Abstract
Background
Despite the significant antitumor activity of pembrolizumab in non-small cell lung cancer (NSCLC), clinical benefit has been less frequently observed in patients whose tumors harbor epidermal growth factor receptor (EGFR) mutations compared to EGFR wild-type patients. Our single center experience on the KEYNOTE-001 trial suggested that pembrolizumab-treated EGFR-mutant patients, who were tyrosine kinase inhibitor (TKI) naïve, had superior clinical outcomes to those previously treated with a TKI. As TKI naïve EGFR-mutants have generally been excluded from pembrolizumab studies, data to guide treatment decisions in this patient population is lacking, particularly in patients with PD-L1 expression ≥50%.
Methods
We conducted a phase II trial (NCT02879994) of pembrolizumab in TKI naive patients with EGFR mutation positive, advanced NSCLC and PD-L1 positive (≥1%, 22C3 antibody) tumors. Pembrolizumab was administered 200mg q3wks. The primary endpoint was objective response rate. Secondary endpoints included safety of pembrolizumab, additional pembrolizumab efficacy endpoints, and efficacy and safety of an EGFR TKI after pembrolizumab.
Results
Enrollment was ceased due to lack of efficacy after 11 of 25 planned patients were treated. 82% of trial patients were treatment naïve, 64% had sensitizing EGFR mutations, and 73% had PD-L1 expression ≥50%. Only 1 patient had an objective response (ORR: 9%), but repeat analysis of this patient’s tumor definitively showed the original report of an EGFR mutation to be erroneous. Observed treatment related adverse events were similar to prior experience with pembrolizumab, but two deaths within 6 months of enrollment, including one attributed to pneumonitis, were of concern.
Conclusions
Pembrolizumab’s lack of efficacy in TKI naïve, PD-L1+, EGFR-mutant patients with advanced NSCLC, including those with PD-L1 expression ≥50%, suggests that it is not an appropriate therapeutic choice in this setting.
Keywords: non-small cell lung cancer (NSCLC), programmed death 1 (PD-1), epidermal growth factor receptor (EGFR), tumor immunology, EGFR tyrosine kinase inhibitor (TKI), pembrolizumab, programmed death ligand 1 (PD-L1)
Introduction
Programmed death 1 (PD-1) axis inhibition has resulted in durable responses in non-small cell lung cancer (NSCLC) patients whose tumors harbor mutations in the epidermal growth factor receptor (EGFR) gene. However, data to date suggests that responses are considerably less frequent in this patient population compared to EGFR wild type (WT) patients [1–4].
Approximately 10% of patients in North America and approximately 30-50% of patients of East Asian decent have mutations in the EGFR gene, of which 90% have sensitizing mutations [5]. Although tumors with EGFR sensitizing mutations are generally responsive to tyrosine kinase inhibitors (TKIs) directed against EGFR [5–9], the benefits are transient, and recurrence inevitably occurs. As patients with EGFR mutations are typically younger than EGFR WT patients [10], this population would derive particular benefit from the durable responses seen with PD-1 axis inhibitors [3].
There has been much speculation regarding the limited benefit of PD-1 axis inhibitors in EGFR-mutant NSCLC [2] [11]. Higher nonsynonymous tumor mutational burden is associated with improved benefit from anti-PD-1 therapy [12], and tumors from EGFR-mutant patients have less mutations than those in EGFR WT patients [13]. While PD-1 axis inhibitors have shown greater benefit among patients with high expression of programmed death ligand 1 (PD-L1), EGFR TKIs downregulate PD-L1 expression in a laboratory setting [14–19]. The relevance of this finding is unclear as tumor PD-L1 expression levels in some clinical series have been largely unaffected by TKI administration [2].
