Abstract
Enediynes are a highly cytotoxic class of compounds. However, metallation of these compounds may modulate their activation, and thus their cytotoxicity. We previously demonstrated that cytotoxicity of 2 different metalloenediynes, including (Z)-N,N′-bis[1-pyridyl-2-yl-meth-(E)-ylidene]octa-4-ene-2,6-diyne-1,8-diamine] (PyED), is potentiated when the compounds are administered to HeLa cells during hyperthermia treatment at concentrations that are minimally or not cytotoxic at 37 °C. In this study, we further characterized the concentration-, time-and temperature-dependence of cytotoxicity of PyED on human U-1 melanoma cells. We also investigated the potential mechanisms by which PyED cytotoxicity is enhanced during hyperthermia treatment. Cell killing with PyED was dependent upon concentration, temperature during treatment, and time of exposure. Potentiation of cytotoxicity was observed when cells were treated with PyED at temperatures ≥39.5 °C, and enhancement of cell killing increased with temperature, and with increasing time at a given temperature. All cells exposed to PyED were shown to have DNA damage, but substantially more damage was observed in cells treated with PyED during heating. DNA repair was also inhibited in cells exposed to the drug during hyperthermia treatment. Thus, potentiation of PyED cytotoxicity by hyperthermia may be due to enhancement of drug-induced DNA lesions, and/or the inhibition of repair of sublethal DNA damage. While the selective thermal activation of PyED supports the potential clinical utility of metalloenediynes as cancer thermochemotherapeutic agents, therapeutic gain could be optimized by identifying compounds that produce minimal toxicity at 37 °C but which become activated and show enhancement of cytotoxicity within a tumor subjected to localized hyperthermic or thermal ablative treatment, or which might act as bifunctional agents. We thus also describe the development and initial characterization of a novel cofactor complex of PyED, platinated PyED (Pt-PyED). Pt-PyED binds to DNA like cisplatin, and much like PyED, cytotoxicity is greatly enhanced after treatment with the drug at elevated temperatures. However, in contrast to PyED, Pt-PyED is only minimally cytotoxic at 37 °C at concentrations at which cytotoxicity is enhanced by hyperthermia. Further development of cisplatin-based enediynes may result in compounds which, when activated, will possess multiple DNA binding modalities similar to cisplatin, but produce less side effects in tissues at normothermic temperatures.
Keywords: Metalloenediyne, melanoma, thermal activation, potentiation
Introduction
Since the discovery of the enediyne moiety in a group of natural anticancer antibiotics, natural and synthetic enediynes have elicited increased interest over the last two decades. The natural calicheamicin, esperamicin, and dynemicin A families all contain the unique (Z)-1,5-diyne-3-ene (“enediyne”) moiety, which, as observed by Bergman and co-workers, undergoes cyclization through the production of a 1,4-benzoid diradical intermediate (1, 2). The mechanism responsible for the observed biological activity of these compounds involves preferential binding to the minor grove in the DNA backbone (3, 4). Bergman cyclization of the enediyne moiety then produces a reactive intermediate that abstracts an H-atom from the ribose ring of the DNA backbone, leading to the formation of a double strand break (DSB)(5).
With the hope of increasing the therapeutic potential of enediynes, much effort has been made to control their cyclization. The geometry of the compound plays a critical role in compound activation by influencing steric strain on the enediyne-containing ring system and the distance between the terminal alkyne carbons. Distances < 3.1 Å resulted in unstable compounds, whereas distances > 3.4 Å required significant thermal energy for activation (5). To better control these variables, metal ions have been employed as ligands, affording specific geometries that can either activate or inhibit the natural stability of a given compound (6, 7).
Metalloenediynes appear to have increased therapeutic potential due to the degree of activation control afforded by their metal ligands. Metal center geometry, ligand flexibility, and the steric bulk of the groups adjacent to the alkyne termini profoundly affect the activation energy required for enediyne cyclization (8). With these factors in mind, a number of stable metalloenediynes have been produced which can be easily induced to undergo cyclization, thus triggering their cytotoxic effects (4).
Multiple strategies have been employed to preferentially induce enediyne cyclization; the two most common are photo- and thermal activation. Although both methods have shown promise, thermal activation may be preferable for clinical applications, due to the therapeutic effects of mild to moderate hyperthermia and the evolving areas of application of heat-induced chemosensitization, notably in tandem with thermal ablation. Even heat treatments that produce little or no cell killing have been shown to enhance cytotoxicity of certain chemotherapeutic agents or types of radiation that produce DNA damage (9); in some cases, this potentiation of cytotoxicity is believed to be mediated through the inhibition of a variety of DNA repair mechanisms or enhancement of initial DNA damage from increased drug influx (9–13). However, the mechanism by which heat potentiates enediyne cytotoxicity has not been definitively elucidated.
The compound PyED is a bis(pyridine)enediyne ligand which forms metalloenediyne complexes with divalent metals. Upon complexation with the Mg(II) ion present in cell culture medium, a geometric change occurs in the compound, allowing it to remain stable at ambient temperatures and to become activated upon exposure to elevated temperatures (8). During the initial characterization of the biological effects of this compound on HeLa cells, PyED cytotoxicity was shown to be potentiated by hyperthermia (42.5 °C for 1h) when cells were treated with concentrations of the compound that were minimally cytotoxic after treatment at 37 °C (14). Cells exposed to PyED during heating showed an enhancement in apoptotic death, and interestingly, a significant reduction in the G2/M block normally observed after heat treatment alone or drug treatment at 37 °C (14).
Greater utility of PyED and novel metal co-factor complexes of this enediyne as chemotherapeutic agents could potentially be realized by “tuning” of their toxicity through better-controlled diradical reactivity; this could theoretically allow these compounds to be administered systemically, but their cytotoxicity would be confined to regions of localized heating. We therefore conducted further studies to characterize the effects of PyED and a platinated form of the compound (Pt-PyED) at normothermic and hyperthermic temperatures. The time-temperature relationship for enhancement of cytotoxicity of these compounds was determined using a clonogenic cell survival assay, and comet and DNA binding assays were used to elucidate the potential mechanisms by which hyperthermia enhances metalloenediyne cytotoxicity. Human melanoma cells were selected for these studies, since melanomas are tumors that have been treated previously with hyperthermia alone with curative intent, with well-defined heat doses having been successfully delivered to the tumor volume (15).
