Abstract
Background:
Poor medication adherence is common among individuals with Bipolar Disorder (BD). Understanding the sources of heterogeneity in clinical net benefit (CNB) and how it is related to psychotropic medications can provide new insight into ways to improve adherence.
Methods:
Data come from the baseline assessments of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Latent class analysis identified groups of CNB, and validity of this construct was assessed using the SF-36. Adherence was defined as taking 75% or more of medications as prescribed. Associations between CNB and adherence were tested using multiple logistic regression adjusting for socio-demographic characteristics.
Results:
Five classes of CNB were identified: High (24%), Moderately high (12%), Moderate (26%), Moderately low (27%) and Low (12%). Adherence to psychotropic medications did not differ across classes (71% to 75%, χ2 = 3.43, p = 0.488). Medication regimens differed by class: 57% of the High CNB were taking ≤ 2 medications, whereas 49% of the Low CNB were taking ≥ 4. CNB classes had good concordance with the SF-36.
Limitations:
Missing data limited measures used to define CNB. Participants’ perceptions of their illness and treatment were not assessed.
Conclusions:
This novel operationalization of CNB has construct validity as indicated by the SF-36. Although CNB and polypharmacy regimens are heterogeneous in this sample, adherence is similar across CNB. Studying adherent individuals, despite suboptimal CNB, may provide novel insights into aspects influencing adherence.
Keywords: Adverse effects, Medication adherence, Polypharmacy, Bipolar Disorder
1. Introduction
Bipolar Disorder (BD) is among the leading causes of disability-adjusted life-years lost worldwide (Bloom et al., 2011). Effective treatment with psychotropic medication, often in combination with psychotherapy, can help individuals with BD manage their illness (Yatham et al., 2005,2013,2018).
Despite advances in pharmacotherapy, adherence to medication among individuals with BD has not markedly improved since the 1950’s when medications with serious adverse effects were the primary treatment modalities (Clatworthy et al., 2009). Approximately 20–60% of individuals with BD will be non-adherent to their medication at some point in their treatment (Kutzelnigg et al., 2014); medication non-adherence contributes to elevated relapse, suicidal behavior and greater healthcare costs (Svarstad et al., 2001; Velligan et al., 2010). Poor adherence is thought to stem from multiple sources, including effects of the illness itself (e.g., “lack of insight” about the condition (Crowe et al., 2011; Ketter, 2010), adverse effects of medications (e.g., heart disease, somnolence (Bates et al., 2010; Clatworthy et al., 2009; Kemp, 2014)), and complexity of medication regimens (e.g., multiple pills take multiple times per day (Ketter, 2010; Vieta, 2005)). In addition, individuals’ attitudes toward their continued risks of exacerbated symptoms and perceived benefits and burdens of treatments for BD effect whether individuals will adhere to their medications (Sajatovic et al., 2008). Adapting self-management strategies to include the individual’s desired locus of control (e.g., active or passive roles in the patient-provider relationship) may be considered as additional support for adherence (Berk et al., 2004). Psychological reactance has been suggested as a response to the need for adherence to prescribed medication because it can be considered a reduction of freedom of choice, resulting in non-adherence (De Las Cuevas et al., 2014).
When considering prescribing medications, practitioners routinely weigh the benefits versus risks of each treatment, seeking a positive balance between expected benefits and risk of adverse effects (Yatham et al., 2013). For example, the Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines (Yatham et al., 2018) provide hierarchical rankings of medications for first-, second-, and third-line recommendations during acute mania, acute depression and maintenance treatment for BD. Recommendations are supported by levels of evidence for efficacy, tolerability profiles and treatment emergent switch risks informed by previous research. Changes between medications are driven by inefficacy of symptom management and tolerability. Sedation and weight gain are frequent reasons for medication non-adherence in BD. Close monitoring is recommended for possible metabolic or other adverse effects; replacing a high metabolic risk medication with a lower metabolic risk medication is recommended if the efficacy is similar. At the population level, this concept of benefit versus risk is quantified in two main ways: (1) Number Needed to Treat (i.e., if the outcome is a desirable, number of persons to be treated with Treatment 1 in order to find one more preferred response than the same number of persons treated with Treatment 2, and (2) Number Needed to Harm (i.e., if the outcome is harmful, negative number needed to treat (Kraemer et al., 2011)). However, these existing notions of benefit versus risk are limited in two important ways. First, although clinical guidelines for maintenance treatment identify the importance of preventing relapse and promoting quality of life and functioning (Yatham et al., 2005), their practical focus is on efficacious symptom management. This approach, along with Number Needed to Treat, reduces the benefit-risk ratio to a single unidimensional quantity (Kraemer et al., 2011). This does not appropriately capture the complexity of what benefit versus risk objectively looks like for the patient; Clinical Net Benefit (CNB) of treatment can be conceptualized as the complex intersection between psychiatric symptoms, adverse effects, and overall functioning.
Second, there has been only limited discussion of how the experience of CNB for individuals with BD relates to their medication adherence. Instead, focus has been on how to remedy non-adherence with clinician-administered psychoeducation (Vieta, 2005) and identifying individual’s perception of their providers’ confidence in their medication regimen as some of the possible methods (Cochran and Gitlin, 1988). Much research has focused on why people do not adhere, but new insight can be found by focusing in individuals who do adhere. A handful of studies explored how perspectives of individuals with BD relate to medication adherence. Using the Beliefs about Medication Questionnaire (Horne et al., 1999), Clatworthy et al. (2009) found that perceptions of higher concern and lower necessity regarding medication were associated with lower adherence. Using components of the Rating of Medication Influences Scale (ROMI) (Weiden et al., 1994), Adams and Scott (2000) found that participants’ perceived benefits-to-risks for medications differentiated those who were highly adherent and partially adherent. Other descriptive studies of individuals with BD have identified treatment of depression, improved functioning, and management of adverse effects as factors most important to CNB, but these studies did not examine the relationships between these factors and medication adherence (Morselli et al., 2003; Yatham et al., 2013). These reports were also limited in scope (i.e., small samples, limited to one type of medication) and one relied on self-administered mail-in questionnaires with lower validity relative to clinical assessments (Bowling, 2005; McIntyre, 2009; Morselli et al., 2003).
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) overcame many limitations of these prior studies. It was a large (N = 4360), 5-year longitudinal randomized clinical trial (RCT) designed to test the utility of different treatment modalities (medications and psychotherapy) for individuals with BD. Participants were also given a battery of clinician- and self-administered psychological assessments as well as detailed clinician-determined medication adherence measures (Sachs et al., 2003).
The objective of this study was to use STEP-BD to identify and characterize subgroups of CNB. Due to the complex, multi-dimensional nature of CNB, this project employed two latent variable approaches, exploratory factor analysis (EFA) and latent class analysis (LCA), to quantify CNB in the context of BD treatment (Lanza et al., 2007). Latent variable modeling is ideal for quantifying a complex construct such as CNB (Krueger et al., 2007; Woolston et al., 2012), and can effectively classify people into discrete subgroups. Classes of CNB were characterized according to objective indicators of symptom management, adverse effects, and overall functioning. Validity of the CNB construct was analyzed using the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36; (McHorney et al., 1993)), a commonly used self-report metric of health and functioning. Further, the association between these CNB classes with characteristics of medication treatment (i.e., type of medication, polypharmacy) and medication adherence was assessed. We hypothesized that LCA will identify unique classes of individuals who systematically differ in objective characteristics of CNB. We also hypothesized that these distinct classes will be differentially associated with medication adherence.