The limited benefit of PD-1 axis inhibitors in EGFR-mutant patients has led to alternate approaches, including combining agents targeting both pathways. Yet synergy has not been observed between EGFR TKIs and anti-PD-1 therapy in a PBMC co-culture system [16]. Clinical trials evaluating concurrent administration of an EGFR TKI and a PD-1 axis inhibitor in EGFR-mutant NSCLC patients have been conducted (NCT02364609, 02630186, 02039674, 02013219, 02088112, 02143466). A number of these studies have run into concerns related to toxicity. Specifically, grade 3 or higher AEs were observed in >50% of patients receiving combination therapy in two phase I studies, with interstitial lung disease (ILD) occurring in 38% of patients receiving both durvalumab and osimertinib [20, 21]. Further, on Arm E of CheckMate-012, which evaluated the combination of nivolumab and erlotinib, the observed clinical outcomes were not clearly superior to what would be expected with erlotinib alone [22]. Due to the high response rate with EGFR TKIs in EGFR-mutant patients [23], PD-1 axis inhibition has not been formally evaluated prior to TKI administration.
We previously reported our single center experience on the KEYNOTE-001 trial at the University of California, Los Angeles (UCLA). Four EGFR-mutant patients that had not received an EGFR TKI prior to pembrolizumab had improved clinical outcomes [Objective response rate (ORR) 50%, median progression-free survival (PFS) 157.5 days, median overall survival (OS) 559 days] compared to the 26 EGFR-mutant patients with a history of TKI therapy prior to pembrolizumab (ORR 4%, median PFS 56 days, median OS 120 days), with a median follow-up for surviving patients of 42.4 months [24, 25]. That experience was limited by small patient numbers, but formed the basis for a trial (NCT02879994) to evaluate the hypothesis that pembrolizumab prior to EGFR TKI therapy in patients with advanced NSCLC whose tumors harbored an EGFR mutation and were PD-L1 positive (≥1% 22C3 antibody) would be superior to the current strategy in which PD-1 axis inhibitors are used after failure of an EGFR TKI. We were reassured by the typical rapid efficacy of EGFR TKIs, which we anticipated could quickly salvage patients who were progressing on pembrolizumab. The planned enrollment was 25 patients.
Methods
Patients
Eligible patients (≥18 years of age) had advanced NSCLC, adequate organ function, and an Eastern Cooperative Oncology Group performance status of ≤1. Key inclusion criteria included the following two tumor specific factors (identified in a CLIA certified lab); 1) EGFR mutation positive (sensitizing or non-sensitizing) 2) PD-L1 positive, defined as ≥1% tumor membranous staining by immunohistochemistry (IHC) using the 22C3 pharmDx test. Key exclusion criteria included prior therapy with an EGFR TKI, prior PD-1 axis inhibitor therapy or any other drug specifically targeting T-cell co-stimulation or immune checkpoint pathways, active autoimmune disease, or history of interstitial lung disease or pneumonitis. (Appendix 1: NCT02879994 Protocol)
Study Oversight
As the study was conducted at a single center, the protocol and its amendments were approved by the UCLA Institutional Review Board (IRB), Internal Scientific Peer Review Committee (ISPRC), and Medical Radiation Safety Committee (MSRC). The study was monitored by the Jonsson Comprehensive Cancer Center Data Safety and Monitoring Board. Good Clinical Practice guidelines were followed throughout the study. Patients were required to provide written informed consent before all study-related activities.
Study Design and Treatment
The primary end point of the study was objective response rate (ORR) to pembrolizumab, per modified Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1). Secondary objectives included safety of pembrolizumab and additional efficacy endpoints [PFS, OS]. Patients received pembrolizumab 200mg by IV infusion every three weeks for up to 35 treatments. After completion of pembrolizumab, patients were followed for evaluation of subsequent EGFR TKI safety and efficacy, as additional secondary endpoints. (Supplementary Figure S1)
Study Assessments
Dedicated computed tomography imaging was performed every 9 weeks +/- 1 and evaluated by investigator-assessed modified RECIST 1.1 [26]. Treatment discontinuation occurred at time of radiographically identified disease progression, investigator decision, toxicity, or withdrawal of consent. Investigators reported adverse events (AEs) and graded them according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 (CTCAE). Investigator assessed AE attribution to study drug was documented with one of the following terms: not related, unlikely, possibly, probably, and related. Treatment related AEs (trAEs) were those AEs labeled possibly, probably, or related to study drug. Although data on subsequent EGFR TKI therapy was specifically sought as part of the trial, to avoid missing relevant data related to treatment after study drug discontinuation, retrospective chart review was performed to supplement collection of data on subsequent TKI efficacy and safety.
