Abstract
Visual stimuli are often used for obsessive-compulsive (OC) symptom provocation in research studies. We tested the induction of anxiety and OC checking symptoms across different types of checking provocation stimuli in three populations: individuals with obsessive compulsive disorder (OCD), individuals with checking symptoms but without a diagnosis of OCD, and control individuals with neither checking symptoms nor a clinical diagnosis. One set of provocative images depicted objects that are commonly associated with checking anxiety.
Another set (‘enhanced provocative images’) depicted similar objects but also included contextual cues suggesting a specific harmful scenario that could occur. As expected, the enhanced provocative images were more effective at inducing anxiety and OC symptoms than the standard provocative images. Future studies requiring checking symptom provocation should therefore consider incorporating similarly suggestive images. Individuals with clinical OCD reported the greatest provocation in response to these images, followed by those with nonclinical checking, followed by control individuals. Thus, these stimuli are able to provoke OC checking symptoms and anxiety differentially across groups, with the intensity of provocation reflecting diagnostic status. All groups demonstrated a similar qualitative pattern of provocation across images. Finally, in all groups, reported anxiety closely tracked intrusive thoughts and checking urges.
Keywords: Obsessive-Compulsive Disorder [F03-080-600], Compulsive Behavior [F01-145-527-100], Obsessive Behavior [F01.145.126.950], Anxiety [F01.470.132]
1. Introduction
Symptom induction using provocative stimuli is commonly used in studies of obsessive-compulsive disorder (OCD). For example, many studies of the neurobiological basis of OCD have employed symptom provocation in a neuroimaging context (Adler et al., 2000; Agarwal et al., 2013; An et al., 2009; Cottraux et al., 1996; Gilbert et al., 2009; Mataix-Cols et al., 2003; Mataix-Cols et al., 2004; Nakao et al., 2005; Rauch et al., 1994; Scheinost et al., 2013; Schienle et al., 2005).
Symptom provocation paradigms can use either personalized or standard stimuli. As OCD symptoms can be quite idiosyncratic, personalized stimuli have the advantage of being highly relevant to a participant’s particular symptomatology. They may include words/sentences chosen by the participant for their association with symptom anxiety (Cottraux et al., 1996; Nakao et al., 2005), photographs taken by the participant of anxiety-inducing scenes from their own life (Schienle et al., 2005), or other personally relevant objects/images (Adler et al., 2000; Rauch et al., 1994). However, because of their idiosyncratic nature, such personalized stimuli can vary on multiple dimensions across participants. While past research has succeeded in minimizing unwanted variation in personalized images (Morgieve et al., 2014; Simon et al., 2010; Simon et al., 2012), these image sets still require creation and validation on a participant-by-participant basis.
When stimuli need to be balanced across participants or conditions, as in comparative functional brain imaging studies, standardized stimulus sets allow for straightforward comparisons across groups and confer the added advantage of avoiding lengthy image collection and validation for each participant. One such set is the Maudsley Obsessive-Compulsive Stimuli Set (MOCSS)(Mataix-Cols et al., 2009). This image set has been very useful for the OCD research community (Agarwal et al., 2013; An et al., 2009; Gilbert et al., 2009; Hampson et al., 2012; Mataix-Cols et al., 2003; Mataix-Cols et al., 2004). Such an image set typically needs to be tailored and balanced for a specific application. For example, the MOCSS is often adapted (Agarwal et al., 2013; Gilbert et al., 2009) or expanded (Hampson et al., 2012) to suit the needs of particular studies.
The development and adaptation of OCD-provocative stimulus sets is facilitated by an understanding of the characteristic image features that affect the intensity of symptom provocation. Different images are used to provoke different categories of OCD symptoms, such as contamination and fear-of-harm/checking symptoms. Here, we examine the characteristics of images that provoke checking symptoms; while contamination symptoms are readily provoked by visual images, we have anecdotally found the reliable provocation of checking symptoms to be more challenging. In the MOCSS, images used to provoke checking symptoms depict objects that individuals with OCD commonly check repeatedly, such as light switches, stoves, and electrical outlets; participants are instructed to imagine that they are in the presence of these objects but are unable to check them. We tested whether such images are more provocative if they more explicitly suggest the type of harm that could arise – for example, if a flammable object is seen on a stove (Figure 1). In this manuscript, we refer to the more explicitly suggestive images as ‘enhanced provocative images’ and the standard, more subtly suggestive images simply as ‘provocative images’. We examined the ability of these images to produce self-reported anxiety, intrusive thoughts, and compulsive urges to check.
Figure 1:

Two examples of provocative images (left panel) and their matched enhanced provocative images (right panel).