2. Methods
2.1. Sample
All eligible participants in the STEP-BD trial aged 18 years and older, who completed baseline assessments, and were taking medication were included in the current study, as medications taken by STEP-BD participants were only approved for this population when the study began (Smarty and Findling, 2007; Thomas et al., 2011). The details of the original study design were described elsewhere (Sachs et al., 2003). Briefly, STEP-BD was a 5-year RCT of individuals treated for bipolar spectrum disorders. It was designed to simulate the “real world” experiences in treatment of individuals with BD. STEP-BD was not solely a RCT, as eligible participants could choose to enter either the Randomized Care Pathways (RCPs) or Standardized Care Pathway (SCP). At baseline, participants who met the symptom inclusion criteria for one of the three RCPs (i.e., acute depression, refractory depression, or relapse prevention) could then choose to enter those pathways or stay in the SCP. In the RCPs, participants were randomly assigned to specific medications (i.e., mood stabilizers, antipsychotics, antidepressants or placebos) to minimize self-selection bias. In the SCP, participants maintained current treatment (i.e., treatment as usual). In addition, participants underwent a battery of clinician- and self-administered psychological assessments, including medication adherence. Although STEP-BD is a longitudinal trial, we are conducting a cross-sectional analysis with these data since we are only using data from the baseline assessments before individuals have participated in STEP-BD.
Although 4360 participants enrolled in the original study, this study further excluded 321 participants with incomplete data on the psychological assessments and physical measures with less than 10% missing data used in this analysis, and 301 individuals who were less than 18 years of age. Missing data < 10% was imputed using Full Information Likelihood Estimation (Dong and Peng, 2013). The final analytic sample size was 3738 (Supplemental Figure 1).
2.2. Outcomes
2.2.1. Clinical net benefit
CNB incorporates three main effects of treatment experienced by the individual: (1) symptom reduction; (2) adverse effects; and (3) overall functioning. CNB can be conceptualized as a 3-dimensional construct lying at the intersection of these axes. Individuals differentially experience these components of treatment, depicted as points in Fig. 1. These different experiences, or coordinates, may in turn uniquely relate to medication adherence. To conceptually define and quantitatively measure these three dimensions of CNB, we used the following variables. Three EFAs empirically reduced measures to only those necessary for the three dimensions of CNB. LCA then grouped participants into distinct subgroups of CNB.
Fig. 1.
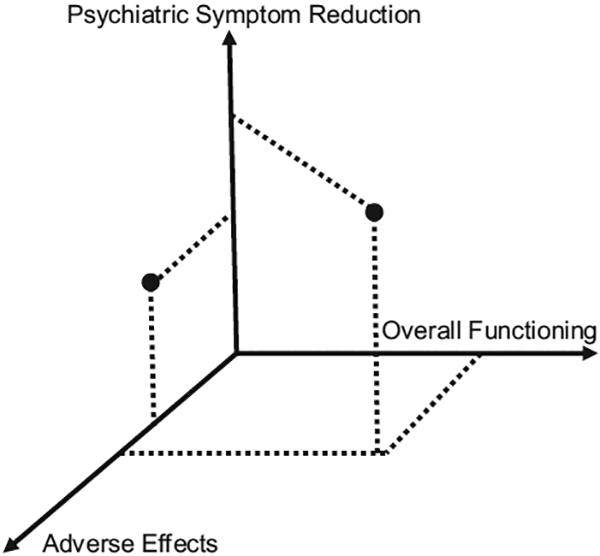
Dots represent different hypothetical CNB groups and their relative coordinates of psychiatric symptoms, adverse medication effects, and overall functioning.
2.2.2. Psychiatric symptoms
Nine symptom scales and psychiatric diagnoses administered and identified at baseline were explored as potential indicators of this component of CNB (Yatham et al., 2013). All symptom indicators were reverse coded such that higher scores indicated lower symptomatology. The treating psychiatrist-administered Clinical Monitoring Form (CMF; (Sachs et al., 2002)) indicated current (i.e., at baseline) binary (yes/no) comorbid DSM-IV diagnoses of alcohol abuse, substance abuse/dependence, binge/purge, and panic disorder; the number of caffeine cups per day (mean: 1.83, SD: 2.35) and number of cigarettes per day (mean: 6.04, SD: 10.96) were transformed into binary variables above and below the sample mean. Current mania and depression were measured using the participant self-reported, 20-item Beck Hopelessness Scale (BHS) (range: 0 = none to 20 = severe; mean: 11.49, SD: 5.75); the clinician-rated, 11-item Young Mania Rating Scale (YMRS) (range: 0 = absent to 60 = severe; mean: 32.00, SD: 6.53); and the clinicianrated, 10-item Montgomery–Asberg Depression Rating Scale (MADRS) (0 = absent to 50 = severe; mean: 33.19, SD: 10.90). Externalizing symptoms (i.e., alcohol abuse, substance abuse/dependence, binge/purge, caffeine cups per day, cigarettes per day) (Krueger et al., 2007) were combined into an externalizing count variable.
2.2.3. Adverse effects
Ten current (i.e., at baseline) adverse effects from the CMF were explored as potential indicators of this dimension of CNB. Each was scored on a 4-point scale ranging from 0 = none to 4 = severe. All of these indicators were reverse-coded so that higher scores indicated fewer effects. These included tremor (mean: 3.77, SD: 0.60); dry mouth (mean: 3.75, SD: 0.63); sedation (mean: 3.70, SD: 0.69); constipation (mean: 3.90, SD: 0.43); diarrhea (mean: 3.88, SD: 0.45); headache (mean: 3.78, SD: 0.60); poor memory (mean: 3.74, SD: 0.64); sexual dysfunction (mean: 3.80, SD: 0.63); increased appetite (mean: 3.80, SD: 0.60); and extrapyramidal symptoms (mean: 3.99, SD: 0.17).
2.2.4. Overall functioning
Four scales were explored as potential indicators of current (i.e., baseline) CNB. All items were reverse-coded so higher scores indicated better functioning: (1) participant self-reported, 16-item Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ) (mean: 42.53, SD: 10.87); (2) clinician-rated LIFE Range of Impaired Functioning Tool (LRIFT), (Leon et al., 1999) (mean: 39.88, SD: 6.45); (3) three indicators from the clinician-rated Work Impact Form (WIF), were combined creating a weighted work impairment score (totally unable to work/carry out normal activities score X 2; able to work/carry out normal activities but had to cut down score X 1.5; extreme effort to perform up to usual level of work/normal daily activities score X 1) (ranging from 0 = no impact to 9 = high impact; mean: 4.18, SD: 3.11); 4) the CMF Global Assessment of Functioning (GAF) for the past week (mean: 62.40, SD: 11.07). Demographic characteristics that could be considered functional outcomes (i.e., employment status, accommodation and marital status) were not included in the overall functioning dimension because these were either only measured at baseline or had large amounts of missing data longitudinally. We could not identify how these characteristics changed longitudinally as we could with the other measures in this dimension.
