Abstract
The whitefly, Bemisia tabaci, is a species complex of more than 40 cryptic species and a major agricultural pest. It causes extensive damage to plants mainly by transmitting plant viruses. There is still a lack of genomic data available for the different whitefly species found in Brazil and their bacterial endosymbionts. Understanding the genetic and transcriptomic composition of these insect pests, the viruses they transmit and the microbiota is crucial to sustainable solutions for farmers to control whiteflies. Illumina RNA-Seq was used to obtain the transcriptome of individual whiteflies from 10 different populations from Brazil including Middle East-Asia Minor 1 (MEAM1), Mediterranean (MED) and New World 2 (NW2). Raw reads were assembled using CLC Genomics Workbench and subsequently mapped to reference genomes. We obtained whitefly complete mitochondrial genomes and draft genomes from the facultative bacterial endosymbiont Hamiltonella for further phylogenetic analyses. In addition, nucleotide sequences of the GroEL chaperonin gene from Hamiltonella from different populations were obtained and analysed. There was concordance in the species clustering using the whitefly complete mitogenome and the mtCOI gene tree. On the other hand, the phylogenetic analysis using the 12 ORF’s of Hamiltonella clustered the native species NW2 apart from the exotics MEAM1 and MED. In addition, the amino acid analysis of GroEL chaperonin revealed a deletion only in Hamiltonella infecting NW2 among whiteflies populations analysed which was further confirmed by PCR and Sanger sequencing. The genomic data obtained in this study will aid understanding the functions that Hamiltonella may have in whitefly biology and serve as a reference for further studies regarding whiteflies in Brazil.
Introduction
The whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is an insect pest of significant economic importance of a wide variety of agricultural crops such as tomatoes, beans, cassava, cotton and ornamentals [1]. B. tabaci causes damage directly through feeding and indirectly through the transmission of plant pathogenic viruses, belonging to the genera Begomovirus, Carlavirus, Crinivirus, Ipomovirus and Torradovirus [2]. Currently, B. tabaci is classified as a cryptic species complex composed of at least 40 morphologically indistinguishable species on the basis of the mtCOI gene [3]. In Brazil, four B. tabaci species have been reported to date, the natives species from the Americas, New World 1 (NW1) and New World 2 (NW2) and the exotic species Middle East-Asia Minor 1 (MEAM1) and Mediterranean (MED) [4–6]. The exotics, MEAM1 and MED are globally distributed and the most challenging to control [7,8]. Species of the complex B. tabaci differ in several aspects including the range of host plants utilized, the capacity to cause plant disorders, attraction of natural enemies, response to pesticides and plant virus transmission capabilities [1,9]. Brazil it’s a country with extensive territory and high diversification of the agricultural systems which makes the identification and tracking of pests more complex. The first report of MED in Brazil [4] followed by a second incursion of this species associated to ornamentals plants [10] has highlighted the need of reference data, such as complete mitochondrial genome, to prevent and track the introduction of exotic pests to the country. In addition, full mitogenome comparisons provides a better understanding of evolutionary genetic relationships between members of B. tabaci [11]. Currently, there are 13 complete whitefly mitochondrial genomes characterized and available on GenBank [11–14].
The complexity of B. tabaci might also depend on the inherited bacterial endosymbionts whose functions are not fully understood [15]. The obligatory endosymbionts are essential for insects to live on nutritionally poor diets [16,17]. In whiteflies, the obligatory endosymbionts is Portiera aleyrodidarum [13]. In addition, insects often harbor facultative endosymbionts that can play different roles on the vector such as enhancing insecticide susceptibility [18,19], facilitating virus transmission [20,21], conferring tolerance to high-temperature [22] and resistance to natural enemies like parasitic wasps [23]. These bacteria have probably been acquired more recently than obligatory endosymbionts [16,24]. In B. tabaci, different facultative symbionts have been described, including Arsenophonus, Hamiltonella, Wolbachia, Cardinium, Fritschea and Rickettsia [8,20]. Among the facultative symbionts, Hamiltonella defensa is a maternally transmitted gamma-proteobacterium found sporadically in sap-feeding insects, including aphids, psyllids, and whiteflies [25–27]. Previous studies in Brazil have shown that different species of B. tabaci, such as MEAM1, MED and NW2 harbor Hamiltonella [10,28]. More recently, a survey has reported that Hamiltonella is highly distributed throughout the Brazilian territory and was detected in 89,5% of the MEAM1 specimens and approximately 50% of the MED specimens analyzed [29]. The high incidence of Hamiltonella in populations of whiteflies in Brazil may have serious implications to virus transmission. The GroEL proteins encoded by Hamiltonella has been found in Israeli B. tabaci (MEAM1) populations interacting with the coat protein of begomovirus and therefore facilitating virus transmission [20,24]. The GroEL produced by other symbionts of B. tabaci (MEAM1 and MED) did not interact with the virus and therefore were not involved in virus transmission [20]. Hamiltonella can play different roles depending on the vector species. In pea aphids, Acyrthosiphon pisum (Harris), Hamiltonella can block larval development of the solitary endoparasitoid wasps Aphidius ervi and Aphidius eadyi, rescuing the aphid host [30].