We examined provocative and enhanced provocative images in individuals with clinical OCD, individuals with checking obsessions and compulsions but without a diagnosis of OCD, and control individuals with neither checking symptoms nor a clinical diagnosis. Comparison of these three groups tests the ability of a standardized stimulus set to provoke OC symptoms in populations with clinical and nonclinical OCD symptoms. It also assesses the validity of piloting and testing stimuli in subclinical or nonclinical populations, which is often desirable in a research context. From a theoretical perspective, this study has bearing on whether subclinical obsessive-compulsive anxiety and clinical OCD lie along a continuum or are qualitatively different phenomena. Previous work has suggested that individuals with subclinical obsessive-compulsive symptoms share many traits with the OCD population, including distinctive cognitive and personality profiles (Gibbs, 1996) and overlapping brain activation patterns during symptom provocation (Mataix-Cols et al., 2003) – an idea that is consistent with the Research Domain Criteria (RDoC) initiative (https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml). Here, we extend this work by exploring whether self-reported checking provocation induced by different stimuli is qualitatively similar across groups.
We had several a priori expectations. First, we predicted that enhanced provocative images would induce greater anxiety than standard provocative images, which would induce greater anxiety than neutral images, confirming that explicit suggestion of harm amplifies the provocation efficacy of checking images. We expected to see this pattern in all groups (participants with OCD, participants with nonclinical checking, and control participants). In response to these provocative and enhanced provocative images, we predicted that participants with OCD would report the greatest anxiety and OC symptoms, followed by participants with nonclinical checking, followed by control participants. Second, we predicted that the anxiety induced by specific images would correlate across participants with OCD and participants with nonclinical checking. This prediction is consistent with a dimensional view of OCD, wherein participants with nonclinical checking would be expected to present qualitatively similar obsessive-compulsive experiences to clinical patients, differing only in intensity or the level of distress or impairment they produce. Finally, we predicted that stimulus-induced obsessions and compulsions would correlate well with anxiety, confirming that anxiety is a reasonable proxy for OCD symptoms in this context.
2. Methods
2.1. Participants
Fifteen individuals diagnosed with OCD (5 males, age 25.20 ± 5.17 years [S.D.]; 10 females, age 29.50 ± 11.47) were recruited through the Yale OCD Research Clinic (ocd.yale.edu). These individuals had all undergone structured psychiatric evaluation using the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998), met criteria for a primary diagnosis of OCD according to DSM-5, had a minimum score of 16 on the Yale-Brown Obsessive Compulsive Scale (Goodman et al., 1989a; Goodman et al., 1989b) at screening, and endorsed checking symptoms on the YBOCS Symptom Checklist. Participants were excluded if they met criteria for active substance or alcohol use disorder within the past 6 months, a psychotic disorder, bipolar, current significant suicidal ideation, or a pervasive developmental disorder.
A ‘nonclinical’ group consisting of ten participants who endorsed checking symptoms but had no self-reported diagnosis of OCD was recruited from the community (5 males, age 22.8 ± 5.93; 5 females, age 23.8 ± 4.21). These individuals were administered the checking subscale (items 14–23) of the Padua Inventory (Burns et al., 1996), and a minimum score of 8 was required for participation. This group had scores on this checking subscale of the Padua inventory that were comparable to the OCD group (indeed they were numerically, though not statistically, higher); the absence of a clinical diagnosis of OCD suggests that these individuals were less impaired by their checking behaviors, as the hallmark of a clinical diagnosis of OCD is the impairment or distress caused by the symptoms.
Lastly, eleven control participants with minimal checking symptoms (less than 8 on the Padua checking subscale) were recruited from the community (3 males, age 38.67 ± 17.62; 8 females, age 27.50 ± 12.20). Control participants underwent structured psychiatric evaluation using the (MINI; Sheehan et al., 1998) and were excluded if they met criteria for any current DSM-5 diagnosis.
See Table 1 for demographic data. Due to an oversight, the Padua data from one participant with OCD was missing, so that patient was excluded in the calculation of descriptive statistics for this scale. All participants provided written consent in accordance with a protocol reviewed and approved by the Yale Human Research Protection Program.
Table 1.
| Control Participants (n = 11) | Participants with Nonclinical Checking (n = 10) | Participants with OCD (n = 15) | |
| Age | 30.55 (13.91) | 23.30 (4.88)# | 28.07 (9.83) |
| Padua Checking Subscale | 1.73 (1.74) | 14.80 (4.96) | 12.29 (11.76) |
| YBOCS | ------ | ------ | 23.60 (6.08) |
M (SD)
one missing value
2.2. Stimuli
Images were drawn from a variety of sources – including the MOCSS (Mataix-Cols et al., 2009) the IAPS (Lang et al., 2008), purchased stock photos, and photos taken by the author HB – to create three stimulus sets with 15 images in each set. The first set was composed of neutral images, unrelated to the major dimensions of OCD symptomatology. The second set, which we refer to as ‘provocative’ images, depict objects often associated with checking symptoms, such as stoves and light switches (similar to those used in the MOCSS, although we generated most of our own images for this study). The third image set, which we refer to as ‘enhanced provocative’ images, depicts the same types of objects as the second image set, but with the addition of a situational or contextual cue that more clearly implies greater potential threat, such as a stove with an apron near a burner, or a light switch set halfway between the on and off positions. Thirteen pairs of provocative and enhanced provocative images were photographs taken by HB and are publicly available upon request (the other two pairs involved images we do not have the right to disseminate). Efforts were made to avoid images that could induce any form of anxiety other than checking anxiety. The three sets of images were balanced in terms of smoothness, contrast, brightness, saturation, and red, green and blue color. See Figure 1 for example images.
