Abstract
Currently, no medical therapies exist to augment stroke recovery. Stem cells are an intriguing treatment option being evaluated, but cell-based therapies have several challenges including developing a stable cell product with long term reproducibility. Since much of the improvement observed from cellular therapeutics is believed to result from trophic factors the stem cells release over time, biomaterials are well-positioned to deliver these important molecules in a similar fashion. Here we show that essential trophic factors secreted from stem cells can be effectively released from a multi-component hydrogel system into the post-stroke environment. Using our polymeric system to deliver VEGF-A and MMP-9, we improved recovery after stroke to an equivalent degree as observed with traditional stem cell treatment in a rodent model. While VEGF-A and MMP-9 have many unique mechanisms of action, connective tissue growth factor (CTGF) interacts with both VEGF-A and MMP-9. With our hydrogel system as well as with stem cell delivery, the CTGF pathway is shown to be downregulated with improved stroke recovery.
Keywords: stroke, biomaterials, stem cells, hydrogel, stroke recovery, connective tissue growth factor
1. Introduction
Stroke has devastating consequences for survivors and their caregivers [1]. Stroke’s societal cost of greater than $100 billion per year is also staggering [2]. Despite this, no medical treatments exist for stroke recovery. Stem cell transplantation is a promising stroke therapy showing efficacy in animal models and in multiple early phase clinical trials [3–7]. Human neural progenitor cells (hNPCs) are a type of stem cell derived from embryonic or fetal cells which are predisposed to a neural fate; they have shown promise in stroke recovery [8].
Unfortunately, several drawbacks remain to developing long-term, cell-based therapies. First, typically only 1–8% of transplanted stem cells survive, due in part to the highly hypoxic and inflamed post-stroke environment [9–11]. Second, the time, cost, and infrastructure required to prepare an adequate number of stem cells for transplantation limits the ability to maintain a large-scale, stable product. Finally, the theoretical safety risks associated with transplantation of stem cells requires the development of rigorous protocols to insure cell homogeneity, quality assurance, and absence of tumorgenicity.
Bioengineering cell-free, stem cell mimics offers a solution to these limitations. Stem cells are thought to enhance stroke recovery largely through trophic factor release [12, 13]. A polymeric system is well suited to deliver factors without the restrictions of cell-based therapies. Recently developed, multi-component hydrogel systems are promising for biocompatible, controlled molecule delivery into biologic systems [14].
Based upon analysis of trophic factors produced by hNPCs, hNPC-secreted VEGF-A and MMP-9 were found to be important for hNPC-enhanced stroke recovery [12, 13]. If given at early time points after stroke, VEGF-A, a factor involved in angiogenesis and neural recovery, weakens blood vessels and is detrimental to stroke recovery [15–17]; but if delivered in a sustained manner, VEGF-A improves healing [13, 16, 18]. Similarly, MMP-9, a molecule that increases active VEGF during angiogenesis and impacts extracellular matrix remodeling, is detrimental in the acute period of stroke [19]; but if delivered in the subacute timeframe (>7 days) is beneficial [20, 21]. The dosing of these molecules is also critical, as higher concentrations can be detrimental to recovery [22]. It is thought that stem cells react to cues in the post-stroke environment to deliver these molecules over time [23, 24]. Given the importance of timing and dose, a controlled-release system is necessary to design an acellular therapy with trophic factor effects for stroke recovery.
In our experiments, we utilize a composite, protein-based hydrogel system to incorporate VEGF-A and MMP-9 [14, 25]. We use this polymeric system to release the factors in a pre-clinical stroke model and investigate the effect on functional recovery. The hydrogel-based treatments delivering VEGF-A, MMP-9, and VEGF-A with MMP-9 (VEGF-A+MMP-9) were compared to animals with embryonic stem cell-derived hNPC treatment. By controlling the release profiles of VEGF-A and MMP-9, we observed similar stroke recovery to animals receiving stem cell transplantation. These studies demonstrate the feasibility of creating a polymeric, stem cell mimic.
2. Materials and Methods
2.1 Hydrogel preparation
The C7 recombinant protein and PEG-peptide polymers were synthesized and purified as reported previously[26]. Phosphate buffered saline (PBS) solutions of both components were prepared at a concentration of 13.3 wt% for C7 and 6.7 wt% for PEG-peptide. 30 μL hydrogel was created by mixing 15 μL C7 with 15 μL PEG-peptide to achieve a final polymer concentration of 10 wt% and a C:P ratio of 1:1. Hydrogel delivering VEGF-A was prepared by encapsulating recombinant human VEGF-A-165 (Novoprotein). VEGF-A-165 was first mixed with the PEG-peptide component and subsequently with C7. The final concentration of VEGF-A within the hydrogel was 1 μg per 30 μL gel. Similarly, hydrogel delivering MMP-9 was prepared by encapsulating MMP-9 (Biolegend) at a concentration of 1 μg per 30 μL gel.
Dynamic light scattering microrheology was performed using a Malvern Zetasizer ZS (630 nm laser) and analyzed using custom software, as previously described [27]. Briefly, 1.0 μm diameter polystryene beads (Polysciences #08226-15) functionalized with poly (ethylene glycol) were dispersed into each hydrogel component at a final concentration of 0.25% w/v. 15 μL of hydrogel was prepared in a quartz cuvette (Malvern ZEN2112) by mixing each component in situ. After 15 minutes of incubation at 37° C, raw scattering autocorrelation functions were collected in back-scatter detection for 15 minutes at a fixed measurement position of 4.2 mm, followed by a sweep over measurement positions ranging from 3.6 mm to 5.2 mm in 0.1 mm increments to obtain the ensemble-averaged scattering intensity. The particle mean-squared displacement was extracted from the raw scattering autocorrelation function and the ensemble-averaged scattering intensity according to the previously described broken-ergodicity correction procedure [27].
The particle mean-squared displacement was transformed to the frequency-dependent complex shear modulus G*(ω) according to the generalized Stokes-Einstein relation [28], , where kB is Boltzmann’s constant, T is the absolute temperature, a is the particle diameter, i is the imaginary unit, and 〈Δr2(ω)〉 is the unilateral Fourier transform of the particle mean-squared displacement. The unilateral Fourier transform of the mean-squared displacement was performed using a local power law analysis, as described previously [27, 28].
