Abstract
BACKGROUND/OBJECTIVES
To provide a reliable assessment of the hypothesized association of fish consumption with stroke risk accumulatively, an updated meta-analysis of published prospective cohort studies was conducted.
SUBJECTS/METHODS
Prospective cohort studies through April 2012 in peer-reviewed journals indexed in MEDLINE and EMBASE were selected. Additional information was retrieved through Google or a search of the reference list in relevant articles. The main outcome measure was the weighted hazards ratio (HR) and corresponding 95% confidence interval (CI) for incident stroke according to fish consumption using a random-effects model.
RESULTS
A database was derived from 16 eligible studies (19 cohorts), including 402 127 individuals (10 568 incident cases) with an average 12.8 years of follow-up. Compared with those who never consumed fish or ate fish <1/month, the pooled adjusted HRs of total stroke risk were 0.97 (95% CI, 0.87–1.08), 0.86 (0.80–0.93), 0.91 (0.85–0.98) and 0.87 (0.79–0.96) for those who consumed fish 1–3/month, 1/week, 2–4/week and ≥5/week, respectively (Plinear trend = 0.09; Pnonlinear trend = 0.02). Study location was a modifier. An inverse association between fish intake and stroke incidence was only found by studies conducted in North America. The modest inverse associations were more pronounced with ischemic stroke and were attenuated with hemorrhagic stroke.
CONCLUSIONS
Accumulated evidence generated from this meta-analysis suggests that fish intake may have a protective effect against the risk of stroke, particularly ischemic stroke.
Keywords: fish, stroke, cohort studies, meta-analysis
INTRODUCTION
Fish consumption has been hypothesized to be associated with a reduced risk of stroke, but epidemiological studies showed inconsistent results and most of them have not separated ischemic stroke from hemorrhagic stroke, which have different etiopathogenesis. Our previous meta-analysis of eight prospective cohort studies published in 2004 suggested that there was a beneficial association between fish consumption and the risk of stroke, especially ischemic stroke.1 Since then, two more meta-analyses,2,3 including one recent brief report,3 have also addressed this topic. However, both of them included some case–control studies with prospective cohort studies under a seemingly more flexible criterion, and assumed a linear association between fish intake and stroke risk. Importantly, neither of them standardized fish intake or performed detailed stratified analysis. Because the range of fish consumption varies substantially across study populations, and the levels of fish intake in the reference groups are significantly different among some studies (for example, between Western and Eastern populations), the combined results might be biased without standardizing fish intake. To date, eight more prospective cohort studies evaluating fish intake and stroke risk have been published, indicating a need to update our previous findings and fill in the gap that lacks detailed subgroup analysis under standardized fish consumption categories left by other studies. In the present meta-analysis, we focused on evaluating potential heterogeneity by population and study characteristics. To standardize fish consumption, we define five categories based on two published large cohort studies, that is, the Nurses’ Health Study (NHS),4 and the Health Professional Follow-Up Study (HPFS),5 and did our best to obtain de novo results from the authors of primary studies.
MATERIALS AND METHODS
Data sources and searches
All prospective cohort studies published in English-language journals through April 2012 that reported the association between fish consumption and incidence of stroke were included. We searched MEDLINE and EMBASE using the terms ‘fish’, ‘seafood’, ‘animal product’, combined with ‘stroke’, ‘cerebrovascular accident’, ‘cerebral or brain infarction’ or ‘cardiovascular disease’. Additional information was retrieved through Google and a search of the references from relevant articles. Papers were included if they were prospective cohort studies, and the studies presented the hazards ratio (HR) and the corresponding 95% confidence intervals (CIs) of stroke relating to fish intake with the lowest category as the reference, or such information could be recalculated. Two of our authors (PX and BQ) independently reviewed all relevant papers identified eligible studies, and extracted the data. Discrepancies were resolved by group discussion.
Study selection
We identified eight new prospective cohort studies6–13 that reported the associations between fish consumption and the risk of stroke in addition to the eight prospective studies4,5,14–19 included in our previous meta-analysis.1 One study from the Physicians’ Health Study (PHS)15 was updated by obtaining the de novo results based on the same data set from the authors with extending the average follow-up time from 4 to 20 years. De novo results from another study were obtained as the original study did not use the lowest fish consumption category as the reference group.8 Three studies,9,13,16 which reported the results for men and women separately, were counted as two separate cohorts. Therefore, a total of 16 studies (19 cohorts) were included in this updated meta-analysis.
Data extraction
The data that we collected included the first author’s name, year of publication, study name, country of origin, number of participants, range of participants’ age, proportion of men, duration or average years of follow-up, categories of fish intake and the amount for each category, methods for measuring fish intake, number of stroke events (by subtypes, if available), covariates as well as HRs and 95% CIs of incident stroke in the corresponding categories. HRs and 95% CIs transformed to their natural logarithms (ln) were used to compute the corresponding s.e. To be consistent, we used the same categories for standardizing fish intake as we did in the previous study.1 We first converted frequency into grams per day. The amount of fish consumption (g/day) was estimated by multiplying the frequency of consumption (serving/day) by the corresponding portion size (g/serving). For example, the derived average portion size in He et al.5 was 105 g/serving. The range of fish consumption for 1–3/month was converted to 3.5 g/day (105 × 1/30) to 10.5 g/day (105 × 3/30). The median or mean value between two adjacent categories was used as the cutoff point. When the portion size of fish intake in an individual study was not available from the published paper, the information was acquired from authors of the primary study or the value was determined based on data from the NHS and the HPFS and considering that the food frequency questionnaire used in these three studies has been validated. If the upper-bound of the highest fish intake category was uncertain, for instance, fish intake was ≥5/week, we assigned 1 serving/day of fish as the upper limit.
