Abstract
Purpose
To assess whether continuous embryo culture involves better embryological and/or clinical outcomes than sequential.
Methods
Prospective study at a private IVF center. All consecutive IVF cycles (September 2013–2015) fulfilling the inclusion criteria underwent embryo culture in either Continuous-Single-Culture-Media (CSCM, n = 972) or sequential media (Quinn’s Advantage, n = 514), respectively. ICSI, blastocyst culture in either standard (MINC) or undisturbed (Embryoscope) incubation, transfer (until September 2016), and pregnancy follow-up (until September 2017) were performed. When aneuploidy testing was required, trophectoderm biopsy and qPCR were performed. Sub-analyses and logistic regression corrected for confounders were performed. The primary outcomes were overall blastocyst rate per oocyte and mean blastocyst rate per cycle. The sample size was defined to reach 95 and 80% statistical power for the former and the latter outcome, respectively. Secondary outcomes were euploidy (if assessed), cumulative delivery rates, gestational age, and birthweight.
Results
Continuous embryo culture resulted into a higher overall blastocyst rate per inseminated oocyte than sequential (n = 2211/5841, 37.9% vs. 1073/3216, 33.4%; p < 0.01), confirmed also from a cycle-based analysis (mean blastocyst rate: 38.7% ± 29.7% vs. 34.3% ± 29.4%; p = 0.01). The continuous media (OR = 1.23), the undisturbed incubation system (OR = 1.22), the maternal age (OR = 0.92), and the sperm factor (OR = 0.85) were outlined as positive predictors of blastulation. However, the cumulative delivery rates per ended cycle (i.e., delivery achieved or no blastocyst produced or left; > 90%) were comparable in the two groups (n = 244/903, 27.0% vs. 129/475, 27.2%). The neonatal outcomes were similar.
Conclusions
Continuous culture involves better embryological but similar clinical outcomes than sequential. This large prospective study supports the absence of clinical disparity among the two approaches.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1195-4) contains supplementary material, which is available to authorized users.
Keywords: Continuous culture, Sequential culture, Single-step media, Sequential media, Blastocyst
Introduction
The standardization of embryo culture conditions and culture media composition has been an important concern in IVF across the last decades [1, 2]. To this regard, time-lapse incubators were introduced and provided the IVF laboratories with the possibility to implement an undisturbed culture system and limit both embryo manipulation and the systematic changes in temperature and gas concentrations [3–5]. Regarding embryo culture to blastocyst, instead, two different approaches have been theorized and adopted across the years, namely the continuous (or single step) and the sequential one, without a clear definition of superiority (if any) of one with respect to the other. The former approach involves embryo growth in a single type of media (it is based on the “let the embryo choose” theory) with or without a refresh with a new drop of the same media at the cleavage stage, while the latter approach requires a changeover of the kind of media adopted for the first 3 days of culture with a different one, meant to better sustain embryo development to blastocyst due to the modification of its nutritional requirements (it is based on the “back to nature” theory) [6–8]. Several kinds of culture media have been commercialized to date with different compositions, but all of them are based on either the former or the latter theory.
When compared in prospective observational or randomized controlled trials (RCTs), single-step and sequential embryo culture approaches showed conflicting results in terms of blastocyst formation and embryo morphological quality. For instance, in a study published in 2002, Macklon and colleagues reported no difference in both the blastulation rate and the ongoing pregnancy rate through continuous embryo culture, with or without refresh at the cleavage stage, with respect to sequential approach [9]. Similarly, more recently in 2013, Summers conducted a morphological assessment study of sibling zygotes cultured either in a continuous medium without changeover in day 3 or in a sequential one. Also in this case, no difference was observed in terms of blastulation rates, inner cell mass, and trophectoderm scores [10]. Conversely, some other authors highlighted a higher blastulation and morphological quality by using a continuous media rather than a sequential one [11].
Recently, Sfontouris and colleagues performed a meta-analysis of the main studies related to this topic and concluded that a higher blastocyst rate, as well as superior morphological quality, may be achieved when a continuous culture media is used [12]. Yet, this did not result into better clinical outcomes, as supported also by the meta-analysis published by Diemant and colleagues in 2017 [12, 13].
All the studies previously cited were performed by using a conventional incubation system. Instead, very few studies compared the two approaches in a time-lapse-based undisturbed incubation environment. The main paper has been published by Basile and colleagues in 2013, where the authors reported the absence of differences among the morphokinetic parameters up to day 3 of preimplantation development between continuous and sequential embryo culture [14]. However, they did not follow the embryo culture up to the blastocyst stage.