2.2 Release kinetics of VEGF-A and MMP-9
Release kinetics of VEGF-A and MMP-9 were measured by first preparing 30 μL hydrogel+VEGF-A or MMP-9 in microcentrifuge tubes (n ≥ 3). The mixture was allowed to gel for 15 minutes at 37 °C before adding 1 mL of PBS. Release kinetics were determined by sampling and replenishing 10 μL of the PBS supernatant over a period of 14 days. Samples were frozen at −80 °C immediately after collection and thawed prior to quantification. The amount of VEGF-A and MMP-9 present in the supernatant at each time point was quantified using Human VEGF-A Quantikine ELISA Kit (R&D Systems) and Human MMP-9 Quantikine ELISA Kit, according to the manufacturer’s protocol. Results are normalized to the initial amount of protein incorporated into each hydrogel.
2.3 Cell culture
hMVECs culture: human microvascular endothelial cells (Lonza) were cultured in EBM-2 endothelial basal medium supplemented with EGM2-MV bullet kit supplements (Lonza) in a humidified incubator at 37°C and 5% CO2. Cells received regular media replenishment every two days and were passaged using TrypLE Express (Thermo Fisher Scientific). Passages 2–7 were used in subsequent experiments.
Human neural progenitor cell culture
All stem cell procedures were approved by Stanford’s Stem Cell Research Oversight committee. As previously described [12], hNPC (passages 17–22) are derived from embryonic stem cells and then were cultured in neural maintenance media supplemented with 1X B27 and N2 along with LIF (10 μg/ml), EGF (20 μg/ml), bFGF (10 ng/ml, all Invitrogen, Waltham, MA except for EGF and LIF from Millipore, Darmstadt, Germany), and pooled human serum albumin (1%, Mediatech Inc, Pittsburgh, PA).
2.4 In vitro Measurement of VEGF-A activity assay on hMVECs
To measure the in vitro activity of released VEGF-A (Novoprotein), 30 μL of hydrogel or 1 μg of VEGF-A encapsulated in 30 μL of hydrogel samples were prepared and incubated in 1 mL EBM-2 endothelial basal medium supplemented with 2% fetal bovine serum (Gibco) in microcentrifuge tubes (n=4). 100 μL of the supernatant was collected on day 6, and replenished with equal volume of fresh EBM-2. hMVECs were seeded into a 48-well tissue culture plate in EGM2-MV and allowed to attach for 4 hours. Media in each well were then replaced with 500 μL EBM-2 basal medium supplemented with 2% fetal bovine serum and 10 μL of collected supernatant. CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) was used to quantify cell metabolic activity at days 1 and 4 according to the manufacturer’s protocol. hMVEC metabolic activity values were normalized to the value measured in the media control group.
2.5 In vitro MMP-9 activity test after release from hydrogel
To measure the in vitro activity of released MMP-9 (Biolegend), 1 μg of MMP-9 was encapsulated in 30 μL of hydrogel, allowed to gel at the bottom of microcentrifuge tubes, and incubated in 1 mL of PBS (Gibco) (n=3). 100 μL of the supernatant was collected on day 6 and replenished with equal volume of fresh PBS. A 100-fold dilution of collected samples was used to measure MMP-9 activity using the Fluorokine® E Human Active MMP-9 kit (R&D Systems) according to manufacturer’s protocol.
For proteolytic activity of released MMP-9, MMP-9 supernatants collected from hydrogel with MMP-9 or VEGF-A+MMP-9 at day 3 release were separated on a zymography gel (Thermo Fisher). Two MMP-9 standards with concentrations of 50 and 100 ng/μL were also included. Proteolytic activity of the released MMP-9 was qualitatively evaluated as light bands on the otherwise dark zymography gel, as reported previously [29].
2.6 Behavior analysis
Behavior testing was performed by individuals blinded to animal grouping. Animals were divided into groups based on pre-transplant behavior testing (n = 12 per group), such that all groups had a similar mean and standard deviation at this time point. Functional recovery was investigated using the vibrissae-forepaw test [30]. Animals were trained on 3 separate days prior to recording their baseline behavior. After baseline, the animals underwent distal middle cerebral artery (dMCA) occlusion and were tested 1 week after stroke prior to microinjection of hydrogels or stem cells, depending on the group. Animals without a significant deficit (significant deficit = vibrissae-forepaw score prior to implantation at <30% of baseline) were removed. Behavior testing was continued for 7 weeks post-stroke.
2.7 dMCA occlusion and microinjections of hydrogel and stem cells
All animal procedures were approved by Stanford University’s Administrative Panel on Laboratory Animal Care. T cell-deficient adult male nude rats (NIH-RNU, 230 ± 30 g) underwent permanent dMCA occlusion model with 30 min bilateral CCA occlusion under isoflurane anesthesia as previously described [12, 13]. Buprenorphine was administered subcutaneously for analgesia. Ampicillin was in cage water 1 day prior to surgery (1 mg/ml) and for 7 days after transplantation.
One week after stroke, animals were randomized by vibrissae-whisker paw score, and hydrogel delivering MMP-9, VEGF-A, or VEGFA+MMP9, hydrogel alone, and stem cell microinjection surgeries were performed by a blinded individual. hNPC cells were dissociated for transplantation. Three 1.0 μl of hydrogels or cell transplants (1 × 105 cells/μl) were injected into the ipsilesional cortex at 7 days post-dMCA occlusion. First injection for anterior–posterior (A–P), medial–lateral (M–L), and dorsal–ventral (D–V) was +1.6, −2.4, and −2.4, respectively; Second injection for A-P, M-L, and D-V was +0.7, −2.4, and −2.4, respectively; Third injection for A-P, M-L, and D-V was −0.3, −2.4, and −2.4, respectively.
2.8 Slice Immunostaining and Stroke Volume
After behavior analysis at 7 weeks post-stroke (6 weeks post-transplantation), rats were perfused and coronal slices (40 μm) and sectioned. Primary antibodies (anti-GFAP (1:500, Abcam), anti-CTGF (1:100, Abcam), and anti-β-dystroglycan (1:100, Abcam)) were incubated overnight at 4 °C as described previously [12]. Secondary antibodies were added and imaged using Keyence microscope (BZ-X700) with BZX analyzer.
For quantification of blood vessels labeled with anti-β-dystroglycan antibodies and GFAP staining as above at 6 weeks after microinjection, two representative peri-infarct areas (0.34 mm × 0.45 mm) were selected from each ipsilateral slice at 400-μm intervals from the genu of the corpus collosum to its splenium (resulting in generally 14–16 slices per animal). One of the areas evaluated is located in the peri-infarct area near the scaffold ~0.3 mm from the ventral surface, and the second is located more medially in the center of the peri-infarct area near the center of the stroke arc. These were selected at the same point in each slice by a blinded individual. Thresholds and exposure times were identical for all groups. ImageJ software was used by a blinded individual to calculate average vessel density.