In this study, we sequenced ten transcriptomes of single whitefly specimens from different Brazilian populations and characterized the full mitochondrial genome belonging to three different species (MEAM1, MED and NW2) of the B. tabaci complex followed by phylogenetic analysis. In addition, the diversity of the facultative endosymbiont Hamiltonella was inferred analyzing 12 different ORF’s. The GroEL amino acid sequences of Hamiltonella from different B. tabaci species were also analyzed. Our goal was to add further details concerning phylogenetic diversity of mitochondrial genome and Hamiltonella among Brazilian populations of B. tabaci. In addition, a GroEL protein analysis was carried out that may give further insights about the functions that Hamiltonella may have in whitefly biology.
Methods
Whitefly sampling
Samples were obtained from pure colonies and straight from the field. Four different B. tabaci populations (153, 154, 156 and 320) were analysed, including two exotics species: Middle East-Asia Minor (MEAM1) and Mediterranean (MED); and a native species: New World 2 (NW2). Populations from colonies were previously identified by sequencing and analysis of the mtCOI gene using the primers C1-J-2195 and TL2-N-3014 [31].
RNA extraction
The RNA extraction of a single individual whitefly was carried out using the ARCTURUS PicoPure kit with modifications [32]. Extracted RNA was subjected to DNase treatment using the TURBO DNA free kit as described by the manufacturer (Ambion Life Technologies CA, USA). Subsequently, the RNA was concentrated using a vacuum centrifuge (Eppendorf, Germany) at 25°C for one hour. The pellet was resuspended in 18 μl of RNase free water and stored at—80 C waiting further analysis. Integrity of RNA was quantified by 2100 Bio-analyser (Aligent Technologies).
cDNA and Illumina library preparation
Total RNA from each individual whitefly sample was used for cDNA library preparation using the Illumina TruSeq Stranded Total RNA Preparation kit as described by the manufacturer (Illumina, San Diego, CA, USA). Later on, sequencing of 10 samples was carried out using the HiSeq2000 on a rapid run mode generating 2x50 bp paired end reads. Base calling, quality assessment and image analysis were conducted using the HiSeq control software v1.4.8 and Real Time Analysis v1.18.61 at the Macrogen Korea.
Trimming and de novo sequence assembly
The raw transcriptome data was trimmed using the software CLC Genomics Workbench v8.5.1 (CLCGW) with quality scores limit set to 0.01, ambiguous limit set to 2. Trimmed reads were then assembled into contigs using de novo sequence assembly tool in CLCGW. The assembly parameters consisted of mismatch cost (2), insertion cost (3), deletion cost (3), length fraction (0.5), similarity fraction (0.9) and minimum contig length of either 500 bp or 1000 bp.
Obtaining and analysing complete mitochondrial genomes
The whiteflies complete mitochondrial genome was obtained by mapping of the assembled contigs to reference genomes from GenBank using the software Geneious v9.1.3 [33]. For each B. tabaci species of the complex, a different reference mitogenome was used: KU877168 for MEAM1, JQ906700 for MED and AY521259 for NW2. When the mapped contigs did not covering the full length of the mitochondrial genome of the reference sequence, we resorted to mapping trimmed reads to the reference sequence and thus the whole length of the reference was covered. Mapping was performed with the following setting in Geneious software; minimum overlap 10%, minimum overlap identity 80%, allow gaps 10%, fine tuning set to iterate up to 10 times at custom sensitivity. A consensus between the mapped trimmed reads and the reference was used to form new mitochondrial genome. Improvements on the draft mitochondrial genomes were carried out using the software Pilon, a tool for genome assembly improvement [34]. Subsequently, mitochondrial genomes were annotated by MITOS [35]. Other whiteflies mitogenomes sequences were downloaded from GenBank (KJ778614, KX714967, KY951451, KF734668, KR819174, KY951448, JQ906700, KY951447, KU877168, KY951449. KY951450, KY951452 and AY521259) [11,12,14,15,36–38] and added to the analysis. Sequences obtained were aligned using MAFFT v7.309 [39] followed by visualization and analysis in Geneious software. A total of 19 sequences were aligned and analysed.
Analysing Hamiltonella genetic diversity
Assembled contigs as well as trimmed reads from each sample were mapped to a Hamiltonella reference genome (CP016303) to obtain the facultative endosymbiont draft genome. Mapping was performed with the following setting in Geneious software; minimum overlap 10%, minimum overlap identity 80%, allow gaps 10%, fine tuning set to iterate up to 10 times at custom sensitivity. Afterward, draft genomes were aligned to the reference using the whole genome alignment tool LASTZ version 1.02.00 [40] within Geneious v. 9.1.8. Genes with full coverage for all the samples were selected for further Bayesian phylogenetic analysis using the software ExaBayes version 1.4.1 [41].
Chaperonin GroEL gene analysis
Trimmed reads were mapped onto the reference sequence for chaperonin GroEL gene from Hamiltonella of B. tabaci (AF130421). As the mapped contigs did not cover the full length of the coding region of the reference sequence AF130421, we resorted to mapping trimmed reads to the reference sequence to get the full coverage of the whole length from the reference. A consensus between the mapped trimmed reads and the reference was used to form new chaperonin GroEL sequences, open reading frames (ORF) were predicted in Geneious. In addition, other chaperonin GroEL sequence from A. pisum was downloaded from Genbank (CP001277) and added to the analysis. Sequences obtained were aligned using MAFFT v7.309 [39] followed by visualization and analysis in Geneious software. A total of 9 sequences were aligned and analysed. In addition, primers were designed (GroEL 1,354 For- CCTC TGCG TCAG ATTG TGGT and GroEL 1,663 Rev–TCAT ACCA TTCA TTCC GCCC A) for a PCR reaction (95°C for 5min, 35 cycles at 95°C for 30 secs, 59.5°C for 30 secs, 72°C for 30 secs and 72°C for 10 min) followed by nucleotide sequencing to confirm the results obtained by NGS.