Statistical Analysis
Patient demographics, safety data, and AE occurrence were collected on all patients. ORR, PFS, and OS were calculated in the intention to treat population of 11 patients. Data cut-off was November 15, 2017 for all analyses.
Results
Patients
Eleven of the planned 25 patients were enrolled on trial between October 2016 and September 2017. All received at least 1 dose of pembrolizumab. One additional patient was screened, but chose to pursue standard of care EGFR TKI instead. Baseline clinical characteristics demonstrated that the majority of enrolled patients were treatment naïve (82%), had sensitizing EGFR mutations (64%), and had PD-L1 expression levels >50% (73%) (Table S1). At the time of the data cut-off, the median duration of follow-up was 233 days and 2 patients remained on pembrolizumab.
Efficacy of Pembrolizumab
One of eleven patients on trial had an objective response to pembrolizumab (ORR: 9%) at the time of the data cut-off. However, after 10 cycles of therapy, a repeat analysis of the responding patient’s original tumor specimen failed to reveal the EGFR exon 19 deletion initially identified. A forensic analysis revealed that the EGFR mutation originally identified in this patient’s tumor was the result of an error in which the patient’s sample was swapped with another sample during the original mutational analysis, which had been performed as a clinical test in a CLIA certified laboratory. Nonetheless, the PD-L1 assessment was confirmed to be correct in this patient.
Thus, in the 10 patients on trial with documented EGFR mutations the ORR was 0% (0/10). Stable disease was the best response for 7 patients including one patient who remains on pembrolizumab. Of the remaining EGFR-mutant patients, progressive disease was seen as the best response in 3 patients, one of whom technically went off trial due to an adverse event before the first imaging assessment, but did have evidence of progression on a scan shortly after trial discontinuation (Table 1). Only one of the 10 EGFR-mutant patients experienced tumor regression ≥10% (Figure 1). Median PFS with pembrolizumab in the 10 patients with documented EGFR mutations was 119 days, while median OS was not reached at time of data cut-off. (Figure 2)
Table 1.
Patient characteristics including demographics, clinical outcomes, and adverse events stratified by trial patient.
| Age (y) | Sex | Race | Smoker | Histology | EGFR mutation | PD-L1 (%) | trAE (grade) | Best Response | Time on Trial (m) | Reason off trial | TKI after trial | TKI-related AE (grade) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 54 | F | Asian | Never | ADC | L858R | 5 | Decreased TSH (1) | SD | 3.2 | PD | Y | Transaminitis (1, 3) Diarrhea (3) |
| 61 | F | Caucasian | Prior | ADC | E330K | 95 | Rash (1) Flu-like symptoms (1) Chills (1) Diarrhea (1, 2) Transaminitis (1-3) |
SD | 3.0 | trAE | N | N/A |
| 35 | M | Asian | Never | SCC | Exon 20 ins | 50 | None | PD | 2.0 | PD | N | N/A |
| 56 | M | Asian | Prior | ADC | Exon 19 del | 55 | None | SD | 4.0 | PD | Y | Rash (2) |
| 71 | F | Asian | Never | ADC | L858R | 10 | Rash (1, 1) Fatigue (2) Adrenal insufficiency (2) |
SD | 4.0 | PD | Y | Rash (2) |
| 61 | M | Caucasian | Prior | ADC | Exon 19 del | 30 | None | SD | 4.0 | PD | Y | Rash (1) |
| 67 | M | Asian | Active | ADC | None* | 80 | Rash (1) Flu-like symptoms (1) |
PR | 8.2 | OT | N/A | N/A |
| 52 | F | Caucasian | Never | ADC | Exon 19 del | 90 | Diarrhea (1) | N/A† | 1.4 | trAE | Y | Pneumonitis (5), Diarrhea (2) |
| 61 | F | Caucasian | Never | ADC | L858R | 55 | None | SD | 4.1 | PD | Y | Rash (1) |
| 65 | F | Caucasian | Never | ADC | Exon 20 ins | 70 | None | PD | 1.9 | PD | N | N/A |
| 63 | F | Caucasian | Prior | ADC | L858R | 80 | Rash (1) | SD | 2.1 | OT | N/A | N/A |
ADC – adenocarcinoma, AE – adverse event, del – deletion, F – female, inc – increased, ins – insertion, M – male, m – month(s), N – no, N/A – not applicable, OT – continues on trial, PD – progressive disease, SCC – squamous cell carcinoma, SD – stable disease, TKI – tyrosine kinase inhibitor, trAE – treatment-related AE, TSH – thyroid stimulating hormone, Y – yes, y – year(s).