2.2.5. Adherence
The CMF recorded milligrams missed for each medication participants took in the past seven days. Adherence to all medications, and specifically to psychotropic medications, was defined as missing 25% or less of participants’ medication regimens as prescribed in the past week; participants who missed more than 25% of all of their medications, and of all of their psychotropic medications as prescribed were considered non-adherent. For example, individuals prescribed four psychotropic medications who missed less than 25% of the dose prescribed of one of these medications was considered adherent. This is consistent with the definitions used in STEP-BD studies (Perils et al., 2010).
2.2.6. Demographic characteristics
Demographic characteristics included age; gender; race (White, Black, and Other); educational attainment (≤ high school, high school diploma or GED, some college, Bachelor’s degree, and Graduate or professional degree); current marital status (married or living as though married, divorced or separated, never married, or widowed); whether participants lived alone; primary residence (private home, group home or something else); income (greater or less than $50,000); whether participants received disability insurance or welfare; employment status (employed, unemployed, disabled or something else); and history of suicidal ideation. In addition, whether individuals entered the Standard Care Pathways or Randomized Care Pathways were identified. Participants selected their treatment pathway (i.e., either the Standard Care or Randomized Care pathways) and this choice can be used to examine participant characteristics that are correlated with this choice. However, the two pathways are not “balanced” on those characteristics as it would be if people were randomly assigned.
2.2.7. Medication
Medications were listed by name (either generic or brand) on the CMF. All medications were identified and grouped into six families: (1) antidepressants, (2) mood stabilizers, (3) antipsychotics, (4) sedatives/hypnotics, (5) stimulants, and (6) other using the U.S. Food & Drug Administration National Drug Code Directory (National Drug Code Directory, 2016). A regimen count variable was created indicating whether a participant was taking one (monotherapy), two, three, four or five or more medications (polypharmacy).
2.2.8. Validation of CNB
The SF-36 was administered to 2920 participants in STEP-BD. This is a general measure of well-being that has been administered in large naturalistic samples as well as clinical samples of individuals with chronic diseases including chronic pain, mood disorders and schizophrenia and has been shown to have construct validity (Arnold et al., 2000). There are eight scales included in the SF-36 to measure different domains: (1) Physical Functioning; (2) Role-Physical; (3) Bodily Pain; (4) General Health; (5) Vitality; (6) Social Functioning; (7) Role-Emotional; and (8) Mental Health. The final scores for each of these domains are transformed into a range of 1–100, where higher scores indicate better well-being (McHorney et al., 1993).
2.3. Analytic approach
Analyses took place in three steps. First, EFAs reduced the number of measures to only those necessary to comprise each of the three dimensions of CNB (symptoms, adverse effects, and functioning). Second, LCA grouped the participants into distinct classes (subgroups) of CNB. We characterized and examined the correlates of those subgroups in terms of demographic characteristics, medication regimens and medication adherence. Finally, we assessed the construct validity of the CNB construct by examining the mean SF-36 scores across the distinct classes.
2.3.1. Exploratory factor analysis
We conducted three EFAs, (psychiatric symptoms, adverse effects and overall functioning) using the previously described indicators. Using Equamax rotation, (Finch, 2011) eigenvalues > one indicated the number of factors to retain. We only retained indicators meeting the definition of simple structure (factor loadings exceeding 0.50 and a cross loading of at least 0.15 less than the items’ highest factor loading) (Cattell, 1966; DeVellis, 1991; Goldberg and Velicer, 2006; Tabachnick and Fidell, 2007; Worthington and Whittaker, 2006).
2.3.2. Latent class analysis
To improve interpretability of the classes, we dichotomized all continuous and ordinal indicators retained from the EFAs based on the participants’ mean scores (Arnau et al., 2001; Beck et al., 1996), with 1 = above the mean (better outcomes). The number of distinct latent classes of CNB were determined by comparing model fit using the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), Sample-Size Adjusted BIC (BICn), Bootstrapped Likelihood Ratio Test (BLRT) and Entropy; for each of these indices smaller values, and Entropy values closest to 1, indicate better relative fit. Measures of model fit, prevalence of class membership and model interpretability were all used to determine the final number of classes (McCutcheon, 1987; Mezuk and Kendler, 2012). Most likely class membership for each participant was determined from their posterior probabilities.
2.3.3. Characterizing the latent classes of CNB
The demographic characteristics, standard or randomized care pathway membership, adherence to medication regimens, types of medication regimens (i.e., monotherapy versus polypharmacy) and makeup of these medication regimens (i.e., percent antidepressants versus mood stabilizers) of the latent classes of CNB were compared using ANOVA for continuous measures and Chi-square analyses for categorical measures.
2.3.4. Validating CNB
We followed instructions for scoring the SF-36 detailed in Ware et al. (1993). Scores for items 8 and 10 were missing in the sample, so the scales using those items were not included in this analysis (i.e., Bodily Pain and Social Functioning). The scale scores were transformed as indicated by the scoring instructions, and means for the full sample, and for each class were calculated for each of these scales.
A post-hoc analysis using the Anticholinergic Drug Scale (ADS) was conducted to further characterize the differences between the classes. To conduct this analysis, we used the methods and anticholinergic effects detailed in Carnahan et al. (2006).
Descriptive statistics, SF-36 scores and ADS were calculated using SAS version 9.4 (SAS Institute Inc.). EFAs and LCAs were conducted using Mplus version 7 (Muthen and Muthen, 1998–2015).
3. Results
Table 1 describes the characteristics of the sample. Mean age was 40 years, a little over half (58%) were female and 91% were non-Hispanic white. Only 1% lived in group homes, and most lived with at least one other person (73%). Over 15% received Social Security Disability Insurance. Two medications were the most common regimen, and 74% of participants were adherent to their psychotropic medication regimens and 71% were adherent to all medications they were taking. Only 5% of the sample entered a Randomized Care Pathway. Approximating one third of the sample (37%) had a history of suicidal ideation.
Table 1.