Materials and Methods
Synthesis of (Z)-N,N′-bis[1-pyridyl-2-yl-meth-(E)-ylidene]octa-4-ene-2,6-diyne-1,8-diamine (PyED) and (Z)-N,N′-bis[1-pyridin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-diyne-1,8-diamineplatinum(II) hexafluorophosphate ([Pt(PyED)](PF6)2)
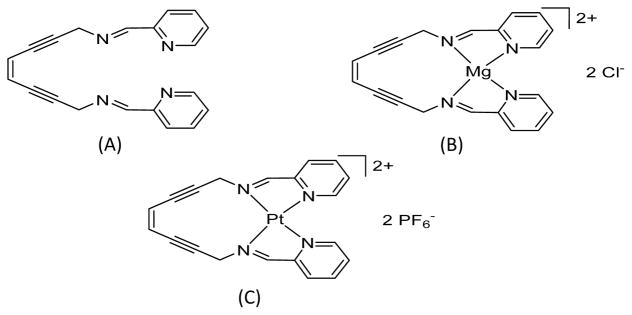
All reagents were purchased from commercial suppliers and used as received unless otherwise stated. PyED was synthesized as described previously by Rawat and Zaleski (8). 1H spectra were recorded on VXR 400 MHz and i400 MHz NMR spectrometers and the residual proton resonance of the solvent was used as an internal reference. Differential scanning calorimetry traces were recorded on a General V4.1C DuPont 910 differential scanning calorimeter coupled to a DuPont Thermal Analyst 2100 at a heating rate of 10 °C min−1. Infrared spectra were recorded on a Thermo Electron Corporation Nicolet 6700 FT-IR spectrometer. As noted previously (14), PyED may readily form a complex with Mg upon reacting with MgCl2 in equal molar ratios, and therefore, the presence of Mg2+ in the cell culture medium used for dilution of the compound and treatment of cells is expected to promote formation of metalloenediyne constructs in situ. However, formation of metallic complexes with other metal ions (e.g. trace metals such as Zn) introduced by supplementation of the cell culture medium with calf serum is also possible (unpublished data). The structure of PyED is shown in Figure 1(A and B) before and after complexation with Mg2+.
Figure 1.
Structures of (Z)-N,N′-bis[1-pyridin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-diyne-1,8-diamine (PyED) before and after complexation with Mg2+ (Mg2+-PyED), and (Z)-N,N′-bis[1-pyridin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-diyne-1,8-diamineplatinum(II) hexafluorophosphate (Pt-PyED). A. PyED; B. Mg2+-PyED; C. Pt-PyED. See Materials and Methods for syntheses of compounds. The metallation of PyED, a bis(pyridine)enediyne ligand, with Mg2+ occurs when it forms a Mg(II) complex upon dilution in cell culture medium (8,14).
To synthesize the Pt-PyED complex in situ, AgPF6 in methanol was added to a stirring suspension of cis-bis(acetonitrile)dichloroplatinum(II) in methanol. The reaction was stirred at 60 °C for 4 h in the absence of light, and the resulting AgCl precipitate removed by gravity filtration. The filtrate was incubated with PyED at 0 °C for 8 h while stirring. Solvent was removed in vacuo at the same temperature to yield a brown solid. The solid was washed with Et2O (3 × 20 ml) and hexanes (3 × 20 ml) to yield the final product as a shiny brown solid containing a Ag(PyED) impurity and used as prepared. Due to solution and thermal reactivity of the product, a 13C NMR spectrum and elemental analysis could not be obtained. The structure of this compound is shown in Figure 1C.
After synthesis and purification in the Zaleski laboratory, compounds were shipped to the Dynlacht laboratory on dry ice and stored at −20 °C until use.
Cell Culture and Drug Preparation
Human U-1 melanoma cells were cultured in McCoy’s 5A modified medium with L-glutamine (Mediatech, Manassas, VA) supplemented with 10% iron-supplemented bovine calf serum (Hyclone, Logan, UT) and maintained in a 37 °C incubator (5% CO2). Three days prior to each experiment, 2.5 × 105 cells were plated into 25 cm2 (T-25) flasks, containing 5 ml cell culture medium and allowed to grow to ~90% confluency (logarithmic growth). Aliquots of PyED or Pt-PyED were dissolved in DMSO the day of experimentation and kept at 4 °C until use. These aliquots were then used to prepare final concentrations of each compound in cell culture medium immediately before experiments.
Clonogenic Cell Survival Assays
To determine the effect of PyED or Pt-PyED on cell survival, stock solutions of each compound in DMSO were diluted directly into pre-warmed (37 °C) cell culture medium in T-25 flasks, to yield final drug concentrations of 0–50 μM PyED or up to 106 μM Pt-PyED. The total volume of drug-containing medium used to treat cells in each T25 flask was 5 ml, with DMSO representing 1.5% of the total volume. Cells exposed to drug or vehicle only (DMSO) were either maintained at 37 °C or subjected to hyperthermia treatment (38.5–42.5 °C) for 1 h by placing flasks in a precision-controlled water bath (± 0.1 °C). After treatment, the medium was aspirated, and cells were washed with 5 ml of fresh medium (37 °C). Cells were then trypsinized, resuspended in 37 °C medium, and counted using en electronic cell counter (Z2 Particle Count and Size Analyzer, Beckman Coulter, Brea, CA). Cells were diluted and plated into new flasks containing 5 ml medium in triplicate. Flasks were returned to the 37 °C incubator for 10–14 days to allow for colony formation. Colonies were fixed with 3:1 methanol:glacial acetic acid, stained with crystal violet, and counted if containing greater than 50 cells. Surviving fractions were calculated and normalized to the plating efficiency of flasks with DMSO only at the respective treatment temperature, or to the plating efficiency of flasks treated at 37 °C with their respective drug treatment. In some experiments, cells were treated with drug or DMSO alone for 0, 15, 30, or 60 min at a specific temperature. Surviving fractions were calculated and normalized to the plating efficiency of cells that were only washed with the treatment medium and then washed with fresh medium before trypsinization. All clonogenic survival experiments were repeated at least once and the standard error of the mean (SEM) was calculated for each normalized surviving fraction.
Quantification of DNA Damage
Cells treated with drug or vehicle only, at 37 °C or at elevated temperatures, were analyzed using the alkaline Comet Assay following manufacturer’s specifications as described in the Trevigen Comet Assay® Starter Kit (Trevigen, Gaithersburg, MD), but with some modifications. Briefly, one day prior to treatment, fresh 1X alkaline electrophoresis buffer (200mM NaOH, 1mM EDTA, pH >13) was prepared and stored at 4°C. Prior to analysis, lysis solution (2.5M NaCl, 100mM EDTA, 10mM Tris, 1% Triton X-100, pH 10) was chilled to 4 °C, LMAgarose aliquots were warmed to 37 °C, and 2-well comet slides were also warmed to 37 °C. Cells were exposed to 50 μM PyED or vehicle (1.5% DMSO) at either 37 °C or 42.5 °C for 30 min, and allowed to recover up to 2 h in 37 °C incubator. Cells were then trypsinized and re-suspended in cold McCoy’s 5A medium (containing 10% iron-suppelmented calf serum by volume). Cell concentration was then determined, and ~1.3 × 105 cells were transferred to microcentrifuge tubes. Cells were then pelleted (1,000 rpm, 5 min), washed in cold 1X PBS (without Ca2+ or Mg2+), and resuspended in fresh, cold 1X PBS. A 1:10 cell suspension:LMAgarose was then prepared and 75μl of this mixture spread evenly into a comet slide well.