Subject found not to have EGFR mutation.
Subject only received one dose of study treatment.
Figure 1. Best Response for Target Lesions by Patient While on Trial.
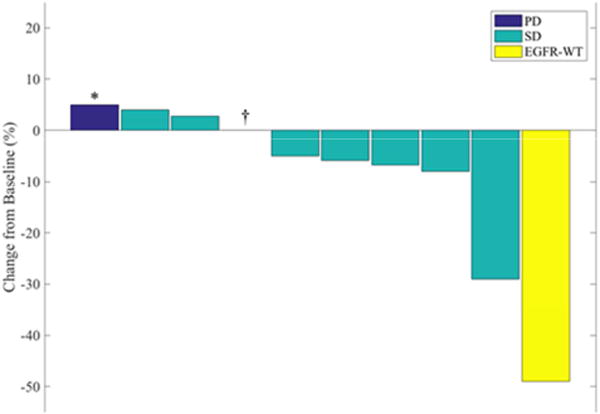
Waterfall plot for patients on trial with evaluable radiographic images.
PD - progressive disease, SD - stable disease, EGFR-WT - epidermal growth factor receptor wild type.
*Subject deemed to have progression based on dural thickening on brain MRI.
†Subject with complete response of target lesion but non-target progression on first scan
Figure 2. Time on Trial and Subsequent Therapies.
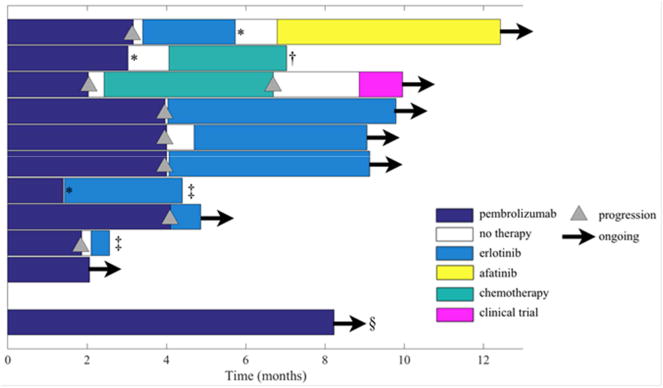
Swimmer plot of time on treatment with reason(s) for treatment discontinuation.
*Adverse event leading to treatment discontinuation.
†Completed treatment and now on surveillance, still alive at time point of data collection.
‡Death while on erlotinib.
§Subject without EGFR mutation.
Adverse Events with Pembrolizumab
46% (5/11) of treated patients experienced a trAE with pembrolizumab and treatment related grade 3 transaminitis led to trial discontinuation in one of these five patients. One additional patient discontinued therapy due to grade 3 hypercalcemia that was deemed to be related to widespread osseous disease. The most common trAEs experienced on trial were rash, diarrhea, and myalgias/chills occurring in 3, 2, and 2 patients, respectively. Of note, grade 2 pembrolizumab associated adrenal insufficiency observed in one patient continues to require hydrocortisone replacement therapy at time of data cut-off. There were no grade 4-5 trAEs reported with pembrolizumab (Table 1 and Table S2)
Efficacy of Subsequent TKI after Trial Discontinuation
Of the 9 patients who received subsequent therapy, 2 were treated with chemotherapy and radiation after trial discontinuation, and the remaining 7 received erlotinib as their next line of therapy. Five of the seven patients treated with erlotinib after progression on pembrolizumab remained on an EGFR TKI at the time of the data cut-off with a median duration on therapy of 109 days, while the other 2 died while on the EGFR TKI therapy. One of these deaths occurred in a patient with an exon 20 insertion who chose erlotinib over chemotherapy at the time of progression. That death was felt to be from respiratory failure related to lack of TKI efficacy. The other 6 patients had sensitizing EGFR mutations.