We recruited a convenience sample (n = 6 individuals without OCD) to confirm that we were successful in generating the intended difference between the provocative and enhanced provocative stimuli. Using a 1 (strongly disagree) to 7 (strongly agree) Likert scale, participants were asked to rate each image (neutral, provocative, and enhanced provocative) according to the following statement: ‘This image suggests that something potentially damaging or dangerous could occur.’ Paired-sample t-tests showed the enhanced provocative images were more suggestive of a dangerous/damaging scenario (M = 4.31, SD = 1.30) in comparison to the provocative images (M = 2.39, SD = 1.12), t (5) = 5.24, p < 0.01, and neutral images (M = 1.72, SD = 1.15), t(5) = 6.19, p<0.01.
2.3. Procedure
The experiment, which took place in a quiet room with a single researcher present, was administered using E-Prime (Psychology Software Tools, Inc.) on a laptop computer.
Participants were directed to follow instructions on the screen and progress at their own pace. The software then presented a randomized sequence of 45 neutral, provocative, and enhanced provocative images. Initial instructions read by participants stated, ‘Imagine the objects pictured are yours, and you aren’t sure whether you turned them off, locked them, or otherwise left them in a state you are comfortable with.’ Using the same Likert scale format as previously described, they were asked to rate each image according to three statements. The first statement was, Ί feel anxious.’ The second was, Ί have a desire to check.’ The final statement was, Ί have intrusive thoughts about possible harm.’ Ratings were provided for each image. The task was self-paced, except for a fixed inter-trial interval of 2.5s between trials, to slow participants down and clear their minds. During this inter-trial interval, to prepare participants for the upcoming image, we displayed the text ‘For the next image, we will start by asking you about your anxiety’. See Figure 2 for a visual depiction of the task.
Figure 2:
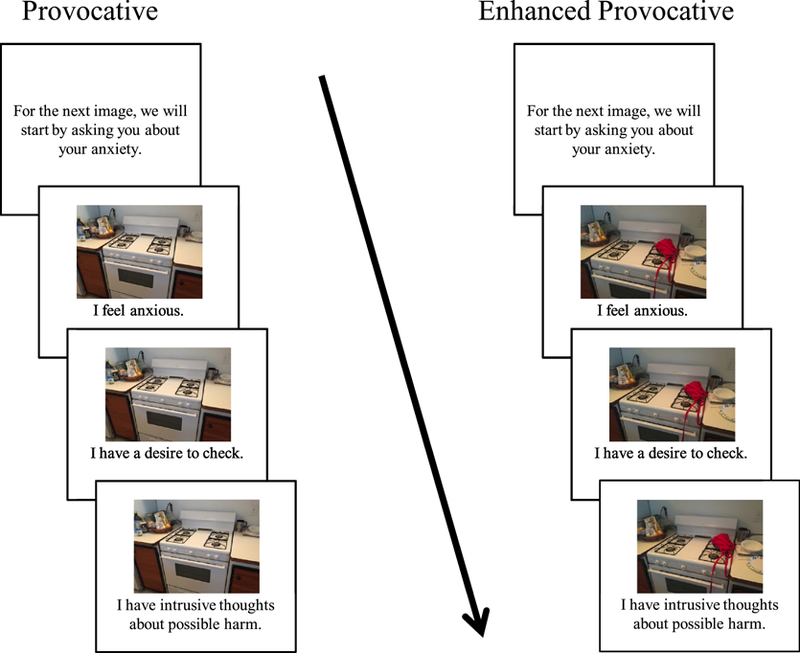
Illustration of a single trial for a provocative (left side) or enhanced provocative (right side) image. Each trial began with instructions (2.5 s), which were immediately followed by three presentations of a picture along with rating prompts (anxiety, intrusive thoughts, compulsive urges). Participants were instructed to respond to each statement using a Likert scale from 1–7 where 1 = Strongly Disagree and 7 = Strongly Agree. Presentation order was random.
