Abstract
Background:
Recent studies have suggested that the quantity and quality of adipose tissue and muscle, assessed on non-contrast CT, may serve as imaging biomarkers of survival in patients with and without neoplasms.
Purpose:
To assess body composition measures that could serve as predictors of therapy response in patients with extremity soft tissue sarcomas treated with radiation therapy and surgery.
Material and Methods:
The study was IRB-approved. Sixty patients had a history of extremity soft tissue sarcoma and underwent FDG-PET/CT prior to radiation therapy and surgical resection. Cross sectional areas (CSA) and CT attenuation (HU) of abdominal subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT) and psoas muscle were assessed on non-contrast CT. Clinical information on predictors of tumor recurrence and post-surgical wound infections were recorded. Cox proportional hazard models were used to determine longitudinal associations between body composition and tumor recurrence/wound infections.
Results:
Twenty-three tumor recurrences occurred over a follow-up period of 43±35 months. Higher SAT and lower psoas attenuation were associated with tumor recurrence which remained significant after adjustment for covariates (p≤0.01). There were 13 post-surgical wound infections. Higher VAT and SAT attenuation were associated with post-surgical wound infections (p<0.04), however, VAT attenuation lost significance after adjustment for covariates.
Conclusion:
Abdominal adipose tissue and psoas muscle attenuation assessed on non-contrast CT may predict tumor recurrence and post-surgical infections in patients with extremity soft tissue sarcomas.
Keywords: soft tissue sarcoma, computed tomography (CT), muscle attenuation, adipose tissue attenuation, tumor recurrence
Introduction
Soft tissue sarcomas represent 1% of all adult malignancies and approximately 10,000 patients are diagnosed in the United States with a soft tissue sarcoma each year (1). About 50% to 60% of soft tissue sarcomas occur in the extremities and these tumors often recur and metastasize, despite complete resection with negative margins (2). Predicting outcome and designing management approaches in patients with soft tissue sarcomas is challenging given the multiple subtypes with different biological behaviors, some showing early progression, recurrence and death, while others presenting with multiple or late recurrences over decades (3).
While in the past soft tissue sarcomas were routinely treated with amputation, advances in radiation therapy and surgical techniques have made limb-sparing surgery the standard treatment for the majority of localized extremity soft tissue sarcomas (4). External beam radiation therapy is usually administered preoperatively, intraoperatively, postoperatively, or in combination (5). Advantages of preoperative over postoperative radiation therapy include a decrease in radiation dose and radiation volume, improved tissue oxygenation, and decrease of metastatic potential of cells that may be spread at the time of surgery (6–8). However, radiation therapy may increase the risk of postoperative wound infections (9–11). Identification of biomarkers that could predict tumor recurrence and post-surgical wound infections would be valuable in the management of patients with extremity soft tissue sarcomas.
Recent studies have suggested that the quantity and quality of adipose tissue and muscle assessed on non-contrast CT may serve as imaging biomarkers of survival in patients with and without neoplasms. In these studies abdominal adiposity and high CT attenuation of abdominal fat and lower attenuation of muscle were associated with increased mortality (12–15).
Patients with soft tissue sarcomas often undergo FDG-PET/CT for staging and surveillance, and adipose tissue and muscle measures, assessed on the non-contrast attenuation-correction CT, may be used as imaging biomarkers to predict tumor recurrence and post-surgical infections in these patients. In addition, adipose tissue and muscle characteristics could provide insights into cancer-related body composition abnormalities, such as cancer cachexia. The purpose of our study was therefore to assess abdominal adipose tissue cross sectional areas and CT attenuation of abdominal adipose tissue and psoas muscle on non-contrast CT in patients with soft tissue sarcomas of the extremities undergoing radiation therapy and surgical resection. We hypothesized that increased CT attenuation of abdominal adipose tissue and decreased attenuation of muscle may serve as imaging biomarkers of tumor recurrence and post-surgical wound infections.
Material and Methods
Our study was IRB approved and complied with HIPAA guidelines with exemption status for individual informed consent.
