Introduction
Stroke is a major cause of death and disability worldwide. The inflammatory response is pivotal to the pathophysiology of ischemic stroke. It begins in the vasculature directly after arterial occlusion, continues in the brain, and systemically throughout all disease stages. Immune responses are tightly regulated and have both beneficial and detrimental properties after stroke; inflammation can result in considerable brain damage and/or inhibition of brain repair, including neurogenesis.1 Variability in different inflammatory processes render the immune response a strong determinant of brain restoration and patient survival following stroke.2 Directed modulation of the immune response could therefore be designed as a potential therapeutic approach to induce stroke recovery.
Modulation can be achieved with stem cell (SCs) therapy, and is now a widely investigated approach with multiple clinical trials for different diseases, including stroke (www.clinicaltrials.gov).3, 4 Certain types of SCs are pluri- or multipotent and have the potential to create many neural cells, which may be important after stroke-based neuronal loss. Exogenous SC transplantations, primarily with neural stem/precursor cells (NSPCs) and mesenchymal-derived stem cells (MSCs), have been examined using different administration routes in various stroke animal models; increased functional recovery was often observed.5–8 Besides NSPCs and MSCs, mixed adult stem cell populations from bone marrow or umbilical cord blood have been examined, showing improved outcomes as well. 9–11 However, the therapeutic time window of these mixed cell populations appears to be narrower, restricting their use to the acute and subacute stages after stroke as compared to the NSPCs and MSCs, which can be used in chronic stroke as well. 12 Therefore, the focus of this Topical Review lies on NSPC and MSC therapy in stroke. Despite many studies, their exact mechanisms behind the brain restoring effects are not completely understood. It is thought that SC-based approaches can induce post-stroke recovery via mechanisms such as neuronal replacement, promotion of angiogenesis, induction of brain plasticity, reduction of cell death or immunomodulation.13 This Topical Review is the first to link SC-induced immunomodulation to different pro-regenerative processes; understanding these interactions is essential to develop successful stroke therapies.
Stem cell-based modulation of inflammation in stroke
The brain cytokine environment
The effects of cytokines released from resident and infiltrating leukocytes in stroke pathology are numerous; they include additional leukocyte recruitment to the site of injury, leukocyte activation and apoptosis induction.14 SC transplantation has been demonstrated to modulate this cytokine environment, both at the injury site and in the periphery. For example, early SC administration after stroke (within 48 hours) decreases pro-inflammatory cytokine brain levels and increases anti-inflammatory cytokine levels.15, 16 Liu et al. showed that cortical MSC administration following distal middle cerebral artery occlusion (dMCAO) decreased the infarct area and improved neurological function, likely through upregulated gene and protein expression of the anti-inflammatory cytokine IL-10, and a decrease in the pro-inflammatory cytokine TNF.16 Decreased pro-inflammatory gene expression, including TNF, IL-1β and IFN-γ, was also observed after intravenous NSPCs transplantation.17 These data show the anti-inflammatory effects of SCs, as confirmed by microarray analysis on mouse brain after intra-hippocampal MSC administration one day post-stroke (Table 1).18
Table 1.
Stem cell-induced immunomodulatory actions in in vivo ischemic stroke models
| Model | Host | Cell type | Timing | Findings | Ref |
|---|---|---|---|---|---|
| Acute (>48 h) administration intravenous | |||||
| Transient MCAO | LE rat | MSCs c.m. allograft |
0 dps | <7 dpt recovery ↑ Microglia/macrophages ↓ Trend toward decreased infarct size Neural progenitor cells ↑ |
31 |
| Permanent MCAO | SD rat | MSCs allograft |
30 min ps | 1−14 dpt recovery ↑ Brain gene expression GFAP ↓, VEGF, SYP, Olig-2, NF ↑ 14 dpt apoptosis penumbra ↓ |
49 |
| Transient MCAO | Wistar rat | MSCs xenograft |
1 dps | Iba1+ and GFAP+ cells in core and penumbra ↓ Brain gene expression iNOS, MCP1, COX-2 ↓, IL-4 ↑ |
27 |
| Permanent MCAO |
SD rat | MSC-derived MVs allograft |
2 dps | 3−7 dpt recovery ↑ Striatal GFAP+ cells ↓ Anti-inflammatory cytokines ↑ Infarct size ↓ Angiogenesis ↑ Neurogenesis ↑ |
43 |
| Acute (>48 h) administration intracerebral | |||||
| Transient MCAO | C57Bl/6, C57Bl/6/SCID mice | MSCs in hippocampus xenograft |