Slides were dried for 1 h and then immersed in cold lysis solution for 1 h at 4 °C. The lysis solution was removed and replaced with freshly prepared alkaline unwinding solution (200mM NaOH, 1mM EDTA, pH >13) at room temperature for 1 h. Slides were then removed and electrophoresed for 30 min in cold alkaline electrophoresis buffer at 1V/cm and 300mA. Slides were rinsed three times with dH2O for 10 min per rinse, then once with 70% EtOH for 30 min, and three additional times with dH2O for 5 min before being allowed to dry at room temperature overnight.
Once dry, gels were stained with SYBR green diluted 1:10,000 in Tris-EDTA (TE) buffer (1 mM EDTA, 10 mM Tris, pH 7.5). Four or five high quality TIFF images were taken of different areas of each gel using a RTKE SPOT 7.3 3-Shot Color camera (Diagnostic Instruments Inc., Sterling Heights, MI) mounted on a DM4000 B microscope (Leica Microsystems Inc., Buffalo Grove, IL). Adobe Photoshop® was then used to convert all images to grayscale BMP files. Fifty cells from each treatment were analyzed using CometScore software (TriTek Corp., Sumerduck, VA). The percent DNA in the tail of each cell, and tail moment, were measured and compared. Analyzed cells were also grouped into 5 comet score classifications, 0, 1, 2, 3 and 4, based on the % DNA in the comet tail, and corresponding to 0–19%, 20–39%, 40–59%, 60–79%, and 80–100% DNA in the tail, respectively. Thus, a score of 0 represents little to no DNA damage and a score of 4 represents maximum damage. This assay was repeated at least once for each treatment condition, and the SEM calculated for each comet parameter.
DNA Intercalation Fluorescence Displacement Assay
The analysis of DNA binding of the Pt-PyED compound was performed as described previously (16,17). Briefly, a competitive DNA intercalation assay was performed using SYBR Green I (Sigma-Aldrich, St. Louis, MO) and sonicated salmon sperm DNA (8.3 ng/μl)(Fisher) in 25 mM MOPS (pH 6.5). Doxorubicin, a known noncovalent DNA binding chemotherapeutic, was used as a positive control. Varying concentrations of the indicated compounds were mixed with reaction components in a 96-well plate in a final volume of 110 μl. Fluorescence (excitation wavelength=485 nm, emission wavelength=528 nm), and a read height of 7 mm was measured using a BioTek Synergy H1 Hybrid Multi-mode Microplate Reader using BioTek Gen5 reader software. Reactions were incubated a maximum of 5 min before measurements were collected. Data represent the mean and range of duplicate determinations.
Results
Cytotoxic Effects of PyED Treatment and Enhancement of PyED Cytotoxicity by Hyperthermia
The presence of magnesium in cell culture medium promotes formation of the metalloenediyne construct of PyED as shown in Figure 1. When U-1 melanoma cells were treated for 1 h with PyED at 37 °C, cell killing was observed only after treatment with drug concentrations of 25–50 μM (Figure 2); treatment with 5 μM PyED was not cytotoxic, while 25 μM was somewhat cytotoxic. However, potentiation of PyED cytotoxicity was observed over the entire range of concentrations when cells were treated with drug at 42.5° C. Cell survival was reduced by ~30 to 50% when cells were treated with 5 or 25 μM, respectively, at 42.5 °C compared to 37 °C. The enhancement of drug-induced cell killing by hyperthermia was dose-dependent over the range of concentrations tested, with hyperthermia potentiating cell killing at 50 μM by nearly an order of magnitude. Thus, we chose this concentration for further investigation of the cytotoxic effects of the compound and the mechanisms by which heat enhances cytotoxicity.
Figure 2.
Cytotoxic effects of PyED and the enhancement of PyED cytotoxicity by hyperthermia. U-1 melanoma cells were treated with various concentrations of PyED or 1.5% DMSO (0 μM PyED) for 1h at either 37 or 42.5 °C. Surviving fractions were normalized to the samples treated with DMSO (vehicle) at each of the respective temperatures. The plating efficiencies for cells treated with DMSO only at 37 or 42.5° C were 0.74 or 0.16, respectively. Error bars represent the SEM for two to four experiments.
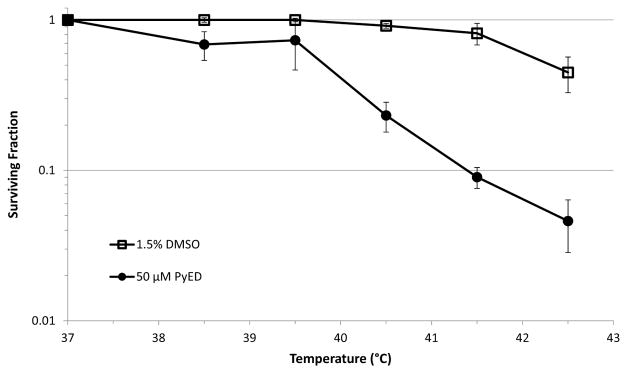
Time- and Temperature-dependence of Potentiation of PyED Cytotoxicity
The temperature-dependence of potentiation of PyED cytotoxicity was investigated by determining the surviving fraction of U-1 cells treated with 50μM PyED for 1 h at temperatures ranging from 37 to 42.5 °C (Figure 3). Hyperthermia treatments up to 41.5 °C with vehicle alone (1.5% DMSO) were, at most, only minimally cytotoxic. Heating cells at temperatures up to 39.5 °C during PyED treatment did not potentiate toxicity compared to treatment at 37° C. However, heating cells at temperatures of 40.5 °C and higher during PyED treatment significantly enhanced cell killing, with enhancement increasing with temperature. The greatest potentiation of PyED cytotoxicity occurred when cells were treated with the compound at 42.5° C.
Figure 3.
Temperature-dependence of potentiation of PyED cytotoxicity. U-1 cells were treated with either 50μM PyED or DMSO (vehicle) for 1 h at various temperatures. Surviving fractions were normalized to the DMSO or PyED treatments at 37 °C. The plating efficiencies for cells treated with DMSO or PyED at 37 °C were 0.30 or 0.07, respectively. Treatment at 38.5 °C without drug was performed only once due to a lack of significant difference in normalized surviving fraction in treatments below 40.5 °C. Error bars represent the SEM for two to seven experiments.