Data synthesis and analysis
Fish consumption was categorized into five standardized intervals: ‘never or <1/month’, ‘1–3/month’, ‘1/week’, ‘2–4/week’ and ‘≥5/week’. We assigned each HR into its corresponding standardized interval according to the range or median amount of fish intake in the category. If the median amount of fish consumption from more than one category in a single study fell into the same standardized category of fish intake in our meta-analysis, we pooled the HRs and used the combined estimate for that category. On the other hand, if the range of fish intake covered more than one standardized category, we allocated the HR based on the median fish intake.
We estimated the weighted HRs and 95% CIs of stroke for each standardized category of fish consumption compared with the lowest category using a random-effects model proposed by DerSimonian and Laird.20 In addition, Cochran’s test was used to test for heterogeneity among studies and I2 was computed to determine the degree of inconsistency across studies. A variance-weighted random-effects meta-regression model was used to test linear trend by modeling the ln HR of stroke as a linear function of fish intake where the median level for each intake category was derived. P for interaction was detected by adding ‘continuous fish intake × subgroup’ term in the model. Publication bias was assessed by the funnel plot and the Egger asymmetry test.21 We also examined the associations for subtypes of stroke (ischemic and hemorrhagic) because of their different etiopathogenesis.
To explore potential sources of heterogeneity, we did subgroup analysis based on gender, duration of follow-up (<12.8 vs ≥12.8 years), dietary assessment (interview-based vs self-administered food frequency questionnaire) and stroke end point (incidence vs mortality) using random-effects models.
Several sensitivity analyses were conducted to test the robustness of our findings: whether the results would be markedly affected by a single study, whether repeated analysis by a fixed-effects model would generate similar results and whether excluding the study with only two fish consumption groups would change the results significantly.
All analyses were performed using STATA statistical software (Version 11.0, STATA Corp., College Station, TX, USA). All statistical tests were two sided and a P-value of ≤0.05 was considered statistically significant.
RESULTS
Characteristics of included studies
The final data set for our meta-analysis of fish consumption and incident stroke included 19 independent cohorts from 16 prospective studies and comprised 402 127 individuals (10 568 incident stroke cases) aged between 30 and 103 years (Table 1). It has an increase of 201 552 individuals (7077 incident cases) as compared with our previous meta-analysis.
Table 1.
Characteristics of 19 cohorts from 16 prospective cohort studies included in this updated meta-analysis
| Study | Participants | Age | Men (%) | Duration (average) of follow-up, year | Exposure assessment | Exposure categories | Outcome (no. of events) | Adjusted variables |
|---|---|---|---|---|---|---|---|---|
| Keli et al.14 Zutphen Study The Netherlands | 552 | 50–69 | 100 | 15 (11.4) | Interview based on Burke’s diet history method | Fish (g/day): ≤20; >20. | Total stroke (n =42) ICD-8: codes 430–438 |
Age, SBP, cigarette smoking, serum TC, energy intake, alcohol consumption and prescribed diet. |
| Orencia et al.17 Chicago Western Electric Study USA | 1847 | 40–55 | 100 | 30 (25.1) | Interview based on Burke’s diet history method | Fish (g/day): 0; 1–17; 18–34; ≥35. | Stroke mortality (n =222) ICD-8 or ICD-9: codes 430–434, 436–438 |
Age, SBP, cigarette smoking, serum TC, diabetes, ECG abnormalities, table salt use, alcohol intake, iron, thiamine, riboflavin, niacin, vitamin C, β-carotene, retinol, polyunsaturated fatty acids, carbohydrates, total protein and total energy. |
| Gillum et al.16 Female participants NHANES I USA | 2351 | 45–74 | 0 | 12 (NA) | Interview based on questionnaire | Fish (/week): Never; <1; 1; >1. | Total stroke (n =251) ICD-9: codes 433–434.9 436 or 437.0–437.1 |
Age, smoking, history of diabetes, history of heart disease, education less than high school graduate, SBP, serum albumin concentration, serum TC, BMI, alcohol intake and physical activity. |
| Gillum et al.16 Male participants NHANES I USA | 2059 | 45–74 | 100 | 12 (NA) | Interview based on questionnaire | Fish (/week): Never; <1; 1; >1. | Total stroke (n =262) ICD-9: codes 433–434.9 436 or 437.0–437.1 |
Age, smoking, history of diabetes, history of heart disease, education less than high school graduate, SBP, serum albumin concentration, serum TC, BMI, alcohol intake and physical activity. |