1 dps | 1−4 dpt recovery ↑ Hippocampal M2 microglia & gene expression YM1, IGF-1, Gal-3 ↑ 1−4 dpt neuronal death hippocampus ↓ |
18 |
| Permanent MCAO | SD rat | MSCs in lateral ventricle allograft |
1 dps | 14 dpt recovery ↑ Brain gene expression IL-10 ↑, TNF ↓ 1, 4 dpt infarct volume ↓ |
16 |
| Transient MCAO | Nude rat | NSPCs in striatum xenograft |
2 dps | 6−12 wpt recovery ↑ Striatal inflammation ↓ Activated microglia ↓ Striatal neurogenesis ↑ SVZ proliferation ↑ |
7 |
| Sub-acute (3–14 days) administration intravenous | |||||
| Transient MCAO | C57Bl/6 mice | NSPCs allograft |
3 dps | > 18 dpt recovery ↑ Brain gene expression TNF, IL-1β, IL-6, IFN-γ ↓ Brain immune cell infiltrate ↓ Reactive astrocytes ↓ Neuronal death ↓ |
17 |
| Sub-acute (3–14 days) administration intracerebral | |||||
| Permanent MCAO | Nude rat | NSPCs ipsilesional xenograft |
7 dps | 1−4 wpt recovery ↑ Iba1+ cells ↓ VEGF neovascularization VEGF ↑ BBB integrity ↑ |
6 |
MCAO: middle cerebral artery occlusion; LE: Lewis Evans rat; SD: Sprague Dawley rat; dps: days post-stroke; ps: post-stroke; wpt: weeks post-transplantation; dpt: days post-transplantation; c.m.: conditioned-medium; MVs: microvesicles; SVZ: subventricular zone; SYP: synaptophysin; Olig-2: oligodendrocyte; NF: neurofilament; GFAP: glial fibrillary acidic protein.
Microglia/macrophage polarization
It is currently thought that pro-inflammatory M1 microglia/macrophages can exacerbate brain injury, whereas anti-inflammatory M2 microglia/macrophages are neuroprotective.19 This dual role makes them an exciting target to enhance post-stroke brain recovery by shifting their balance from the detrimental M1 to the beneficial M2 phenotype. Accumulating evidence indicates that SCs can alter the polarization status of microglia/macrophages.20, 21 In vitro, primary microglia/macrophages co-cultured with MSCs increased mRNA and protein expression of M2 markers such as Arg1, CD206, IL-10 and decreased expression of M1 markers such as IL-12 and TNF. This occurred both in a direct cell-contact and an indirect transwell environment, indicating the presence of paracrine factors.22–25
Ohtaki et al. first described M2 microglia induction by MSC administration in a transient MCAO model.18 After MSC transplantation into the dentate gyrus at one day post-stroke, M2 protein expression of YM-1, IGF-1 and Galectin-3 was increased which correlated with improved neurological recovery. A later study suggested that MSC transplantation into the lateral ventricle of stroked rats decreased infarct volume and increased functional recovery by increasing IL-10 and decreasing TNF expression in the brain.16 These M2-polarizing actions have been confirmed in several experimental stroke studies, which were all associated with improved neurological function.26, 27 Thus, the observed beneficial actions of SCs are likely partly due to skewing microglia/macrophages toward a neuroprotective and neuroregenerative phenotype (Table 1).
Brain immune cell infiltration
The acute phase (first 48 hours) after stroke is characterized by cytokine and chemokine secretion, and blood-brain barrier disruption; this results in massive immune cell infiltration into the brain.28 This first phase has devastating effects on stroke outcome.29, 30 Acute and subacute (3–14 days) administration of both NSPCs and MSCs diminished Iba1+ cells, which are either the resident microglia or infiltrated macrophages.6, 17, 27, 31 Activated ED1+ microglia/macrophage numbers also decreased in the striatum of NSPCs-transplanted ischemic rats.7 In contrast, other studies showed increased numbers of microglia/macrophages in stroked animal models intracerebrally transplanted with NSPCs. They suggest this induces brain recovery by increased secretion of brain remodeling factors such as insulin growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF) and transforming growth factor-β1 (TGF-β1).32, 33 These data suggest the beneficial effects of transplanted SCs are partly due to the inhibition of leukocyte infiltration (Table 1).
Stem cell-induced effects on the systemic immune response
Splenic contraction, a reduction in splenic cells and a corresponding increase in brain monocytes have been observed after transient MCAO,34 indicating the importance of systemic immune responses. Systemically administered NSPCs in the acute phase post-stroke restored neurological function, and decreased brain edema and infarct volume. IL-1β, TNF, IL-23 and IL-17 expression levels in the ischemic hemisphere and blood were decreased, whereas TGF-β and IL-10 were increased in blood; an increase in blood T regulatory cells was also observed.35 This suggests that peripheral immunomodulation can improve brain recovery post-stroke.