2.4. Analyses
Cronbach’s alpha was moderate to high for all three ratings for each of the three stimulus sets, suggesting strong internal consistency (Table 2). We used mixed-factor ANOVAs to compare subjective reactions to neutral, provocative, and enhanced provocative stimuli across groups. Diagnostic status (control vs. nonclinical vs. OCD) served as the between-subjects factor, and stimulus type (neutral, provocative, and enhanced provocative) served as the within-subjects factor. Two separate analyses were carried out to measure the effects on 1) subjective anxiety and 2) a composite score that reflected ‘OC symptoms’, which was derived by averaging the checking and intrusive thoughts ratings. While checking and intrusive thoughts were assessed via separate questions in order to best capture two distinct domains of symptoms, we were interested primarily in OC symptoms as a whole. As such, our analyses of these data were focused solely on the composite score rather than its individual constituents. Greenhouse-Geisser estimates of degrees of freedom were used to adjust for violations of sphericity.
Table 2.
Descriptive data and reliability estimates for stimulus types.
| Rating | ||||||
|---|---|---|---|---|---|---|
| Anxiety | Checking | Intrusive | OC Sx. | |||
| Stimulus Type | Neutral | HC | 1.15 (0.25) | 1.10 (0.16) | 1.00 (0.00) | 1.05 (0.08) |
| NON | 1.83 (0.90) | 1.97 (1.00) | 1.63 (0.64) | 1.80 (0.76) | ||
| OCD | 2.44 (0.91) | 2.41 (0.91) | 2.08 (0.63) | 2.25 (0.68) | ||
| α | 0.91 | 0.90 | 0.87 | 0.93 | ||
| Provocative | HC | 1.48 (0.46) | 1.73 (0.56) | 1.11 (0.13) | 1.42 (0.33) | |
| NON | 2.47 (0.83) | 3.17 (1.03) | 2.44 (0.83) | 2.81 (0.86) | ||
| OCD | 3.67 (1.07) | 3.85 (1.20) | 3.26 (1.12) | 3.56 (1.08) | ||
| α | 0.93 | 0.92 | 0.93 | 0.96 | ||
| Enhanced Provocative | HC | 2.50 (1.14) | 3.08 (1.29) | 1.70 (.80) | 2.39 (1.01) | |
| NON | 3.85 (1.13) | 4.49 (0.89) | 3.71 (0.95) | 4.10 (0.86) | ||
| OCD | 4.86 (0.79) | 4.97 (0.97) | 4.19 (1.05) | 4.58 (0.82) | ||
| α | 0.93 | 0.91 | 0.93 | 0.95 | ||
M(SD)
For each image, the average anxiety rating and composite OC symptom score was computed for each participant group. Three linear regression analyses including anxiety ratings from each pair of groups (OCD-nonclinical, nonclinical-control, and OCD-control) were then used to test whether images were similarly provocative across groups (that is, if the qualitative pattern of provocation across images was preserved across groups). These analyses were repeated on the OC symptom scores.
A second set of regression analyses were run separately for the three groups, comparing OC symptom ratings with anxiety across images.
To control for effects of stimulus type, these regressions were run again with addition of two binary dummy regressors coding for image type (Draper and Smith, 1998). Statistical analyses were conducted in SPSS 24 (IBM Corp, Armonk, NY).
3. Results
3.1. Statistical Assumptions
In preparation for ANOVAs, data were inspected for normality and outliers. Normal Q-Q plots suggested that residuals were relatively normal. Kolmogorov-Smirnov tests of the residuals confirmed normality for the provocative and enhanced provocative images, but not the neutral images, possibly due to floor effects in the data. Inspection of boxplots revealed two outliers (both members of the OCD group), one for neutral anxiety and another for neutral checking ratings. Mixed ANOVAs were repeated with these two cases removed and results were not substantively changed.
Data were inspected for normality and homoscedasticity in preparation for regression analyses. Inspection of P-P plots suggested the distributions of residuals were generally normal. However, Kolmogorov-Smirnov tests suggested residuals from several regression equations including control participant ratings were significantly non-normal. This may again represent a floor effect. Inspection of residual scatterplots suggested that variance across residuals was homoscedastic for nearly all regression equations, although there was some evidence of heteroscedasticity for several equations that included control participant ratings as dependent variables. These modest departures from normality and homoscedasticity may limit conclusions drawn using parametric analyses in the control group, but they do not affect analyses of the other two groups.