For CTGF staining, four representative peri-infarct areas (2 proximal peri-infarct and 2 medial peri-infarct) as above were evaluated with 4 slices per animal and 4 animals evaluated for each condition as detailed above. Thresholds and exposure times were identical for all groups. Assessments were performed by a blinded individual.
Cresyl violet staining was used to assess stroke volume as previously described [12]. Serial slices 400 μm apart from the genu of the corpus callosum to the splenium were taken and analyzed using ImageJ software. Assessments were performed by a blinded individual.
2.9 Cell counting for transplanted hNPCs
Rats were perfused with 1 X PBS and 4% PFA at day 1, day 3 and day 7 after Cell transplantation. Brains were carefully removed, post-fixed overnight and equilibrated in 30% sucrose. Coronal sections were cut at 30 μm from the beginning of AP +1.2 through the AP −2.0. Serial sections were collected in 24 well plates filled with cryoprotectant. One out of six serial sections, 0.18mm apart starting at A-P +1.1, were taken per brain, as this encompassed the transplantation site. All the selected section was processed using standard immunohistochemistry procedures.
Primary antibodies were incubated overnight, 4°C with an antihuman antibody (anti HuNu, 1:1000; MAB1281, Millipore, Inc.). Secondary antibodies were incubated 2 h at room temperature with biotinylated secondary antibody (1:1000, Vector Laboratories), washed, incubated with an avidin-biotin-peroxidase complex (30 min, ABC, Vectastain Elite; Vector Laboratories). The 3,3′-diaminobenzidine (DAB) staining was used and the human stem cell was stained in brown color. Sections were then mounted in anterior-posterior order on a glass slide.
The optical fractionator stereological method was used to obtain estimates of the total number of HuNu-positive nuclei using Stereo Investigator software (MBF Bioscience, Williston, VT, USA). The Cortex and striatum was counted separately. In brief, every 6th section extending rostral and caudal from AP+1.2 to AP −2.0 (sections spaced 180 μm apart) was sampled so that measurements spanned a total of 3.2 mm (about 18 sections per animal). The transplanted HuNu cells was delineated at a 2.5× objective and generated counting grid of 75×75 um. An unbiased counting frame was placed on the first counting area and systemically moved through all counting areas until the entire delineated area was sampled. Actual counting was performed using a 63× oil objective.
Estimates of the total number of cells were obtained using the following formula:
where E is the estimate of the total number of stained cells in each animal, ΣN is the sum of n values in all the sections analyzed, and k indicates that every kth section was considered.
2.10 Statistics
All the data are presented as the mean ± S.E. of independent experiments. N values indicate the number of independent experiments conducted or the number of individual experiments. Group differences were considered statistically significant at p < 0.05 (* and **, p < 0.05 and 0.01, respectively). An analysis of variance (ANOVA) test was used for multicomponent comparisons and Tukey-HSD post-hoc test was used to confirm the statistical significances between groups. For the animal behavior study, a non-parametric method (Kruskal-Wallis H test) was used for multicomponent comparisons and Dunn’s multiple comparisons test was used to confirm the statistical significance between groups. Image analyses and cell counting were blinded and performed by independent investigators.
3. Results
3.1 Protein-Based Hydrogel Incorporates VEGF-A and MMP-9
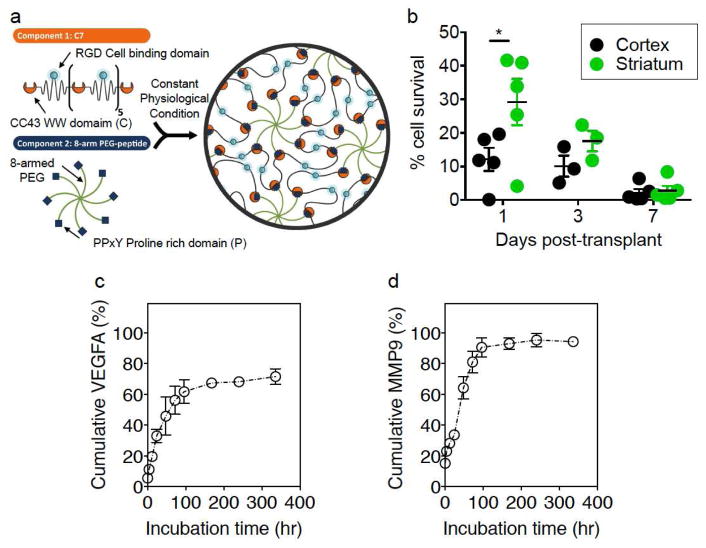
VEGF-A and MMP-9 are two important molecules secreted from stem cells to enhance stroke recovery [12, 13]. To mimic the controlled release of these factors from stem cells, a two-component composite, protein-based hydrogel was used to incorporate these proteins and provide gradual release of these factors into the post-stroke animals (Fig. 1a.).
Figure 1.
(a) Schematic illustration of hydrogel for injectable encapsulation of VEGF-A and MMP-9. (b) Percentage of hNPC survival after transplantation. Cell survival rate decreased after transplantation. After 7 days post-transplantation of hNPCs, most cells are not detectable. (c) and (d) Cumulative release profiles of proteins MMP-9 in (b) and VEGF-A in (c) over incubation time normalized to the total amount of protein encapsulated. Data shown as mean±SEM, n=3 per group.
The first hydrogel component is an engineered recombinant protein composed of 7 repeats of the CC43 WW domain, termed C7. The second hydrogel component is an 8-arm, 20 kDa polyethylene glycol (PEG) that has a single proline-rich peptide attached to the end of every arm. Simple mixing of the two components at constant physiological conditions forms a hydrogel via hydrogen-bond mediated, guest-host interaction between C7 and the PEG-peptide. Further rheological characterization demonstrated that the hydrogel does not change greatly when loaded with proteins (Suppl. Fig. 1). This hydrogel is both shear-thinning and self-healing, allowing hand injection of the hydrogel through a syringe-needle and rapid reformation of the gel upon ejection from the needle [14]. Previous work has demonstrated that encapsulation of peptides and growth factors within this hydrogel system can significantly reduce their diffusivity, resulting in a much longer, sustained release profiles of the encapsulated growth factors [14, 31]. This prior work also demonstrated that the primary mechanism of protein release was surface erosion. The biodegradable hydrogel incorporated with VEGF-A and MMP-9 was designed to be injected into the cortex similar to stem cell therapy to determine its efficacy on functional recovery after stroke.