Patients
A retrospective search was performed to identify patients with a history of extremity soft tissue sarcoma who had undergone FDG-PET/CT prior to onset of any therapy at our institution between January 2005 and June 2016. Inclusion criteria were age ≥ 18 years, pathologically proven soft tissue sarcoma of the extremities, diagnostic attenuation-correction non-contrast PET/CT, and radiation therapy followed by surgical resection of the soft tissue sarcoma. Exclusion criteria were malignancy other than soft tissue sarcoma, abdominal surgery or other pathology which could affect abdominal adipose tissue and psoas muscle measurements, such as ascites or metastases. We identified 127 patients with extremity soft tissue sarcoma who had a diagnostic PET/CT available. Forty patients were excluded because of therapy regimen (palliative chemotherapy/radiation therapy without surgical resection or surgical resection and/or chemotherapy but no radiation therapy). Twenty-seven patients were excluded due to tumor recurrence and/or metastatic disease at time of initial PET/CT. Sixty patients met inclusion criteria and comprised the study group.
Clinical data
Clinical data were obtained from electronic medical records: age, sex, BMI, types of pre-existing comorbidities (cardiovascular disease, renal disease, type II diabetes mellitus or a combination of those), sarcoma type (adipocyte, fibroblastic, smooth muscle, skeletal muscle, extraskeletal chondrogenic, vascular, or uncertain differentiation), sarcoma grade (grades 1 to 3, French Federation of Cancer Centers Sarcoma Group), sarcoma size (cm) determined as largest diameter on cross-sectional imaging, sarcoma stage (stage I to IV, TNM system of the American Joint Committee on Cancer), treatment regimen including radiation dose and use of adjuvant chemotherapy, presence of positive resection margins, local tumor recurrence, development of wound infection, and time between PET/CT and tumor recurrence/wound infection.
Body composition assessment
Whole-body 18-F-FDG-PET/CT (Siemens Biograph 16 or 64, Siemens, Erlangen, Germany or GE Discovery, GE Healthcare, Milwaukee, WI, USA) was performed per standard clinical protocol. Attenuation-correction CT was performed in the mid-expiration phase without intravenous contrast (slice thickness 5 mm; table feed per rotation, 18 mm; time per table rotation, 0.5 s; tube voltage, 120 kVp; tube current, 11 mAs; field of view, 50 cm). Images were reconstructed to a slice thickness of 2.4 mm. The CT scanners used in this study were tested on an annual basis according to American Association of Physicists in Medicine (AAPM) and American College of Radiology (ACR) guidelines (AAPM report #74 and #96 and ACR CT QC manual) and standard clinical quality assurance measures were performed to assess for reproducibility of scans over time.
Non-contrast attenuation-correction CTs were used for assessment of abdominal adipose tissue cross-sectional areas (CSA) (cm2) and abdominal adipose tissue and right psoas muscle attenuation (Hounsfield Units, HU). Measurements were performed in the abdomen at the mid-portion of the 4th lumbar vertebra remote from the site of sarcoma, to avoid the influence of the primary tumor and changes from prior biopsy on body composition measures. In addition, measurements performed at the L4 level have been shown to correlate with whole body adiposity (16). Automated thresholding methods were applied using a threshold set for −50 to −250 HU to identify abdominal adipose tissue (17), and −29 to 150 HU to identify muscle tissue (18) (Osirix software version 3.2.1; www.osirix-viewer.com/index.html) (Supplemental Fig.). Intra-reader variability coefficients of variation (CV) for these measurements at our institution are 0.6% to 3.8% and interclass correlation coefficients (ICC) are 0.98 to 1.0 (95% confidence interval (CI) 0.83 to 1.0). For inter-reader variability CVs are 3.1% to 3.3% and ICCs are 0.98 to 1.0 (95% CI 0.85 to 1.0). This was determined by analyzing five cases twice by the same reader and by analyzing five cases by two different readers (SAB and JV, 1 year of experience under the supervision of MAB, 11 years of experience).
Statistical analysis
Statistical analysis was performed using JMP statistical software version 11.0 (SAS Institute Inc., Cary, NC, USA). For assessment of tumor recurrence and post-surgical wound infection, Cox proportional hazard models were used to assess the longitudinal association between abdominal adipose tissue and psoas muscle measures and tumor recurrence and post-surgical wound infections and the results are presented as hazard ratios (HR) with 95% confidence intervals (CI). Multivariate analysis was performed by subsequently adding three different covariate models, which included known risk factors for sarcoma recurrence (3, 19, 20). The first model adjusted for age and pre-existing comorbidities, model 2 consisted of model 1 plus sarcoma type, size, and stage, model 3 included model 2 plus pre-operative radiation dose and the presence of positive resection margins. For post-surgical wound infection three different covariate models which included known risk factors for post-surgical wound infections were added (11). The first model adjusted for age and BMI. Model 2 included Model 1 plus co-morbidities and model 3 included model 2 plus tumor stage, size, and preoperative radiation dose. For all analyses, a two-sided P-value of < 0.05 was considered statistically significant. Data are shown as mean ± standard deviation (SD).