Demographic characteristics of the full sample, and by LCA class. Includes logistic regression results testing association of classes with adherence*.
| Full sample | High benefit | Moderately high benefit | Moderate benefit | Moderately lowbenefit | Low benefit | P-values | |
|---|---|---|---|---|---|---|---|
| N (%) | 3738 | 889 (23.78) | 432 (11.56) | 961 (25.71) | 1010 (27.02) | 446 (11.93) | |
| Age (Mean, SD) | 40.45 (12.78) | 41.13 (14.33) | 41.04 (13.18) | 39.54 (12.93) | 40.50 (11.39) | 40.46 (11.65) | 0.091 |
| N = 3568 | N= 837 | N= 417 | N= 930 | N= 958 | N= 426 | ||
| Female—no./total no. (%) | 2054/3563 (57.65) | 445/837 (53.17) | 266/417 (63.79) | 530/929 (57.05) | 553/955 (57.91) | 260/425 (61.18) | 0.004 |
| Race—no./total no. (%) | 0.702 | ||||||
| White | 2531/2789 (90.75) | 565/621 (90.98) | 281/305 (92.13) | 656/724 (90.61) | 722/798 (90.48) | 307/341(90.03) | |
| Black | 162/2789 (5.81) | 33/621 (5.31) | 12/305 (3.93) | 45/724 (6.22) | 53/798 (6.64) | 19/341 (5.57) | |
| Other | 96/2789 (3.44) | 23/621 (3.70) | 12/305 (3.93) | 23/724 (3.18) | 23/798 (2.88) | 15/341 (4.40) | |
| Education—no./total no. (%) | <0.001 | ||||||
| Less than high school diploma | 105/3448 (3.05) | 16/800 (2.00) | 6/401 (1.50) | 29/907 (3.20) | 44/926 (4.75) | 10/414 (2.42) | |
| High school diploma or GED | 521/3448 (15.11) | 100/800 (12.50) | 42/401 (10.47) | 127/907 (14.00) | 168/926 (18.14) | 84/414 (20.29) | |
| Some college | 1296/3448 (37.59) | 256/800 (32.00) | 143/401 (35.66) | 335/907 (36.93) | 390/926 (42.12) | 172/414 (41.55) | |
| College diploma (Bachelor’s degree) | 911/3448 (26.42) | 239/800 (29.88) | 118/401 (29.43) | 265/907 (29.22) | 197/926 (21.27) | 92/414 (22.22) | |
| Graduate or professional degree | 615/3448 (17.84) | 189/800 (23.63) | 92/401 (22.94) | 151/907 (16.65) | 127/926 (13.71) | 56/414 (13.53) | |
| Marital status—no./total no. (%) | <0.001 | ||||||
| Married/living as married | 1300/3531 (36.82) | 305/829 (36.79) | 174/414 (42.03) | 336/923 (36.40) | 313/942 (33.23) | 172/423 (40.66) | |
| Divorced/separated | 888/3531 (25.15) | 171/829 (20.63) | 98/414 (23.67) | 214/923 (23.19) | 298/942 (31.63) | 107/423 (25.30) | |
| Never married | 1285/3531 (36.39) | 341/829 (41.13) | 137/414 (33.09) | 357/923 (38.68) | 317/942 (33.65) | 133/423 (31.44) | |
| Widowed | 58/3531 (1.64) | 12/829 (1.45) | 5/414 (1.21) | 16/923 (1.73) | 14/942 (1.49) | 11/423 (2.60) | |
| Lives alone—no./total no. (%) | 956/3526 (27.11) | 232/828 (28.02) | 113/414 (27.29) | 226/922 (24.51) | 287/940 (30.53) | 98/422 (23.22) | 0.015 |
| Primary residence—no./total no. (%) | 0.402 | ||||||
| Private home | 3310/3459 (95.69) | 767/801 (95.76) | 375/400 (93.75) | 869/910 (95.49) | 898/932 (96.35) | 401/416 (96.39) | |
| Group home/assisted living facility | 37/3459 (1.07) | 8/801 (1.00) | 4/400 (1.00) | 9/910 (0.99) | 11/932 (1.18) | 5/416 (1.20) | |
| Other | 112/3459 (3.24) | 26/801 (3.25) | 21/400 (5.25) | 32/910 (3.52) | 23/932 (2.47) | 10/416 (2.40) | |
| Income—no./total no. (%) | <0.001 | ||||||
| $50,000 or less | 1968/3261 (60.35) | 413/760 (54.34) | 201/382 (52.62) | 505/851 (59.34) | 603/873 (69.07) | 246/395 (62.28) | |
| More than $50,000 | 1293/3261 (39.65) | 347/760 (45.66) | 181/382 (47.38) | 346/851 (40.66) | 270/873 (30.93) | 149/395 (37.72) | |
| Other sources of income | |||||||
| SSD—no./total no. (%) | 523/3405 (15.36) | 78/786 (9.92) | 55/395 (13.92) | 119/894 (13.31) | 184/921 (19.98) | 87/409 (21.27) | <0.001 |
| Welfare—no./total no. (%) | 65/3405 (1.91) | 5/786 (0.64) | 8/395 (2.03) | 7/894 (0.78) | 30/921 (3.26) | 15/409 (3.67) | <0.001 |
| Employment—no./total no. (%) | <0.001 | ||||||
| Employed | 1623/3504 (46.32) | 467/818 (57.09) | 202/414 (48.79) | 432/917 (47.11) | 372/934 (39.83) | 150/421 (35.63) | |
| Unemployed | 816/3504 (23.29) | 157/818 (19.19) | 87/414 (21.01) | 229/917 (24.97) | 226/934 (24.20) | 117/421 (27.79) | |
| Disabled | 629/3504 (17.95) | 74/818 (9.05) | 62/414 (14.98) | 138/917 (15.05) | 250/934 (26.77) | 105/421 (24.94) | |
| Other | 436/3504 (12.44) | 120/818 (14.67) | 63/414 (15.22) | 118/917 (12.87) | 86/934 (9.21) | 49/421 (11.64) | |
| History of suicidal ideation—no./total no. (%) | 1348/3613 (37.31) | 211/868 (24.31) | 137/421 (32.54) | 356/950 (37.47) | 455/951 (47.84) | 189/423 (44.68) | <0.001 |
| Medication regimens – no./total no. (%) | <0.001 | ||||||
| Monotherapy | 620/3393 (18.27) | 202/785 (25.73) | 57/429 (13.29) | 150/869 (17.26) | 158/875 (18.06) | 53/435 (12.18) | |
| Two medications | 863/3393 (25.43) | 244/785 (31.08) | 85/429 (19.81) | 249/869 (28.65) | 208/875 (23.77) | 77/435 (17.70) | |
| Three medications | 738/3393 (21.75) | 145/785 (18.47) | 87/429 (20.28) | 203/869 (23.36) | 210/875 (24.00) | 93/435 (21.38) | |
| Four medications | 504/3393 (14.85) | 85/785 (10.83) | 69/429 (16.08) | 130/869 (14.96) | 145/875 (16.57) | 75/435 (17.24) | |
| Five or more medications | 668/3393 (19.69) | 109/785 (13.89) | 131/429 (30.54) | 137/869 (15.77) | 154/875 (17.60) | 137/435 (31.49) | |
| Anticholinergic drug score (Mean, SD) | 2.83 (1.91) | 2.36 (1.76) | 3.14 (1.92) | 2.75 (1.84) | 2.87 (1.92) | 3.40 (2.06) | <0.001 |
| Adherence—no./total no. (%) | |||||||
| Psychiatric medications | 2464/3352 (73.51) | 587/780 (75.26) | 319/423 (75.41) | 627/861 (72.82) | 628/864 (72.69) | 303/424 (71.46) | 0.488 |
| All medications | 2421/3392 (71.37) | 557/785 (70.96) | 315/429 (73.19) | 626/869 (72.04) | 620/875 (70.86) | 304/434 (70.05) | 0.838 |
|
Pathway Standardized care |
<0.001 | ||||||
| Standardized care | 3344/3537 (94.54) | 816/821 (99.39) | 416/431 (96.52) | 869/918 (94.66) | 844/926 (91.14) | 399/441 (90.48) | |
| Randomized care | 193/3537 (5.46) | 5/821 (0.61) | 15/431 (3.48) | 49/918 (5.34) | 82/926 (8.86) | 42/441 (9.52) | |
| Predicting adherence to* | All medications | Psychiatric only | |||||
| Classes (Ref = High benefit) | OR (95% CI) | OR (95% CI) | |||||
| Moderately high benefit | 0.87 (0.62–1.22) | 0.92 (0.64–1.31) | |||||
| Moderate benefit | 0.93 (0.71–1.22) | 1.14 (0.86–1.51) | |||||
| Moderately low benefit | 1.00 (0.76–1.31) | 1.14 (0.86–1.52) | |||||
| Low benefit | 1.00 (0.72–1.38) | 1.18 (0.84–1.65) |
Adjusted for medication regimens, gender, education, marital status, lives alone, income, social security disability insurance, welfare, employment, and pathway.