We performed experiments to determine hNPC survival after implantation. We found that a majority of the cells did not survive 3 days after implantation (Fig. 1b). MMP-9 and VEGF-A release from the hydrogel was quantified using ELISA of the supernatant over time. While cumulative MMP-9 release over 2 weeks was nearly 100%, the cumulative amount of VEGF-A delivered was about 70% of the encapsulated dose, presumably due to inactivation of VEGF-A, which is known to have a short half-life (Fig. 1c, d). Both factors had similar sustained release profiles, with the majority of the delivery occurring within 100 hours (i.e. about 4 days). Absolute hourly release for VEGF-A for the first 100hrs was 9.1±0.6 ng/hr and for MMP-9 was 6.1±0.8 ng/hr. Moreover, quantification of the supernatant from polymer alone gives non-detectable levels of both VEGF-A and MMP-9 (Suppl. Fig. 2). Because decreased survival is observed in transplanted stem cells after 7 days, these controlled release profiles are likely similar to the timeframe stem cells are most actively releasing trophic factors [32]. While active mechanisms in vivo likely alter kinetics to a certain degree, release of proteins in a similar profile over the first several days of implantation is expected. This indicates that both the polymer and the hNPCs likely release a majority of the factors within the first few days after implantation.
3.2 VEGF-A and MMP-9 Remain Active After Release from Protein-Based Hydrogel
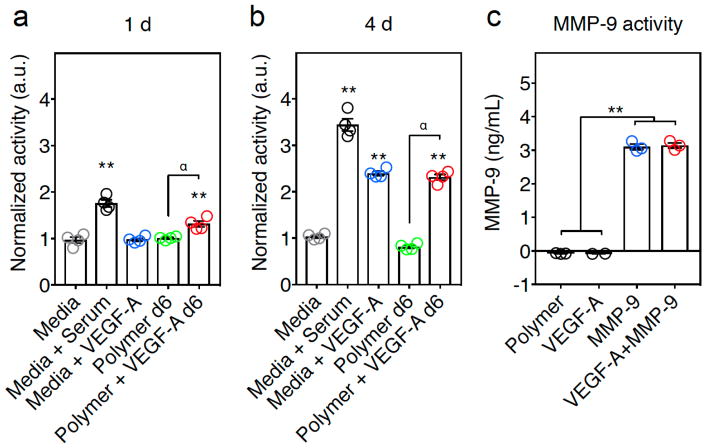
To insure VEGF-A and MMP-9 remain active throughout their encapsulation and delivery from the hydrogel, independent functional assays were performed. To determine functional VEGF-A release, the cell proliferation in response to VEGF-A in human dermal microvascular endothelial cells (hMVEC) was measured by evaluating metabolic activity. hMVEC cells were exposed for 1 day or 4 days to the 6-day supernatant of the hydrogels containing VEGF-A (Polymer + VEGF-A) or control hydrogels (Polymer). After 1 day incubation, hMVEC cells exposed to Polymer+VEGF-A supernatant showed a small, but significant increase in activity compared to the hMVEC exposed to control hydrogel supernatants (Fig 2a). After 4 days of incubation the VEGF-A hydrogel supernatant group showed similar activity to the controls containing either media+serum or media+VEGF-A, and significantly higher activity than the control hydrogel supernatants (Polymer) or media-alone (Media) (Fig. 2b, Suppl Fig. 3). The Media group was used as a negative control and Media+Serum and Media+VEGF-A were used as a positive control. This indicates the continued metabolic activity of the VEGF-A encapsulated in the gels over time.
Figure 2.
VEGF-A and MMP-9 released from polymer hydrogels are biologically active. (a, b) In vitro measurement of VEGF-A activity assay on hMVECs. (a) After 1 day of exposure, hMVECS exposed to Polymer+VEGF-A supernatant had more activity than hydrogel alone (Polymer) supernatant. (α, p ≤ 0.01). The 6-day Polymer+VEGF-A supernatant and control containing Media+Serum showed significantly higher than Media alone (**, p ≤ 0.01). (b) After exposure in culture for 4 days, the 6-day Polymer+VEGF-A supernatant showed similar outgrowth to the controls containing Media+Serum and Media+VEGF-A and significantly higher than Media alone (**, p ≤ 0.01) or the day 6 Polymer alone supernatant group (α, p ≤ 0.01) (ANOVA analysis showed at 1d and 4d F = 72.68 and 404.5, p ≤ 0.0001 and 0.0001, respectively. Post-hoc Tukey HSD test shows that Polymer+VEGF-A at 1d and 4d was statistically significant compared to the Polymer group (α, p ≤ 0.01), and ** indicates p ≤ 0.01 as compared to the Media control, error bars are SEM, n=4). (c) MMP-9 activity after release from the hydrogel. Polymer with MMP-9 (MMP-9) or VEGF-A+MMP-9 had significantly more activity than the controls of Polymer alone or polymer with VEGF-A (VEGF-A), (ANOVA analysis showed F = 810 and p ≤ 0.0001. Post-hoc Tukey HSD test showed statistical significance, ** indicates p ≤ 0.01, error bars are SEM, n=3).
In a similar fashion, continued MMP-9 activity in the hydrogels was assessed utilizing a fluorometric antibody based-assay designed to quantitatively measure the level of active MMP-9. The results confirmed that VEGF-A+MMP-9 gels and MMP-9-alone gels had significantly higher active MMP-9 concentrations compared to the polymer alone (Polymer) or the polymer containing VEGF-A (VEGF-A) (Fig. 2c). These results demonstrate the ability of MMP-9 to remain active after release from the gel.