Results
The study group comprised 60 patients, 32 men and 28 women with a mean age at time of FDG-PET/CT of 50±18 years (Table 1). Over a mean follow-up time of 43±35 months there were 23 tumor recurrences (median time to tumor recurrence: 16 months).
Table 1. Patient characteristics.
Data presented as mean ± SD for continuous variables and n (%) for categorical variables
| n =60 | |
|---|---|
| Females | 28 (46.7) |
| Males | 32 (53.3) |
| Age at PET/CT (years) | 50±18 |
| BMI at PET/CT | 27.4 ± 6.0 |
|
Sarcoma type adipocytic fibroblastic smooth-muscle skeletal muscle uncertain diff. chondrogenic vascular |
9 (15.0) 14 (23.3) 5 (8.3) 3 (5.0) 26 (43.3) 2 (3.3) 1 (1.7) |
|
Sarcoma stage 1 2 3 4 |
6 (10.0) 29 (48.3) 20 (33.3) 5 (8.3) |
|
Sarcoma grade 1 2 3 |
7 (11.7) 39 (65.0) 14 (23.3) |
| Sarcoma size (cm) | 7.7 ± 6.5 |
|
Comorbidities none cardiovascular (CV) diabetes mellitus (DM) CV + DM |
31 (51.7) 24 (40.0) 2 (3.3) 3 (5.0) |
|
Therapy Pre-operative radiation Post-operative radiation Pre-operative radiation dose (Gy) Post-operative radiation dose (Gy) Adjuvant chemotherapy |
60 (100) 20 (33.3) 46.3±11.6 40.9±18.5 13 (22.0) |
| Positive resection margins | 19 (32.8) |
There was a significant association between CT attenuation of SAT and tumor recurrence (Table 2 and Fig. 1). Increased attenuation of SAT was associational with tumor recurrence (p=0.001), which remained significant after adjustment for covariates (p≤0.02). No associations between VAT attenuation, SAT or VAT CSAs and tumor recurrence were identified (p≥0.4).
Table 2. Association of adipose tissue and muscle measures and tumor recurrence.
Data are presented as hazard ratio (95% CI) per 1 SD increase in cm2 and HU, respectively.
| VAT CSA (cm2) | p | VAT attenuation (HU) | p | SAT CSA (cm2) | p | SAT attenuation (HU) | p | Psoas attenuation (HU) | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| No Covariates | 1.00 (0.99–1.00) | 0.9 | 1.00 (0.96–1.04) | 1.0 | 1.00 (1.00–1.00) | 0.4 | 1.07 (1.03–1.11) | 0.001 | 0.92 (0.89–0.96) | 0.0002 |
| Model 1 | 0.99 (0.99–1.00) | 0.07 | 1.02 (0.97–1.07) | 0.4 | 1.00 (0.99–1.00) | 0.08 | 1.09 (1.04–1.13) | 0.0002 | 0.92 (0.89–0.96) | 0.0002 |
| Model 2 | 0.99 (0.98–1.00) | 0.09 | 1.01 (0.94–1.07) | 0.8 | 0.99 (0.99–1.00) | 0.5 | 1.12 (1.04–1.22) | 0.002 | 0.92 (0.86–0.98) | 0.01 |
| Model 3 | 1.00 (0.99–1.01) | 0.7 | 1.03 (0.96–1.11 ) | 0.4 | 1.01 (0.99–1.01) | 0.2 | 1.10 (1.01–1.19) | 0.02 | 0.89 (0.82–0.97) | 0.004 |
Model 1 Adjusted for age and comorbidities
Model 2 Adjusted for Model 1 plus sarcoma type, size, and stage.