A spleen-dependent neuroprotective effect was observed after systemic administration of NSPCs in ischemic rats.36 Intravenous injection of NSPCs 2 hours after stroke improved functional recovery, reduced infarct size and edema, and decreased brain inflammatory infiltration. Cytokine analysis demonstrated decreased pro-inflammatory expression of TNF, IL-6 and NF-κB in brain and spleen. Numerous NSPCs were also observed in the spleen; splenectomy eliminated the effects on brain edema and immune cell infiltration. A decrease in pro-inflammatory gene expression was confirmed in the MASTERS trial, a phase II randomized, double-blind, placebo-controlled trial evaluating SC treatment in acute strokes, at 7 days post-transplantation. Furthermore, a reduction in spleen size was prevented, indicating suppression of the peripheral immune response. 37, 38 These data suggest that solely modulating peripheral immunity could promote neurorestorative effects.
Chronic inflammation of the brain
The chronic stroke phase (>1 month) is characterized by persistent immune cell infiltration. B and T cells are present in the stroke core at 4–12 weeks post-stroke in different experimental models. In contrast to lymphocytic localization in the core, activated microglia/macrophages have been detected in the thalamus, striatum and internal capsule.39 This immune cell infiltration has also been detected in postmortem human brain samples, even decades after stroke.40 Despite this apparent chronic immune response, most SC transplantations have been performed during the earlier phases post-stroke. However, it was shown that intravenous MSC administration in MCAO rats at 60 days post-stroke reduces brain and splenic inflammation.41 Clinical studies with SC transplantation in the chronic stroke phase, like the Sanbio SB623 and PISCES trials, showed some promising neurological improvements, however its potential underlying immunomodulatory effects remain to be defined.12 The Sanbio study demonstrated a transient FLAIR signal starting one week after MSC transplantation, which correlated with neurological recovery.3 This signal may represent a beneficial inflammatory response, which could attenuate the chronic inflammatory response.
Stem cell-based immunomodulation of non-inflammatory repair processes after stroke
SCs also directly influence important brain repair processes, and multiple studies show that transplanted SCs induce angiogenesis.6, 42–44 The expression and secretion of angiogenic factors such as VEGF, BDNF and fibroblast growth factor (FGF) are of key importance. SC transplantation also enhances brain plasticity by increasing axonal and dendritic sprouting.33, 45, 46 Studies have shown that VEGF and thrombospondins 1 and 2 partially mediate these effects.5, 47 Brain plasticity modulation also occurs at the synaptic level after transplantation, with changes in the number of excitatory and inhibitory synapses in different cortical layers of the brain. 48–50 Furthermore, SC transplantation increases the survival of endogenous glial and neuronal progenitors after ischemia.17, 18, 51 Decreases in cell death are often accompanied by increased secretion of neurotrophic factors such as BDNF, FGF and VEGF,13, 52 which generate survival signals in glia and neurons and can increase cellular resistance against oxidative stress.51
Numerous preclinical and a few clinical studies have shown beneficial SC-induced effects on angiogenesis, brain plasticity and brain cell death after stroke; these details are beyond the scope of this review, but are extensively reviewed elsewhere.53–56 Despite the evidence demonstrating the beneficial effects of post-stroke SC transplantation on these processes, their exact mechanisms of action are unclear. It is most likely that stem cell-induced immunomodulation plays a central role.
Immunomodulation of angiogenesis
SC transplantation can polarize microglia/macrophages toward the anti-inflammatory and angiogenic M2 phenotype, and a classical factor secreted by M2 microglia/macrophages is VEGF.57, 58 VEGF-dependent suppression of inflammation after intracerebral NSC transplantation was demonstrated in ischemic rats and associated with enhanced angiogenesis and functional recovery.6 TGF-β, another prototypical M2 mediator induced after SC transplantation, also plays a prominent role in angiogenesis induction.59 VEGF and TGF-β also interact to control angiogenesis, thereby strengthening each other’s function.60 Moreover, increased M2 polarization could result in less M1 microglia/macrophages and therefore less expression of their key cytokine IFN-γ; this cytokine has strong anti-angiogenic potential in diseases like cancer and atherosclerosis, and so could also be important in stroke.61, 62
The complement cascade is now recognized as more than a ‘component’ of the innate immune system; it is implicated in CNS development and regeneration, and is known to influence stroke.63, 64 The effects of SC transplantation on the complement system are currently unknown; however the active complement factors C3a and C5a are associated with M2 microglia/macrophages and they can stimulate angiogenesis,65, 66 making them interesting research targets. These data suggest SC-induced angiogenesis is mediated not only by expression of remodeling factors like VEGF, BDNF and FGF, but also induced by the anti-inflammatory effects of SCs (Figure 1a).