Bayesian phylogenetic analyses
All the phylogenetic analyses were run on 384 nodes on the Magnus supercomputer (Pawsey Centre, Western Australia). Mitochondrial genome phylogenetic analysis were performed by MrBayes 3.2.2. [42]. Analyses were run for 30 million generations with sampling every 1000 generations. Each analysis consisted of four independent runs, utilizing four coupled Markov chains. The run convergence was assessed by finding the plateau in the likelihood scores (standard deviation of split frequencies < 0.0015). In each of the runs, the first 25% trees were discarded as burn-in and the posterior probability is shown on each node.
In addition, the Hamiltonella phylogenetic analysis was performed on DNA sequences of 12 protein-coding genes for a dataset with 9 taxa using ExaBayes version 1.4.1; [41]. Bayesian analysis was carried out for four independent runs for 1 million generations, with trees sampled every 500 generations. The run convergence was monitored by finding the plateau in the likelihood scores (standard deviation of split frequencies < 0.0015). In each of the runs, the first 25% trees were discarded as burn-in for the estimation of a majority rule consensus topology and posterior probability for each node. Bayesian run files are available from the authors upon request. Trees were visualized, edited and rooted using FigTree v1.4.3.
Results
The sequenced number of reads from the 10 samples ranged from 29,840,288 to 64,080,188 among the ten samples and the number of contigs assembled ranged from 7,730 to 41,165 (Table 1).
Table 1. Next generation sequencing data from single whitefly transcriptomes of Bemisia tabaci populations collected in Brazil.
| Sample ID | Species | Colony / Open Field | Reference | Number of Reads | Number of Reads After Trimming | CLC minimum contig length | Number of contigs | Contig average length |
|---|---|---|---|---|---|---|---|---|
| 153_1 | MEAM1 | Colony | KU877168 | 36,449,340 | 36,449,269 | Trimmed reads | - | 143.9 |
| 500 | 25,640 | 1,240 | ||||||
| 1000 | 10,079 | 2,087 | ||||||
| 153_2 | MEAM1 | Colony | N/A | 44,093,422 | 41,041,633 | Trimmed reads | - | 146.0 |
| 500 | 31,072 | 1,228 | ||||||
| 154_1 | MED | Colony | JQ906700 | 37,206,396 | 37,206,313 | Trimmed reads | - | 144.7 |
| 1000 | 9,737 | 1,983 | ||||||
| 154_2 | MED | Colony | JQ906700 | 29,840,288 | 29,840,226 | Trimmed reads | - | 144.1 |
| 1000 | 7,730 | 1,947 | ||||||
| 320_1 | MED | Open Field | N/A | 64,080,188 | 57,947,512 | Trimmed reads | - | 145.5 |
| 500 | 41,165 | 1,240 | ||||||
| 320_3 | MED | Open Field | JQ906700 | 39,894,296 | 39,894,200 | Trimmed reads | - | 145.1 |
| 1000 | 9,719 | 1,969 | ||||||
| 156_2 | NW2 | Colony | AY521259 | 40,826,418 | 40,826,334 | Trimmed reads | - | 145.2 |
| 1000 | 11,961 | 2,232 | ||||||
| 156_3 | NW2 | Colony | AY521259 | 43,194,264 | 42,194,175 | Trimmed reads | - | 145.0 |
| 1000 | 12,802 | 2,244 | ||||||
| 156_4 | NW2 | Colony | N/A | 48,800,318 | 45,102,719 | Trimmed reads | - | 145.9 |
| 500 | 34,090 | 1,298 | ||||||
| 156_5 | NW2 | Colony | N/A | 24,568,460 | 22,491,230 | Trimmed reads | - | 145.7 |
| 500 | 21,597 | 1,310 |
Hamiltonella genetic diversity
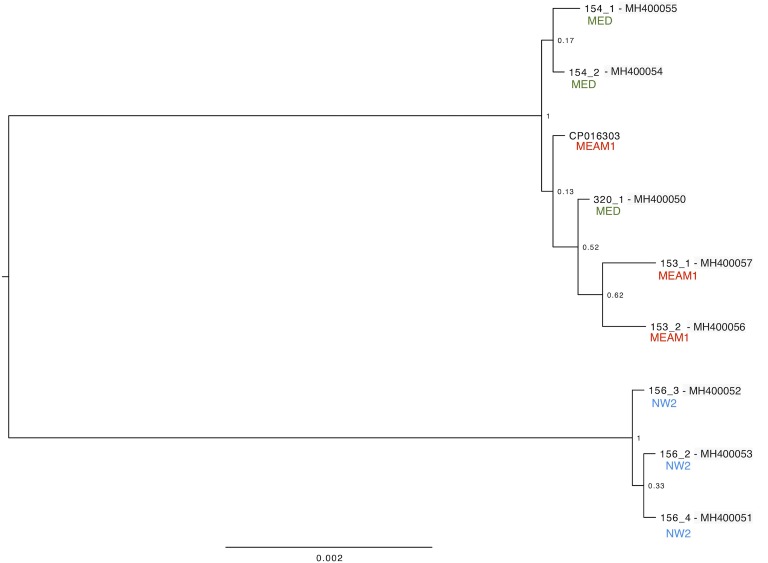
The diversity of Hamiltonella in B. tabaci populations from Brazil was carried out analysing 12 ORF’s from eight single whitefly transcriptomes totalizing an alignment of 6,378bp length. The analysed ORF’s were: DNA transformation protein tfoX, porin OmpA, acyl carrier, 50S ribosomal protein L3, 50S ribosomal protein L23, 30S ribosomal protein S17, 50S ribosomal protein L24, 50S ribosomal protein L5, rpsN, nucleotide exchange factor GrpE, porin and DNA-binding protein. The phylogenetic analysis from the sequenced accessions separate into two deeply divergent clades representing the native species from the Americas (NW2) and the exotics (MEAM1 and MED) (Fig 1). Furthermore, the identity percentages among the 12 ORF’s from Hamiltonella were obtained (S1 Table).