| Yuan et al.18 Shanghai China | 18 244 | 45–64 | 100 | 12 (9.8) | Interview based on questionnaire | Fish (g/week): <50; 50–<100; 100–<150; 150–<200; ≥200. | Total stroke death (n =480) ICD-9: codes 430–438 |
Age, total energy intake, level of education, BMI, current smoker, average no. of cigarettes smoked per day, no. of alcoholic drinks consumed per week, history of diabetes and history of hypertension. |
| Iso et al.4 Nurses’ Health Study USA | 79 839 | 34–59 | 0 | 14 (13.6) | Self-administered questionnaire | Fish: <1/month; 1–3/month; 1/week; 2–4/week; ≥5/week. | Total stroke (n =574) Ischemic stroke (n =303) Hemorrhagic stroke (n =181) ICD codes are not available. |
Age, BMI, alcohol intake, menopausal status and postmenopausal hormone use, vigorous exercise, usual aspirin use, multivitamin use, history of hypertension and frequency of total fruit and vegetable servings and for nutrient intake of saturated fat, trans-unsaturated fat, linoleic acid, animal protein and calcium. |
| He et al.5 Health Professional Follow-Up Study USA | 43 671 | 40–75 | 100 | 12 (10.6) | Self-administered questionnaire | Fish: <1/month; 1–3/month; 1/week; 2–4/week; ≥5/week. | Total stroke (n =608) Ischemic stroke (n =377) Hemorrhagic stroke (n =106) ICD codes are not available. |
Age, smoking status, BMI, physical activity, history of hypertension, aspirin, multivitamins, total energy, total fat, saturated fat, trans-unsaturated fat, alcohol, potassium, magnesium, fruits and vegetables and hypercholesterolemia. |
| Sauvaget et al.19 Hiroshima /Nagasaki Life Span Study Japan | 37 130 | 34–103 | 38 | 16.5 (NA) | Self-administered questionnaire | Fish: Low; Moderate; High. | Total stroke (n =1462) Ischemic stroke (n =655) Hemorrhagic stroke (n =354) ICD-9: codes 430–438; ICD-10: codes I60-I68; I69.0-I69.4, I69.8 |
Age, sex, birth cohort, smoking, alcohol, BMI, education, histories of diabetes or hypertension, radiation dose and city. |
| Folsom and Demissie Iowa Women’s Health Study USA6 | 41 836 | 55–69 | 0 | 11(10.6) | Self-administered questionnaire | Fish (/week): <0.5; 0.5–<1.0; 1.0–1.5; >1.5–<2.5; ≥2.5. | Total stroke death (n =313) ICD-9: codes 430–438 ICD-10: codes I60-I69, G45 |
Age, energy intake, educational level, physical activity level, alcohol consumption, smoking status, pack-years of cigarette smoking, age at first live birth, estrogen use, vitamin use, BMI, waist/hip ratio, diabetes, hypertension, intake of whole grains, fruit and vegetables, red meat, cholesterol and saturated fat. |
| Nakamura et al.8 NIPPON Japan | 8879 | ≥30 | 44 | 19 (17.3) | Self-administered questionnaire | Fish: Seldom; 1–2/week; 0.5/day; 1/day; ≥2/day. | Total stroke death (n =288) Ischemic stroke (n =165) Hemorrhagic stroke (n =63) ICD-9 and ICD-10 codes not available |
Age, sex, smoking, alcohol drinking, hypertension, BMI, diabetes and TC. |
| Mozaffarian et al.7 Cardiovascular Health Study USA | 4778 | 65–98 | 42 | 12 (NA) | Self-administered questionnaire | Tuna/other fish: <1/month; 1–3/month; 1–4/week; ≥5/week. Fried fish/fish sandwich: <1/month; 1–3/month; ≥1/week. | Total stroke (n =626) Ischemic stroke (n =537) Hemorrhagic stroke (n =73) Based on medical records and death certificate. ICD codes are not available |
Age, sex, education, diabetes, prevalent coronary heart disease, smoking status, pack-years of smoking, aspirin use, BMI, leisure-time physical activity, alcohol use, total caloric intake, SBP, LDL, HDL, triglyceride and CRP. |
| Myint et al.9 Female participants EPIC Study UK | 13 340 | 40–79 | 0 | 11 (8.5) | Self-administered questionnaire | Fish (/week): <1; 1–2; >2. | Total stroke (n =204) ICD-10: codes I60 to I69 |
Age, SBP, BMI, smoking, cholesterol and diabetes, fish oil supplement use, physical activity, alcohol consumption, and total energy intake. |
| Myint et al.9 Male participants EPIC Study UK | 10 972 | 40–79 | 100 | 11 (8.5) | Self-administered questionnaire | Fish (/week): <1; 1–2; >2. | Total stroke (n =217) ICD-10: codes I60 to I69 |
Age, SBP, BMI, smoking, cholesterol and diabetes, fish oil supplement use, physical activity, alcohol consumption and total energy intake. |
| Yamagishi et al.10 JACC Study Japan | 57 972 | 40–79 | 39 | 14.5 (12.7) | Self-administered questionnaire | Fish (median intake in quintiles, g/day): 20, 33, 45, 62 and 86 for men; 21, 33, 46, 62 and 85 for women. | Total stroke death (n =972) Ischemic stroke (n =319) Intraparenchymal hemorrhagic stroke (n =223) Subarachnoid hemorrhagic stroke (n =153) ICD-10: codes I60 to I69 |