3.2. Effects of stimulus type and participant group
For anxiety ratings, there was a significant main effect of stimulus type [F (1.79, 58.99) = 97.28, p < 0.01, η2ρ = 0.75]. Post-hoc contrasts showed that participants reported substantially more anxiety in reaction to the enhanced provocative stimuli than neutral [F (1, 33) = 142.22, p < 0.01, η2ρ = 0.51] and provocative [F (1, 33) = 84.91, p < 0.01, η2ρ = 0.72] stimuli, and more anxiety in reaction to provocative than neutral stimuli [F (1, 33) = 34.73, p < 0.01, η2ρ = 0.51]. A significant main effect of group showed that subjective anxiety significantly differed across the three groups, [F (2, 33) = 21.99, p < 0.01, η2ρ = 0.57]. Post-hoc contrasts showed that participants with OCD reported significantly greater subjective anxiety than control participants (t = 6.62, p < 0.01) and participants with nonclinical checking (t = 3.11, p < 0.01); participants with nonclinical checking also reported significantly greater anxiety than control participants (t = 3.11, p < 0.01). There was a significant group by stimulus type interaction [F (3.58, 58.99) = 3.25, p < 0.05, η2ρ = 0.17], suggesting that differences in anxiety ratings between stimulus types varied as a function of group. Post-hoc contrasts revealed that differences in anxiety ratings across stimulus types significantly differed between control participants and participants with OCD, [F (1.77, 42.36) = 5.46, p < 0.01, η2ρ = 0.19], but did not significantly differ between controls and participants with nonclinical checking, [F (1.52, 28.79) = 1.76, p = 0.20, η2ρ = 0.09], or between participants with nonclinical checking and participants with OCD, [F (1.82, 41.89) = 1.79, p = 0.18, η2ρ = 0.07]. See Figure 3A and Figure 3B for illustration of these results.
Figure 3.
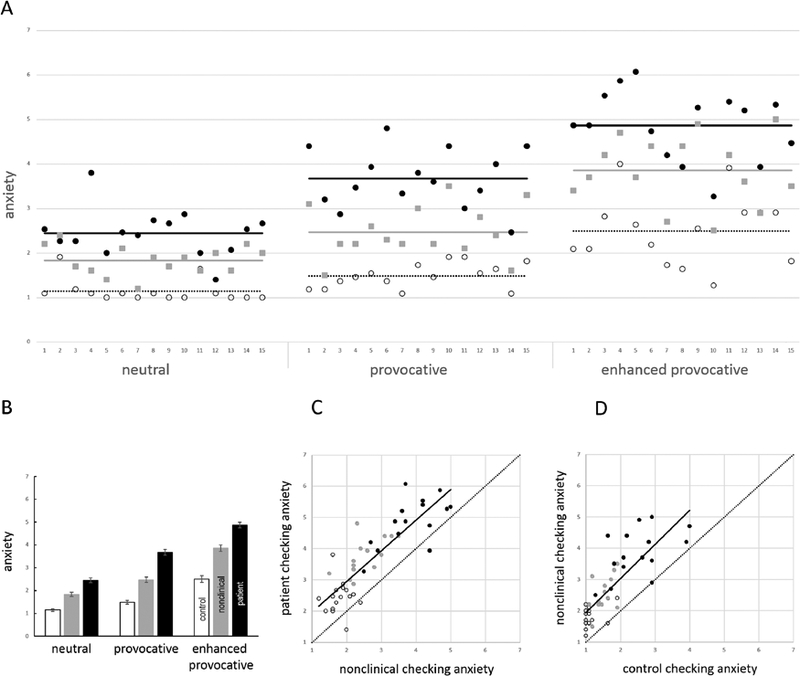
Effects of group and stimulus type on anxiety (panels A and B) and correlations of anxiety ratings between groups (panels C and D). (A) Plot showing anxiety ratings of control participants (empty circles), participants with nonclinical checking (grey squares), and participants with OCD (black circles) across all images in the neutral, provocative, and enhanced provocative categories (left to right). (B) Mean anxiety ratings for control participants (white), participants with nonclinical checking (grey), and participants with OCD (black) for images in the neutral, provocative, and enhanced provocative categories (left to right). (C, D) Scatter plots of anxiety ratings of participants with OCD versus participants with nonclinical checking (panel C), and of anxiety ratings of participants with nonclinical checking versus control participants (panel D). The solid line indicates the line of best fit; the dotted line shows the line x=y. In both cases, note that the line of best fit is approximately parallel to x=y, but raised, indicating consistently greater anxiety both in the OCD group compared to the nonclinical checking group (panel C), and in the nonclinical checking group compared to the control group (panel D). In both panels C and D, anxiety ratings for the different stimulus types are color coded, with enhanced provocative images (black circles) tending to elicit the most anxiety, followed by provocative images (grey circles), followed by neutral images (empty circles).