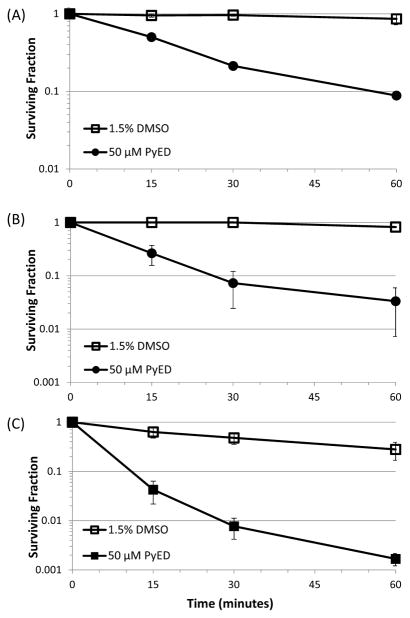
Since significant potentiation of PyED cytotoxicity was observed during 1 h treatments at 41.5 °C and 42.5 °C, these temperatures were used to determine how the enhancement of cytotoxicity changed as a function of drug treatment time. Cells were exposed to either 50 μM PyED or vehicle (1.5% DMSO) at each temperature for 0–60 min (Figure 4). PyED treatment for as little as 15 min resulted in cytotoxicity for both temperatures tested. Enhancement of cytotoxicity by 41.5 °C heating reached a plateau after 30 min; however, potentiation of cytotoxicity was observed to increase with duration of treatment with the drug at 42.5 °C. Due to the significant enhancement of cell killing after 30 min PyED treatments at 42.5 °C when compared to treatment at 37° C, this time and temperature combination was selected for investigating the potential mechanisms of enhancement of cell killing.
Figure 4.
Time-dependence of potentiation of cytotoxic effects of PyED with hyperthermia. U-1 cells were treated with either 50 μM PyED or DMSO (vehicle) for various times at 37 °C (A), 41.5 °C (B), and 42.5 °C (C). Surviving fractions were normalized to 0 min treatments where the cell monolayer was only washed with the treatment medium. Plating efficiencies were as follows: (A) 0.70 for cells treated with DMSO or 0.63 for cells treated with PyED at 37 °C, (B) 0.66 for cells treated with DMSO or 0.66 for cells treated with PyED at 41.5 °C, (C) 0.79 for cells treated with DMSO or 0.84 for cells treated with PyED at 42.5 °C. Error bars represent the SEM for 2 experiments.
Hyperthermia Increases and Inhibits Repair of Drug-induced DNA Damage
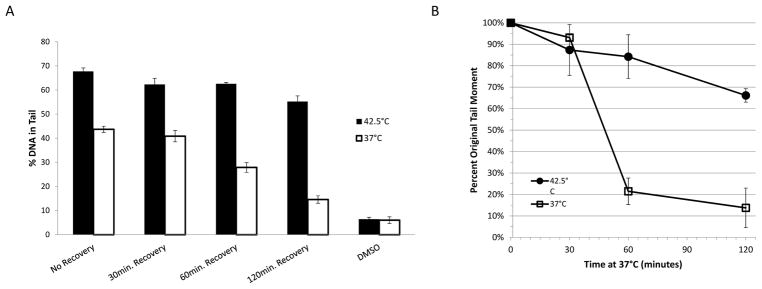
DNA damage in U-1 cells treated with PyED at normal physiological or elevated temperatures was determined using the alkaline Comet Assay (18). Cells were exposed to 50 μM PyED for 30 min at either 37 or 42.5 °C and were then prepared for analysis immediately after treatment or allowed to recover in fresh medium at 37 °C for various times prior to processing. The average percent DNA in the tail of 50 cells from each treatment was used as an indicator of DNA damage. The significantly higher amount of DNA in the tails of cells exposed to PyED during hyperthermia treatment compared to cells exposed to drug alone (at 37 °C) suggest that heat potentiates PyED-induced DNA damage (Figure 5A).
Figure 5.
Hyperthermia enhances DNA damage induced by PyED and inhibits DNA repair in U-1 melanoma cells. (A) Cells were treated with either 50μM PyED or DMSO (vehicle) alone for 30 min at 37 or 42.5 °C. Cells were then allowed to recover at 37° C for various times prior to preparing samples for analysis by alkaline comet assay. The average %DNA in the tail of 50 random cells from each treatment was determined. Error bars represent SEM (n=2 for recovery treatments; n=3 for all other treatments). Cells treated with DMSO alone were prepared immediately after treatment. (B) Cells were treated with 50 μM PyED for 1 h at 37° or 42.5 °C and then incubated up to 120 min at 37 °C prior to processing for analysis by alkaline Comet Assay. Average tail moments (ATM) of 50 cells/sample were calculated, and residual DNA damage determined by calculating percentage of initial damage remaining (% of ATM of cells treated & incubated for various times at 37 °C prior to processing, relative to initial ATM of cells treated/processed immediately). Error bars represent the SEM for 2–3 experiments.
Comet tail moment (the product of the tail length and percent DNA in the tail) is frequently used as a more conservative measure of DNA damage. Average tail moments for cells treated with PyED and allowed to recover at 37 °C were plotted in Figure 5B as percentages of the average tail moment without recovery as a function of time at 37 °C following treatment. Significant repair occurred in cells treated with drug at 37 °C, especially between 30–60 min after treatment. Cells treated with PyED at 42.5 °C exhibited a slower rate of repair; cells exposed to hyperthermia showed only a 34% decrease in tail moment after 2 h of incubation at 37 °C, compared to an 86% decrease without hyperthermia. Thus, in addition to potentiating PyED-induced DNA damage, the lack of recovery in tail moment to near-control values after treatment for cells exposed to PyED at 42.5 °C indicates that hyperthermia inhibits repair of PyED-induced DNA damage.
The distribution of DNA damage (from non-existent to severe compared to untreated controls, as indicated by percent DNA in the tail) across the populations of cells analyzed at various times after each treatment was determined by grouping the comet tails of treated cells into 5 comet score classifications, 0, 1, 2, 3 and 4, corresponding to 0–19%, 20–39%, 40–59%, 60–79%, and 80–100% of DNA in the tail, respectively. The number of cells in each category was tabulated for each treatment, and the data are shown in Figure 6. A greater number of cells that were heated during PyED treatment showed more extensive DNA damage at all times after treatment when compared to cells that were treated with PyED at 37 °C.
Figure 6.
Classification of DNA damage in cells treated with PyED for 30 min at 37 or 42.5 °C. The number of cells falling into each of 5 comet damage levels (see Materials and Methods) was tabulated for each treatment. DMSO treatment with and without hyperthermia are shown in (A) and (F), respectively. Initial damage after drug treatment at 37 or 42.5 °C is shown in (B) and (G), respectively. Cells receiving PyED treatments at 42.5 °C that were allowed to recover for 30, 60 or 120 min are shown in panels C, D, and E, respectively. Cells receiving PyED treatments at 37 °C and allowed to recover for 30, 60 or 120 min are shown in panels H, I, and J, respectively. Error bars represent SEM (n=2 for recovery treatments; n=3 for treatments without recovery).