Age, gender, history of hypertension and diabetes mellitus, smoking status, alcohol consumption, BMI, mental stress, walking, sports, education levels, total energy, and dietary intakes of cholesterol, saturated and omega-6 polyunsaturated fatty acids, vegetables, and fruit. |
| Montonen et al.11 Finnish Mobile Clinic Health Examination Survey Finland | 3958 | 40–79 | 52 | 28 (NA) | Interview based on questionnaire | Fish (median intake in quintiles, g/day): 6, 18, 32 and 72. | Total stroke (n =659) Thrombotic or embolic occlusions (n =364) Intracerebral hemorrhages (n =80) ICD-8: codes 430 to 438 code 344 |
Age, sex, energy intake, smoking, BMI, physical activity, geographic area, occupation, diabetes, use of post-menopausal hormones, hypertension, serum TC, and consumptions of butter, vegetables, fruits and berries. |
| Larsson et al.12 Swedish Mammography Cohort Sweden | 34 670 | 49–83 | 0 | 11 (10.4) | Self-administered questionnaire | Fish (/month): <1; 1–1.4; 1.5–2.0; 2.1–3.0; >3.0. | Total stroke (n =1680) Ischemic stroke (n =1310) Hemorrhagic stroke (n =233) ICD-10: codes I60, I61, I36 and I64 |
Age, smoking, education, BMI, total physical activity, history of diabetes, history of hypertension, aspirin use, family history of myocardial infarction, and intakes of total energy, alcohol, processed meat, unprocessed red meat, fruit and vegetables. |
| de Goede et al.13 Female participants Monitoring Project on Chronic Disease Factors The Netherlands | 11 081 | 20–65 | 0 | 13 (10.5) | Self-administrated FFQ | Fish (g/day): <3.0; 3.0–7.2; 7.3–14.0; >14.0. | Total stroke (n =106) Ischemic stroke (n =64) Hemorrhagic stroke (n =31) ICD-9: codes I60-I66 and G45. |
Age, smoking, BMI, education, parental history of myocardial infarction, alcohol intake, total energy intake, dietary fiber, vitamin C, β-carotene, saturated fatty acids, trans fatty acids, monounsaturated fatty acids, linoleic acid and α-linolenic acid. |
| de Goede et al.13 Male participants Monitoring Project on Chronic Disease Factors The Netherlands | 8988 | 20–65 | 100 | 13 (10.5) | Self-administrated FFQ | Fish (g/day): <3.3; 3.3–7.4; 7.5–14.0; >14.0. | Total stroke (n =115) Ischemic stroke (n =80) Hemorrhagic stroke (n =16) ICD-9: codes I60–I66 and G45. |
Age, smoking, BMI, education, parental history of myocardial infarction, alcohol intake, total energy intake, dietary fiber, vitamin C, β-carotene, saturated fatty acids, trans fatty acids, monounsaturated fatty acids, linoleic acid, and α-linolenic acid. |
| (De novo) Physicians’ Health Study USA | 21 040 | 40–84 | 100 | 20 (19.9) | Self-administered questionnaire | Fish: <1/month;1–3/ month; 1/week; 2–4/week; ≥5/week. | Total stroke (n =1487) Ischemic stroke (n =1232) Hemorrhagic stroke (n =251) ICD-9:codes 430, 431, 434, 436 ICD-10 codes not available |
Age, smoking, alcohol consumption, BMI, history of diabetes, physical activity, history of hypertension, history of elevated cholesterol, family history of myocardial infarction prior age 60, vitamin intake, saturated fat and randomized treatment assignments. |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; ECG, electrocardiography; EPIC, European Prospective Investigation into Cancer; HDL, high-density lipoprotein; HR, hazards ratio; ICD, International Classification of Diseases; JACC, Japan Collaborative Cohort Study for Evaluation of Cancer Risk; LDL, low-density lipoprotein; NA, not available; NHANES, National Health and Nutrition Examination Survey; NIPPON, National Integrated Projects for Prospective Observation of Non-Communicable Diseases; SBP, systolic blood pressure; TC, total cholesterol.
Of the 19 cohorts, sample sizes varied across studies from 552 (Zutphen Study)14 to 79 839 (NHS).4 The average duration of follow-up was 12.8 years (range 8.5–28.0 years) calculated based on the total person-year of included studies. Fish consumption was assessed either by self-administered (13 cohorts) or interview-based food frequency questionnaire (6 cohorts). Fish intake was classified into two to five categories in primary studies. All reported HRs (95% CIs) of stroke were adjusted for multiple covariates (Table 1).
Association of fish consumption with the incidence of stroke
After pooling data from the identified studies, fish consumption was significantly associated with the incidence of stroke. Compared with those who never consumed fish or ate fish less than once per month, the pooled weighted HRs of incident total stroke were 0.97 (95% CI, 0.87–1.08), 0.86 (0.80–0.93), 0.91 (0.85–0.98) and 0.87 (0.79–0.96) for fish consumption 1–3/month, 1/week, 2–4/week, and ≥5/week, respectively (P for linear trend =0.09; Table 2). A nonlinear trend was detected after logarithm transformation of continuous fish intake (P for nonlinear trend =0.02).
Table 2.