Adverse Events Experienced on EGFR TKI Therapy after Discontinuation of Pembrolizumab
86% (6/7) of the patients that received an EGFR TKI as their next line of therapy experienced an adverse event that was presumed to be associated with the TKI by treating physician. The one patient who did not experience a TKI associated adverse event only received erlotinib for 14 days. Most notable TKI associated adverse events reported were grade 3 transaminitis, leading to a dose reduction of erlotinib and eventually a transition to afatinib, and grade 5 pneumonitis leading to respiratory failure and death. (Table 1)
The patient who experienced grade 5 pneumonitis underwent imaging after approximately 6 weeks of erlotinib therapy which showed improvement in disease burden, and the patient exhibited clinical improvement (Figure 2). She died of respiratory failure 16 days after the onset of hypoxia deemed to be related to pneumonitis. The death occurred 89 days after initiating erlotinib and 132 days after the last and only dose of pembrolizumab. The pneumonitis was attributed to erlotinib, but a role of pembrolizumab cannot be ruled out. (Table 1)
Decision regarding study discontinuation
The study was designed as a small study to assess the feasibility of pembrolizumab administration prior to an EGFR TKI in EGFR-mutant patients with PD-L1 expression. It was designed to show an ORR of ≥26%. Within an approximately one month period, the responding patient was found to not harbor an EGFR mutation, one patient developed fatal pneumonitis on erlotinib and an additional patient died. Despite enrollment of only 11 of 25 planned patients, the study was closed to further enrollment based on futility.
Discussion
This study evaluated the hypothesis that treatment of PD-L1 positive, EGFR-mutant advanced NSCLC with pembrolizumab, prior to an EGFR TKI, would lead to better clinical outcomes than have been observed with pembrolizumab after progression on an EGFR TKI. It was designed to demonstrate an ORR ≥26%. Thus, the trial could meet its primary end-point with an ORR substantially lower than that historically observed with an EGFR TKI in EGFR-mutant NSCLC [23], and the consent form specifically acknowledged this likelihood. In cancer therapy, a treatment that maximizes response rate is generally the favored approach, but not always. In BRAF mutant melanoma, immunotherapy prior to BRAF TKI-directed treatment is a validated treatment option despite substantially lower response rates [27]. Three criteria would have to be fulfilled to validate a strategy of administering pembrolizumab prior to an EGFR TKI. First, the clinical benefit of pembrolizumab would have to be greater when given prior to an EGFR TKI rather than after. Second, the benefits of an EGFR TKI would have to be unaffected by the sequencing of pembrolizumab, and finally, the safety profile of pembrolizumab followed by an EGFR TKI would need to be similar to what is seen when the order is reversed.
Although the number of patients treated on trial is limited due to premature closure for futility, the data do not support the idea of a meaningful increase in benefit of pembrolizumab when given prior to an EGFR TKI. Since tumoral PD-L1 expression levels are predictive of response to PD-1 inhibition in NSCLC, most clearly in those patients with expression levels ≥50%, the trial was designed to enrich the evaluated patient population, restricting enrollment to only those patients whose tumors were PD-L1 positive (≥1%) [3]. In fact, 70% of the EGFR mutation positive patients treated on trial had PD-L1 expression levels ≥50%. The lack of an objective response in this PD-L1 enriched population suggests that pembrolizumab is not an appropriate therapeutic choice for the treatment of advanced, EGFR mutation positive, NSCLC prior to an EGFR TKI, even among patients with PD-L1 ≥50%.