3.3 Protein-Based Hydrogel Mimics hNPC-Mediated Improvement in Stroke Recovery
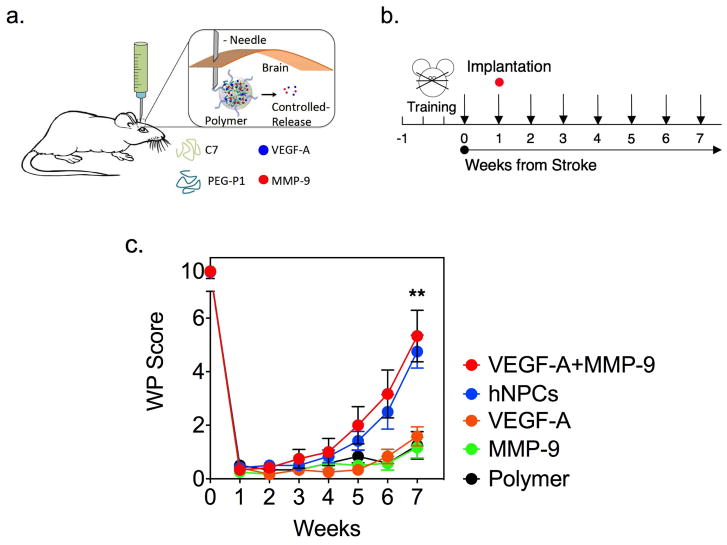
To determine if the hydrogel system delivering VEGF-A and MMP-9 could mimic stem cell-enhanced stroke recovery, a distal middle cerebral artery (dMCA) occlusion rodent stroke model was utilized. This model results in a cortical stroke with surrounding peri-infarct tissue. One week after animals had received a dMCA occlusion stroke, the polymer or hNPCs were transplanted into the brain using stereotactic technique. Concentrations that were used in the in vitro functional assays were used for in vivo implantation. Functional behavior assays were performed for 7 weeks following the stroke to monitor for recovery (Fig. 3a, b).
Figure 3.
Engineered stem cell mimics enhance stroke recovery. (a) Schematic representation of polymer injection. The polymer releases proteins in a controlled manner. (b) Experimental timeline with training three times in the week prior to dMCA occlusion. Arrows indicated behavioral testing. (c) Vibrissae-forepaw behavioral testing. VEGF-A+MMP-9 group and the hNPC group exhibited statistically significantly greater recovery at 7 weeks post-stroke compared to other groups (Kruskal-Wallis: H = 27.3, p ≤ 0.0001; and Dunn’s multiple comparisons test, ** indicates p ≤ 0.01, Data shown as mean±SEM, n=12). There was no statistical significance difference in recovery between hNPCs and hydrogel releasing VEGF-A+MMP-9, p=0.978.
Animals that received the VEGF-A+MMP-9 hydrogel had improved functional recovery compared to the polymer alone (Polymer), polymer delivering VEGF-A only (VEGF-A), and polymer delivering MMP-9 only (MMP-9) groups at 7 weeks after stroke (Fig. 3c). The animals that received the VEGF-A+MMP-9 hydrogel recovered similarly to the animals that received hNPCs (no statistical significance, p=0.978). Of note, the hydrogel system had similar functional recovery to that seen with hNPCs only when the two factors were combined, but not when individual factors were delivered.
3.4 CTGF Is Reduced in Enhanced Stroke Recovery
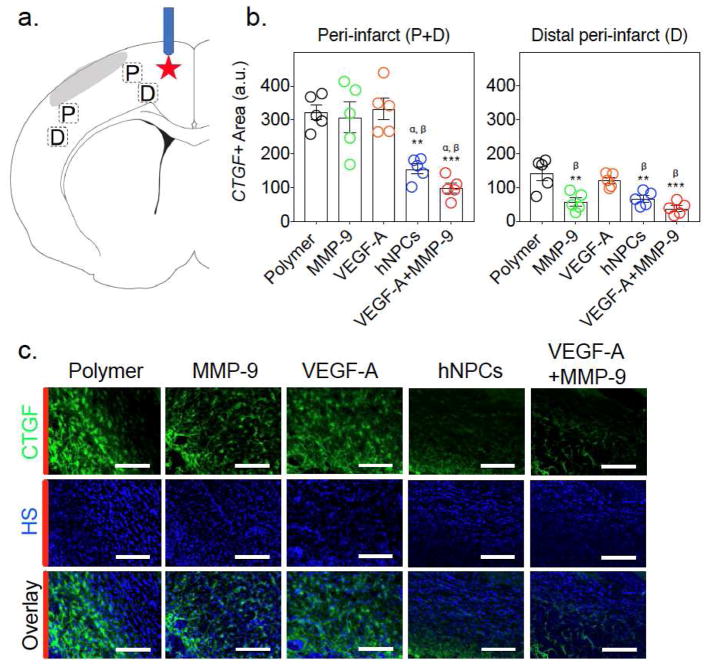
To evaluate why the combination of VEGF-A and MMP-9 was required for functional improvement after stroke (when either factor alone was not sufficient), we focused on connective tissue growth factor (CTGF). CTGF interacts with both VEGF-A and MMP-9 and plays an important role in tissue remodeling; however, its role in stroke recovery is unknown [33–37]. To investigate CTGF expression, brain slices were stained for CTGF, and the area of staining was evaluated in four peri-infarct areas (2 proximal peri-infarct (P) and 2 distal peri-infarct (D), 4 slices per animal) (Fig. 4a).
Figure 4.
Both stem cells and VEGF-A+MMP-9 hydrogels decrease CTGF expression after stroke. (a) Schematic with areas for quantitative analysis indicated by rectangles. P and D indicate proximal peri-infarct and distal peri-infarct, respectively. Red star indicates the injection site of cells or varying polymer groups. (b) Quantitative measurements of CTGF area in peri-infarct (P+M) and distal peri-infarct (D). ANOVA analysis showed F = 14.5, p < 0.0001. Tukey-HSD post-hoc analysis indicates that hNPCs and VEGF-A+MMP-9 are statistically significant as compared to the Polymer group (** and *** indicates p < 0.01 and 0.0001). Additionally, α and β indicate statistical significances compared to MMP-9 and VEGF-A alone groups, respectively (p < 0.01). Data shown as mean±SEM, n=5. (c) Immunofluorescent CTGF staining of brain slices in proximal peri-infarct (P) (Scale bar indicates 100 μm). HS indicates Hoechst nuclear staining. Red bars indicate where the lesion is located.
Animals that received the VEGF-A+MMP-9 polymer or hNPCs had significantly reduced CTGF expression compared to the animals in the other groups (Fig. 4b,c). Given MMP-9 cleaves CTGF, it was expected that CTGF would be reduced in the MMP-9 alone group. While the MMP-9 alone group did not show reduced CTGF overall, when the distal peri-infarct tissue was examined, decreased CTGF was observed compared to the polymer alone groups (Fig. 4b). Thus, the distal peri-infarct CTGF levels of animals receiving MMP-9 alone were similar to those seen by the combined VEGF-A+MMP-9 group and the hNPCs group. However, the proximal peri-infarct tissue did not show decreased CTGF in the MMP-9 alone groups compared to polymer alone (Suppl. Fig. 4). Representative CTGF images (green) of brain slices from the proximal peri-infarct region demonstrate substantial reduction of CTGF expression in the hNPCs and VEGF-A+MMP-9 groups (Fig. 4c). Secondary alone and sections without secondary served as controls and did not show any appreciable signal (Suppl Fig 5). It was also found that macroscopically brains were similar, and no significant polymer remained at the injection site at 7 weeks after stroke (Suppl Fig. 6).