Pooled weighted hazard ratios and 95% confidence intervals of stroke according to fish consumption
| No. of cohorts | No. of participants (events) | Fish consumption
|
Ptrend | Pinteraction | |||||
|---|---|---|---|---|---|---|---|---|---|
| <1/month | 1–3/month | 1/ week | 2–4/week | ≥5/week | |||||
| All studies | 19 | 402 127 (10 568) | 1.0 | 0.97 (0.87–1.08) | 0.86 (0.80–0.93) | 0.91 (0.85–0.98) | 0.87 (0.79–0.96) | 0.09 | |
| Subtype of stroke | |||||||||
| Ischemic stroke | 11 | 311 106 (5406) | 1.0 | 0.96 (0.84–1.11) | 0.82 (0.73–0.93) | 0.89 (0.81–0.97) | 0.83 (0.75–0.92) | 0.07 | 0.24 |
| Hemorrhagic stroke | 11 | 311 106 (1764) | 1.0 | 1.08 (0.85–1.39) | 0.96 (0.83–1.11) | 0.97 (0.84–1.10) | 0.92 (0.80–1.07) | 0.28 | |
| Gender | |||||||||
| Men | 9 | 111 363 (4048) | 1.0 | 0.96 (0.84–1.09) | 0.89 (0.77–1.02) | 0.93 (0.79–1.09) | 0.90 (0.76–1.07) | 0.16 | 0.19 |
| Women | 6 | 182 825 (3139) | 1.0 | 1.00 (0.81–1.24) | 0.83 (0.74–0.93) | 0.88 (0.78–0.99) | 0.74 (0.46–1.17) | 0.09 | |
| Follow-up, year | |||||||||
| <12.8 | 13 | 250 334 (5876) | 1.0 | 0.98 (0.86–1.11) | 0.85 (0.76–0.95) | 0.92 (0.84–1.00) | 0.88 (0.79–0.98) | 0.11 | 0.57 |
| ≥12.8 | 6 | 151 793 (4692) | 1.0 | 0.94 (0.76–1.18) | 0.88 (0.78–0.99) | 0.90 (0.78–1.04) | 0.83 (0.67–1.02) | 0.07 | |
| Location | |||||||||
| Europe | 7 | 83 381 (3023) | 1.0 | 1.00 (0.77–1.29) | 0.88 (0.75–1.03) | 0.94 (0.80–1.11) | 0.91 (0.76–1.08) | 0.21 | 0.22 |
| North | 8 | 196 521 (4343) | 1.0 | 0.97 (0.86–1.11) | 0.86 (0.75–0.99) | 0.87 (0.74–1.03) | 0.79 (0.65–0.96) | 0.03 | |
| America | |||||||||
| Asia | 4 | 122 225 (3202) | 1.0 | 0.93 (0.72–1.21) | 0.81 (0.69–0.95) | 0.91 (0.82–1.00) | 0.89 (0.72–1.10) | 0.21 | |
| Dietary assessment | |||||||||
| Interview-based | 6 | 29 011 (1916) | 1.0 | 0.95 (0.79–1.13) | 0.94 (0.81–1.09) | 0.90 (0.70–1.14) | 1.05 (0.88–1.25) | 0.95 | 0.12 |
| Self-administered | 13 | 373 116 (8652) | 1.0 | 0.98 (0.86–1.12) | 0.84 (0.77–0.92) | 0.91 (0.86–0.97) | 0.82 (0.75–0.91) | 0.06 | |
| Stroke end point | |||||||||
| Incidence | 14 | 273 349 (8293) | 1.0 | 0.95 (0.84–1.08) | 0.86 (0.79–0.95) | 0.88 (0.79–0.98) | 0.83 (0.74–0.93) | 0.047 | 0.18 |
| Mortality | 5 | 128 778 (2275) | 1.0 | 1.02 (0.83–1.24) | 0.85 (0.69–1.04) | 0.95 (0.86–1.06) | 0.97 (0.83–1.14) | 0.40 | |
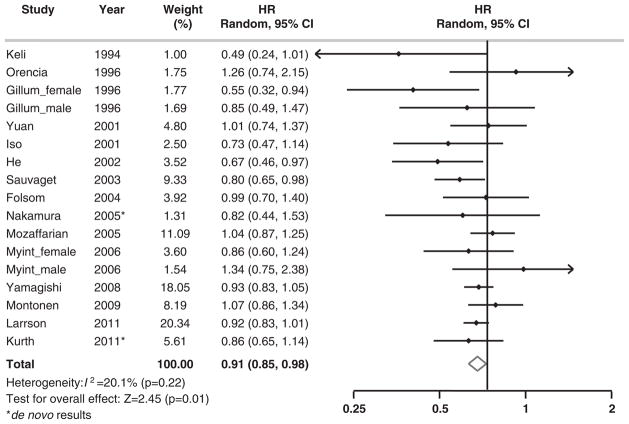
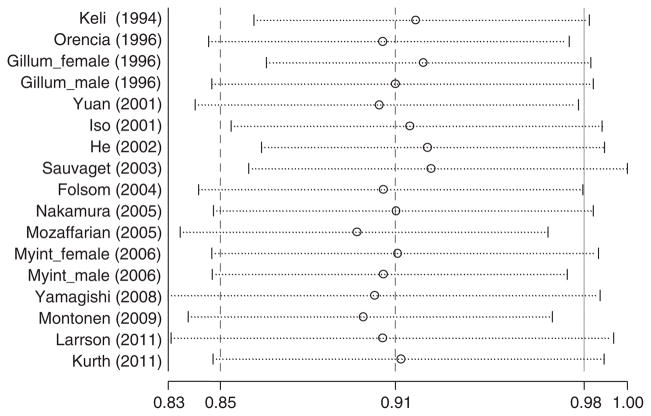
We tested the heterogeneity among the included cohorts by calculating I2 for each category of fish intake with the lowest group as the reference. I2 ranged from 0.0 to 20.1% for fish consumption. As shown in Figure 1, there was no significant heterogeneity among studies (I2 =20.1%, P =0.22) when comparing participants who consumed fish 2–4/week with those in the reference group. The sensitivity analysis revealed that none of the studies significantly influenced the pooled estimate (Figure 2). The weighted HR comparing those who consumed fish 2–4/week with those in the reference group ranged from 0.90 (95% CI, 0.83–0.97) when we excluded Mozaffarian et al.7 to 0.93 (0.86–0.998) when we excluded Sauvaget et al.19 When the analysis was repeated using a fixed-effects model, the results were essentially the same. When we further excluded one study with only two fish consumption categories,14 the results were not materially altered.
Figure 1.