The trial does not have sufficient power to definitively assess effects of prior pembrolizumab on the benefit of a subsequent EGFR TKI. Yet, 5 of the 7 patients that received an EGFR TKI after trial discontinuation, remain on a TKI at time of data cut-off. Of the other two patients, one experienced disease response after 6 weeks but died from an adverse event, and the other, who had a non-sensitizing EGFR mutation, died of presumed lack of TKI efficacy after 14 days of therapy. While this data suggests that TKI efficacy is not affected by preceding PD-1 axis inhibition, conclusions regarding the durability of this efficacy cannot be drawn at this time, given the short median duration of TKI therapy in these patients (109 days) [23]. Although our belief that patients with sensitizing EGFR mutations would subsequently respond rapidly to a TKI was correct, we failed to recognize the degree to which it would be emotionally difficult for both patients and physicians to withhold an intervention (EGFR TKI) that was nearly certain to work.
Whether administering pembrolizumab prior to an EGFR TKI increases the degree of toxicity experienced by patients compared to the reversed order is not clear, but the results are not entirely reassuring. Significant toxicity was observed on trials evaluating concurrent administration of an EGFR TKI and a PD-1 axis inhibitor, leading to study discontinuations [20, 21]. On the present trial, the rate of treatment related toxicity was similar to what has generally been observed with PD-1 axis inhibition in NSCLC, with approximately 50% of EGFR-mutant patients experiencing a trAE, leading to study discontinuation in one patient [1, 3] [28]. However, it is important to recognize that one patient developed pembrolizumab associated adrenal insufficiency, likely necessitating lifelong corticosteroids. Had she received a TKI as her initial therapy, this toxicity would have at the very least been delayed, lessening the impact on her quality of life.
Fatal pneumonitis, which was presumed to be TKI associated, developed in one Caucasian patient that received erlotinib after progression on pembrolizumab. Although erlotinib associated pneumonitis has been reported, the incidence is <1% and is even less common in Caucasians [29]. It is difficult to know if the preceding administration of pembrolizumab contributed to the development of this case of pneumonitis, but its occurrence certainly raises questions about the safety of this sequencing of therapies. Interestingly, on the TATTON trial, which evaluated the combination of durvalumumab and osimertinib, the incidence of ILD was 38% in patients receiving both drugs, leading to trial discontinuation [20]. When designing the current study, we were reassured by data from the CheckMate-012 study in which the combination of nivolumab and erlotinib appeared to be better tolerated than durvalumab and osimertinib [22]. However, now that data supports osimertinib as a frontline option in EGFR-mutant NSCLC [30], the significant toxicity observed on the TATTON trial become relevant, since for many patients osimertinib would be the anticipated subsequent therapy after progression on a PD-1 axis inhibitor.
In summary, immature data suggests EGFR TKI efficacy is similar whether given before or after pembrolizumab. However, the lack of an objective response in 10 PD-L1 positive, EGFR-mutant patients, inclusive of 7 with PD-L1 expression >50%, was sobering. Further, the occurrence of one lifelong pembrolizumab associated adverse event, adrenal insufficiency, and the potential contribution of pembrolizumab to a case of fatal pneumonitis that occurred during subsequent TKI therapy, raise significant concern regarding this sequencing of therapies. Without greater ability to select appropriate patients, even among patients with EGFR mutations and PD-L1 expression of ≥50%, pembrolizumab is not an appropriate therapy prior to an EGFR TKI in patients with sensitizing EGFR mutations.
Supplementary Material
Acknowledgments
Funding/Support: Dr. Garon was in part supported by R01 CA208403.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Dr. Lisberg reports compensated AstraZeneca advisory board attendance.
Dr. Garon reports funds to his institution from AstraZenca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Genentech, Mirati, Merck, and Novartis.
Dr. Goldman reports grants from BMS and Medimmune/AstraZeneca during the conduct of the study.
Dr. Mendenhall reports compensation for Merck speaker’s bureau.
The remaining authors have no conflicts of interest to report.