Model 3 Adjusted for Model 2 plus pre- operative radiation dose and positive resection margins
VAT = visceral adipose tissue; CSA = cross-sectional area; SAT = subcutaneous adipose tissue;
Fig. 1.
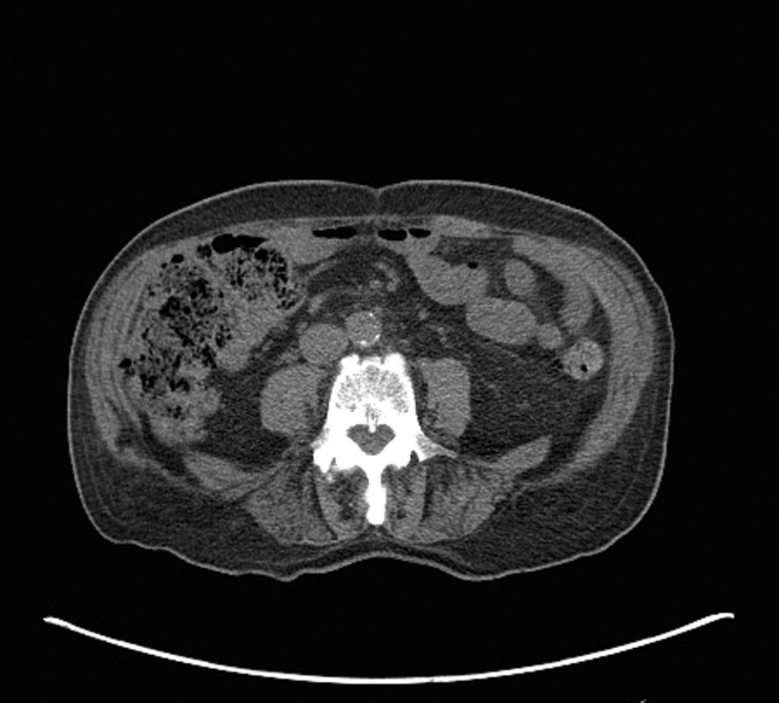
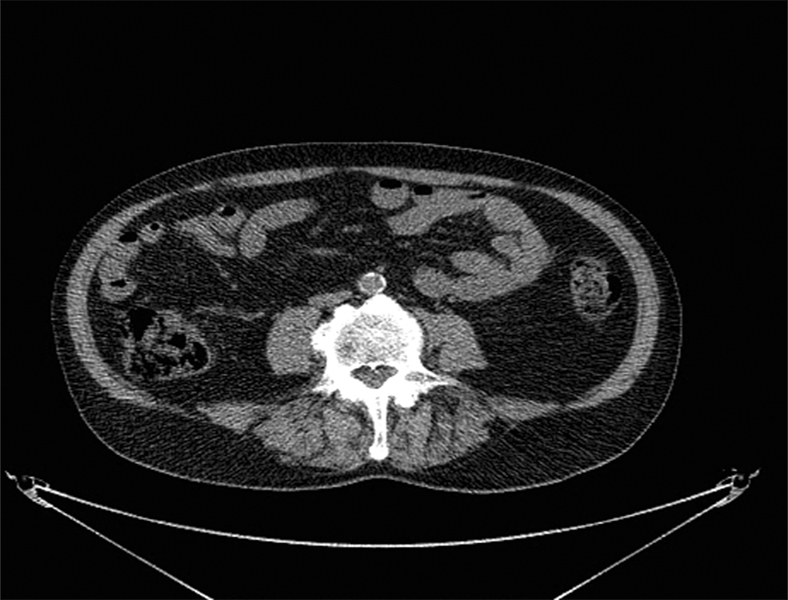
Axial non-contrast CTs at the level of L4 in two men with thigh liposarcoma treated with pre-operative radiation therapy followed by surgical resection. (A) 76 year-old man who developed tumor recurrence demonstrates increased adipose tissue attenuation (−76 HU) and low psoas muscle attenuation (33 HU) compared to (B) 72 year-old year old man who did not developed tumor recurrence and who had lower CT attenuation of adipose tissue (−103 HU) and higher psoas muscle attenuation (52 HU). Images are presented using the same window and level.
There was a significant association between CT attenuation of psoas muscle and tumor recurrence (Table 2 and Fig. 1). Decreased attenuation of psoas muscle was associated with tumor recurrence (p=0.0002). The association remained significant after adjusting for covariates (p≤0.01).