3.1. Exploratory factor analysis
Table 2 provides factor loadings for the three EFAs. Eigenvalues for the psychiatric symptoms EFA indicated one factor (first factor: 1.981, and second factor: 0.950). Although the factor loading for YMRS was less than 0.50 (0.312), BHS and MADRS only measure depressive states, therefore YMRS was retained to account for mania. The final psychiatric symptoms EFA retained one factor with three indicators: MADRS, BHS and YMRS. The overall functioning EFA eigenvalues indicated a one factor model (first factor: 2.090 and second factor: 0.704), therefore the final overall functioning EFA retained one factor with four indicators: QLESQ, LRIFT, GAF and Work Impairment. Although the eigenvalues for the adverse effects EFA indicated a two-factor model (first factor: 4.179 and second factor: 1.123), the second factor had only one measure. Therefore, the one factor model was retained, and the final adverse effects EFA included: memory difficulties, dry mouth, sexual dysfunction, headache, constipation, sedation, diarrhea, and tremor.
Table 2.
Results from exploratory factor analysis. Factor loadings are in order of importance.
| Measures | Factor loadings |
|---|---|
| Psychiatric symptoms | |
| MADRS | 0.808 |
| BHS | 0.652 |
| Panic | 0.404 |
| YMRS | 0.312 |
| Externalizing disorders* | −0.199 |
| Adverse events | |
| Memory difficulties | 0.804 |
| Dry mouth | 0.733 |
| Sexual dysfunction | 0.678 |
| Headache | 0.644 |
| Constipation | 0.601 |
| Sedation | 0.600 |
| Diarrhea | 0.537 |
| Tremor | 0.535 |
| Appetite increase | 0.487 |
| EPS | 0.230 |
| Functioning | |
| QLESQ | 0.703 |
| LRIFT | 0.629 |
| GAF past week | 0.557 |
| Work impact score** | 0.523 |
Bold = Kept in model.
Count that combined: Alcohol Abuse (Y/N); Current Substance Abuse or Dependence (Y/N); Binge Purge (Y/N); Caffeine Cups Per Day (cont.); Nicotine Packs Per Day (cont.).
Weighted combination: Unable to work or carry out normal activities; Had to cut down on what you did; Extreme effort to perform usual level of normal activities.
3.2. Latent class analysis
Model fit statistics indicated that both the five and six class models had comparable fit (Supplemental Table 1). However, the smallest class in the five class model consisted of N = 432 (12%) of the participants, whereas the smallest class in the six class model consisted of only N = 259 (7%) of the participants. Thus, the five class model was chosen due to the best balance of interpretability and model fit.
Results for the five class model of CNB defined by their responses on the three dimensions of CNB (psychiatric symptoms, overall functioning, and adverse effects) are in Fig. 2 and Supplemental Table 2. The five classes were: (1) high benefit (low symptoms, low adverse effects and high functioning; class prevalence: 24%); (2) moderately high benefit (moderately low symptoms, moderate adverse effects and moderately high functioning; class prevalence 12%); (3) moderate benefit (moderate symptoms, low adverse effects and moderate functioning; class prevalence 26%); (4) moderately low benefit (high symptoms, low adverse effects and low functioning; class prevalence 27%); and (5) low benefit (high symptoms, moderate adverse effects, and low functioning; class prevalence 12%).
Fig. 2.
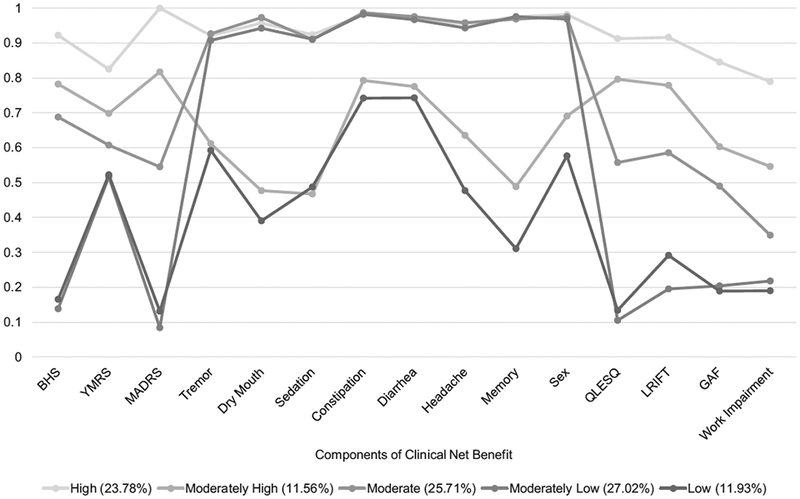
Results of the latent class analysis depicting the five classes of CNB. BHS, YMRS and MADRS are the psychiatric symptoms dimension. Tremor through sex are the adverse effects dimension. QLESQ, LRIFT, GAF and work impairment are the overall functioning dimension.
3.3. Characterizing the classes
The results of both the ANOVA and Chi-square tests between the classes are indicated as P-values in Table 1. Classes differed in all characteristics except in terms of age (F = 2.01; p = 0.09), race (χ2 = 5.51, p = 0.70) and primary residence (χ2 = 8.33, p = 0.40). The high benefit class had the highest proportion with graduate education (N = 189, 24%) and employment (N = 467, 57%) and the lowest percentage entering the Randomized Care Pathway (N = 5, 0.61%) and history of suicidal ideation (N = 211, 24%), while the low benefit class had the highest proportion unemployed (N = 117, 28%), receiving social security disability insurance (N = 87, 21%) and entering the Randomized Care Pathway (N = 42, 10%). This supports the external validity of these classes.