Bibliography/References Cited
- 1.Borghaei H, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gainor JF, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer (NSCLC): A Retrospective Analysis. Clinical Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 4.Hellmann MD, et al. MINI03.05 Efficacy of Pembrolizumab in Key Subgroups of Patients with Advanced NSCLC. Journal of Thoracic Oncology. 2015;10(9):S261–S406. [Google Scholar]
- 5.Sharma SV, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 6.Lund-Iversen M, et al. Clinicopathological Characteristics of 11 NSCLC Patients with EGFR-Exon 20 Mutations. Journal of Thoracic Oncology. 2012;7(9):1471–1473. doi: 10.1097/JTO.0b013e3182614a9d. [DOI] [PubMed] [Google Scholar]
- 7.Lynch TJ, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non–Small-Cell Lung Cancer to Gefitinib. New England Journal of Medicine. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 8.Paez JG, et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. The Lancet Oncology. 2012;13(1):e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 10.KIM HJ, et al. Relationship Between EGFR Mutations and Clinicopathological Features of Lung Adenocarcinomas Diagnosed via Small Biopsies. Anticancer Research. 2014;34(6):3189–3195. [PubMed] [Google Scholar]
- 11.Gettinger S, Politi K. PD-1 axis inhibitors in EGFR and ALK Driven Lung Cancer: Lost cause? Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(18):4539–4541. doi: 10.1158/1078-0432.CCR-16-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi NA, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu-Lieskovan S, et al. 16th World Conference on Lung Cancer. Denver, Colorado: 2015. High Intratumoral T Cell Infi ltration Correlated with Mutational Load and Response to Pembrolizumab in Non-Small Cell Lung Cancer. [Google Scholar]
- 14.Akbay EA, et al. Activation of the PD-1 Pathway Contributes to Immune Escape in EGFR-Driven Lung Tumors. Cancer Discovery. 2013;3(12):1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azuma K, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Annals of Oncology. 2014;25(10):1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 16.Chen N, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. Journal of Thoracic Oncology. 2015;10(6):910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 17.Im JS, et al. Immune-Modulation by Epidermal Growth Factor Receptor Inhibitors: Implication on Anti-Tumor Immunity in Lung Cancer. PLoS ONE. 2016;11(7):e0160004. doi: 10.1371/journal.pone.0160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin C, et al. Programmed Death-Ligand 1 Expression Predicts Tyrosine Kinase Inhibitor Response and Better Prognosis in a Cohort of Patients With Epidermal Growth Factor Receptor Mutation-Positive Lung Adenocarcinoma. Clinical Lung Cancer. 2015;16(5):e25–e35. doi: 10.1016/j.cllc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. 2015;2015 doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn MJ, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. Journal of Thoracic Oncology. 2016;11(4):S115. [Google Scholar]
- 21.Gibbons DL, et al. 57O: Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. Journal of Thoracic Oncology. 2016;11(4):S79. [Google Scholar]
- 22.Hellmann MD, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. The Lancet Oncology. 18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mok TS, et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. New England Journal of Medicine. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 24.Garon EBWB, Lisberg A, Kim KY, Horton JM, Kamranpour N, Chau K, Abarca P, Spiegel ML, Han M, Sago W, Hu-Lieskovan S, Das K, Wallace WD, Slamon DJ, Dubinett SM, Goldman JW. Prior TKI Therapy in NSCLC EGFR Mutant Patients Associates with Lack of Response to Anti-PD-1 Treatment. J Thorac Oncol. 2015;10:S269. [Google Scholar]
- 25.Lisberg A, et al. MA 02.07 A Phase II Study of Pembrolizumab in EGFR Mutant, PD-L1+, Tyrosine Kinase Inhibitor (TKI) Naïve Patients with Advanced NSCLC. Journal of Thoracic Oncology. 12(11):S1805. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) European Journal of Cancer. 45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? The Lancet Oncology. 2013;14(2):e60–e69. doi: 10.1016/S1470-2045(12)70539-9. [DOI] [PubMed] [Google Scholar]
- 28.Brahmer J, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepherd FA, et al. Erlotinib in Previously Treated Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 30.Soria JC, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. New England Journal of Medicine. 0(0) doi: 10.1056/NEJMoa1713137. null. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