For the composite score of OC symptoms, there was a significant main effect of stimulus type [F (1.67, 55.21) = 114.92, p < 0.01, η2ρ = 0.78]. Post-hoc contrasts showed that participants reported substantially more OC symptoms in reaction to the enhanced provocative stimuli than neutral [F (1, 33) = 159.61, p < 0.01, η2ρ = 0.83] and provocative [F (1, 33) = 97.39, p < 0.01, η2ρ = 0.75] stimuli, and more OC symptoms in reaction to provocative stimuli than neutral stimuli [F (1, 33) = 54.24, p < 0.01, η2ρ = 0.62]. A significant main effect of group suggested that OC symptoms significantly differed across the three groups, [F (2, 33) = 25.89, p < 0.01, η2ρ = 0.61]. Post-hoc contrasts showed that participants with OCD reported significantly more OC symptoms than control participants (t = 7.10, p < 0.01) and participants with nonclinical checking (t = 2.09, p = 0.05); participants with nonclinical checking also reported greater OC symptoms than control participants (t = 4.49, p < 0.01). There was a significant group by stimulus interaction [F (3.35, 55.21), = 3.77, p < 0.05, η2ρ = 0.19], suggesting that differences in OC symptom ratings between stimulus types varied as a function of group. Post-hoc contrasts revealed that differences in OC symptoms across stimulus types significantly differed between control participants and participants with OCD, [F (1.69, 40.63) = 5.80, p ≤ .01, η2ρ = 0.20], between controls and participants with nonclinical checking, [F (1.44, 27.36) = 4.82, p < .05, η2ρ = .20], but not between participants with nonclinical checking and participants with OCD, [F (1.51, 34.81) = 0.56, p = .53, η2ρ = 0.02]. See Supplementary Figure 1A and Supplementary Figure 1B for illustration of these results and Table 2 for ratings for each stimulus type and group.
3.3. Correlation of anxiety ratings between groups
Anxiety ratings of individual images from participants with OCD were significantly related to the anxiety ratings from participants with nonclinical checking (β = 0.85, p < 0.01; see Figure 3C) and control participants (β = 0.78, p < 0.01); anxiety ratings from participants with nonclinical checking were also significantly related to anxiety ratings from control participants (β = 0.80, p < 0.01; see Figure 3D). After controlling for stimulus type, these relationships were still significant (range β = 0.40 – 0.55, p < 0.01).
Similarly, OC symptom ratings of images from participants with OCD were significantly related to the ratings from the participants with nonclinical checking (β = 0.92, p < 0.01; see Supplemental Figure 1C) and control participants (β = 0.86, p < 0.01); OC symptom ratings of participants with nonclinical checking and control participants were also significantly related (β = 0.89, p < 0.01; see Supplemental Figure 1D). These relationships remained significant when controlling for stimulus type (range β = 0.57 – 0.89, p < 0.01).
3.4. Correlations between anxiety ratings and composite OC symptoms
The anxiety and composite OC symptom ratings of images are plotted against each other in Figure 4. In the data from control participants, the anxiety and composite OC symptom ratings of images were significantly related (β = 0.93, p < 0.01, see Figure 4A); this relationship was still significant when controlling for stimulus type, (β = 0.83, p < 0.01). In the data from the participants with nonclinical checking, the anxiety and composite OC symptom ratings of images were significantly related (β = 0.95, p < 0.01, see Figure 4B); this relationship was still significant when controlling for stimulus type, (β = 0.86, p < 0.01). In participants with OCD, the anxiety and composite OC symptom ratings were significantly related (β = 0.96, p < 0.01, Figure 4C); this relationship was still significant when controlling for stimulus type, (β = 0.89 p < 0.01).
Figure 4:
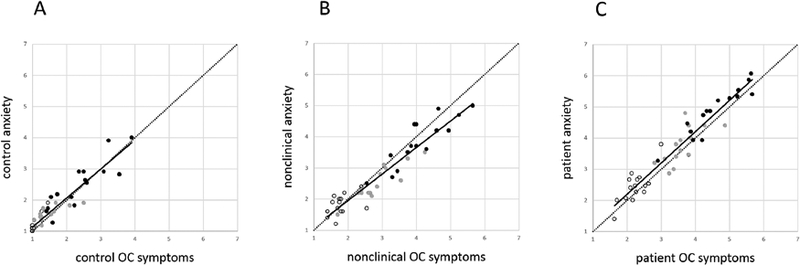
The average anxiety rating for each image plotted against the average OC symptom rating for the same image in (A) control group, (B) the nonclinical checking group, and (C) the OCD group. Neutral images are shown with empty circles, provocative images with grey circles, and enhanced provocative images with black circles. In all groups, strong relationships are apparent between anxiety ratings and composite OC ratings. As the scales of the axes are held constant, the spread away from the origin in panel C reflects greater anxiety and OC symptoms in the OCD group
4. Discussion
As hypothesized, the provocative intensity of visual stimuli intended to induce fear-of-harm/checking symptoms was enhanced when elements were added that more clearly suggested a specific harmful scenario. This was true across groups, where images designed to suggest a harmful scenario (‘enhanced provocative’ images) induced higher levels of both reported anxiety and OC symptoms. Thus, for applications in which intense provocation of obsessive-compulsive checking symptoms is needed, it is recommended that these suggestive images be used. For example, if an application requires brain activation in every participant in response to a standard stimulus set, suggestive stimuli are recommended.