Similarly, very few data have been published also dealing with the definition of the euploidy rate after either continuous or sequential culture. To this regard, the main study published to date is the paired RCT by Werner and colleagues [15], where sibling 2PN zygotes were cultured with either a continuous or a sequential approach. Here, the authors did not report any difference in terms of euploidy rate per biopsied blastocyst and euploid blastocysts’ reproductive competence. However, a significantly higher euploidy rate per 2PN zygote was reported with the sequential media, mainly because of a higher blastocyst rate with respect to the single-step one.
Finally, to our knowledge, few or no data are available to date to compare the cumulative pregnancy rate per cycle and the obstetrical and perinatal outcomes among the two approaches. Nevertheless, this information is pivotal to define the safety of embryo culture performed following either the “let the embryo choose” or the “back to nature” theory.
In this prospective study, we aimed at assessing whether the blastocyst rate per metaphase (MII) oocyte is different among IVF cycles conducted in either a single-step (Irvine Scientific, Australia; Continuous-Single-Culture-Media, CSCM) or a sequential (Cooper Surgical Fertility Companies, USA; Quinn’s Advantage Cleavage and Blastocyst media) media from both an oocyte-based and a cycle-based perspective. Part of the cycles underwent undisturbed embryo culture in a time-lapse incubator and/or preimplantation genetic testing for aneuploidies (PGT-A). This allowed us to perform some important sub-analyses and to assess the primary outcome also in different incubation conditions (undisturbed versus standard culture environment), as well as to evaluate the euploidy rate in the cycles addressed to PGT-A. The oocyte retrievals were conducted for the first 2 years of the study, then 1 year of follow-up of the related frozen embryo transfers (ETs) was included. Therefore, we could evaluate both the live birth rate per ET and the cumulative delivery rate per completed cycle for almost all the treatments performed during the study period. A further year was included for sensing both the gestational age and the birthweight of the newborns among the deliveries from the two study groups.
Materials and methods
Study design
This is a prospective study performed at a single private IVF center in Italy. All the consecutive oocyte retrievals (September 2013–September 2015) where at least one MII oocyte was obtained were included. The exclusion criteria were PGT cycles conducted for monogenic diseases (PGT-M) or structural rearrangements (PGT-SR) and cycles requiring a surgical retrieval of the sperm due to azoospermia. The allocation of the cycles into each study group was quasi-randomized: single-step embryo culture approach (Irvine CSCM) was adopted for all IVF cycles performed starting on Monday, Wednesday, Thursday, and Friday, while sequential embryo culture approach (Quinn’s Advantage) was adopted for all IVF cycles performed starting on Tuesday or Saturday. The protocols, schedules, and operators involved in the clinical activity did not vary from day to day. The clinicians were blinded to the allocation among the two study groups. All frozen ETs performed up to September 2016 were included, and the pregnancies were followed up to September 2017. Blastocyst culture in either standard benchtop (MINC, Cook Medical, USA) or undisturbed incubator (Embryoscope, Vitrolife, Sweden) was conducted without any specific indication and depending on the availability of free chambers in the latter. PGT-A was conducted when indicated and/or requested from the patients, who signed an informed consent after extensive counseling. The Institutional Review Board of the clinic approved the study.
Outcome measures
The primary outcome measure was defined as the blastocyst rate per inseminated MII oocyte. The a priori sample size calculation highlighted that 8562 MII oocytes (5708 in the Irvine CSCM and 2854 in the Quinn’s Advantage arm, respectively) were required to achieve a 95% statistical power (α = 0.05) in assessing down to 4% difference in the blastocyst rate per MII oocyte between the study groups. From a cycle-based perspective, by estimating an average of 6 MII oocytes per treatment, such sample size corresponded to 1427 cycles (951 in the Irvine CSCM and 476 in the Quinn’s Advantage arm, respectively) and to 80% power in assessing down to 4% difference in the mean blastocyst rate per cycle between the study groups.
The secondary outcomes included all the embryological data to investigate putative disparities among the two culture strategies, from both an oocyte-based and a cycle-based perspective (e.g., overall and mean fertilization rate per cycle or overall and mean euploid blastocyst rate per PGT-A cycle). The rate of cycles without fertilized oocyte, blastocyst, and euploid blastocyst (among PGT-A cycles) produced, as well as the clinical outcomes (i.e., biochemical pregnancy rate, miscarriage rate and live birth rate per ET), were all monitored.