Figure 1. Stem cell-based immunomodulation of non-inflammatory repair processes after stroke.
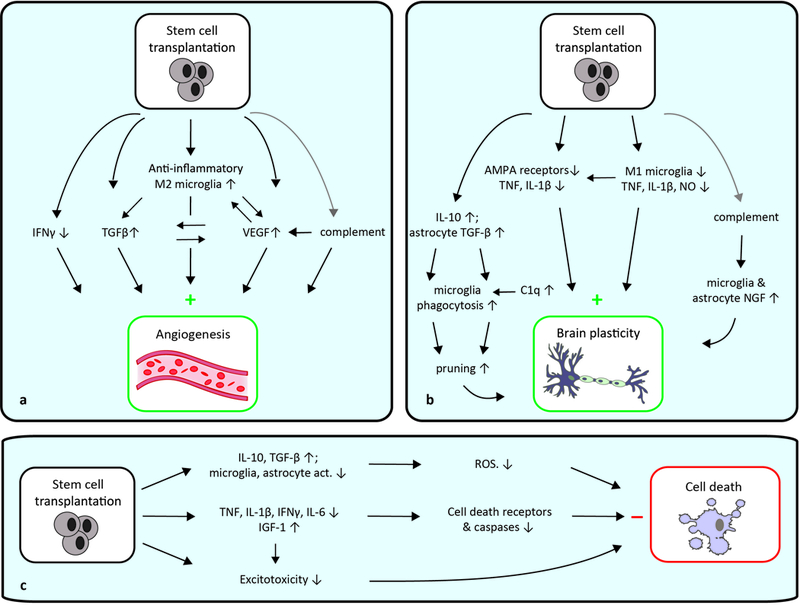
a, SC-induced immunomodulation of angiogenesis is mediated by anti-inflammatory actions; they induce M2 microglia/macrophages with strong angiogenic potential via secretion of anti-inflammatory cytokines and growth factors, and by inhibition of pro-inflammatory cytokines. Increased VEGF expression and secretion acts either directly on endothelial cells for induction of angiogenesis, or indirectly through anti-inflammatory actions. b, SC-induced immunomodulation of brain plasticity is mediated by decreased M1 microglia activity, thereby decreasing inflammatory cytokine production and subsequently inducing brain plasticity. Decreased inflammatory expression results in less AMPA receptor surface expression, enhancing brain plasticity. Astrocyte-microglia interactions can increase synapse pruning, and complement activation may also affect plasticity. c, The anti-inflammatory actions of transplanted SCs decrease cell death receptor expression, inhibit the caspase cascade and decrease excitotoxicity, all decreasing brain cell death. Grey arrows indicate possible connections. ROS: reactive oxygen species; act.: activity.
Immunomodulation of brain plasticity
Brain plasticity - in the form of neurogenesis, synaptic remodeling, axonal sprouting and dendritic branching - is essential for brain repair and functional recovery after stroke. Neuroinflammation, and activated M1 microglia in particular, can have detrimental effects on brain plasticity which can be reversed by administration of anti-inflammatory drugs.67, 68 These effects are thought to be mediated by pro-inflammatory factors including TNF, IL-1β and NO.68–70 TNF controls synaptic plasticity by regulating neuronal surface expression of excitatory AMPA and inhibitory GABAA receptors.71, 72 IL-1β influences the surface expression of AMPA receptors in a similar fashion, albeit with lower efficacy. Post-stroke SC transplantation decreases microglia activation and secretion of pro-inflammatory cytokines, including TNF and IL-1β. Additionally, SC transplant after stroke enhances neurogenesis in the acute and subacute phase.7, 31, 43 This suggests that decreased microglial activation can increase brain plasticity and improve functional recovery.
Cytokine-mediated interactions between microglia and astrocytes could also affect brain plasticity. After SC transplantation, increased IL-10 expression stimulates production of TGF-β by astrocytes, which decreases microglial activation and increases their phagocytotic capacity.73 TGF-β secretion by astrocytes induces neuronal complement protein C1q expression, thereby targeting them for elimination by phagocytotic microglia.73–75 Unwanted synapses are then pruned by microglia, crucial for brain remodeling. This demonstrates an indirect role for astrocytes in microglial-synapse elimination. Astrocytes can also monitor and modify synapse function directly, making their effects on brain plasticity context-specific.74
If the complement system is affected after SC transplantation, then microglia and astrocytes would likely respond to complement stimulation with the production of trophic molecules necessary for neuronal proliferation and renewal.63 It is thus also possible that SC-induced complement activation induces brain plasticity (Figure 1b).