The validation of a standardized provocative stimulus set is especially important in the realm of OC checking symptoms. Anecdotally, we have found checking symptoms to be more difficult to reliably provoke in OCD samples than contamination symptoms - especially when using a standardized, non-personalized stimulus set. Whereas contamination anxiety can be reliably induced by showing images of bodily fluids, for example, the production of checking anxiety seems to be more dependent on participants’ cognitive engagement as they actively envision a scenario of how harm might occur. Our enhanced provocative images may make these scenarios more obvious, and thus require less cognitive engagement and effort by participants. We suggest that such images will more reliably produce checking symptoms in studies of OCD than do standard provocative stimuli that simply depict objects involved in checking anxiety (without any contextual cues suggesting harmful scenarios that could unfold).
While the enhanced provocative images examined in this study were successful in eliciting increased anxiety and OC symptoms on a general level, they must be able to do so in a manner that is consistent with diagnostic status if they are to be an effective tool for symptom provocation. Consistent with this goal, the stimulus set succeeded in eliciting increased anxiety and OC symptoms in participants with OCD relative to both nonclinical and control participants. Participants with nonclinical checking, while reporting less symptoms than the OCD group, still exhibited greater anxiety and OC symptoms than did control participants. This differential response demonstrates the ability of the images to provoke symptoms in accordance with participant diagnostic status. Taken together, the findings outlined thus far suggest that a standardized set of enhanced provocative images is capable of a) targeting anxiety and OC symptoms in a manner that is consistent with diagnostic status and b) maximizing the intensity of this symptom provocation, perhaps by decreasing the necessary threshold for cognitive engagement with the stimuli.
Although significant group effects indicated differences in the amount of provocation experienced by each group, the pattern of symptoms reported across stimuli was qualitatively similar in all three groups. In other words, the same images tended to bother all participants, regardless of whether they were participants with OCD, participants with nonclinical checking, or control participants. Thus, while differences were found, as expected, in the magnitude of anxiety experienced, this study provides evidence for a qualitative similarity in the pattern of response exhibited across the three groups. This suggests that provocative images can be piloted on individuals with nonclinical checking - or even control individuals - when piloting on individuals with OCD is not practical. The possibility that standard and enhanced stimuli engage similar responses that differ only in degree and that clinical, nonclinical, and control participants have qualitatively similar responses to both sets of stimuli may be further explored using functional neuroimaging to characterize brain responses, in parallel with the behavioral outcome data described here.
Finally, self-reported anxiety was found to track intrusive obsessive thoughts and compulsive urges closely. Thus, when developing and piloting stimulus sets for symptom provocation, self-reported anxiety may be a reasonable proxy for OC symptoms, as long as the stimuli are selected properly. It is of course important to consider other possible sources of anxiety that may be induced by an image, and to avoid stimuli with potentially confounding sources of anxiety. For example, an image that includes an angry or scornful face within a dangerous scenario could engender social anxiety as well as checking anxiety and is not ideal for use in a standardized OCD stimulus set. Efforts were made in this study to avoid such confounded sources of anxiety, which likely contributed to the observed relationship between anxiety and obsessive compulsive symptomatology.
It is important to note that our nonclinical checking group was recruited in a different manner than the OCD and control groups. OCD and control participants were evaluated using a semi-structured clinical interview to confirm OCD diagnosis in patients and identify potential comorbid diagnoses that might be exclusionary. The nonclinical checking sample, on the other hand, was drawn from the general population based on a minimum score on the checking subscale of the Padua Inventory, with comorbid diagnoses being characterized only by selfreport. These recruitment methods gave rise to two particularities. First, there were slightly (but not significantly) higher Padua scores in the nonclinical checking group than in the OCD group, and greater variability in the Padua scores amongst participants with OCD. This was not surprising considering the fact that participants with OCD, in contrast with the nonclinical group, were not required to have a minimum score on the checking subscale of the Padua Inventory. Second, there may be undetected comorbidity (and potentially undiagnosed OCD) in the nonclinical checking group. In spite of this, however, the expected effects were still seen, with the OCD group reporting the highest provocation, followed by the nonclinical checking group and the control group. Across the three groups, we also observed a similar qualitative pattern of provocation across images. As such, we note that this limitation can also be interpreted as a strength, as it shows that a lightly screened study group drawn from the general population can be used for stimulus development, with significant confidence that findings in this more convenient population will generalize to more carefully characterized clinical populations.
This study has several other limitations. First, the data from neutral images and control participants did not meet all the statistical assumptions (likely due to floor effects in symptoms induced) and thus must be considered tentative. However, the primary findings of the study, which focused on provocative and enhanced provocative images in nonclinical checking and patient groups, are not subject to this concern as data in these groups did not violate statistical assumptions. Finally, while this study offers insight into the development and implementation of standardized stimulus sets aimed at provoking OC checking symptoms, we examined only a single stimulus set in a small number of participants; replication with different stimuli and a larger sample is therefore needed.