Multivariable adjusted HRs and 95% CIs of incidence of total stroke according to fish consumption in 19 cohorts from 16 prospective cohort studies. The summary estimates were obtained by using a random-effects model. The dots indicate the adjusted HRs by comparing fish consumption 2–4 times per week (only 17 cohorts had data in this category) to less than once per month. The horizontal lines represent 95% CIs. The diamond markers indicate the pooled estimate.
Figure 2.
Influence of removing studies one by one on multivariable adjusted HRs of incident stroke comparing fish consumption 2–4 times per week to less than once per month. Circles are HRs and horizontal dotted lines 95% CIs for meta-analysis of the studies listed excluding the study listed in the left. The dash vertical line in the center is the summary estimate of the HR including all included studies.
Based on the 11 cohorts that provided data on stroke subtypes,4,5,7,8,10–12,15,19 we found a significant modest beneficial association between fish intake and incidence of ischemic stroke (P for linear trend =0.07; P for nonlinear trend =0.01). The incidence of ischemic stroke was significantly lower when consuming fish once per month or more as compared with eating fish less than once per month. For hemorrhagic stroke, we did not observe any significant association with fish intake (Table 2).
Stratified analysis
In the stratified analyses, the inverse association between fish intake and risk of stroke was slightly attenuated among studies with relatively short follow-up period (<12.8 years), conducted in Europe or Asia, and among studies using in-person interview for dietary assessment. The observed magnitude of inverse association was also slightly lowered among men. Nevertheless, none of the interactions between fish consumption and these potential effect modifiers were statistically significant (Table 2).
Publication bias
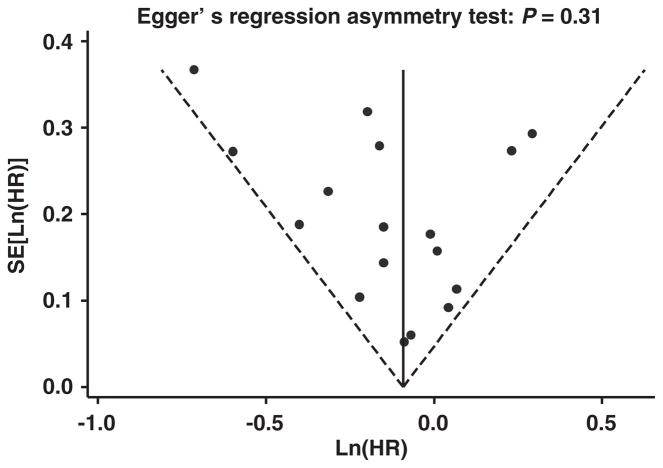
We assessed publication bias by visually examining a funnel plot of precision against ln (HR) (‘fish consumption at 2–4/week vs <1/ month’ listed as an example in Figure 3). The asymmetry was formally assessed by Egger’s test. No evidence of significant publication bias was found (P =0.31). Neither of the P-values for public bias from Egger’s test in other three higher groups of fish consumption was statistically significant (P-values ranged from 0.36 to 0.98).
Figure 3.
Funnel plot with pseudo 95% CIs for HR of incident stroke comparing fish consumption 2–4 times per week to less than once per month in 17 cohorts from 15 prospective cohort studies. The funnel plot shows s.e. of ln(HR) against ln(HR) for the 17 separated cohorts included in the meta-analysis. The vertical line indicates the fixed-effects summary estimate of the HR with sloping lines representing the expected 95% CIs for a given s.e., assuming no heterogeneity between studies.
DISCUSSION
Our updated review quantitatively summarized the current literature evaluating fish consumption and the risk of stroke by including 16 additional prospective cohort studies since our previous meta-analysis. Overall, fish consumption was inversely associated with the risk of stroke. A significant modest beneficial association was observed between increasing fish consumption and the risk of ischemic stroke, whereas no significant relation was found with hemorrhagic stroke. Gender, follow-up length and dietary assessment method did not significantly modify the associations.
Several strengths need to be highlighted. First, this meta-analysis comprised >380 000 male and female adults with a wide age range, which increased the statistical power, especially in subgroup analysis, to examine the association between fish intake and risk of stroke compared with our original meta-analysis published in 2004. Second, the prospective nature of the cohort studies reduced the possibility of selection bias and recall bias. Even though randomized, placebo-controlled clinical trials are preferred to evaluate causal relations, this study design would not be practical to study the primary prevention of stroke because of prolonged compliance needed for fish intake. In addition, we obtained de novo data from some primary studies, standardized fish intake into five categories, and performed detailed subgroup analysis. Of note, considering that different etiologies exist between ischemic and hemorrhagic stroke,22 and most newly published studies have data on stroke subtype, we analyzed data separating ischemic stroke from hemorrhagic stroke. As there were 5406 ischemic stroke cases and 1764 hemorrhagic stroke cases from 311 106 participants in 11 cohorts, the statistical power was also sufficient. Moreover, we assessed stroke mortality separately, which provided additional information on fish and fatal stroke. Furthermore, we did not find significant heterogeneity among included studies, indicating that combining these studies was reasonable.
We found no consistent benefits of fish consumption on stroke risk by gender and location. Regular fish consumption was only significantly related with decreased stroke risk on females but not on males. It might be because of the different lifestyles associated with two genders, such as more smoking or alcohol drinking in men, which interact with the intake and the metabolism of nutrients in fish. The difference in fish benefits by locations might be due to the different dietary patterns, fish types or preparation methods by regions. Various degrees of measurement error might explain at least part of the inconsistent results for dietary assessment.
