypertensive damage, causing ischemia and oxidative stress, which lead to activation of growth factors and release of inflammatory and profibrotic cytokines. Renal injury, whether nephrotoxic or ischemic, in animal models of PKD acts as a “third hit” to accelerate cystogenesis [39-41]. Consistent with this hypothesis, in HALT Study A urinary albumin excretion, an indicator of microvascular and glomerular damage, decreased only in the low BP arm but increased in the standard BP arm [19].
We also examined whether achieved BP level was associated with percent increase in TKV or slope of eGFR decline. There was no correlation between time-varying systolic, diastolic or mean home BP with TKV growth. However, for every 10 mmHg higher SBP there was a significantly steeper decline in eGFR. Although the difference of 0.2 ml/min/1.73 m2 per year appears to be very small, it adds up over a lifetime and does suggest that low BP helps to preserve renal function in ADPKD. Because BP was treated to a goal of less than 130/80 mmHg in all participants, we cannot assess the effects of a higher BP goal, e.g. < 140/90 mmHg as recommended by the Eighth Joint National Committee (JNC 8) guidelines of 2014 [42].
Other investigators have assessed the relationships between achieved BP and kidney disease progression. A pooled patient-level analysis of 11 randomized trials involving 1860 subjects with nondiabetic chronic kidney disease suggested that an achieved SBP of 110-129 mmHg was associated with the lowest risk for doubling of serum creatinine or ESRD [43], and people with diabetic nephropathy who achieved SBP 120-130 mmHg had improved patient and renal survival [44]. In the Chronic Renal Insufficiency Cohort (CRIC) Study a time-updated SBP of less than 120 mmHg was associated with a significantly lower hazard of developing ESRD than higher SBP [45]. Similar findings were reported from the VA NEPHRON-D (Veteran Affairs Nephropathy in Diabetes) Trial [46]. Although these observations are derived from secondary analyses that may suffer from confounding by disease severity [47], they all show that low SBP levels are associated with slower progression of various kidney diseases. Our post hoc analysis of HALT Study A suggests that this may also be true for ADPKD.
Limitations of this study are the relatively small number of participants (n = 477), the differences in baseline characteristics between the low and high DED groups, and the limited follow-up time for a disease that progresses over decades. The target range for systolic BP was very low and narrow (95-130 mmHg), limiting the magnitude of differences in outcomes. We did not assess the effects of uncontrolled hypertension.
conclusion
In conclusion, the primary intention-to-treat analysis of HALT PKD Study A supports a very low BP target (95/60 to 110/70 mmHg) for young to middle-aged adults with ADPKD and preserved renal function [19]. As shown here, the observed benefit on TKV enlargement was not simply due to allowing the use of higher doses of RAAS blocking drugs in the low BP arm and occurred despite the greater use of diuretics [19] which stimulate the RAAS. The relationship between the severity of hypertension and renal cystic disease is complex and depends on gender, among other factors. Achievement of low systolic blood pressure in early ADPKD is associated with a slower loss of renal function.
Disclosure
Dr. Perrone has received consulting fees from Sanofi–Genzyme and Vertex Pharmaceuticals and consulting fees and grant support through his institution from Otsuka Pharmaceuticals and Kadmon; Drs. Torres and Harris, grant support from Otsuka Pharmaceuticals; Dr. Steinman, grant support from Kadmon, Fibrogen and AMAG Pharmaceuticals and a consulting fee from Sanofi-Genzyme; Dr. Rahbari-Oskoui, fees for serving on advisory boards from Otsuka, Kadmon, and Astute Medical, and also research support from Otsuka; Dr. Bae, consulting fees from Kadmon; Dr. Chapman, consulting fees from Kadmon, Otsuka and Pfizer, and also grant support from Otsuka; and Dr. Hogan receives research funds from Novartis USA and is a co-Investigator on multiple Otsuka-sponsored tolvaptan studies in ADPKD (at Mayo Clinic site). No other potential conflict of interest relevant to this article was reported.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Boards.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were in accordance with the standards set forth in the Declaration of Helsinki https://www.wma.net/policiespost/wma-declaration-of-helsinki-ethicalprinciples-for-medical-research-involving-human-subjects/principles of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
CONSENT FOR PUBLICATION
All patients gave informed consent.