Immunomodulation of brain cell death
Acute and subacute SC transplantation decreases brain cell death and increases functional recovery, which is associated with decreased secretion of inflammatory mediators such as TNF, IL-1β, IL-6 and IFN-γ.17, 18, 76 These cytokines are known apoptosis inducers, acting through the caspase cascade and expression of cell death receptors in several disease conditions including stroke.77, 78 Accordingly, blocking TNF or IFN-γ prevents secondary infarct growth after stroke.79 Expression of IL-10 and TGF-β is upregulated after SC transplantation, which reduces microglial and astrocytic activation. Reduced cellular activation decreases reactive oxygen species levels, which are known to induce cell death.80 Therefore, a direct link between cytokine levels and brain cell death may exist.
Excitotoxicity is a form of neurotoxicity and a major contributor of post-stroke neuronal injury; this phenomenon could also explain the decreased brain cell death observed after early post-stroke SC transplantation.81 As described, TNF and IL-1β regulate synaptic plasticity by stimulating excitatory neurotransmission; when deregulated, neurotoxicity can result.82 As SC transplantation reduces TNF and IL-1β levels, this could reduce excitotoxicity and thereby reduce brain cell death (Figure 1c).
Stem cells as a therapeutic strategy for stroke: future directions
Ischemic stroke is complex and affects a variety of brain regions, involving multiple interactions with the vasculature and immune system. Altering central or peripheral immune responses often improves functional recovery following stroke (Table 1), and there is strong evidence that SCs could be used as a clinically relevant therapy to target multiple pathways.
As described in this Topical Review, both the tissue specific NSPCs and the non-tissue specific MSCs can have advantageous effects with regard to immunomodulation of pro-regenerative processes. For both SC types, these immunomodulatory effects are mainly due to their bystander effect; the secretion of important proteins such as cytokines and trophic factors. Their mechanism of action seems to differ slightly when transplanted intravascularly, as MSCs primarily seem to control the immune response in the periphery, while NSPCs are prone to specifically home to the lesion site to exert their immunomodulatory effects there. 83 For both NSPCs and MSCs, true tissue restoration by integrating into the brain and differentiating into correctly functioning cells, such as neurons and glia, or endothelial cells is believed to have only a minor contribution to functional recovery. 84, 85 Indeed, evidence for this is scarce, although it has been shown that NSPCs can integrate into the brain and acquire neuronal characteristics, such as expression of synaptic proteins, synapse formation and appropriate electrophysiological aspects. 84 Whether this then is regulated via their immunomodulatory properties remains to be determined. Overall, a better understanding of the similarities between NSPCs and MSCs in the immunomodulation of pro-regenerative processes is needed, as this might reveal the essential immunomodulators for stroke recovery. In contrast, a better understanding of the differences between them will show SC specific actions in stroke recovery, which could help determine which particular SC type is needed for an optimal therapeutic effect, for example according to time post-stroke.
In addition, fundamental questions remain regarding the optimal route and dosage of SC transplantation, and why few (or no) transplanted cells engraft in the brain.53 The survival rates of transplanted cells can vary,86 which may indicate that secreted factors from transiently surviving or dying SCs have immunomodulatory roles. Clinical studies would benefit greatly from non-invasive imaging techniques to track the transplanted SCs longitudinally and repeatedly. 87 In this way, one can monitor their survival, migration, proliferation and the immune reactions they elicit. This all might help understand how their pro-regenerative effects are generated. Additionally, in vivo brain imaging using MRI or PET would be useful to monitor the immunomodulatory response of the brain to the SCs. Altogether, this would be of great value to determine a patient-specific therapeutic approach, for example based on their lesion size and location. Despite the enormous potential of SC tracking and in vivo brain imaging, several problems exist regarding these technologies. It is essential to understand whether the tracking agents affect cellular functions and viability before this can be applied in the clinic. 88 Regarding in vivo brain imaging, each imaging technique has its own advantages and disadvantages, for example concerning spatial resolution and the use of contrast agents. Ideally, one should combine multiple imaging techniques to make this a non-invasive, safe and efficient way to serve as a qualitative and quantitative technique. 89
We also consider combinatorial approaches to be of importance in future clinical studies. This rapidly emerging treatment option encompasses for example co-treatment with growth factors, or transplantation of genetically modified SCs. 90, 91 This approach will enable an improved understanding about the immunomodulation of pro-regenerative processes suggested in this Topical Review. Its effectiveness indeed was shown by a recent study in which MSCs were genetically modified to secrete abundant IL-10, which improved its therapeutic effects as compared to treatment with MSCs alone. 92 Another promising combination therapy is a tissue engineering approach using biomaterials. Biomaterials can serve as protective scaffold to ensure better survival of the graft and can among others enhance cellular infiltration into the lesion to stimulate regeneration. 93, 94 Interestingly, they can also be used for targeted delivery and sustained release of growth factors or cytokines, thereby serving as a promising tool to assess the benefits of immunomodulation on pro-regenerative processes post-stroke. However, before use in the clinic, one should be confident these combination therapies do not affect the SCs properties or induce adverse effects such as an inflammatory response.