Fig 1. Phylogenetic analysis from 12 ORF’s of Hamiltonella from Brazilian populations of Bemisia tabaci.
Analysis was carried out using the software ExaBayes version 1.4.1. MEAM1, Middle East-Asia Minor-1; MED, Mediterranean; NW2, New World 2.
GroEL chaperonin analysis
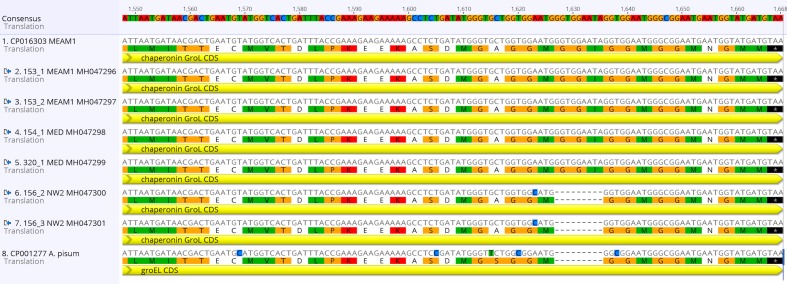
The GroEL (Chaperonin 60) gene was obtained from six B. tabaci single whitefly transcriptomes of Brazil. In addition to the data obtained in this study, the analysis also included Hamiltonella GroEL sequences downloaded from GenBank from the pea aphid A. pisum (CP001277) and from B. tabaci MEAM1 (CP016303). Analysis of the translated proteins revealed a three amino acids deletion present only in the B. tabaci NW2 species and in the pea aphid A. pisum (Fig 2). The deletion present in Hamiltonella from NW2 and pea aphid was a sequence of two glycine and one isoleucine. The nucleotide deletion was confirmed by PCR, which amplified an 300bp amplicon, followed by nucleotide sequencing.
Fig 2. The chaperonin GroEL protein analysis of the Hamiltonella endosymbiont.
Analysis was visualized on Geneious v9.1.3 and revealed a three amino acids deletion only in the Bemisia tabaci New World 2 species and the pea aphid Acyrthosiphon pisum.
Mitochondrial genome
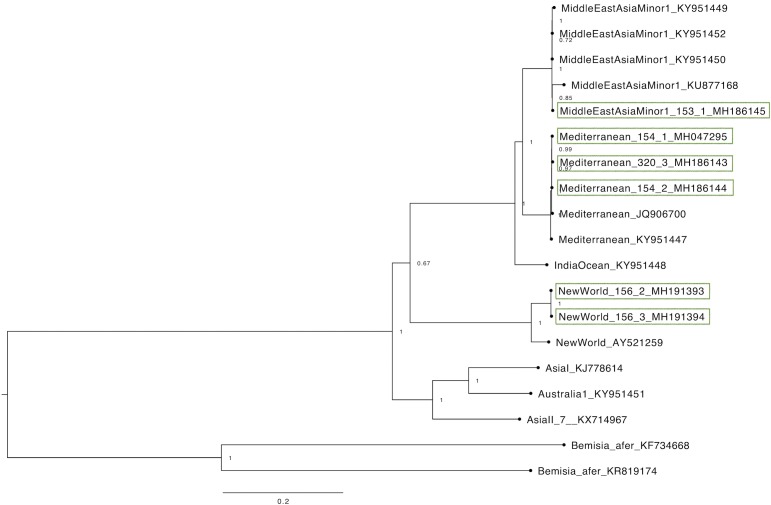
Six complete mitochondrial genomes were obtained from three different species found in Brazil (NW2, MEAM1 and MED). Phylogenetic analysis of the complete mitochondrial genomes separated different species of the complex in distinct clades (Fig 3). Furthermore, the identity percentages among all the mitogenomes from B. tabaci were obtained (S2 Table).
Fig 3. Phylogenetic analysis of the complete mitochondrial genome of whiteflies populations from Brazil obtained from single whitefly transcriptomes.
The alignment totalized 19 samples and 16073bp length including references from GenBank. Brazilian samples obtained in this study were highlighted in green.