Acknowledgements
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK62402 to Dr. Schrier, DK62411 to Dr. Perrone, DK62410 to Dr. Torres, DK082230 to Dr. Moore Patterson, DK62408 to Dr. Chapman, and DK62401 to Washington University in St. Louis) and the National Center for Research Resources General Clinical Research Centers (RR000039 to Emory University, RR000585 to the Mayo Clinic, RR000054 to Tufts Medical Center, RR000051 to the University of Colorado, RR023940 to the University of Kansas Medical Center, and RR001032 to Beth Israel Deaconess Medical Center), National Center for Advancing Translational Sciences Clinical and Translational Science Awards (RR025008 and TR000454 to Emory University, RR024150 and TR00135 to the Mayo Clinic, RR025752 and TR001064 to Tufts University, RR025780 and TR001082 to the University of Colorado, RR025758 and TR001102 to Beth Israel Deaconess Medical Center, RR033179 and TR000001 to the University of Kansas Medical Center, and RR024989 and TR000439 to Cleveland Clinic), by funding from the Zell Family Foundation (to the University of Colorado), and by the grant from the PKD Foundation.
Mutation analysis was supported by DK62410-S1 to Dr. Harris and the Mayo Translational PKD Center (DK090728). Study drugs were donated by Boehringer Ingelheim Pharmaceuticals Inc (telmisartan and matched placebo) and Merck & Co Inc (lisinopril).
Most of all, we thank the hundreds of patients who took part in the HALT-PKD trials and the dedicated study coordinators who guided them through the years of participation.
We also thank Diane Comer, Center for Research on Health Data at the University of Pittsburgh, for expert assistance with the statistical analyses.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Iglesias C.G., Torres V.E., Offord K.P., Holley K.E., Beard C.M., Kurland L.T. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935-1980. Am. J. Kidney Dis. 1983;2:630–639. doi: 10.1016/s0272-6386(83)80044-4. [DOI] [PubMed] [Google Scholar]
- 2.Somlo S., Chapman A.B. Autosomal dominant polycystic kidney disease. In: Coffman T.M., Falk R.J., Molitoris B.A., et al., editors. Schrier’s Diseases of the Kidney. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2012. pp. 519–563. [Google Scholar]
- 3.Ong A.C., Devuyst O., Knebelmann B., et al. Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet. 2015;385:1993–2002. doi: 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 4.Ecder T., Schrier R.W. Hypertension in autosomal-dominant polycystic kidney disease: early occurrence and unique aspects. J. Am. Soc. Nephrol. 2001;12:194–200. doi: 10.1681/ASN.V121194. [DOI] [PubMed] [Google Scholar]
- 5.Cadnapaphornchai M.A., McFann K., Strain J.D., et al. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74:1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman A.B., Stepniakowski K., Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv. Chronic Kidney Dis. 2010;17:153–163. doi: 10.1053/j.ackd.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman A.B., Johnson A., Gabow P.A., Schrier R.W. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1990;323:1091–1096. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 8.Torres V.E., Donovan K.A., Scicli G., et al. Synthesis of renin by tubulocystic epithelium in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;42:364–373. doi: 10.1038/ki.1992.297. [DOI] [PubMed] [Google Scholar]
- 9.Loghman-Adham M., Soto C.E., Inagami T., Cassis S. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2004;287:F775–F788. doi: 10.1152/ajprenal.00370.2003. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca J.M., Bastos A.P., Amaral A.G., et al. Renal cyst growth is the main determinant for hypertension and concentrating deficit in Pkd1-deficient mice. Kidney Int. 2014;85:1137–1150. doi: 10.1038/ki.2013.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogborn M.R., Sareen S., Pinette G. Cilazapril delays progression of hypertension and uremia in rat polycystic kidney disease. Am. J. Kidney Dis. 1995;26:942–946. doi: 10.1016/0272-6386(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 12.Kennefick T.M., Al Nimri M.A., Oyama T.T., et al. Hypertension and renal injury in experimental polycystic kidney disease. Kidney Int. 1999;56:2181–2190. doi: 10.1046/j.1523-1755.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 13.Zafar I., Tao Y., Falk S., et al. Effect of statin and angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2007;293:F854–F859. doi: 10.1152/ajprenal.00059.2007. [DOI] [PubMed] [Google Scholar]
- 14.Orskov B., Sorensen V.R., Feldt-Rasmussen B., Strandgaard S. Improved prognosis in patients with autosomal dominant polycystic kidney disease in Denmark. Clin. J. Am. Soc. Nephrol. 2010;5:2034–2039. doi: 10.2215/CJN.01460210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patch C., Charlton J., Roderick P.J., Gulliford M.C. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: A population-based study. Am. J. Kidney Dis. 2011;57:856–862. doi: 10.1053/j.ajkd.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Alam A., Perrone R.D. Left ventricular hypertrophy in ADPKD: Changing demographics. Curr. Hypertens. Rev. 2013;9:27–31. doi: 10.2174/1573402111309010005. [DOI] [PubMed] [Google Scholar]