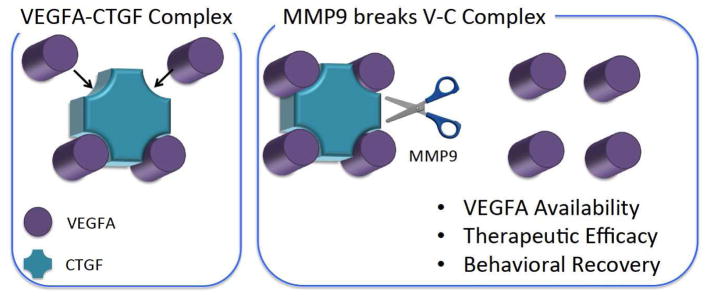
These findings suggest a possible mediator for improved recovery for the VEGF-A+MMP-9 hydrogels through the CTGF pathway (Fig. 5). CTGF is known to bind VEGF-A, thus limiting the functional bioactivity of VEGF-A [33]. MMP-9 is able to cleave CTGF and allows VEGF-A, which is resistant to degradation by the MMP-9, to become active again [20, 38–43]. As increased local VEGF-A activity from transplanted cells is known to correlate with beneficial effects post-stroke [12, 13], an increase in local VEGF-A activity achieved through an acellular, MMP-9/CTGF mediated mechanism is also expected to improve therapeutic outcome. Further studies are required to investigate causality of the CTGF pathway for stroke recovery.
Figure 5.
Proposed model for engineered stem cell mimics to enhance stroke recovery. VEGF-A forms a VEGF-A-CTGF complex, resulting in low efficacy of VEGF-A delivery at the infarct lesion. In the presence of MMP, it breaks the V-C complex by targeting the CTGF domain.
To evaluate alternative pathways, we analyzed astrocytes, blood vessel density in the peri-infarct region, and infarct size. At 7 weeks post-stroke, no change in blood vessel density, the amount of glia, or stroke size was observed (Suppl. Fig. 7 and 8). The inflammatory response was also evaluated by examining B and T cells at the stroke area (Suppl. Fig. 9). No difference was found in these inflammatory responses between groups.
4. Discussion
The delivery of important trophic factors, VEGF-A and MMP-9, via a two-component protein hydrogel enhances stroke recovery. The hydrogel-based delivery was able to mimic stem cell-based functional improvement in animals who had experienced a stroke. Both molecules are necessary to increase behavioral outcomes, as animals who received VEGF-A or MMP-9 only did not experience a recovery benefit. This reinforces the concept that hNPCs are releasing multiple trophic molecules over time, resulting in neural repair. While hNPCs likely effect numerous pathways in addition to VEGF-A and MMP-9, the hydrogel based system was able to improve recovery to a similar degree with delivery of these two proteins. The degradation of CTGF by MMP-9 to release VEGF-A is a candidate pathway to explain the increased recovery. Further experimentation to determine if CTGF has a causal role is necessary. The ability to identify important pathways from stem cell biology and act on those pathways with biomaterials is a powerful tool to develop therapies for neural repair after ischemia.
Once important factors are determined, such as VEGF-A and MMP-9, biomaterials allow for tunable, controlled-release of these molecules. A polymeric drug delivery system allows for more precise control of molecule release and bypasses many of the pitfalls of cell-based treatments. This reduces the biological variability seen with producing and maintaining a therapeutic cell line, which will be required for many patients over many years. In addition, the relatively simple and low-cost scale-up of polymer production relative to cell manufacturing would greatly increase the availability of the therapy, which is required given the frequency of stroke world-wide. Finally, the reduced risk of tumorgenicity or additional unwanted side effects of cell therapy also make a polymeric-based molecule delivery system more advantageous for sustained treatment of stroke victims.
Prior studies have shown that release of trophic factors, such as VEGF-A, can improve stroke outcomes [44, 45]. To our knowledge, this is the first study to compare a polymeric-based controlled release of critical repair molecules directly to stem cell therapy. Our finding that the sustained delivery of trophic factors has similar effects on functional improvement as hNPCs suggests that delivering essential factors could serve as an alternative to cell-based therapeutics. While prior studies have implicated trophic factor release as an important mechanism for stem cell therapeutics and our prior work indicated increased levels of VEGF-A and MMP-9 in stem cells, this study does not prove that the exact mechanism are the same between the hNPC- and polymeric-based therapies. These experiments do further strengthen the hypothesis that much of the augmentation seen from stem cell therapies is a result of the paracrine effects of the cells. Our results show that both VEGF-A and MMP-9 were required to increase functional recovery, which suggests that multiple molecular interactions may be needed to see effects similar to hNPCs. It is also interesting that although both the polymer and the hNPCs deliver most of the factors within the first few days after implantation, behavioral recovery is observed at later time points. This suggests that both therapies effect the host environment at earlier time points which alters the tissue response to allow for improved recovery.
Of note, the two-component hydrogel system was able to release an active metalloproteinase into a biological system in a controlled fashion with activity shown at 6 days in vitro and the ability to modify behavior illustrated in vivo. Given the potent proteolytic activity of these molecules, controlling their release profile is an important safety requirement. Our protein-polymer-based system provides a method to further optimize release characteristics to deliver these important molecules. The polymeric system allows for easier manipulation of the delivery of these molecules than a cell-based therapy. By changing the drug loading, hydrogel mesh size, and hydrogel erosion and degradation kinetics, more prolonged or higher concentrations of trophic factors could be delivered, which may have additional therapeutic benefits [31, 46]. As further insight is gained about the identity of important recovery factors, their therapeutic dosage, and their time-window of action; a polymeric system offers greater tunability than a cell-based treatment to deliver these essential therapies in an optimized fashion.