Psychotropic medication adherence did not differ across the classes (χ2 = 3.43, p = 0.488), ranging between 71% and 75%. Adherence to all medications also did not differ between classes (χ2 = 1.14, p = 0.838), ranging between 70% and 73%. This held true after adjusting for all significantly different between class demographic characteristics including medication regimens (i.e., monotherapy versus taking five or more medications; see bottom of Table 1) and standard versus randomized care pathway membership. However, medication regimens did differ between classes (χ2 = 167.39, p < 0.001; see Fig. 3). In the high benefit class over 50% were taking two or fewer medications. In contrast, in the low benefit class almost 50% were taking four or more medications. Only the monotherapy regimens (i.e., proportions of antidepressants, mood stabilizers, etc.) differed between the classes (Supplemental Table 3 and Supplemental Figure 2; χ2 = 39.8, p < 0.001). As the number of medications increased (i.e., two medications to three medications) the percent mood stabilizers decreased and other medications taken increased in all classes (i.e., 84% to 28% and 2% to 34% respectively in the high benefit class).
Fig. 3.
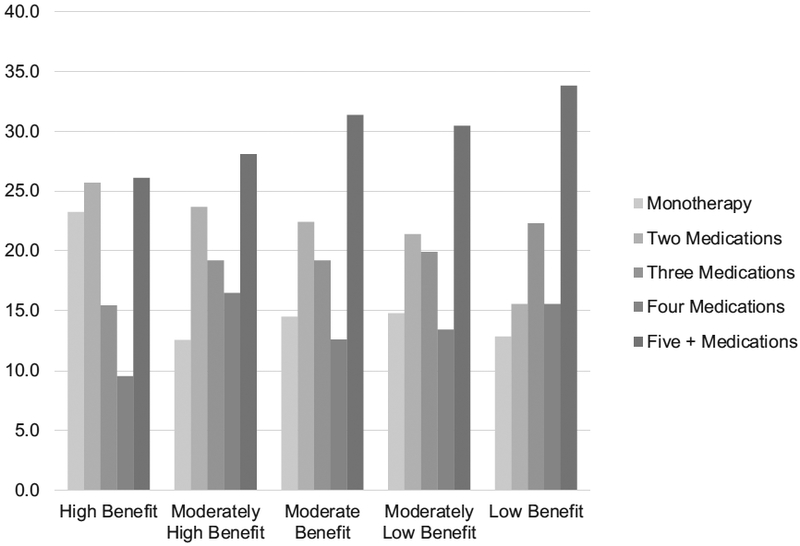
Medication regimens by class.
3.4. Validating CNB
CNB classes differed in SF-36 scores in the expected manner, providing validation support for this novel construct (Table 3). That is, the high benefit class had the highest SF-36 scores on all six scales, the moderately high benefit class had the second highest SF-36 scores on all six scales, etc. and this pattern held for almost all scales for all five classes. Additionally, the ADS score differed across classes in the expected pattern for almost all classes, with the lowest score in the high benefit class (Mean = 2.36, SD = 1.76) and the highest score in the low benefit class (Mean = 3.40, SD = 2.06). However, the moderately high benefit class had the second highest ADS score (Mean = 3.14, SD = 1.92). This reflects the association between the moderately high and low benefit classes that had similar probabilities in the adverse effects dimension (Fig. 2).
Table 3.
Scores on the SF-36 scale for the full sample and by latent classes of CNB.
| Full sample Mean (SD) | High benefit | Moderately high benefit | Moderate benefit | Moderately low benefit | Low benefit | |
|---|---|---|---|---|---|---|
| Physical functioning | 77.26 (25.80) | 83.05 (23.42) | 80.32 (23.76) | 78.21 (25.23) | 72.41 (27.58) | 71.86 (26.39) |
| Role-physical | 52.34 (43.69) | 65.10 (41.26) | 57.82 (42.70) | 51.84 (43.69) | 44.65 (43.52) | 40.89 (42.86) |
| General health | 54.52 (23.43) | 63.82 (22.10) | 57.52 (21.40) | 54.77 (22.68) | 47.97 (23.32) | 47.76 (23.11) |
| Vitality | 38.87 (22.85) | 49.68 (22.36) | 41.63 (21.64) | 39.26 (21.66) | 31.19 (21.63) | 31.82 (21.21) |
| Role-emotional | 34.50 (41.04) | 52.23 (42.60) | 37.83 (41.18) | 34.31 (40.06) | 22.38 (36.27) | 24.40 (37.33) |
| Mental health | 50.51 (24.16) | 64.71 (20.97) | 55.30 (22.95) | 51.30 (21.25) | 39.59 (22.90) | 40.97 (24.07) |
4. Discussion
The primary finding from this study is that the notion of CNB from treatment individuals receive for BD can be expanded beyond traditional metrics using latent variable techniques. We empirically identified subgroups of individuals with distinctly intersecting clinical characteristics of psychiatric symptoms, adverse effects and overall functioning using a novel three-dimensional model. Supporting our hypothesis and the external validity of these classes of CNB, the five subgroups of high, moderately high, moderate, moderately low and low benefit also differed in terms of sociodemographic characteristics such as education, employment, disability status, suicidal ideation, and entry into the Randomized versus Standard Care Pathways in STEP-BD. The construct validity of CNB was supported by the SF-36 analysis and the ADS score.
As indicated by Fig. 2, symptom reduction and global functioning were stronger differentiators of class membership than adverse effects. This is shown by the greater level of variation in both symptoms and functioning across classes. As a result, symptomatology and functioning contribute to differentiation across the classes to a greater degree than adverse effects. As reported by Chakrabarti (2016), many recent studies have found no association between side effects and non-adherence, further supporting the decreased importance of adverse effects.
Importantly, contrary to our hypothesis, although classes differed in the three CNB dimensions, they did not differ in medication adherence. Several reasons may explain this finding. First, adherence to psychotropic medications was approximately 74% and adherence to all medications was approximately 71% for this sample at baseline, which is higher than adherence rates reported in observational studies of individuals with BD (i.e., 30–50%) (Vieta, 2005). This is not unusual, as most clinical trials select for more adherent individuals, and these numbers are consistent with other randomized clinical trials (Sylvia et al., 2014). Second, all participants in STEP-BD were currently receiving treatment by a psychiatrist. Individuals actively seeking treatment with a psychiatrist in this population are more likely to be adherent. Thus, the variability in rates of adherence prior to study entry would be less, due to these characteristics. Third, although we defined adherence as it was originally defined in the study protocol, there are many other methods to define adherence. A very strict definition of adherence, classifying individuals missing any amount of their medications as non-adherent, could be used. Other methods to quantify adherence, as detailed in Chapman and Horne (2013), (i.e., taxonomy composed of initiation, discontinuation, implementation), elucidate how complicated adherence is to measure generally. As the study progressed, it is possible that adherence rates between the classes began to diverge.
Despite low benefit from treatment, individuals in the low benefit class had the same medication adherence as individuals benefiting from their treatment. These results suggest that factors associated with medication adherence identified by prior work (e.g., effects of the illness itself, adverse effects from medications, and complex regimens) are only part of the complex interplay of experiences individuals have of their illness and its treatment. As this is a cross-sectional analysis of participants in a treatment trial, we cannot directly assess if medication adherence improves clinical outcomes. Instead, these findings emphasize that there are factors—beyond adherence—that contribute to variation in CNB among people being treated for BD. Future work should examine whether the relationship between CNB and adherence changes over time.