Discussion
In this study we present the phylogenetic relationship of B. tabaci and its association with the facultative endosymbiont Hamiltonella. We show the evolutionary differences between native populations of B. tabaci species of the Americas and invasive B. tabaci species. From our data the genetic differences are not confined to the mtCOI gene, but extend to the rest of the mitogenome and to the facultative endosymbiont Hamiltonella. These findings will contribute to the understanding about the functions that Hamiltonella may have in B. tabaci biology and will aid the correct identification of B. tabaci specimens.
Six complete mitochondrial genomes were obtained from three different species of the complex B. tabaci: MEAM1, MED and NW2. Complete mitochondrial genomes comparisons are essential for better understanding the evolutionary genetic relationships between members of the B. tabaci species complex [3,11]. There are 13 complete whitefly mitochondrial genomes characterized and available on GenBank to date [11,12,14,15,36–38] which is a valuable contribution to a better understanding of the taxonomy of this global pest. The addition of six more full mitogenomes from B. tabaci to the global database will contribute for more accurate identification and will serve as references for further full mitochondrial genome phylogenies studies. The phylogenetic analysis of the full mitogenome (Fig 3) conducted in this study separated the species in different clades in a similar pattern compared to phylogenetic trees of partial fragment of the mtCOI gene [1]. This reinforces that using a partial fragment of the mtCOI gene to infer phylogeny in B. tabaci is a reliable way to delimitate the species boundaries and it represents the whole mitochondrial genome.
A multilocus phylogenetic analysis was carried out for the facultative endosymbiont Hamiltonella. Previous studies in Brazil phylogenetically analyzed Hamiltonella based on 16S rRNA gene and found genetically homogeneous populations of Hamiltonella from MEAM1 and MED across Brazil [29]. Another phylogenetic study based on 16S rRNA gene, in Southeast Europe, have grouped Hamiltonella from both B. tabaci and T. vaporariorum in the same clades. Our phylogenetic analysis based on 12 ORF’s clustered Hamiltonella from NW2 populations in a different clade from MEAM1 and MED populations (Fig 1) suggesting that native populations are infected with a genetically different Hamiltonella from invasive populations, reinforcing the inexistence of endosymbiont horizontal transmission between invasive and native species.
The differences of Hamiltonella from native and exotic species extends to the GroEL gene. Our analysis revealed an amino acid deletion of two glycine and one isoleucine in the GroEL gene present only in Hamiltonella from native populations (Fig 2). Glycine is a non-essential amino acid and isoleucine is an essential amino acid for Hamiltonella [43]. The absence of these amino acids could be affecting the confirmation of GroEL on populations of Hamiltonella presenting this deletion. Thus, this deletion in Hamiltonella could imply in biological changes in populations of whiteflies harbouring this facultative endosymbiont.
It’s known that facultative bacterial endosymbionts are associated with viral transmission [20,21] and different members of the B. tabaci species complex may transmit the same viruses with different efficiencies [44]. The facultative endosymbiont Hamiltonella has been identified as a key driver in the transmission of begomoviruses [24]. Hamiltonella encodes a GroEL chaperonin homologue protein that is crucial in safeguarding begomoviruses in the haemolymph [24]. Several studies have shown the interaction between the begomoviruses Tomato yellow leaf curl virus (TYLCV) coat protein (CP) and GroEL present in the haemolymph of B. tabaci [20,45]. Disturbing the association GroEL-TYLCV in vivo by feeding insects with an antibody raised against Buchnera GroEL leads to the degradation of the virus and to a markedly decrease in transmission efficiency of the virus [24,45,46].
There is still a lack of knowledge regarding the interactions among vector, symbionts and whitefly-transmitted viruses within Brazilian populations. Previous surveys conducted in Brazil have found that field populations of MEAM1, MED and NW2 analyzed frequently harbor Hamiltonella [28, 41]. Therefore, it would be important to find out if there is a relationship between the diversity of H. defensa found in the current study and biological features of field populations of B. tabaci. The existence of biological and behavioral differences between the native species New World and the exotic MEAM1 have been reported already [5,47]. The New World B. tabaci are found more often colonizing weeds and wild plants [5]. In addition, previous transmission studies comparing NW2 and MEAM1 species, both harboring Hamiltonella, have found a different transmission efficiency of Brazilian whitefly-transmitted viruses between the species [47]. It was found that the begomovirus Euphorbia yellow mosaic virus (EuYMV) is transmitted more efficiently by NW2 compared to MEAM1 and that the crinivirus, Tomato chlorosis virus (ToCV) and the carlavirus, Cowpea mild mottle virus (CpMMV) are transmitted more efficiently by MEAM1 than NW2 [47]. Interestingly, each of these viruses has a different mode of transmission by the whitefly vector. The begomoviruses, EuYMV is transmitted in a persistent manner, the crinivirus, ToCV is transmitted in a semipersistent manner and the carlavirus, CpMMV is transmitted in a non-persistent mode [48,49], which is a very unusual mode of transmission among B. tabaci transmitted viruses.
The reasons for a difference in transmission efficiencies by NW2 and MEAM1 are still unknown but might be related to several factors related to the host, the viruses or other facultative endosymbiont found in the vector. However, the data found in this study of phylogenetic differences between insect symbiotic Hamiltonella from NW2 and MEAM1 added to the deletion of three amino acids present in the homologue GroEL Chaperonin protein might aid to explain the difference in the transmission efficiencies among native and exotic species present in Brazil if further studies were carried out.