There were 13 post-surgical wound infections. Mean time from surgery to infection was 1.7±2.8 months. There was a significant association between CT attenuation of SAT and VAT and post-surgical wound infections. Higher VAT and SAT attenuation were associated with post-surgical wound infections (p=0.04) which remained significant after adjustment for all covariates for SAT attenuation and after adjustment of age and BMI for VAT attenuation. However, the association with VAT attenuation lost significance after adjustment for additional covariates (Table 3). VAT CSA was associated with post-operative wound infections after controlling for age and BMI (p=0.01).
Table 3. Association of adipose tissue and muscle measures and post-operative wound infection.
Data are presented as hazard ratio (95% CI) per 1 SD increase in cm2 and HU, respectively.
| VAT CSA (cm2) | p | VAT attenuation (HU) | p | SAT CSA (cm2) | p | SAT attenuation (HU) | p | Psoas attenuation (HU) | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| No Covariates | 0.99 (0.98–1.01) | 0.09 | 1.05 (1.00–1.11) | 0.04 | 1.00 (0.99–1.00) | 0.5 | 1.05 (1.00–1.10) | 0.04 | 1.02 (0.97–1.08) | 0.4 |
| Model 1 | 0.98 (0.97–1.00) | 0.01 | 1.07 (1.00–1.14) | 0.05 | 1.00 (0.99–1.00) | 0.9 | 1.05 (1.00–1.11) | 0.05 | 0.99 (0.94–1.06) | 0.9 |
| Model 2 | 0.99 (0.97–1.00) | 0.1 | 1.07 (1.00–1.15) | 0.07 | 1.00 (0.99–1.00) | 1.0 | 1.06 (1.00–1.12) | 0.04 | 0.97 (0.90–1.05) | 0.5 |
| Model 3 | 0.99 (0.96–1.01) | 0.2 | 1.01 (0.92–1.11) | 0.8 | 1.00 (0.99–1.01) | 0.7 | 1.21 (1.06–1.44) | 0.004 | 0.97 (0.88–1.06) | 0.5 |
Model 1 Adjusted for age and BMI
Model 2 Adjusted for Model 1 plus comorbidities
Model 3 Adjusted for Model 2 plus tumor stage, size, and preoperative radiation dose
VAT = visceral adipose tissue; CSA = cross-sectional area; SAT = subcutaneous adipose tissue;
Discussion
Our study showed that abdominal adipose tissue and psoas muscle attenuation, assessed on non-contrast attenuation-correction CT, may serve as imaging biomarkers for tumor recurrence and post-surgical infections in patients with extremity sarcomas treated with radiation therapy and surgical resection. Higher abdominal SAT and lower psoas muscle attenuation were associated with increased risk of tumor recurrence and higher abdominal adipose tissue attenuation was associated with post-surgical infections.
Soft tissue sarcomas are a diverse group of malignancies with the majority occurring in the extremities. The different histologic subtypes, varying natural history and response to therapy makes the prediction of outcome and the design of therapeutic regimens challenging (3). Goals of treating patients with soft tissue sarcomas are long-term survival, avoidance of local recurrence, maximizing function and minimizing morbidity (4). Limb sparing surgery combined with radiation therapy is considered to be effective for local control in patients with extremity soft tissue sarcomas (4–6). However, tumor recurrence remains a known risk, especially in the setting of positive resection margins. In addition, post-surgical wound infections are frequently encountered following preoperative radiation therapy (9–11). Therefore, biomarkers that could predict tumor recurrence and post-surgical wound infections would be useful in the management of patients with extremity soft tissue sarcomas.
Recent studies have focused on the assessment of body composition by CT to predict outcome in patients with cancers. Studies in patients with gastrointestinal cancers showed that increased abdominal adiposity and muscle loss were independent predictors of tumor recurrence and mortality (12, 21, 22). A study in patients with pancreatic cancer undergoing pancreatoduodenectomy showed that increased VAT CSA was an independent predictor of post-surgical complications (23).