Supplementary Material
Highlights.
Controls, nonclinical checkers, and OCD patients rated images for their provocation
Images with contextual cues suggesting dangerous scenarios were more provocative
Anxiety and obsessive-compulsive symptoms induced by images were highly correlated
Patients’ ratings of images correlated with control group ratings but were higher
Acknowledgements
This work was supported by NIMH (R01 MH100068, MH; R01 MH095790, CP) and by the State of Connecticut through its support of the Ribicoff Research Facilities at the Connecticut Mental Health Center. The views expressed represent those of the authors, and not of the State of Connecticut. Authors H. Brooks and S. Monahan contributed to this study while participating in the summer internship program organized by the Yale Child Study Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM, 2000. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J. Psychiatr. Res 34 (4–5), 317–324. [DOI] [PubMed] [Google Scholar]
- Agarwal SM, Jose D, Baruah U, Shivakumar V, Kalmady SV, Venkatasubramanian G, et al. , 2013. Neurohemodynamic correlates of washing symptoms in obsessive-compulsive disorder: a pilot fMRI study using symptom provocation paradigm. Indian J. Psychol. Med 35 (1), 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, et al. , 2009. To discard or not to discard: the neural basis of hoarding symptoms in obsessive-compulsive disorder. Mol. Psychiatry 14 (3), 318–331. [DOI] [PubMed] [Google Scholar]
- Burns GL, Keortge SG, Formea GM, Sternberger LG, 1996. Revision of the Padua Inventory of obsessive compulsive disorder symptoms: distinctions between worry, obsessions, and compulsions. Behav. Res. Ther 34 (2), 163–173. [DOI] [PubMed] [Google Scholar]
- Cottraux J, Gerard D, Cinotti L, Froment JC, Deiber MP, Le Bars D, et al. , 1996. A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive-compulsive disorder with checking rituals. Psychiatry Res. 60 (2–3), 101–112. [DOI] [PubMed] [Google Scholar]
- Draper NR, Smith H, 1998. Applied Regression Analysis, (3rd ed.). Wiley, New York. [Google Scholar]
- Gibbs NA, 1996. Nonclinical populations in research on obsessive-compulsive disorder: A critical review. Clin. Psychol. Rev 16 (8), 729–773. [Google Scholar]
- Gilbert AR, Akkal D, Almeida JR, Mataix-Cols D, Kalas C, Devlin B, et al. , 2009. Neural correlates of symptom dimensions in pediatric obsessive-compulsive disorder: a functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry 48 (9), 936–944. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. , 1989a. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch. Gen. Psychiatry 46 (11), 1012–1016. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. , 1989b. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 46 (11), 1006–1011. [DOI] [PubMed] [Google Scholar]
- Hampson M, Stoica T, Saksa J, Scheinost D, Qiu M, Bhawnani J, et al. , 2012. Real-time fMRI biofeedback targeting the orbitofrontal cortex for contamination anxiety. J Vis Exp (59). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual). University of Florida, Gainesville, FL. [Google Scholar]
- Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, et al. , 2003. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol. Psychiatry 53 (6), 482–493. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Lawrence NS, Wooderson S, Speckens A, Phillips ML, 2009. The Maudsley Obsessive-Compulsive Stimuli Set: validation of a standardized paradigm for symptom-specific provocation in obsessive-compulsive disorder. Psychiatry Res. 168 (3), 238–241. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML, 2004. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch. Gen. Psychiatry 61 (6), 564–576. [DOI] [PubMed] [Google Scholar]
- Morgieve M, N’Diaye K, Haynes WI, Granger B, Clair AH, Pelissolo A, et al. , 2014. Dynamics of psychotherapy-related cerebral haemodynamic changes in obsessive compulsive disorder using a personalized exposure task in functional magnetic resonance imaging. Psychol. Med 44 (7), 1461–1473. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. , 2005. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol. Psychiatry 57 (8), 901–910. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. , 1994. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch. Gen. Psychiatry 51 (1), 62–70. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Stoica T, Saksa J, Papademetris X, Constable RT, Pittenger C, et al. , 2013. Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Transl Psychiatry 3, e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Stark R, Walter B, Vaitl D, 2005. Neural responses of OCD patients towards disorder-relevant, generally disgust-inducing and fear-inducing pictures. Int. J. Psychophysiol 57 (1), 69–77. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. , 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Simon D, Kaufmann C, Musch K, Kischkel E, Kathmann N, 2010. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology 47 (4), 728–738. [DOI] [PubMed] [Google Scholar]
- Simon D, Kischkel E, Spielberg R, Kathmann N, 2012. A pilot study on the validity of using pictures and videos for individualized symptom provocation in obsessive-compulsive disorder. Psychiatry Res. 198 (1), 81–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