The genomic data obtained in this study from the facultative endosymbionts, Hamiltonella and the complete mitochondrial of Brazilian B. tabaci populations is unprecedented and essential to serve as a reference for further studies regarding whiteflies in Brazil. The phylogenetic and amino acid analysis revealed genetic diversity between Hamiltonella from native and exotic populations that will aid for better understanding about the functions that Hamiltonella may have in whitefly biology.
Supporting information
The identity percentage was obtained on Geneious software v9.1.8.
(DOCX)
The identity percentage was obtained on Geneious v9.1.8.
(DOCX)
Acknowledgments
The analyses were carried out using computational facilities at Pawsey Supercomputing Centre who are supported by the Australian Government and the Government of Western Australia.
Data Availability
All 20 files are available from the NCBI database (accession number(s) MH400055, MH400054, MH400050, MH400057, MH400056, MH400052, MH400053, MH400051, MH047296, MH047297, MH047298, MH047299, MH047300, MH047301, MH186145, MH047295, MH186143, MH186144, MH191393, MH191394).
Funding Statement
RKS is a grant recipient from Fundação de Amparo à Pesquisa do Estado de São Paulo (http://www.fapesp.br) Process Number 2017/21588-7. BRDM is a grant recipient from Conselho Nacional de Desenvolvimento Científico e Tecnológico (http://cnpq.br) Process Number 200826/2015-8.
References
- 1.De Barro PJ, Liu S-S, Boykin LM, Dinsdale AB. Bemisia tabaci: A Statement of Species Status. Annu Rev Entomol. Annual Reviews; 2011;56: 1–19. 10.1146/annurev-ento-112408-085504 [DOI] [PubMed] [Google Scholar]
- 2.Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S. Emerging Virus Diseases Transmitted by Whiteflies. Annu Rev Phytopathol. Annual Reviews; 2011;49: 219–248. 10.1146/annurev-phyto-072910-095235 [DOI] [PubMed] [Google Scholar]
- 3.Boykin Boykin, Armstrong Karen, Kubatko Laura, De Barro Paul. Species Delimitation and Global Biosecurity. Evol Bioinforma. 2011; 1 10.4137/EBO.S8532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Fonseca Barbosa L, Yuki VA, Marubayashi JM, De Marchi BR, Perini FL, Pavan MA, et al. First report of Bemisia tabaci Mediterranean (Q biotype) species in Brazil. Pest Manag Sci. 2015;71 10.1002/ps.3909 [DOI] [PubMed] [Google Scholar]
- 5.Barbosa LFF, Marubayashi JM, De Marchi BR, Yuki VA, Pavan MAMA, Moriones E, et al. Indigenous American species of the Bemisia tabaci complex are still widespread in the Americas. Pest Manag Sci. 2014;70: 1440–1445. 10.1002/ps.3731 [DOI] [PubMed] [Google Scholar]
- 6.Marubayashi JM, Yuki VA, Rocha KCG, Mituti T, Pelegrinotti FM, Ferreira FZ, et al. At least two indigenous species of the Bemisia tabaci complex are present in Brazil. J Appl Entomol. Blackwell Publishing Ltd; 2013;137: 113–121. 10.1111/j.1439-0418.2012.01714.x [DOI] [Google Scholar]
- 7.Gauthier N, Clouet C, Perrakis A, Kapantaidaki D, Peterschmitt M, Tsagkarakou A. Genetic structure of Bemisia tabaci Med populations from home-range countries, inferred by nuclear and cytoplasmic markers: impact on the distribution of the insecticide resistance genes. Pest Manag Sci. 2014;70: 1477–1491. 10.1002/ps.3733 [DOI] [PubMed] [Google Scholar]
- 8.Hu J, De Barro P, Zhao H, Wang J, Nardi F, Liu S-S. An Extensive Field Survey Combined with a Phylogenetic Analysis Reveals Rapid and Widespread Invasion of Two Alien Whiteflies in China. Roberts RG, editor. PLoS One. Public Library of Science; 2011;6: e16061 10.1371/journal.pone.0016061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, De Barro PJ, Liu SS. Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex. Bull Entomol Res. 2010;100: 359–366. 10.1017/S0007485310000015 [DOI] [PubMed] [Google Scholar]
- 10.Aparecida de Moraes L, Marubayashi Massaharu J, Yuki Atsushi V, Ghanim M, Bello VHVH, Rossitto De Marchi B, et al. New invasion of Bemisia tabaci Mediterranean species in Brazil associated to ornamental plants. Phytoparasitica. Springer Netherlands; 2017;45: 1–9. 10.1007/s12600-017-0607-9 [DOI] [Google Scholar]
- 11.Tay WT, Elfekih S, Court L, Gordon KH, De Barro PJ. Complete mitochondrial DNA genome of Bemisia tabaci cryptic pest species complex Asia I (Hemiptera: Aleyrodidae). Mitochondrial DNA. 2014;1736: 1–2. 10.3109/19401736.2014.926511 [DOI] [PubMed] [Google Scholar]