Our meta-analysis also has limitations. First, we cannot rule out the possibility of residual confounding due to inclusion of different factors or bias caused by measurement errors, or unmeasured factors could be present in each primary study. However, most studies were well designed and adjusted for major lifestyle and dietary variables, which alleviates our concern about the residual confounding. Also, although all the studies had information about portion size and the range for each fish intake category, misclassification of fish consumption is still possible when we standardized fish consumption, although it is unlikely. Publication bias is not a serious concern in our meta-analysis, as we found no strong evidence for it, either by visualizing the funnel plot or conducting statistical tests. Nevertheless, a potential bias resulting from excluding studies published in other languages (if any) is possible.
Since the publication of our previous study, two studies have attempted to combine the available evidence to quantitatively assess the association between fish intake and stroke risk.2,3 They both used the dose-response model in pooling the estimates, whereas the linear assumption between fish intake and risk of stroke had to be made. In the quantitative analysis published in 2005,2 the intercept interpretation as relative risk of stroke at low levels of fish consumption compared with no fish intake at all may not be accurate as a combined reference group ( 0 with <1/ month) was used. In addition, one case–control study23 was inappropriately mingled with the other selected prospective cohort studies. The recent quantitative analysis3 briefly reported the pooled estimate of fish and stroke without performing detailed subgroup or sensitivity analysis. Also, a nested case–control study24 reported odds ratio was not so appropriately combined with other prospective cohort studies with relative risk reported in that meta-analysis as the controls are from different sources. We included it in a sensitivity analysis, and all the conclusion remained.
The beneficial effect of fish consumption on stroke risk has been suggested to be related to the antithrombotic activity and anti-inflammatory property of long-chain omega-3 polyunsaturated fatty acids (LCn3PUFAs), the key nutrients in fish.22,25 LCn3PUFA intake was found to shift the balance of prostaglandin I/thromboxane A to a more antithrombotic state.25–27 Although not conclusive,22 the anti-inflammatory effect of LCn3PUFAs was speculated to alleviate the progression of stroke by stabilizing the atherosclerotic plaque by decreasing the infiltration and activity of the inflammatory cells around the plaque.1,28 Other favorable effects such as lowering blood pressure and improving the lipid profile may also contribute to the beneficial effect of LCn3PUFAs on the cardiovascular system.29,30 It makes biological sense that fish intake was found to be inversely related to the risk of ischemic but not hemorragic stroke because of the different etiopathogenesis of these two stroke subtypes. The antiplatelet activity of LCn3PUFAs that protects against ischemic stroke development may be a risk factor for hemorrhagic stroke development.22 However, we did not observe an increased risk of hemorrhagic stroke in higher categories of fish consumption. The potential adverse effect of high fish consumption on the risk of hemorrhagic stroke may be masked by the other beneficial effects of fish intake.
Although it is reasonable to assume that the beneficial effect of fish intake on stroke risk comes from the LCn3PUFAs intake, a meta-analysis of nine randomized clinical trials published in 2006 did not find any effect of LCn3PUFA intake on stroke risk.31 However, short-duration clinical trials based on patients focusing on fish oil supplement use cannot rule out a long-term beneficial effect of fish intake on the risk of stroke in the general population as found in this meta-analysis. Of note, it is also possible that the observed benefit of fish consumption is because of the nutrient package of whole fish.22 Therefore, studying LCn3PUFA from fish intake alone might not fully explain the benefit of fish on stroke risk. An overall healthy lifestyle of the fish consumers may also account for part of our observation. For example, people with higher fish intake tend to have a healthier lifestyle such as being more likely to be normal weight, less likely to smoke and more likely to exercise, which may protect against the occurrence of stroke.32–35 However, almost all of the included studies considered body mass index, smoking status and physical activity as potential confounders in the model.
In summary, our updated meta-analysis of all relevant prospective cohort studies provided evidence of a modest beneficial association between fish consumption and the risk of stroke, in particular ischemic stroke. Consuming fish once per week may significantly reduce the incidence of ischemic stroke. No evidence was found that high fish consumption would increase the risk of hemorrhagic stroke. Further studies are required to better understand the potentially different mechanisms of fish consumption on the risk of stroke subtypes.
Acknowledgments
This study was partially supported by Grants R21DK073812 and R21NS056445 from the National Institutes of Health. Dr Djousse received research funding from the National Institutes of Health. Dr He received research funding from the National Institutes of Health and the American Cancer Society.
Footnotes
Contributors: PX reviewed all relevant papers, identified eligible studies, extracted the data, analyzed data, drafted the manuscript and contributed to the critical revision of the manuscript; BQ reviewed all relevant papers, identified eligible studies, extracted the data, drafted the manuscript and contributed to the critical revision of the manuscript; YS contributed to the critical revision of the manuscript; YN contributed to de novo data analysis of NIPPON DATA80 study and the critical revision of the manuscript; TK contributed to de novo data analysis of Physicians’ Health Study and the critical revision of the manuscript; SY contributed to the critical revision of the manuscript; LD contributed to the critical revision of the manuscript; KH made study concept and design, drafted the manuscript and contributed to the critical revision of the manuscript; PX and BQ had the primary responsibility for final content. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
Dr Xun and Dr Nakamura declare no conflict of interest. Mrs Qin received Sanofi-Aventis/UNC Global Nutrition Scholarship. Dr Song received research funding from the National Institutes of Health. Dr Kurth has received within the past 2 years investigator-initiated research funding from the French National Research Agency, the US National Institutes of Health, Merck, the Migraine Research Foundation, and the Parkinson’s Research Foundation. Furthermore, he is a consultant to World Health Information Science Consultants, LLC; he has received honoraria from the American Academy of Neurology and Merck for educational lectures and from MAP Pharmaceutical for contributing to a scientific advisory panel. Mrs Yaemsiri received American Heart Association’s pre-doctoral fellowship.