CTGF regulates extracellular matrix synthesis and proliferation and binds to the important angiogenic molecule VEGF-A [33, 36, 47, 48]. When VEGF-A is bound to CTGF, its angiogenic properties are inhibited, and VEGF-A’s angiogenic and other non-angiogenic contributions to stroke recovery are also likely prevented as well. MMP-9 is known to degrade CTGF and allow VEGF-A to become active again [40, 41]. Since VEGF-A is resistant to MMP-9 degradation, it is able to resume its biological functions after the complex is cleaved [20, 38, 39, 42, 43]. While vascular and inflammatory changes were not seen at 7 weeks after stroke in our model, these changes can be transient, and VEGF-A has been shown to have numerous other effects on stroke recovery including enhancing neural plasticity and neural repair [13, 49]. Our results show downregulation of CTGF correlates with improved behavioral recovery in the combined VEGF-A+MMP-9 polymer and hNPC groups but fail to prove a causative mechanism. Further investigation into the causative role of the CTGF pathways appears warranted and may provide a new avenue for stroke therapeutics. Although MMP-9 and VEGF-A each are known to have beneficial effects on neural repair, individually in our stroke model they failed to have an effect on functional recovery. This indicates the importance of providing multiple molecular interactions to improve stroke outcomes.
In summary, we have developed a two-component hydrogel system which provides controlled release of important molecules to enhance stroke recovery. The VEGF-A+MMP-9 hydrogel mimics stem cell effects on functional recovery after ischemia. Although a potential advantage of intracerebral stem cell transplant after stroke is the interaction of the cells with the stroke environment to signal optimal temporal release of various beneficial molecules, it may be possible to replicate this temporal pattern with a hydrogel system. Further studies to characterize the CTGF pathway are required to determine the importance of this molecule in neural repair and to further investigate the mechanism of action. Additionally, critical factors for recovery can be determined from additional studies of hNPC biology. By delivering these molecules, the hydrogel-based delivery system can provide a sustainable method to develop future, scalable stroke therapeutics and further investigate stroke recovery processes.
Supplementary Material
Highlights.
Multi-component hydrogels deliver stem cell trophic factors into post-stroke models
A polymeric system delivering VEGF-A and MMP-9 improved recovery after stroke
Hydrogel system enhanced functional recovery to a similar degree as stem cells
Connective Tissue Growth Factor (CTGF) pathway important for improved recovery
Acknowledgments
The authors would like to thank Cindy Samos for editing and Nicholas Suhar for assistance with artwork. The work was supported in part by the National Institutes of Health (NIH) grant K08-NS089976 to P.M.G; NIH grant R01-NS058784, California Institute of Regenerative Medicine (CIRM) grant RB5-07363, Bernard and Ronni Lacroute, and the William Randolph Hearst Foundation to G.K.S; the Stanford Neurosciences Institute and Stroke Collaborative Action Network to G.K.S and S.C.H; and NIH R21-EB020235, National Science Foundation DMR-1508006, and CIRM RT3-07948 to S.C.H.
Footnotes
Author Contributions: P.M.G contributed to conception and design of experiments, assembly, analysis and interpretation of data, performing the stroke surgeries and animal procedures, writing and final approval of the paper. B.O. contributed to conception and design of experiments, performing in vitro assays and characterization of polymer, performing immunofluorescent studies, analysis and interpretation of data, writing of manuscript. R.D. contributed to development and fabrication of the polymer, performing in vitro assays and characterization of the polymer, and writing of the manuscript. T.H. contributed to design of experiments, performing stroke surgeries and animal procedures and analysis. L.C. contributed to fabrication of the polymer, in vitro characterization of the polymer and design of experiments. A.L. contributed to processing and analysis of animal tissue. X.L. contributed to performing the cell survival surgery, B.A.K. contributed to performing the microrheological experiments. T.M.B. contributed to conception and design of the experiments, manuscript writing and final approval. S.C.H. contributed to conception and design of experiments, interpretation of data, manuscript preparation and final approval. G.K.S. contributed to conception and design of experiments, interpretation of data, writing and final approval of the paper.
Competing Interests: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Gooch CL, Pracht E, Borenstein AR. The Burden of Neurological Disease in the United States: A Summary Report and Call to Action. Annals of neurology. 2017 doi: 10.1002/ana.24897. [DOI] [PubMed] [Google Scholar]
- 3.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain: a journal of neurology. 2011;134(Pt 6):1777–89. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee S, Williamson DA, Habib N, Chataway J. The potential benefit of stem cell therapy after stroke: an update. Vascular health and risk management. 2012;8:569–80. doi: 10.2147/VHRM.S25745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George PM, Steinberg GK. Novel Stroke Therapeutics: Unraveling Stroke Pathophysiology and Its Impact on Clinical Treatments. Neuron. 2015;87(2):297–309. doi: 10.1016/j.neuron.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke; a journal of cerebral circulation. 2016;47(7):1817–24. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao J, Nan Z, Motooka Y, Low WC. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem cells and development. 2005;14(6):722–33. doi: 10.1089/scd.2005.14.722. [DOI] [PubMed] [Google Scholar]
- 8.Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet (London, England) 2016;388(10046):787–96. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 9.Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke; a journal of cerebral circulation. 2007;38(2 Suppl):817–26. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi S, Sakaguchi M, Kuroiwa T, Yamasaki M, Kanemura Y, Shizuko I, Shimazaki T, Onodera M, Okano H, Mizusawa H. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. Journal of neuroscience research. 2004;78(2):215–23. doi: 10.1002/jnr.20246. [DOI] [PubMed] [Google Scholar]
- 11.Toda H, Takahashi J, Iwakami N, Kimura T, Hoki S, Mozumi-Kitamura K, Ono S, Hashimoto N. Grafting neural stem cells improved the impaired spatial recognition in ischemic rats. Neuroscience letters. 2001;316(1):9–12. doi: 10.1016/s0304-3940(01)02331-x. [DOI] [PubMed] [Google Scholar]