- 12.Wang H-L, Yang J, Boykin LM, Zhao Q-Y, Li Q, Wang X-W, et al. The characteristics and expression profiles of the mitochondrial genome for the Mediterranean species of the Bemisia tabaci complex. BMC Genomics. 2013;14: 401 10.1186/1471-2164-14-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thao ML, Baumann P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl Environ Microbiol. American Society for Microbiology; 2004;70: 3401–6. 10.1128/AEM.70.6.3401-3406.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay WT, Elfekih S, Polaszek A, Court LN, Evans GA, Gordon KHJ, et al. Novel molecular approach to define pest species status and tritrophic interactions from historical Bemisia specimens. Sci Rep. 2017;7: 429 10.1038/s41598-017-00528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrella G, Nappo AG, Manco E, Greco B, Giorgini M. Invasion of the Q2 mitochondrial variant of Mediterranean Bemisia tabaci in southern Italy: possible role of bacterial endosymbionts. Pest Manag Sci. 2014;70: 1514–1523. 10.1002/ps.3686 [DOI] [PubMed] [Google Scholar]
- 16.Baumann P. Biology of Bacteriocyte-Associated Endosymbionts of Plant Sap-Sucking Insects. Annu Rev Microbiol. 2005;59: 155–189. 10.1146/annurev.micro.59.030804.121041 [DOI] [PubMed] [Google Scholar]
- 17.Ferrari J, Vavre F. Bacterial symbionts in insects or the story of communities affecting communities. Philos Trans R Soc Lond B Biol Sci. The Royal Society; 2011;366: 1389–400. 10.1098/rstb.2010.0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kontsedalov S, Zchori‐Fein E, Chiel E, Gottlieb Y, Inbar M, Ghanim M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag Sci. 2008;64: 789–792. 10.1002/ps.1595 [DOI] [PubMed] [Google Scholar]
- 19.Kontsedalov S, Gottlieb Y, Ishaaya I, Nauen R, Horowitz R, Ghanim M. Toxicity of spiromesifen to the developmental stages of Bemisia tabaci biotype B. Pest Manag Sci. 2009;65: 5–13. 10.1002/ps.1636 [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Kontsedalov S, Skaljac M, Brumin M, et al. The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J Virol. American Society for Microbiology; 2010;84: 9310–7. 10.1128/JVI.00423-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rana VS, Singh ST, Priya NG, Kumar J, Rajagopal R. Arsenophonus GroEL Interacts with CLCuV and Is Localized in Midgut and Salivary Gland of Whitefly B. tabaci. Oliveira PL, editor. PLoS One. Public Library of Science; 2012;7: e42168 10.1371/journal.pone.0042168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brumin M, Kontsedalov S, Ghanim M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. Blackwell Publishing Asia; 2011;18: 57–66. 10.1111/j.1744-7917.2010.01396.x [DOI] [Google Scholar]
- 23.Mahadav A, Gerling D, Gottlieb Y, Czosnek H, Ghanim M. Parasitization by the wasp Eretmocerus mundus induces transcription of genes related to immune response and symbiotic bacteria proliferation in the whitefly Bemisia tabaci. BMC Genomics. 2008;9: 342 10.1186/1471-2164-9-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorovits R, Czosnek H. Insect Symbiotic Bacterial GroEL (Chaperonin 60) and Plant Virus Transmission. In: Mande SC, Kumar CMS, Sharma A, editors. Moonlighting Cell Stress Proteins in Microbial Infections. 2013. pp. 173–187. 10.1007/978-94-007-6787-4 [DOI] [Google Scholar]
- 25.Rao Q, Wang S, Su YL, Bing XL, Liu SS, Wang XW. Draft genome sequence of “Candidatus Hamiltonella defensa,” an endosymbiont of the whitefly Bemisia tabaci [Internet]. Journal of Bacteriology. 2012. p. 3558 10.1128/JB.00069-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luan J-B, Shan H-W, Isermann P, Huang J-H, Lammerding J, Liu S-S, et al. Cellular and molecular remodelling of a host cell for vertical transmission of bacterial symbionts. Proc R Soc B Biol Sci. 2016;283: 20160580 10.1098/rspb.2016.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luan J, Sun X, Fei Z, Douglas AE. Maternal Inheritance of a Single Somatic Animal Cell Displayed by the Bacteriocyte in the Whitefly Bemisia tabaci. Curr Biol. 2018;28: 459–465.e3. 10.1016/j.cub.2017.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marubayashi JM, Kliot A, Yuki VA, Rezende JAM, Krause-Sakate R, Pavan MA, et al. Diversity and Localization of Bacterial Endosymbionts from Whitefly Species Collected in Brazil. Bourtzis K, editor. PLoS One. Public Library of Science; 2014;9: e108363 10.1371/journal.pone.0108363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Moraes Aparecida L, Muller C, Oliveira de Freitas Bueno RC, Santos A, Bello VH, Rossitto De Marchi B, et al. Distribution and phylogenetics of whiteflies in Brazil and their endosymbiont relationships. unpublised. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Q, Oliver KM, Pan H, Jiao X, Liu B, Xie W, et al. Facultative Symbiont <I>Hamiltonella</I> Confers Benefits to <I>Bemisia tabaci</I> (Hemiptera: Aleyrodidae), an Invasive Agricultural Pest Worldwide. Environ Entomol. 2013;42: 1265–1271. 10.1603/EN13182 [DOI] [PubMed] [Google Scholar]