Initial Development and Testing of a Platinated Enediyne (Pt-PyED)
Since the results from our current and prior studies suggested that metalloenediynes may be useful as anti-cancer agents, we have recently sought to generate cisplatin-based enediynes that could conceivably function in a dual-threat modality to damage DNA and inhibit DNA metabolism in tumor cells. Thus, our interest turned toward the synthesis of cofactor (divalent Pt) complexes of enediynes. Based on our experience with PyED, we synthesized the divalent Pt co-factor complex of PyED (Pt-PyED, Figure 1C). The diradical cyclization reactivity of the Pt-PyED complex is greater than that of the divalent Mg complex (J. Zaleski, unpublished observations).
Pt-PyED was only mildly cytotoxic after treatment for 1 h at 37 °C, and much less cytotoxic than PyED; even after treatment with 106 μM Pt-PyED, surviving fraction remained relatively high (0.646)(one experiment, data above 35 μM not shown). As observed with PyED, we noted a significant concentration-dependent potentiation of cytotoxicity when cells were treated with Pt-PyED during heating at 42.5 °C (Figure 7). It is worth noting that greater enhancement of Pt-PyED cytotoxicity might have been observed at higher drug concentrations. However, cells heated during treatment with Pt-PyED at concentrations greater than 35 μM detached from the surface of the tissue culture flask during the treatment; therefore, surviving fraction of cells that had been treated with higher concentrations of drug during heating could not readily be determined using our clonogenic cell survival assay (data not shown).
Figure 7.
Potentiation of Pt-PyED toxicity by hyperthermia. U-1 cells were treated with various concentrations of Pt-PyED or with 1.5% DMSO (vehicle) only for 1h at 37 °C or 42.5 °C. Surviving fraction was normalized to control samples exposed to DMSO only, for 1 h at each of the respective temperatures. Plating efficiencies for cells treated with DMSO at 37 or 42.5 °C were 0.42 or 0.21, respectively. Error bars represent the SEM for 2–3 experiments.
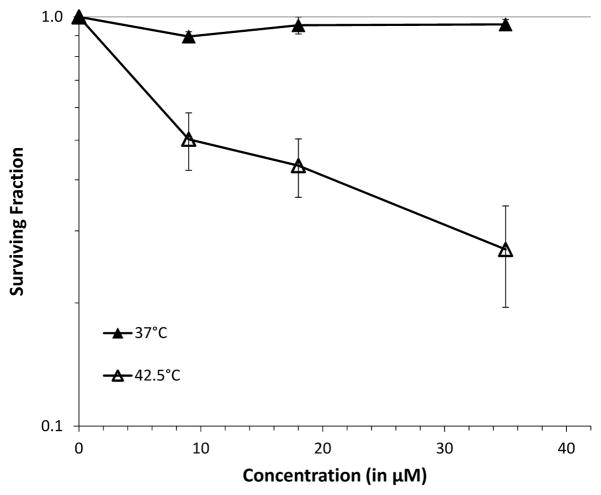
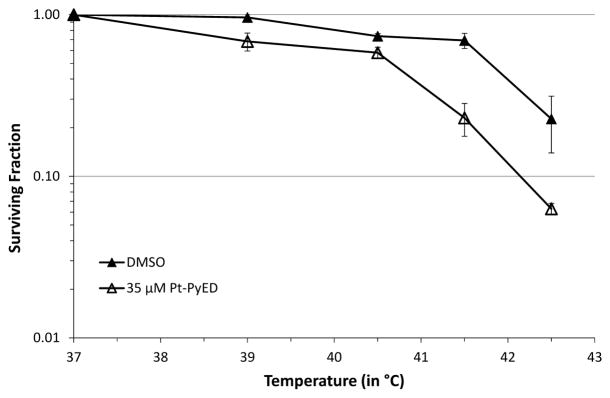
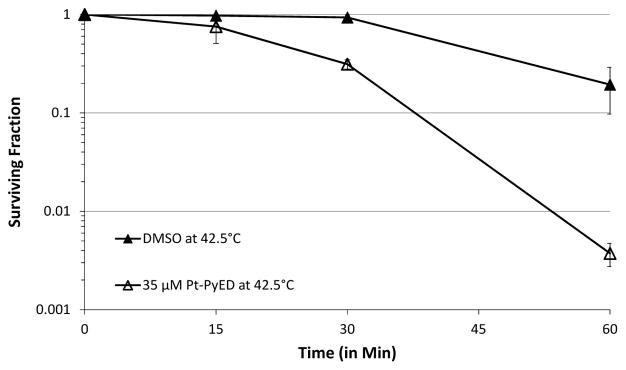
Also, similar to what was observed with PyED, the extent of enhancement of cytotoxicity was both temperature- and time-dependent. Some enhancement of cell killing was noted at temperatures as low as 39 °C, while maximum enhancement was observed when cells were treated at 42.5 °C (Figure 8). When cells were treated with drug from 0–60 min at 42.5 °C, enhancement of Pt-PyED cytotoxicity was noted with increased treatment time (Figure 9), although no potentiation was noted after only a 15 min treatment. There was nearly a 100-fold reduction in cell survival when cells were heated in the presence of drug compared to when they were heated with vehicle only.
Figure 8.
Temperature-dependence of potentiation of Pt-PyED cytotoxicity. U-1 cells were treated with either 35 μM Pt-PyED or DMSO (vehicle) for 1 h at various temperatures. Surviving fractions were normalized to the Pt-PyED or DMSO treatments at 37 °C. Plating efficiencies for cells treated for 1 h at 37 °C with either DMSO or Pt-PyED were 0.29 or 0.22, respectively. Error bars represent the SEM for 2–3 experiments.
Figure 9.
Time-dependence of potentiation of cytotoxic effects of Pt-PyED with hyperthermia. U-1 cells were treated with either 35 μM Pt-PyED or DMSO (vehicle) for various times at 42.5 °C. Surviving fractions were normalized to 0 min treatments where the cell monolayer was only washed with the treatment medium. Plating efficiencies were 0.36 or 0.33 for cells treated with DMSO or PyED at 42.5 °C. Error bars represent the SEM for 2 experiments.
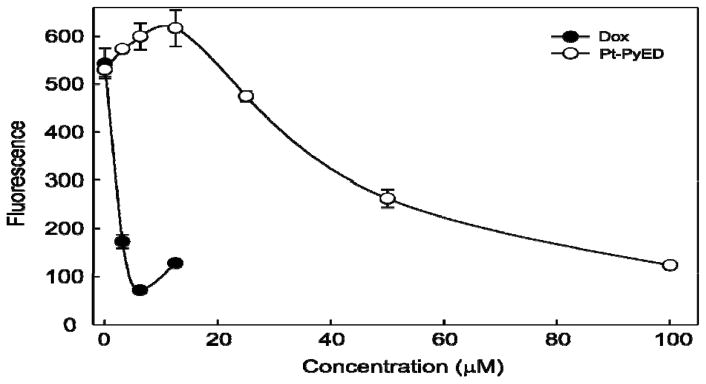
Finally, we wished to determine whether Pt-PyED, our 2nd-generation enediyne, possessed the appropriate functionality for intercalative or alkylative DNA binding modes like cisplatin. Using a conventional fluorescence displacement assay, we determined that Pt-PyED does bind to DNA (Figure 10). The positive control compound, doxorubicin, shows potent displacement of the fluorescent dye from DNA. The small increase in fluorescence observed at the highest concentration tested (15 μM) is attributed to doxorubicin autofluorescence. Pt-PyED displayed a concentration-dependent decrease in fluorescence, indicating a moderate affinity for DNA consistent with damage observed using the comet assay.