References
- 1.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, et al. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke. 2004;35:1538–1542. doi: 10.1161/01.STR.0000130856.31468.47. [DOI] [PubMed] [Google Scholar]
- 2.Bouzan C, Cohen JT, Connor WE, Kris-Etherton PM, Gray GM, Konig A, et al. A quantitative analysis of fish consumption and stroke risk. Am J Prev Med. 2005;29:347–352. doi: 10.1016/j.amepre.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Orsini N. Fish consumption and the risk of stroke: a dose-response meta-analysis. Stroke. 2011;42:3621–3623. doi: 10.1161/STROKEAHA.111.630319. [DOI] [PubMed] [Google Scholar]
- 4.Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 5.He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, et al. Fish consumption and risk of stroke in men. JAMA. 2002;288:3130–3136. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol. 2004;160:1005–1010. doi: 10.1093/aje/kwh307. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Longstreth WT, Jr, Lemaitre RN, Manolio TA, Kuller LH, Burke GL, et al. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005;165:200–206. doi: 10.1001/archinte.165.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura Y, Ueshima H, Okamura T, Kadowaki T, Hayakawa T, Kita Y, et al. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980–99. Am J Med. 2005;118:239–245. doi: 10.1016/j.amjmed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Myint PK, Welch AA, Bingham SA, Luben RN, Wareham NJ, Day NE, et al. Habitual fish consumption and risk of incident stroke: the European Prospective Investigation into Cancer (EPIC)-Norfolk prospective population study. Public Health Nutr. 2006;9:882–888. doi: 10.1017/phn2006942. [DOI] [PubMed] [Google Scholar]
- 10.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Montonen J, Jarvinen R, Reunanen A, Knekt P. Fish consumption and the incidence of cerebrovascular disease. Br J Nutr. 2009;102:750–756. doi: 10.1017/S0007114509274782. [DOI] [PubMed] [Google Scholar]
- 12.Larsson SC, Virtamo J, Wolk A. Fish consumption and risk of stroke in Swedish women. Am J Clin Nutr. 2011;93:487–493. doi: 10.3945/ajcn.110.002287. [DOI] [PubMed] [Google Scholar]
- 13.de Goede J, Verschuren WM, Boer JM, Kromhout D, Geleijnse JM. Gender-specific associations of marine n-3 fatty acids and fish consumption with 10-year incidence of stroke. PLoS One. 2012;7:e33866. doi: 10.1371/journal.pone.0033866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keli SO, Feskens EJ, Kromhout D. Fish consumption and risk of stroke. The Zut-phen Study Stroke. 1994;25:328–332. doi: 10.1161/01.str.25.2.328. [DOI] [PubMed] [Google Scholar]
- 15.Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH. Fish consumption and cardiovascular disease in the physicians’ health study: a prospective study. Am J Epidemiol. 1995;142:166–175. doi: 10.1093/oxfordjournals.aje.a117615. [DOI] [PubMed] [Google Scholar]
- 16.Gillum RF, Mussolino ME, Madans JH. The relationship between fish consumption and stroke incidence. The NHANES I Epidemiologic Follow-up Study (National Health and Nutrition Examination Survey) Arch Intern Med. 1996;156:537–542. [PubMed] [Google Scholar]
- 17.Orencia AJ, Daviglus ML, Dyer AR, Shekelle RB, Stamler J. Fish consumption and stroke in men. 30-year findings of the Chicago Western Electric Study. Stroke. 1996;27:204–209. doi: 10.1161/01.str.27.2.204. [DOI] [PubMed] [Google Scholar]
- 18.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–816. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 19.Sauvaget C, Nagano J, Allen N, Grant EJ, Beral V. Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Int J Epidemiol. 2003;32:536–543. doi: 10.1093/ije/dyg151. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He K. Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease--eat fish or take fish oil supplement? Prog Cardiovasc Dis. 2009;52:95–114. doi: 10.1016/j.pcad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Caicoya M. Fish consumption and stroke: a community case-control study in Asturias, Spain. Neuroepidemiology. 2002;21:107–114. doi: 10.1159/000054807. [DOI] [PubMed] [Google Scholar]
- 24.Wennberg M, Bergdahl IA, Stegmayr B, Hallmans G, Lundh T, Skerfving S, et al. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br J Nutr. 2007;98:1038–1045. doi: 10.1017/S0007114507756519. [DOI] [PubMed] [Google Scholar]
- 25.Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004;107:1–11. doi: 10.1042/CS20040119. [DOI] [PubMed] [Google Scholar]
- 26.von Schacky C, Fischer S, Weber PC. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J Clin Invest. 1985;76:1626–1631. doi: 10.1172/JCI112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988;318:549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- 28.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 29.Nestel PJ. Effects of N-3 fatty acids on lipid metabolism. Annu Rev Nutr. 1990;10:149–167. doi: 10.1146/annurev.nu.10.070190.001053. [DOI] [PubMed] [Google Scholar]
- 30.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–533. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 31.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 33.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34:2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 34.Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007;167:1420–1427. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- 35.Galimanis A, Mono ML, Arnold M, Nedeltchev K, Mattle HP. Lifestyle and stroke risk: a review. Curr Opin Neurol. 2009;22:60–68. doi: 10.1097/WCO.0b013e32831fda0e. [DOI] [PubMed] [Google Scholar]