- 31.Frohlich DR, Torres-Jerez I, Bedford ID, Markham PG, Brown JK. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol Ecol. 1999;8: 1683–1691. 10.1046/j.1365-294x.1999.00754.x [DOI] [PubMed] [Google Scholar]
- 32.Sseruwagi P, Wainaina J, Ndunguru J, Tumuhimbise R, Tairo F, Guo J-Y, et al. The first transcriptomes from field-collected individual whiteflies (Bemisia tabaci, Hemiptera: Aleyrodidae). Gates Open Res. 2017;1: 16 10.12688/gatesopenres.12783.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28: 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. Wang J, editor. PLoS One. Public Library of Science; 2014;9: e112963 10.1371/journal.pone.0112963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, et al. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 2013;69: 313–319. 10.1016/j.ympev.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 36.Tay WT, Elfekih S, Court LN, Gordon KHJ, Delatte H, De Barro PJ. The Trouble with MEAM2: Implications of Pseudogenes on Species Delimitation in the Globally Invasive Bemisia tabaci (Hemiptera: Aleyrodidae) Cryptic Species Complex. Genome Biol Evol. Oxford University Press; 2017;9: 2732–2738. 10.1093/gbe/evx173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H-L, Zhang Z, Bing X-L, Liu Y-Q, Liu S-S, Wang X-W. A complete mitochondrial DNA genome derived from a Chinese population of the Bemisia afer species complex (Hemiptera: Aleyrodidae). Mitochondrial DNA Part A. 2016;27: 3500–3501. 10.3109/19401736.2015.1066367 [DOI] [PubMed] [Google Scholar]
- 38.Wang H-L, Xiao N, Yang J, Wang X-W, Colvin J, Liu S-S. The complete mitochondrial genome of Bemisia afer (Hemiptera: Aleyrodidae). Mitochondrial DNA. 2016;27: 98–99. 10.3109/19401736.2013.873921 [DOI] [PubMed] [Google Scholar]
- 39.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. Oxford University Press; 2002;30: 3059–66. Available: http://www.ncbi.nlm.nih.gov/pubmed/12136088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz S, Kent WJ, Smit A, Zhang Z, Baertsch R, Hardison RC, et al. Human–Mouse Alignments with BLASTZ. Genome Res. 2003;13: 103–107. 10.1101/gr.809403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aberer AJ, Kobert K, Stamatakis A. ExaBayes: Massively Parallel Bayesian Tree Inference for the Whole-Genome Era. Mol Biol Evol. Oxford University Press; 2014;31: 2553–2556. 10.1093/molbev/msu236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, H?hna S, et al. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst Biol. 2012;61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degnan PH, Yu Y, Sisneros N, Wing RA, Moran NA. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proc Natl Acad Sci. 2009;106: 9063–9068. 10.1073/pnas.0900194106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polston JE, De Barro P, Boykin LM. Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag Sci. John Wiley & Sons, Ltd; 2014;70: 1547–1552. 10.1002/ps.3738 [DOI] [PubMed] [Google Scholar]
- 45.Morin S, Ghanim M, Sobol I, Czosnek H. The GroEL Protein of the Whitefly Bemisia tabaci Interacts with the Coat Protein of Transmissible and Nontransmissible Begomoviruses in the Yeast Two-Hybrid System. Virology. 2000;276: 404–416. 10.1006/viro.2000.0549 [DOI] [PubMed] [Google Scholar]
- 46.Morin S, Ghanim M, Zeidan M, Czosnek H, Verbeek M, van den Heuvel JFJM. A GroEL Homologue from Endosymbiotic Bacteria of the WhiteflyBemisia tabaciIs Implicated in the Circulative Transmission of Tomato Yellow Leaf Curl Virus. Virology. 1999;256: 75–84. 10.1006/viro.1999.9631 [DOI] [PubMed] [Google Scholar]
- 47.De Marchi BR, Marubayashi JM, Favara GM, Yuki VA, Watanabe LFLFM, Barbosa LFLF, et al. Comparative transmission of five viruses by Bemisia tabaci NW2 and MEAM1. Trop Plant Pathol. Tropical Plant Pathology; 2017;1 10.1007/s40858-017-0186-9 [DOI] [Google Scholar]
- 48.Marubayashi JM, Yuki VA, Wutke EB. Transmission of the Cowpea mild mottle virus by whitefly Bemisia tabaci biotype B for plants of beans and soy. Summa Phytopathol. Grupo Paulista de Fitopatologia; 2010;36: 158–160. 10.1590/S0100-54052010000200009 [DOI] [Google Scholar]
- 49.Zanardo LG, Carvalho CM. Cowpea mild mottle virus (Carlavirus, Betaflexiviridae): a review. Trop Plant Pathol. Tropical Plant Pathology; 2017; 417–430. 10.1007/s40858-017-0168-y [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The identity percentage was obtained on Geneious software v9.1.8.
(DOCX)
The identity percentage was obtained on Geneious v9.1.8.
(DOCX)
Data Availability Statement
All 20 files are available from the NCBI database (accession number(s) MH400055, MH400054, MH400050, MH400057, MH400056, MH400052, MH400053, MH400051, MH047296, MH047297, MH047298, MH047299, MH047300, MH047301, MH186145, MH047295, MH186143, MH186144, MH191393, MH191394).