Figure 10.
Pt-PyED binds to DNA in vitro. Salmon sperm DNA stained with the dye SYBR Green was incubated in the presence of either Doxorubicin (Dox)(control) or Pt-PyED. The loss of SYBR Green fluorescence is a function of binding (intercalation) of the test compounds to DNA (e.g., displacement of the dye from DNA). Error bars represent the SEM for two experiments.
Discussion
In this study, we have demonstrated that the cytotoxic effects of the enediyne PyEd (which becomes metallated in cell culture medium) and the divalent Pt co-factor complex of PyED, Pt-PyED, may be significantly potentiated when the compounds are administered to U-1 melanoma cells at hyperthermic temperatures. Both PyED (at concentrations less than 50 μM) and Pt-PyED were only somewhat moderately or minimally cytotoxic, respectively, to cells exposed to micromolar concentrations at 37 °C (Figures 2, 7). However, for both compounds, enhancement of cytotoxicity by heat was dependent upon drug concentration, temperature at which the drug was administered, and time of exposure to each drug (Figs. 2–4 and Figs. 7–9). Data shown in Figures 3 and 8 suggest that the compounds likely do not sensitize cells to the hyperthermic treatment; rather, it appears that the hyperthermic treatment is what enhances cytotoxicity of the compounds. If the former scenario were true, it is likely that we would have observed greater enhancement of cell killing at all temperatures above 37 °C. No potentiation of cytotoxicity was observed when cells were heated up to 39.5° C during PyED treatment, while only slight potentiation was observed when cells were treated with Pt-PyED in the 39° to 40.5 °C temperature range (Figure 3 and 7). Higher temperatures (41.5 and 42.5 °C) were required for significant potentiation of PyED and Pt-PyED cytotoxicity, and in the case of PyED, brief treatments (15 min) with the drug at elevated temperatures resulted in greater cell killing than heat or drug treatment alone (Figure 4). Longer treatments at 41.5 or 42.5 °C resulted in greater enhancement of cytotoxicity for both compounds (Figures 4, 9).
We sought to determine the mechanism by which hyperthermia potentiates metalloenediyne toxicity. In a recent study, Zaleski and co-workers (19) tested the ability of PyED or PyED-metalloenediyne complexes containing Cu(II), Fe (II), and Zn (II) to damage supercoiled DNA. They found that efficient nicking of the DNA occurred in the presence of all three divalent metalloenediynes ions, but reported only minimal degradation when DNA was exposed to free PyED; thus, they concluded that the extent of the chelation-induced radical generation event is strongly metal-dependent. However, interestingly, they found that the activity displayed by the divalent reaction at 37 °C is similar to that demonstrated at hyperthermic temperatures (42 °C).
In contrast to the aforementioned experiments with supercoiled DNA, the quantification of DNA damage (using the comet assay) in U-1 cells treated with PyED for 30 min with and without hyperthermia (42.5 °C) resulted in our observation of significant differences not only in the amount of initial DNA damage induced (cells treated with PyED during heating showed more initial damage than cells treated at 37 C), but also in the kinetics of DNA repair. U-1 cells treated with drug at 37° C sustained significant DNA damage, but this damage was nearly completely repaired within 2 h of recovery at 37 °C. The initial damage observed in non-heated cells could be due to the activation of some of the PyED compound after complexation with magnesium, or some other biological activity not yet understood (Figures 5 & 6). Nevertheless, the significant repair (see below) coupled with higher clonogenic survival in non-heated cells might suggest that the DNA damage incurred was less detrimental to cell survival after treatment at 37 °C. We previously observed that PyED treatment at 37 °C in HeLa cells induces a G2/M cell cycle arrest (14). Such an arrest in U-1 cells could explain the ability of these cells to repair significant damage before progressing to mitosis. It is worth noting that drug treatment at 37 °C resulted in cells with nearly a full spectrum of levels of DNA damage; some cells were minimally damaged while others sustained extensive damage (Figure 6). However, 2 h after treatment, nearly all non-heated cells produced comets indicative of complete repair of DNA damage, similar to the extent observed in cells treated with DMSO alone.
In contrast, U-1 cells treated with PyED at 42.5 °C were shown to have significantly more DNA damage immediately after treatment compared to those treated with drug alone, hyperthermia alone, or the additive effect of the two treatments (Figure 6). These results suggest that thermal activation may potentiate PyED toxicity, at least in part, but synergistically, through compound activation. Previous studies showed that when DNA is exposed to Cu(II), Fe(II), and Zn(II) complexes of PyED in the presence of DNA, considerable DNA degradation occurs within 1 h under hyperthermic conditions, with Cu(II) activation producing >50% degradation of DNA within 5 min. The importance of thermally-activated radical production is evidenced by the lack of compromised DNA observed using the cyclized non-radical-generating control complex PyBD, pyridine-2-ylmethyl-(2-{[(pyridine-2-ylmethylene)-amino]-methyl}-benzyl)-amine or free (unmetallated) PyED (19) Additionally, in situ activation of PyED via chelation of Cu(II) and Zn(II) has been shown to result in radical-induced disaggregation of Aβ fibrils at 37 °C relative to the cyclized control PyBD complex. The extent of radical damage is elevated upon treatment under hyperthermic conditions, consistent with the increased radical generation after exposure to elevated temperatures (20). These experiments established not only the activation of these compounds under physiologically relevant conditions, but also the significance that the potent 1,4-diradical species plays in the mechanism of biopolymer degradation.
As noted above, hyperthermia during drug treatment was also shown to inhibit DNA repair; incubation of cells at 37 °C for up to 2 h (to allow for recovery) did not result in a significant decrease in DNA damage similar to what was observed with non-heated cells (Fig. 5B). In cells treated at 37 °C and then incubated for 120 min at 37 °C in drug-free medium, the amount of DNA in the comet tail had nearly returned to that of cells treated with vehicle (DMSO) alone (Figure 5A). Since we previously showed that PyED treatment at 42.5 °C in HeLa cells reduced G2/M cell cycle arrest (14), it is attractive to speculate that progression of U-1 cells to mitosis with DNA damage results in the potentiation of cytotoxicity observed for heated drug-treated cells. Unlike the distribution of DNA damage in non-heated cells, drug treatment with heat yielded cells with greater levels of DNA damage. Even after 2 h at 37 °C, no “level 0” comets were observed, whereas treatment with heat alone yielded only cells with “level 0 or 1” comets.