We believe that SC-induced immunomodulation can be one of the central players in post-stroke recovery, via direct anti-inflammatory effects of the transplanted cells, or via its stimulating effects on angiogenesis and brain plasticity. Therefore, managing post-stroke inflammation through SCs administration is a worthwhile focus for future studies. Given the promising results obtained from pre-clinical and clinical research to date, there is significant belief that a better mechanistic understanding of the complex interactions required to develop successful immunomodulatory SC therapies for stroke is within reach.
Supplementary Material
Acknowledgments
We thank Christine D. Plant and Cindy H. Samos for manuscript preparation.
Sources of funding
The work was supported in part by NIH grant R01 NS058784 and California Institute of Regenerative Medicine grant RB5–07363 (GKS).
Footnotes
Disclosures
Dr. Steinberg is a consultant for Qool Therapeutics, Peter Lazic US, Inc., and NeuroSave.
References
- 1.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. 2016;119:142–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17:796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke. 2016;47:1817–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagpal A, Choy FC, Howell S, Hillier S, Chan F, Hamilton-Bruce MA, et al. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: A systematic review and meta-analysis. Stem Cell Res Ther. 2017;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain : a journal of neurology. 2011;134:1777–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki N, Ando S, Sumida K, Horie N, Saito K. Analysis of altered gene expression specific to embryotoxic chemical treatment during embryonic stem cell differentiation into myocardiac and neural cells. The Journal of toxicological sciences. 2011;36:569–585 [DOI] [PubMed] [Google Scholar]
- 7.Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiology of disease. 2013;52:191–203 [DOI] [PubMed] [Google Scholar]
- 8.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11839–11844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boltze J, Schmidt UR, Reich DM, Kranz A, Reymann KG, Strassburger M, et al. Determination of the therapeutic time window for human umbilical cord blood mononuclear cell transplantation following experimental stroke in rats. Cell Transplant. 2012;21:1199–1211 [DOI] [PubMed] [Google Scholar]
- 10.Newcomb JD, Ajmo CT Jr., Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 2006;15:213–223 [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenmuir CL, Wechsler LR. Update on cell therapy for stroke. Stroke Vasc Neurol. 2017;2:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nature reviews . Neuroscience. 2006;7:395–406 [DOI] [PubMed] [Google Scholar]
- 14.Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017 [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525 [DOI] [PubMed] [Google Scholar]
- 16.Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of il-10 and tnf-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. 2009;6:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain : a journal of neurology. 2009;132:2239–2251 [DOI] [PubMed] [Google Scholar]
- 18.Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14638–14643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giunti D, Parodi B, Usai C, Vergani L, Casazza S, Bruzzone S, et al. Mesenchymal stem cells shape microglia effector functions through the release of cx3cl1. Stem cells. 2012;30:2044–2053 [DOI] [PubMed] [Google Scholar]
- 21.Hsuan YC, Lin CH, Chang CP, Lin MT. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016;6:e00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegyi B, Kornyei Z, Ferenczi S, Fekete R, Kudlik G, Kovacs KJ, et al. Regulation of mouse microglia activation and effector functions by bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2014;23:2600–2612 [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Hjorth E, Zhu M, Calzarossa C, Samuelsson EB, Schultzberg M, et al. Interplay between human microglia and neural stem/progenitor cells in an allogeneic co-culture model. J Cell Mol Med. 2013;17:1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan K, Zhang R, Sun C, Chen L, Li P, Liu Y, et al. Bone marrow-derived mesenchymal stem cells maintain the resting phenotype of microglia and inhibit microglial activation. PLoS One. 2013;8:e84116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M, Dong Q, Yao H, Zhang Y, Yang Y, Dang Y, et al. Induced neural stem cells modulate microglia activation states via cxcl12/cxcr4 signaling. Brain Behav Immun. 2017;59:288–299 [DOI] [PubMed] [Google Scholar]
- 27.Sheikh AM, Nagai A, Wakabayashi K, Narantuya D, Kobayashi S, Yamaguchi S, et al. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: Contribution of fractalkine and il-5. Neurobiology of disease. 2011;41:717–724 [DOI] [PubMed] [Google Scholar]
- 28.Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, et al. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta. 2016;1862:461–471 [DOI] [PubMed] [Google Scholar]