- 12.George PM, Bliss TM, Hua T, Lee A, Oh B, Levinson A, Mehta S, Sun G, Steinberg GK. Electrical preconditioning of stem cells with a conductive polymer scaffold enhances stroke recovery. Biomaterials. 2017;142:31–40. doi: 10.1016/j.biomaterials.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem cells (Dayton, Ohio) 2011;29(2):274–85. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulyasasmita W, Cai L, Hori Y, Heilshorn SC. Avidity-controlled delivery of angiogenic peptides from injectable molecular-recognition hydrogels. Tissue engineering. Part A. 2014;20(15–16):2102–14. doi: 10.1089/ten.tea.2013.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Zechariah A, Qu Y, Hermann DM. Effects of vascular endothelial growth factor in ischemic stroke. Journal of neuroscience research. 2012;90(10):1873–82. doi: 10.1002/jnr.23088. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. The Journal of clinical investigation. 2000;106(7):829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22(4):379–92. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Harrigan MR, Ennis SR, Sullivan SE, Keep RF. Effects of intraventricular infusion of vascular endothelial growth factor on cerebral blood flow, edema, and infarct volume. Acta neurochirurgica. 2003;145(1):49–53. doi: 10.1007/s00701-002-1035-1. [DOI] [PubMed] [Google Scholar]
- 19.Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J Neurophysiol. 2007;98(1):334–44. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature cell biology. 2000;2(10):737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature medicine. 2006;12(4):441–5. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 22.Harrigan MR, Ennis SR, Masada T, Keep RF. Intraventricular infusion of vascular endothelial growth factor promotes cerebral angiogenesis with minimal brain edema. Neurosurgery. 2002;50(3):589–98. doi: 10.1097/00006123-200203000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regenerative medicine. 2010;5(1):121–43. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhasin A, Srivastava MVP, Mohanty S, Vivekanandhan S, Sharma S, Kumaran S, Bhatia R. Paracrine Mechanisms of Intravenous Bone Marrow-Derived Mononuclear Stem Cells in Chronic Ischemic Stroke. Cerebrovascular diseases extra. 2016;6(3):107–119. doi: 10.1159/000446404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong Po Foo CT, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. Two-component protein-engineered physical hydrogels for cell encapsulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22067–72. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai L, Dewi RE, Heilshorn SC. Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Advanced functional materials. 2015;25(9):1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krajina BA, Tropini C, Zhu A, DiGiacomo P, Sonnenburg JL, Heilshorn SC, Spakowitz AJ. Dynamic Light Scattering Microrheology Reveals Multiscale Viscoelasticity of Polymer Gels and Precious Biological Materials. ACS Cent Sci. 2017;3(12):1294–303. doi: 10.1021/acscentsci.7b00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason TG, Ganesan K, van Zanten J, Wirtz D, Kuo S. Particle Tracking Microrheology of Complex Fluids. Phys Rev Lett. 1997;79:3282. [Google Scholar]
- 29.Toth M, Fridman R. Assessment of Gelatinases (MMP-2 and MMP-9 by Gelatin Zymography. Methods in molecular medicine. 2001;57:163–74. doi: 10.1385/1-59259-136-1:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke; a journal of cerebral circulation. 2001;32(11):2682–8. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 31.Mulyasasmita W, Cai L, Dewi RE, Jha A, Ullmann SD, Luong RH, Huang NF, Heilshorn SC. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. Journal of controlled release: official journal of the Controlled Release Society. 2014;191:71–81. doi: 10.1016/j.jconrel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora-Lee S, Sirerol-Piquer MS, Gutierrez-Perez M, Gomez-Pinedo U, Roobrouck VD, Lopez T, Casado-Nieto M, Abizanda G, Rabena MT, Verfaille C, Prosper F, Garcia-Verdugo JM. Therapeutic effects of hMAPC and hMSC transplantation after stroke in mice. PloS one. 2012;7(8):e43683. doi: 10.1371/journal.pone.0043683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. The Journal of biological chemistry. 2002;277(39):36288–95. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 34.Leeuwis JW, Nguyen TQ, Theunissen MG, Peeters W, Goldschmeding R, Pasterkamp G, Vink A. Connective tissue growth factor is associated with a stable atherosclerotic plaque phenotype and is involved in plaque stabilization after stroke. Stroke; a journal of cerebral circulation. 2010;41(12):2979–81. doi: 10.1161/STROKEAHA.110.589036. [DOI] [PubMed] [Google Scholar]
- 35.Lin SC, Chou HC, Chiang BL, Chen CM. CTGF upregulation correlates with MMP-9 level in airway remodeling in a murine model of asthma. Archives of medical science: AMS. 2017;13(3):670–676. doi: 10.5114/aoms.2016.60371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai N, Nakamura M, Lipson KE, Miyake T, Kamikawa Y, Sagara A, Shinozaki Y, Kitajima S, Toyama T, Hara A, Iwata Y, Shimizu M, Furuichi K, Kaneko S, Tager AM, Wada T. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Scientific reports. 2017;7(1):5392. doi: 10.1038/s41598-017-05624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwab JM, Postler E, Nguyen TD, Mittelbronn M, Meyermann R, Schluesener HJ. Connective tissue growth factor is expressed by a subset of reactive astrocytes in human cerebral infarction. Neuropathology and applied neurobiology. 2000;26(5):434–40. doi: 10.1046/j.1365-2990.2000.00271.x. [DOI] [PubMed] [Google Scholar]
- 38.Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development (Cambridge, England) 2003;130(17):4123–33. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer cell. 2003;3(6):589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, Fontijn RD, David G, Hommes DW, Lamers CB, Sier CF. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. European journal of cancer (Oxford, England: 1990) 2008;44(13):1904–13. doi: 10.1016/j.ejca.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 41.Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Investigative ophthalmology & visual science. 2007;48(9):4360–7. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 42.Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26(51):7194–203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. The Journal of cell biology. 2005;169(4):681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bible E, Qutachi O, Chau DY, Alexander MR, Shakesheff KM, Modo M. Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles. Biomaterials. 2012;33(30):7435–46. doi: 10.1016/j.biomaterials.2012.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herz J, Reitmeir R, Hagen SI, Reinboth BS, Guo Z, Zechariah A, ElAli A, Doeppner TR, Bacigaluppi M, Pluchino S, Kilic U, Kilic E, Hermann DM. Intracerebroventricularly delivered VEGF promotes contralesional corticorubral plasticity after focal cerebral ischemia via mechanisms involving anti-inflammatory actions. Neurobiology of disease. 2012;45(3):1077–85. doi: 10.1016/j.nbd.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwood-Goodwin M, Teasley ES, Heilshorn SC. Dual-stage growth factor release within 3D protein-engineered hydrogel niches promotes adipogenesis. Biomaterials science. 2014;2(11):1627–1639. doi: 10.1039/C4BM00142G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Kato H, Nakata N, Kogure K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain research. 1992;586(1):121–4. doi: 10.1016/0006-8993(92)91380-w. [DOI] [PubMed] [Google Scholar]
- 48.Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PloS one. 2007;2(11):e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70(10):1753–61. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.