- 17.Chapman A.B., Devuyst O., Eckardt K.U., et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88:17–27. doi: 10.1038/ki.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman A.B., Torres V.E., Perrone R.D., et al. The HALT polycystic kidney disease trials: Design and implementation. Clin. J. Am. Soc. Nephrol. 2010;5:102–109. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrier R.W., Abebe K.Z., Perrone R.D., et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres V.E., Abebe K.Z., Chapman A.B., et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2014;371:2267–2276. doi: 10.1056/NEJMoa1402686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman A.B., Guay-Woodford L.M., Grantham J.J., et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The consortium for radiologic imaging studies of polycystic kidney disease (CRISP) cohort. Kidney Int. 2003;64:1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 22.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [Erratum in: Ann Intern Med 2011; 155: 408]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyer C.M., Sundsbak J.L., Abebe K.Z., et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2016;27:2872–2884. doi: 10.1681/ASN.2015050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Collaborating Centre for Drug Statistics Methodology ATC classification index with DDDs. 2018 www.whocc.no
- 25.Irazabal M.V., Rangel L.J., Bergstralh E.J., et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf G., Neilson E.G. Angiotensin II induces cellular hypertrophy in cultured murine proximal tubular cells. Am. J. Physiol. 1990;259:F768–F777. doi: 10.1152/ajprenal.1990.259.5.F768. [DOI] [PubMed] [Google Scholar]
- 27.Rüster C., Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J. Am. Soc. Nephrol. 2011;22:1189–1199. doi: 10.1681/ASN.2010040384. [DOI] [PubMed] [Google Scholar]
- 28.Lavoz C., Rodrigues-Diez R., Benito-Martin A., et al. Angiotensin II contributes to renal fibrosis independently of Notch pathway activation. PLoS One. 2012;7:e40490. doi: 10.1371/journal.pone.0040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman J. Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD). Biochim. Biophys. Acta. 2011;1812:1327–1336. doi: 10.1016/j.bbadis.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres V.E., King B.F., Chapman A.B., et al. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2007;2:112–120. doi: 10.2215/CJN.00910306. [DOI] [PubMed] [Google Scholar]
- 31.Meijer E., Rook M., Tent H., et al. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2010;5:1091–1098. doi: 10.2215/CJN.00360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawel-Boehm N., Maceira A., Valsangiacomo-Buechel E.R., et al. Normal values for cardiovascular magnetic resonance in adults and children. J. Cardiovasc. Magn. Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrone R.D., Abebe K.Z., Schrier R.W., et al. Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2011;6:2508–2515. doi: 10.2215/CJN.04610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres V.E., Chapman A.B., Perrone R.D., et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 2012;81:577–585. doi: 10.1038/ki.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabow P.A., Chapman A.B., Johnson A.M., et al. Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int. 1990;38:1177–1180. doi: 10.1038/ki.1990.330. [DOI] [PubMed] [Google Scholar]
- 36.Kelleher C.L., McFann K.K., Johnson A.M., Schrier R.W. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U.S. population. Am. J. Hypertens. 2004;17:1029–1034. doi: 10.1016/j.amjhyper.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Chappell M.C., Marshall A.C., Alzayadneh E.M., Shaltout H.A., Diz D.I. Update on the angiotensin converting enzyme 2-angiotensin (1-7)-MAS receptor axis: fetal programing, sex differences, and intracellular pathways. Front. Endocrinol. (Lausanne) 2014;4:201. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres V.E., Chapman A.B., Devuyst O., et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastos A.P., Piontek K., Silva A.M., et al. Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J. Am. Soc. Nephrol. 2009;20:2389–2402. doi: 10.1681/ASN.2008040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takakura A., Contrino L., Zhou X., et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum. Mol. Genet. 2009;18:2523–2531. doi: 10.1093/hmg/ddp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J., Ouyang X., Schoeb T.R., et al. Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement. J. Am. Soc. Nephrol. 2012;23:1161–1171. doi: 10.1681/ASN.2011050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James P.A., Oparil S., Carter B.L., et al. 2014 evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 43.Jafar T.H., Stark P.C., Schmid C.H., et al. Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition. A patient-level meta-analysis. Ann. Intern. Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 44.Pohl M.A., Blumenthal S., Cordonnier D.J., et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: Clinical implications and limitations. J. Am. Soc. Nephrol. 2005;16:3027–3037. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- 45.Anderson A.H., Yang W., Townsend R.R., et al. Time-updated systolic blood pressure and the progression of chronic kidney disease. A cohort study. Ann. Intern. Med. 2015;162:258–265. doi: 10.7326/M14-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leehey D.J., Zhang J.H., Emanuele N.V., et al. BP and renal outcomes in diabetic kidney disease: The Veterans Affairs Nephropathy in Diabetes Trial. Clin. J. Am. Soc. Nephrol. 2015;10:2159–2169. doi: 10.2215/CJN.02850315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis E.M., Appel L.J., Wang X., et al. Limitations of analyses based on achieved blood pressure: lessons from the African American study of kidney disease and hypertension trial. Hypertension. 2011;57:1061–1068. doi: 10.1161/HYPERTENSIONAHA.111.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]