In our study there were no associations between abdominal fat CSAs and tumor recurrence or post-surgical infection. However, not only the quantity of adipose or muscle tissue but also the quality of these tissues has been shown to independently predict survival in patients with and without cancer. Two large multicenter studies demonstrated increased all-cause mortality in patients with high CT attenuation of abdominal adipose tissue, independent of known risk factors, such as age, smoking, or co-morbidities (13, 14). A study in patients with bone and soft tissue sarcomas undergoing a variety of therapies has demonstrated a positive association between mortality and high CT attenuation of abdominal SAT and low attenuation of psoas muscle, independent of known risk factors, such as age, BMI, co-morbidities, tumor stage and grade (15). In our study, focusing on patients with soft tissue sarcomas of the extremities treated with a standard regimen, radiation therapy and surgery, high abdominal SAT and low psoas muscle attenuation were positive predictors of tumor recurrence, independent of tumor size, stage, radiation dose and other risk factors. In addition, higher abdominal SAT and VAT attenuation were positive predictors of post-surgical infections, independent of known risk factors for SAT attenuation and independent of age and BMI for VAT. These observations might provide insights into the pathophysiology of cancer-related body composition abnormalities, such as cancer cachexia.
The role of adipose tissue in cancer has received recent attention given the positive association between obesity and cancer incidence and mortality (24). Adipocytes present an important part of the tumor microenvironment and studies have shown that cancer cells can cause dedifferentiation of adipocytes into pre-adipocytes. This dedifferentiation is associated with increased lipolysis and loss of lipids, as well as changes in the expression of genes regulating energy turnover, cytoskeleton, and extracellular matrix (25). These cancer-associated adipocytes can have systemic metabolic effects and promote tumor growth (26–28). Studies in monkeys have shown that increased CT attenuation of abdominal adipose tissue corresponds to smaller adipocytes and increased extracellular matrix fibrosis (13). The observed association between increased CT attenuation of adipose tissue, remote from the site of sarcoma, and tumor recurrence may reflect smaller cancer-associated adipocytes and/or an adipocyte microenvironment promoting tumor growth.
Previous studies have elucidated cross talk between adipose tissue and muscle in cancer. Adipose and muscle tissue secrete inflammatory cytokines, myokines, adipokines, and free fatty acids, which regulate adipose and muscle metabolism (25, 29). The observed association between tumor recurrence and low CT attenuation of psoas muscle in addition to the high attenuation adipose tissue may reflect this cross talk. Interestingly, a study in mice has shown that ablation of adipocyte lipase results in preservation of skeletal muscle, suggesting that the breakdown of fat in cancer precedes that of skeletal muscle and that the breakdown of adipocytes affects the breakdown of muscle (25, 30). However, further studies are necessary to investigate molecular mechanisms mediating tissue attenuation and tumor recurrence in patient with soft tissue sarcomas.
The study had several limitations, first is the retrospective study design which limits the ability to infer causality. In addition, we were dependent on medical records for clinical information. Second, we did not obtain adipose tissue or muscle biopsies to investigate underlying molecular pathways of adipose and muscle tissue and attenuation mediating tumor recurrence and post-surgical infections. Third, the study was performed using different CT scanners over a long time period. However, quality assurance measures according to the AAPM and ACR were performed to assess for reproducibility of scans over time. Strengths of the study include the large number of patients with extremity soft tissue sarcomas with pre-therapy imaging available and who underwent a standard treatment regimen, namely surgical resection followed by radiation therapy, and detailed measures of tissue attenuation on a standardized non-contrast CT protocol. In addition, the analyses were controlled for multiple covariates, known to predict tumor recurrence and post-surgical wound infections. We did not aim to assess threshold values to assess the sensitivity and specificity of body composition measures to detect tumor recurrence but rather used this pilot study to shed light into the regulation of tumor cells and body composition.
In conclusion, increased abdominal adipose tissue and lower psoas muscle attenuation are associated with increased risk of tumor recurrence and higher abdominal adipose tissue attenuation is associated with the development of post-surgical infections in patients with soft tissue sarcomas of the extremities. Adipose tissue and muscle CT attenuation can be easily assessed on any clinical workstation and these measurements could provide additional information on prognosis in patients with sarcomas. Shedding light into the biology of adipocytes and muscle cells in the tumor microenvironment may help identify metabolic targets that allow for development of new therapies for the treatment of sarcomas.
Supplementary Material
Acknowledgments
This study was supported by NIH grants 5P30DK040561-18 and K24DK109940.