- 29.Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211 [DOI] [PubMed] [Google Scholar]
- 30.Clark WM, Lauten JD, Lessov N, Woodward W, Coull BM. The influence of antiadhesion therapies on leukocyte subset accumulation in central nervous system ischemia in rats. J Mol Neurosci. 1995;6:43–50 [DOI] [PubMed] [Google Scholar]
- 31.Tsai MJ, Tsai SK, Hu BR, Liou DY, Huang SL, Huang MC, et al. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci. 2014;21:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capone C, Frigerio S, Fumagalli S, Gelati M, Principato MC, Storini C, et al. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS One. 2007;2:e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, et al. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim E, Yang J, Beltran CD, Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab. 2014;34:1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Q, Zhang Z, Zhang S, Yang H, Zhang X, Pan J, et al. Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain Res. 2015;1594:293–304 [DOI] [PubMed] [Google Scholar]
- 36.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain : a journal of neurology. 2008;131:616–629 [DOI] [PubMed] [Google Scholar]
- 37.Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (masters): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368 [DOI] [PubMed] [Google Scholar]
- 38.Yang B, Hamilton JA, Valenzuela KS, Bogaerts A, Xi X, Aronowski J, et al. Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune response from the spleen. Stem cells. 2017;35:1290–1302 [DOI] [PubMed] [Google Scholar]
- 39.Doyle KP, Quach LN, Sole M, Axtell RC, Nguyen TV, Soler-Llavina GJ, et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci. 2015;35:2133–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mena H, Cadavid D, Rushing EJ. Human cerebral infarct: A proposed histopathologic classification based on 137 cases. Acta Neuropathol. 2004;108:524–530 [DOI] [PubMed] [Google Scholar]
- 41.Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. 2015;46:2616–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Q, Zhang ZG, Ding GL, Zhang L, Ewing JR, Wang L, et al. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using mri. Neuroimage. 2005;28:698–707 [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, et al. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Scientific reports. 2016;6:33038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of cd34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, et al. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399 [DOI] [PubMed] [Google Scholar]
- 47.Liauw J, Hoang S, Choi M, Eroglu C, Choi M, Sun GH, et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008;28:1722–1732 [DOI] [PubMed] [Google Scholar]
- 48.Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, et al. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab. 2013;33:1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutierrez-Fernandez M, Rodriguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdan S, et al. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther. 2013;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nature reviews. Neuroscience. 2009;10:861–872 [DOI] [PubMed] [Google Scholar]
- 51.Madhavan L, Ourednik V, Ourednik J. Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem cells. 2008;26:254–265 [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786 [DOI] [PubMed] [Google Scholar]
- 53.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiology of disease. 2010;37:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem cells. 2010;28:1099–1106 [DOI] [PubMed] [Google Scholar]
- 56.Moniche F, Montaner J, Gonzalez-Marcos JR, Carmona M, Pinero P, Espigado I, et al. Intra-arterial bone marrow mononuclear cell transplantation correlates with gm-csf, pdgf-bb, and mmp-2 serum levels in stroke patients: Results from a clinical trial. Cell Transplant. 2014;23 Suppl 1:S57–64 [DOI] [PubMed] [Google Scholar]
- 57.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory m2, but not pro-inflammatory m1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118 [DOI] [PubMed] [Google Scholar]
- 58.Roszer T Understanding the mysterious m2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goumans MJ, Liu Z, ten Dijke P. Tgf-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127 [DOI] [PubMed] [Google Scholar]
- 60.Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P. Transforming growth factor-beta 1 (tgf-beta1) induces angiogenesis through vascular endothelial growth factor (vegf)-mediated apoptosis. J Cell Physiol. 2009;219:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boshuizen MC, de Winther MP. Interferons as essential modulators of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1579–1588 [DOI] [PubMed] [Google Scholar]
- 62.Lindner DJ. Interferons as antiangiogenic agents. Curr Oncol Rep. 2002;4:510–514 [DOI] [PubMed] [Google Scholar]
- 63.Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Fang S, Parsa AT. Complement and the central nervous system: Emerging roles in development, protection and regeneration. Immunol Cell Biol. 2010;88:781–786 [DOI] [PubMed] [Google Scholar]