Finally, of note is the apparent inverse relationship between polypharmacy and CNB. Specifically, the low benefit class had the highest prevalence of polypharmacy, with over half taking four or more different medications concurrently. Previous research has found that polypharmacy is associated with psychiatric and medical comorbidity (e.g., co-occuring anxiety disorder or diabetes). Moreover, while psychotropic polypharmacy has become increasingly common, rates of functional and psychiatric impairment have remained relatively constant (Weinstock et al., 2014).
4.1. Strengths
Strengths of this study include use of a large RCT with rigorous and extensive assessments. STEP-BD was a more heterogeneous sample than most RCTs in that it enrolled individuals with comorbidities, already taking medications, at different stages of illness, from a wide age range, and from the full spectrum of BD; this increases generalizability of the results. By using latent variable techniques, we empirically identified the indicators of CNB rather than relying solely on theoretical conceptualizations. Detailed information on medications allowed us to examine components of complex medication regimens commonly used to treat individuals with BD and their relationship with adherence. Finally, construct validity was supported by results from the SF-36 analysis.
4.2. Limitations
Limitations included the lack of measures of participants’ perceptions of their illness, of medications or other methods used to treat it, or of their individual preferences. Thus, our measure of CNB objectively infers benefit individuals are receiving from their treatment. Medication usage was not confirmed by pill counts or blood serum levels; however, the clinical interview used here is best-practice for large, complex trials like STEP-BD. Missing data limited the number of measures used to define the CNB construct. In addition, due to the cross-sectional nature of this study, and the use of baseline data, multiple forms of treatment received by individuals prior to their study entrance could be affecting their CNB beyond medication. These include psychotherapy, relationship between individuals and their clinicians, types of clinicians they are seeing (social worker versus psychiatrist), as well as other unmeasured factors.
Conclusions
As we hypothesized, our analysis did identify five unique classes of individuals who systematically differed in objective characteristics of CNB; high benefit, moderately high benefit, moderate benefit, moderately low benefit and low benefit. However, we did not find differences in the association between adherence, both to psychotropic and all medications, and these classes. Our findings support the importance of collaborative, person-centered, shared decision-making approaches to treatment to identify targets for supporting medication adherence. Our results are broadly consistent with previous studies of the experience of individuals with BD that highlight the importance of perceived necessity of medication versus concerns about adverse effects; if perceptions of necessity outweigh concerns, individuals may continue taking their medications even if symptom management and functioning is suboptimal. This may contribute to the unexpected finding of high adherence across these groups that differed substantially in CNB. The group of individuals who are adherent despite low benefit from their treatment could provide new insight into CNB. Identifying individuals in this group could help their treating clinicians to initiate changes to their treatment, with the goal of increasing their CNB.
Supplementary Material
Acknowledgments
N. Bareis accessed the STEP-BD limited access dataset, clinical-trials.gov Identifier: NCT00012558, through the NDCT, and conducted the data analysis. J. Lu, C. Kirkwood, S. Kornstein, E. Wu and B. Mezuk provided feedback on the analysis plan, provided writing assistance and proof read the manuscript.
Financial support
This work was supported by the National Institute of Mental Health (B. Mezuk, grant number K01-MH093642), (STEP-BD, grant number N01-MH80001), (N. Bareis, grant number 5T32MH020004–18). This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Footnotes
Author contributors
None.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jad.2018.05.063.
Conflict of interest
None.
References
- Adams J, Scott J, 2000. Predicting medication adherence in severe mental disorders. Acta. Psychiatr. Scand 101, 119–124. [DOI] [PubMed] [Google Scholar]
- Arnau RC, Meagher MW, Norris MP, Bramson R, 2001. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol 20, 112–119. [DOI] [PubMed] [Google Scholar]
- Arnold LM, Witzeman KA, Swank ML, McElroy SL, Keck PE Jr., 2000. Health-related quality of life using the SF-36 in patients with bipolar disorder compared with patients with chronic back pain and the general population. J. Affect. Disord 57, 235–239. [DOI] [PubMed] [Google Scholar]
- Bates JA, Whitehead R, Bolge SC, Kim E, 2010. Correlates of medication adherence among patients with bipolar disorder: results of the bipolar evaluation of satisfaction and tolerability (BEST) study: a nationwide cross-sectional survey. Prim. Care Companion J. Clin. Psychiatry 12 pii: PCC.09m00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W, 1996. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Pers. Assess 67, 588–597. [DOI] [PubMed] [Google Scholar]
- Berk M, Berk L, Castle D, 2004. A collaborative approach to the treatment alliance in bipolar disorder. Bipolar Disord 6, 504–518. [DOI] [PubMed] [Google Scholar]
- Bloom DE, Cafiero ET, Jane-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C, 2011. The Global Economic Burden of Noncommunicable Diseases. World Economic Forum, Geneva. [Google Scholar]
- Bowling A, 2005. Mode of questionnaire administration can have serious effects on data quality. J. Public Health (Oxf) 27, 281–291. [DOI] [PubMed] [Google Scholar]
- Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR, 2006. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J. Clin. Pharmacol 46, 1481–1486. [DOI] [PubMed] [Google Scholar]
- Cattell RB, 1966. The Scree test for the number of factors. Multivariate Behav. Res 1, 245–276. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, 2016. Treatment-adherence in bipolar disorder: a patient-centred approach. World J. Psychiatry 6, 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Horne R, 2013. Medication nonadherence and psychiatry. Curr. Opin. Psychiatry 26, 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy J, Bowskill R, Parham R, Rank T, Scott J, Horne R, 2009. Understanding medication non-adherence in bipolar disorders using a Necessity-Concerns Framework. J. Affect.Disord 116, 51–55. [DOI] [PubMed] [Google Scholar]
- Cochran SD, Gitlin MJ, 1988. Attitudinal correlates of lithium compliance in bipolar affective disorders. J. Nerv. Ment. Dis 176, 457–464. [DOI] [PubMed] [Google Scholar]
- Crowe M, Wilson L, Inder M, 2011. Patients’ reports of the factors influencing medication adherence in bipolar disorder - an integrative review of the literature. Int. J. Nurs. Stud 48, 894–903. [DOI] [PubMed] [Google Scholar]
- De Las Cuevas C, Penate W, Sanz EJ, 2014. The relationship of psychological reactance, health locus of control and sense of self-efficacy with adherence to treatment in psychiatric outpatients with depression. BMC Psychiatry 14, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVellis RF, 1991. Scale Development: Theory and Applications, Sage, Thousand Oaks, CA. [Google Scholar]