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55:10–30. [DOI] [PubMed] [Google Scholar]
- 2.Morrison BA. Soft tissue sarcomas of the extremities. Proc (Bayl Univ Med Cent) 2003; 16:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan MF, Antonescu CR, Moraco N, et al. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg 2014; 260:416–421; discussion 421–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010; 2010:506182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suit HD, Mankin HJ, Wood WC, et al. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985; 55:2659–2667. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen OS, Cummings B, O’Sullivan B, et al. Preoperative and postoperative irradiation of soft tissue sarcomas: effect of radiation field size. Int J Radiat Oncol Biol Phys 1991; 21:1595–1599. [DOI] [PubMed] [Google Scholar]
- 7.Pollack A, Zagars GK, Goswitz MS, et al. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys 1998; 42:563–572. [DOI] [PubMed] [Google Scholar]
- 8.Robinson MH, Keus RB, Shasha D, et al. Is pre-operative radiotherapy superior to postoperative radiotherapy in the treatment of soft tissue sarcoma? Eur J Cancer 1998; 34:1309–1316. [DOI] [PubMed] [Google Scholar]
- 9.Akudugu JM, Bell RS, Catton C, et al. Wound healing morbidity in STS patients treated with preoperative radiotherapy in relation to in vitro skin fibroblast radiosensitivity, proliferative capacity and TGF-beta activity. Radiother Oncol 2006; 78:17–26. [DOI] [PubMed] [Google Scholar]
- 10.Geller DS, Hornicek FJ, Mankin HJ, et al. Soft tissue sarcoma resection volume associated with wound-healing complications. Clin Orthop Relat Res 2007; 459:182–185. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz A, Rebecca A, Smith A, et al. Risk factors for significant wound complications following wide resection of extremity soft tissue sarcomas. Clin Orthop Relat Res 2013; 471:3612–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015; 63:131–140. [DOI] [PubMed] [Google Scholar]
- 13.Murphy RA, Register TC, Shively CA, et al. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J Gerontol A Biol Sci Med Sci 2014; 69:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenquist KJ, Massaro JM, Pedley A, et al. Fat Quality and Incident Cardiovascular Disease, All-Cause Mortality and Cancer Mortality. J Clin Endocrinol Metab 2015; 100:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veld J, Vossen JA, De Amorim Bernstein K, et al. Adipose tissue and muscle attenuation as novel biomarkers predicting mortality in patients with extremity sarcomas. Eur Radiol 2016. [DOI] [PubMed] [Google Scholar]
- 16.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004; 97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 17.Borkan GA, Gerzof SG, Robbins AH, et al. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 1982; 36:172–177. [DOI] [PubMed] [Google Scholar]
- 18.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998; 85:115–122. [DOI] [PubMed] [Google Scholar]
- 19.Haas RL, Miah AB, LePechoux C, et al. Preoperative radiotherapy for extremity soft tissue sarcoma; past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiother Oncol 2016; 119:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang S, Kim HS, Kim W, et al. Comorbidity is independently associated with poor outcome in extremity soft tissue sarcoma. Clin Orthop Surg 2015; 7:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark W, Siegel EM, Chen YA, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 2013; 216:1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamandl D, Paireder M, Asari R, et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol 2016; 26:1359–1367. [DOI] [PubMed] [Google Scholar]
- 23.Schroder FF, de Graaff F, Bouman DE, et al. The Preoperative CT-Scan Can Help to Predict Postoperative Complications after Pancreatoduodenectomy. Biomed Res Int 2015; 2015:824525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371:569–578. [DOI] [PubMed] [Google Scholar]
- 25.Argiles JM, Stemmler B, Lopez-Soriano FJ, et al. Nonmuscle Tissues Contribution to Cancer Cachexia. Mediators Inflamm 2015; 2015:182872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andarawewa KL, Motrescu ER, Chenard MP, et al. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res 2005; 65:10862–10871. [DOI] [PubMed] [Google Scholar]
- 27.Dirat B, Bochet L, Dabek M, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 2011; 71:2455–2465. [DOI] [PubMed] [Google Scholar]
- 28.Nieman KM, Romero IL, Van Houten B, et al. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta 2013; 1831:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012; 16:153–166. [DOI] [PubMed] [Google Scholar]
- 30.Das SK, Eder S, Schauer S, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011; 333:233–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