- 64.D’Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: Implications for neuroprotection. Mol Med. 2001;7:367–382 [PMC free article] [PubMed] [Google Scholar]
- 65.Rutkowski MJ, Sughrue ME, Kane AJ, Ahn BJ, Fang S, Parsa AT. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res. 2010;59:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan MA, Assiri AM, Broering DC. Complement and macrophage crosstalk during process of angiogenesis in tumor progression. J Biomed Sci. 2015;22:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13632–13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765 [DOI] [PubMed] [Google Scholar]
- 69.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by il-4 or ifn-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160 [DOI] [PubMed] [Google Scholar]
- 70.Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, et al. Nitric oxide negatively regulates mammalian adult neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9566–9571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial tnf-alpha. Nature. 2006;440:1054–1059 [DOI] [PubMed] [Google Scholar]
- 72.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of ampa receptor and gaba receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norden DM, Fenn AM, Dugan A, Godbout JP. Tgfbeta produced by il-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol. 2015;7:a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates cns synapse elimination. Cell. 2007;131:1164–1178 [DOI] [PubMed] [Google Scholar]
- 76.Zhu Y, Guan YM, Huang HL, Wang QS. Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacol Sin. 2014;35:585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haase G, Pettmann B, Raoul C, Henderson CE. Signaling by death receptors in the nervous system. Curr Opin Neurobiol. 2008;18:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368 [DOI] [PubMed] [Google Scholar]
- 79.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory t cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199 [DOI] [PubMed] [Google Scholar]
- 80.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ros) in apoptosis induction. Apoptosis. 2000;5:415–418 [DOI] [PubMed] [Google Scholar]
- 81.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188 [DOI] [PubMed] [Google Scholar]
- 82.Pribiag H, Stellwagen D. Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology. 2014;78:13–22 [DOI] [PubMed] [Google Scholar]
- 83.Ottoboni L, De Feo D, Merlini A, Martino G. Commonalities in immune modulation between mesenchymal stem cells (mscs) and neural stem/precursor cells (npcs). Immunol Lett. 2015;168:228–239 [DOI] [PubMed] [Google Scholar]
- 84.Azad TD, Veeravagu A, Steinberg GK. Neurorestoration after stroke. Neurosurg Focus. 2016;40:E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janowski M, Wagner DC, Boltze J. Stem cell-based tissue replacement after stroke: Factual necessity or notorious fiction? Stroke. 2015;46:2354–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalladka D, Muir KW. Brain repair: Cell therapy in stroke. Stem Cells Cloning. 2014;7:31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chao F, Shen Y, Zhang H, Tian M. Multimodality molecular imaging of stem cells therapy for stroke. Biomed Res Int. 2013;2013:849819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng Y, Huang J, Zhu T, Li R, Wang Z, Ma F, et al. Stem cell tracking technologies for neurological regenerative medicine purposes. Stem Cells Int. 2017;2017:2934149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aghayan HR, Soleimani M, Goodarzi P, Norouzi-Javidan A, Emami-Razavi SH, Larijani B, et al. Magnetic resonance imaging of transplanted stem cell fate in stroke. J Res Med Sci. 2014;19:465–471 [PMC free article] [PubMed] [Google Scholar]
- 90.Toyama K, Honmou O, Harada K, Suzuki J, Houkin K, Hamada H, et al. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol. 2009;216:47–55 [DOI] [PubMed] [Google Scholar]
- 91.Yu X, Chen D, Zhang Y, Wu X, Huang Z, Zhou H, et al. Overexpression of cxcr4 in mesenchymal stem cells promotes migration, neuroprotection and angiogenesis in a rat model of stroke. J Neurol Sci. 2012;316:141–149 [DOI] [PubMed] [Google Scholar]
- 92.Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A, et al. Mesenchymal stem cells overexpressing interleukin-10 promote neuroprotection in experimental acute ischemic stroke. Mol Ther Methods Clin Dev. 2017;6:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.George PM, Bliss TM, Hua T, Lee A, Oh B, Levinson A, et al. Electrical preconditioning of stem cells with a conductive polymer scaffold enhances stroke recovery. Biomaterials. 2017;142:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang H, Sun F, Wang J, Xie L, Yang C, Pan M, et al. Combining injectable plasma scaffold with mesenchymal stem/stromal cells for repairing infarct cavity after ischemic stroke. Aging Dis. 2017;8:203–214</References [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