- Dong Y, Peng CY, 2013. Principled missing data methods for researchers. Springerplus 2, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch WH, 2011. A comparison of factor rotation methods for dichotomous data. J. Mod. Appl. Stat. Methods 10, 549–570. [Google Scholar]
- Goldberg LR, Velicer WF, 2006. Principles of exploratory factor analysis, Second edition Springer, New York, NY. [Google Scholar]
- Horne R, Weinman J, Hankins M, 1999. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol. Health 14, 1–24. [Google Scholar]
- Kemp DE, 2014. Managing the side effects associated with commonly used treatments for bipolar depression. J. Affect. Disord 169, S34-S44. [DOI] [PubMed] [Google Scholar]
- Ketter TA, 2010. Strategies for monitoring outcomes in patients with bipolar disorder. Prim. Care Companion J. Clin. Psychiatry 12, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Frank E, Kupfer DJ, 2011. How to assess the clinical impact of treatments on patients, rather than the statistical impact of treatments on measures. Int. J. Methods Psychiatr. Res 20, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD, 2007. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J. Abnorm. Psychol 116 645–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Kopeinig M, Chen CK, Fabian A, Pujol-Luna MG, Shin YC, Treuer T, D’Yachkova Y, Deix C, Kasper S, Doby D, 2014. Compliance as a stable function in the treatment course of bipolar disorder in patients stabilized on olanzapine: results from a 24-month observational study. Int. J. Bipolar Disord 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Collins LM, Lemmon DR, Schafer JL, 2007. PROC LCA: A SAS procedure for latent class analysis. Struct. Equ. Modeling 14, 671–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB, 1999. The Range of Impaired Functioning Tool (LIFE-RIFT): a brief measure of functional impairment. Psychol. Med 29, 869–878. [DOI] [PubMed] [Google Scholar]
- McCutcheon AC, 1987. Latent Class Analysis Sage Publications, Beverly Hills. [Google Scholar]
- McHorney CA, Ware JE Jr., Raczek AE, 1993. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 31, 247–263. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, 2009. Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J. Clin. Psychiatry 70, 5–11. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Kendler KS, 2012. Examining variation in depressive symptoms over the life course: a latent class analysis. Psychol. Med 42, 2037–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli PL, Elgie R, Europe G, 2003. GAMIAN-Europe/BEAM Survey I–global analysis of a patient questionnaire circulated to 3450 members of 12 European advocacy groups operating in the field of mood disorders. Bipolar Disord 5, 265–278. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO, 1998. Mplus User’s Guide, Seventh Edition Muthen & Muthen, Los Angeles. CA. [Google Scholar]
- National Drug Code Directory, 2016. http://www.accessdata.fda.gov/scripts/cder/ndc/default.cfm (accessed 16.Dec 31).
- Perils RH, Ostacher MJ, Miklowitz DJ, Hay A, Nierenberg AA, Thase ME, Sachs GS, 2010. Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J. Clin. Psychiatry 71, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs GS, Guille C, McMurrich SL, 2002. A clinical monitoring form for mood disorders. Bipolar Disord 4, 323–327. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF, 2003. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol. Psychiatry 53, 1028–1042. [DOI] [PubMed] [Google Scholar]
- Sajatovic M, Biswas K, Kilbourne AK, Fenn H, Williford W, Bauer MS, 2008. Factors associated with prospective long-term treatment adherence among individuals with bipolar disorder. Psychiatr. Serv 59, 753–759. [DOI] [PubMed] [Google Scholar]
- Smarty S, Findling RL, 2007. Psychopharmacology of pediatric bipolar disorder: a review. Psychopharmacology (Berl) 191, 39–54. [DOI] [PubMed] [Google Scholar]
- Svarstad BL, Shireman TI, Sweeney JK, 2001. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr. Serv 52, 805–811. [DOI] [PubMed] [Google Scholar]
- Sylvia LG, Reilly-Harrington NA, Leon AC, Kansky CI, Calabrese JR, Bowden CL, Ketter TA, Friedman ES, Iosifescu DV, Thase ME, Ostacher MJ, Keyes M, Rabideau D, Nierenberg AA, 2014. Medication adherence in a comparative effectiveness trial for bipolar disorder. Acta Psychiatr. Scand 129, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, 2007. Using Multivariate Statistics. Pearson, Boston, MA. [Google Scholar]
- Thomas T, Stansifer L, Findling RL, 2011. Psychopharmacology of pediatric bipolar disorders in children and adolescents. Pediatr. Clin. North Am 58, 173–187 xii. [DOI] [PubMed] [Google Scholar]
- Velligan D, Sajatovic M, Valenstein M, Riley WT, Safren S, Lewis-Fernandez R, Weiden P, Ogedegbe G, Jamison J, 2010. Methodological challenges in psychiatric treatment adherence research. Clin. Schizophr. Relat. Psychoses 4, 74–91. [DOI] [PubMed] [Google Scholar]
- Vieta E, 2005. Improving treatment adherence in bipolar disorder through psychoeducation. J. Clin. Psychiatry 66, 24–29. [PubMed] [Google Scholar]
- Ware JE Jr., Snow KK, Kosinski M, Gandek B, 1993. SF-36 Health Survey Manual and Interpretation Guide The Health Institute, New England Medical Center; Massachusetts, Boston. [Google Scholar]
- Weiden P, Rapkin B, Mott T, Zygmunt A, Goldman D, Horvitz-Lennon M, Frances A, 1994. Rating of medication influences (ROMI) scale in schizophrenia. Schizophr Bull 20, 297–310. [DOI] [PubMed] [Google Scholar]
- Weinstock LM, Gaudiano BA, Epstein-Lubow G, Tezanos K, Celis-Dehoyos CE, Miller IW, 2014. Medication burden in bipolar disorder: a chart review of patients at psychiatric hospital admission. Psychiatry Res 216, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolston A, Tu YK, Baxter PD, Gilthorpe MS, 2012. A comparison of different approaches to unravel the latent structure within metabolic syndrome. PLoS One 7, e34410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington RL, Whittaker TA, 2006. Scale development research: a content analysis and recommendations for best practices. Couns. Psychol 34, 806–838. [Google Scholar]
- Yatham LN, Kennedy SH, O’Donovan C, Parikh S, MacQueen G, McIntyre R, Sharma V, Silverstone P, Alda M, Baruch P, Beaulieu S, Daigneault A, Milev R, Young LT, Ravindran A, Schaffer A, Connolly M, Gorman CP, Canadian Network for M, Anxiety T, 2005. Canadian Network for Mood and Anxiety Treatments (CANMAT) Guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord 7, 5–69. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, O’Donovan C, Macqueen G, McIntyre RS, Sharma V, Ravindran A, Young LT, Milev R, Bond DJ, Frey BN, Goldstein BI, Lafer B, Birmaher B, Ha K, Nolen WA, Berk M, 2013. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 15, 1–44. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, Sharma V, Goldstein BI, Rej S, Beaulieu S, Alda M, MacQueen G, Milev RV, Ravindran A, O’Donovan C, McIntosh D, Lam RW, Vazquez G, Kapczinski F, McIntyre RS, Kozicky J, Kanba S, Lafer B, Suppes T, Calabrese JR, Vieta E, Malhi G, Post RM, Berk M, 2018. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 20, 97–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