While hyperthermia likely increases the cytotoxicity of both PyED and Pt-PyED by increasing cyclization and activation of the compounds and subsequently an increase in DNA damage, we propose that potentiation of metalloenediyne cytotoxicity may also be mediated through the inhibition of DNA repair by the heat treatment. There is a precedent for proposing such a mechanism: Hyperthermia potentiates radiation-induced cell killing (21), and this synergistic effect is attributed to inhibition of repair of radiation-induced DNA damage, particularly DSBs (22–26). Inhibition of repair of radiation-induced DNA strand breaks may in turn be due in part to inactivation of critical DNA repair proteins by heat, or masking of damaged DNA sites by aggregated denatured proteins (27, 28, and references therein). It is conceivable that repair of DNA strand breaks induced by chemicals such as the metalloenediynes may be inhibited similarly by hyperthermia. However, it is possible that hyperthermia may have also increased PyED cytotoxicity through other (or multiple) distinct mechanisms. For example, heating during drug treatment might result in different species of DNA damage (e.g., DSBs), may have inhibited specific repair pathways, or perhaps influenced drug influx and efflux into and out of the cell. Some thermochemotherapeutic effects are indeed known to be the result of increased drug influx or decreased efflux, which allows a greater intracellular concentration of a specific drug to induce more DNA damage (12,13).
We have shown that short exposures (as brief as 15 min) to temperatures less than 42.5 °C (and as low as 40.5 °C) result in enhancement of metalloenediyne cytotoxicity. Although much progress has been made in clinical techniques for administering site-specific hyperthermia treatments, lower temperatures and shorter treatment times are still ideal. Longer treatment times increase the possibility of changes in blood flow, altering the delivery of some drugs to the target tissue (15,29). Also, in general, maintaining a target tissue at a specific temperature is more clinically feasible with shorter treatment times and lower temperatures. Thus, understanding the temperature- and time-dependence of metalloenediyne cytotoxicity is critical for providing guidance regarding the minimum temperature and heating times required to significantly potentiate metalloenediyne-induced toxicity. Furthermore, such information may be useful in guiding the development of compounds that may have clinical utility when administered with thermal therapy. Indeed, evolving areas of interest for the utilization of hyperthermia in the clinic include an application of the principles of chemosensitization to thermal ablation. Thermal ablation of tumors involves the local application of extreme temperatures (usually 50 °C or higher). The cells of the tumor are rapidly killed in and around a central zone of coagulation, with normal tissues being largely spared, especially if they lie outside the temperature gradient of the ablation zone. However, typically, there are tumor cells at the periphery of the tumor, within a portion of the temperature gradient outside of the coagulation zone, which may not be heated to the temperature required to elicit immediate cell death; that is, many tumor cells could receive only a sub-lethal heat dose. Any cells that receive a sub-lethal heat dose could ultimately result in the re-growth of the tumor. Potentially, our metalloenediyne compounds could be administered systemically; since they are only minimally to somewhat moderately cytotoxic at normal physiological temperature they would not be expected to produce the side effects normally observed after a typical treatment with chemotherapeutic agents. However, after uptake by tumor cells at the tumor periphery, the compounds could be activated by hyperthermic temperatures that are normally sub-lethal outside the coagulation zone, but still sufficient to sensitize the cells to the drug. Thus, we propose that an evolving and exciting potential application of metalloenediyne therapy may involve the thermal enhancement of cytotoxic effects of the compounds by targeted heat delivery during thermal ablation. If enediyne activation can be sufficiently controlled with little to no detrimental effects to normal tissue, the cytotoxic effects of the compound could be targeted to and activated within tumor tissue to produce selective cytotoxic effects. Such an approach could be developed for treatment of a variety of tumor types (29, 30).
Some of the major limitations to the clinical utility of conventional (non-metallated) enediyne compounds have been their proclivity toward activation and resulting toxicity at physiological temperatures (3). Metallation of enediynes has addressed some of these limitations, as the metal ligand serves to both stabilize the inactive compound and lower the energy required for activation. Although achieving and maintaining a specific tumor volume at an elevated temperature homogeneously for extended periods of time had been quite difficult when moderate hyperthermia treatments were administered alone, technological developments have made localized hyperthermia more clinically feasible, and with simultaneous administration of metalloenediynes, ablative temperatures would not be required to effect local tumor control; even low or moderate-temperature hyperthermia (up to 42.5 °C) that has been used to elicit heat-radiationsensitization (15,31) could be used to achieve significant cell killing.
The results obtained in this study with PyED and Pt-PyED indicate that in vivo testing and further development and investigation of thermally-activated metalloenediyne compounds are warranted. Although PyED exhibits some toxicity at normal body temperature at higher concentrations, the localized activation of each of these novel compounds has the potential to enhance therapeutic gain, since thermal therapy (ablative or moderate, longer duration hyperthermia), administered locally to a defined volume of tumor-containing tissue, could selectively activate compounds and increase cell killing within the tumor without affecting systemic toxicity. The data from our current study demonstrate that manipulation of structure influences activation temperatures of enediyne motifs, and that metal ions are excellent cofactors for activation. However, future efforts will be focused on the development of additional ligand derivatives with improved solubility, and enhanced and better-controlled diradical reactivity, for which activity, toxicity profile, and efficacy can be efficiently tuned, and which display a proclivity for activation after exposure to slightly supra-physiological temperatures. The development of compounds such as Pt-PyED, which bind to DNA (Figure 10) and might potentially function like cisplatin as a dual-threat modality to induce damage DNA and inhibit DNA metabolism in tumor cells, could be of significant potential clinical relevance.
Acknowledgments
This study was supported by grants from the National Cancer Institute (National Institutes of Health) (CA196293), the Experimental and Developmental Therapeutics Program of the Indiana University Melvin and Bren Simon Cancer Center, and the Indiana Lions Cancer Control Fund. EJK was supported by the Undergraduate Research Opportunities Program of the Center of Research and Learning at Indiana University-Purdue University Indianapolis. We would also like to thank Robin DeWalt for her assistance. This paper is dedicated to the memory of Dr. Kurt Hofer, an excellent scientist and a gifted teacher that touched the lives of thousands (including JRD) in both the laboratory and lecture hall.
References
- 1.Bergman RG. Reactive 1,4-dehydroaromatics. Accounts Chem Res. 1973;6(1):25–31. [Google Scholar]
- 2.Jones RR, Bergman RG. p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure. J Amer Chem Soc. 1972;94(2):660–1. [Google Scholar]
- 3.Nicolaou KC, Smith AL, Yue EW. Chemistry and biology of natural and designed enediynes. Proc Nat Acad Sci USA. 1993;90(13):5881–8. doi: 10.1073/pnas.90.13.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi MC, Rawat DS. Recent developments in enediyne chemistry. Chem & biodiversity. 2012;9(3):459–98. doi: 10.1002/cbdv.201100047. [DOI] [PubMed] [Google Scholar]
- 5.Pedro J, Benites DSR, Zaleski Jeffrey M. Metalloenediynes: Ligand field control of thermal Bergman cyclization reactions. J Amer Chem Soc. 2000;122(30):7208–17. [Google Scholar]
- 6.Chandra T, Allred RA, Kraft BJ, Berreau LM, Zaleski JM. Syntheses and thermal reactivities of tetradentate metalloenediynes of Cu(II) and Zn(II) Inorganic Chem. 2004;43(2):411–20. doi: 10.1021/ic030218x. [DOI] [PubMed] [Google Scholar]
- 7.Rawat DS, Benites PJ, Incarvito CD, Rheingold AL, Zaleski JM. The contribution of ligand flexibility to metal center geometry modulated thermal cyclization of conjugated pyridine and quinoline metalloenediynes of copper(I) and copper(II) Inorganic Chem. 2001;40(8):1846–57. doi: 10.1021/ic010014l. [DOI] [PubMed] [Google Scholar]
- 8.Rawat DS, Zaleski JM. Mg2+-induced thermal enediyne cyclization at ambient temperature. J Amer Chem Soc. 2001;123(39):9675–6. doi: 10.1021/ja011215r. [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. Semin Oncol. 2014 Dec;41(6):714–29. doi: 10.1053/j.seminoncol.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Bodell WJ, Cleaver JE, Roti Roti JL. Inhibition by hyperthermia of repair synthesis and chromatin reassembly of ultraviolet-induced damage to DNA. Radiat Res. 1984;100(1):87–95. [PubMed] [Google Scholar]
- 11.Nevaldine B, Longo JA, Hahn PJ. Hyperthermia inhibits the repair of DNA double-strand breaks induced by ionizing radiation as determined by pulsed-field gel electrophoresis. Int J Hyperthermia. 1994;10(3):381–8. doi: 10.3109/02656739409010282. [DOI] [PubMed] [Google Scholar]
- 12.Bull JM. An update on the anticancer effects of a combination of chemotherapy and hyperthermia. Cancer Res. 1984;44(10 Suppl):4853s–6s. [PubMed] [Google Scholar]
- 13.Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61(7):3027–32. [PubMed] [Google Scholar]
- 14.Routt SM, Zhu J, Zaleski JM, Dynlacht JR. Potentiation of metalloenediyne cytotoxicity by hyperthermia. Int J Hyperthermia. 2011;27(5):435–44. doi: 10.3109/02656736.2011.578607. [DOI] [PubMed] [Google Scholar]
- 15.Giaccia AJ, Hall EJ. Radiobiology for the Radiologist. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 16.Gavande NS, VanderVere-Carozza P, Mishra AK, Vernon TL, Pawelczak KS, Turchi JJ. Design and Structure-Guided Development of Novel Inhibitors of the Xeroderma Pigmentosum Group A (XPA) Protein-DNA Interaction. J Med Chem. 2017;60(19):8055–8070. doi: 10.1021/acs.jmedchem.7b00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra AK, Dormi SS, Turchi AM, Woods DS, Turchi JJ. Chemical inhibitor targeting the replication protein A-DNA interaction increases the efficacy of Pt-based chemotherapy in lung and ovarian cancer. Biochem Pharmacol. 2015;93(1):25–33. doi: 10.1016/j.bcp.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Molec Biotech. 2004;26(3):249–61. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 19.Porter MR, Lindahl SE, Lietzke A, Metzger EM, Wang Q, Henck E, Chen C-H, Niu H, Zaleski JM. Metal-mediated diradical tuning for DNA replication arrest via template strand scission. Proc Natl Acad Sci USA. 2017 Sep 5;114(36) doi: 10.1073/pnas.1621349114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter MR, Kochi A, Karty JA, Lim MH, Zaleski JM. Chelation-induced direadical formation as an approach to modulation of the amyloid-b aggregation pathway. Chem Sci. 2015;6(2):1018–26. doi: 10.1039/c4sc01979b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Hur E, Elkind MM, Bronk BV. Thermally enhanced radioresponse of cultured Chinese hamster cells: inhibition of repair of sublethal damage and enhancement of lethal damage. Radiat Res. 1974;58:38–51. [PubMed] [Google Scholar]
- 22.Corry PM, Robinson S, Getz S. Hyperthermic effects on DNA repair mechanisms. Radiology. 1977;123:475–82. doi: 10.1148/123.2.475. [DOI] [PubMed] [Google Scholar]
- 23.Mills MD, Meyn RE. Effects of hypethermia on DNA repair mechanisms. Radiat Res. 1981;87:314–28. [PubMed] [Google Scholar]
- 24.Radford IR. Effects of hyperthermia on the repair of X-ray induced DNA double strand breaks in mouse L cells. Int J Radiat Biol. 1983;5:551–7. doi: 10.1080/09553008314550641. [DOI] [PubMed] [Google Scholar]
- 25.Dikomey E, Franzke J. Effect of heat on induction and repair of DNA strand breaks in X-irradiated CHO cells. Int J Radiat Biol. 1992;61:221–33. doi: 10.1080/09553009214550851. [DOI] [PubMed] [Google Scholar]
- 26.Wong RSL, Dynlacht J, Cedervall B, Dewey WC. Analysis by pulsed field gel electrophoresis of DNA double-strand breaks induced by heat and/or X-irradiation in bulk and replicating DNA of CHO cells. Int J Radiat Biol. 1995;68:141–52. doi: 10.1080/09553009514551041. [DOI] [PubMed] [Google Scholar]
- 27.Dynlacht JR, Batuello CN, Lopez JT, Ki KK, Turchi JJ. Identification of Mre11 as a Target for Heat Radiosensitization. Radiat Res. 2011;176:323–32. doi: 10.1667/rr2594.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerashchenko BI, Gooding G, Dynlacht JR. Hyperthermia alters the interaction of proteins of the Mre11 complex in irradiated cells. Cytometry A. 2010;277:940–52. doi: 10.1002/cyto.a.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habash RW, Krewski D, Bansal R, Alhafid HT. Principles, applications, risks and benefits of therapeutic hyperthermia. Front Biosci. 2011;3:1169–81. doi: 10.2741/e320. [DOI] [PubMed] [Google Scholar]
- 30.Shao RG. Pharmacology and therapeutic applications of enediyne antitumor antibiotics. Curr Molec Pharm. 2008;1(1):50–60. doi: 10.2174/1874467210801010050. [DOI] [PubMed] [Google Scholar]
- 31.Diederich CJ, Hynynen K. Ultrasound technology for hyperthermia. Ultrasound Med Biol. 1999;25(6):871–87. doi: 10.1016/s0301-5629(99)00048-4. [DOI] [PubMed] [Google Scholar]