Two sub-analyses were performed (i) in ICSI and ICSI+PGT-A cycles and (ii) in standard or undisturbed incubation environment.
The gestational age and the birthweight were also reported, clustered according to the WHO guidelines, and compared in the two study arms.
Finally, to assess the efficacy of the two approaches, an analysis of the cumulative delivery rate per completed cycle was performed (defined as the number of cycles with at least one live birth among all the cycles in the study, calculated by including all fresh and/or frozen blastocyst transfers until one delivery was achieved or until all embryos were used [16]).
Laboratory procedures
After controlled ovarian stimulation (conducted as described previously [17]), cumulus-oocyte complexes (COCs) were aspirated 36 h after ovulation induction. COCs were collected from the follicular fluid in either Irvine CSCM or Quinn’s Advantage fertilization medium added with 3% Quinn’s Advantage Human Serum Albumin (HSA) and cultured for 2–3 h at 37 °C in controlled atmosphere and humidity and low oxygen tension (6%CO2 and 5%O2) until ICSI. Briefly, the oocytes were denuded in a HEPES-buffered medium containing 20 IU/μL hyaluronidase (either Irvine Scientific or Quinn’s Advantage). ICSI was performed as described previously [18] in the HEPES-buffered medium (either Irvine Scientific or Quinn’s Advantage). All the embryos obtained from fertilized oocytes (defined by the presence of two equally sized pronuclei after 16–20 h from insemination) were cultured at 37 °C in separate 25-μl single microdrops (either Irvine CSCM or Quinn’s Advantage Cleavage and Blastocyst media both added with 5% Quinn’s Advantage HSA) up to the blastocyst stage (day 5–7) in a humidified atmosphere containing 5%O2 and 6%CO2. If Quinn’s Advantage cleavage media was used, a changeover in day 3 was performed with new 25-μl drops of Quinn’s Advantage blastocyst medium. To ensure stable culture conditions and preserve embryo developmental competence, the temperature and gas concentrations in the incubators were daily monitored (as well as the environmental temperature and humidity), and the pH of the culture media was confirmed to fulfill the ideal ranges according to the manufacturers’ indications. Either a standard benchtop incubator (MINC) or an undisturbed time-lapse one (Embryoscope) was used.
All the blastocysts were categorized in three groups according to the day of full development (day 5, day 6, or day 7), and in four groups according to their morphological quality: excellent (≥ 3AA), good (3, 4, 5, 6, AB, and BA), average (3, 4, 5, 6 BB, AC, and CA), and poor (≤ 3BB), based on the inner cell mass (A = numerous tightly packed cells; B = several and loosely packed cells; C = very few cells) and trophectoderm (A = many cells organized in epithelium; B = several cells organized in loose epithelium; C = few large cells) quality scores (as previously defined in [19, 20]). All embryos were cultured to the fully expanded blastocyst stage, independently from embryo morphological quality.
In the PGT-A cycles, trophectoderm biopsy was performed only on fully expanded blastocysts as previously described [19]. This approach does not entail any hatching procedure at the cleavage stage, and sequential laser-assisted zona opening and mouth-controlled pipette-assisted trophectoderm fragment retrieval are conducted. The biopsy was performed on all embryos that developed as viable blastocysts, independently from the day of full expansion and their morphological quality. Quantitative polymerase chain reaction (qPCR)-based 24-chromosome aneuploidy testing was conducted according to Treff and colleagues [21], a method which was internally validated in our laboratory in 2015 [22]. This methodology was designed to specifically identify constitutive whole chromosome but not segmental aneuploidies.
Vitrification was performed for either supernumerary blastocyst after fresh ET or any blastocyst obtained in freeze-all cycles with or without PGT-A. In the latter case, the blastocysts were cryopreserved soon after biopsy. Vitrification and warming were performed according to Cobo and colleagues [23] by using Cryotop devices and solutions (Kitazato BioPharma Co., Japan). Only blastocyst transfers were performed. Soon after warming and until transfer, the blastocyst was placed into the same culture medium used for embryo culture. In PGT-A cycles, only euploid blastocysts were selected for transfer and only frozen ET were performed. Endometrial preparation and transfer procedures were performed as previously described [24].
Statistical analysis
The a priori sample size analysis (as well as the post hoc power analysis) was conducted through the software G*Power v3.1. Patient, cycle, embryological, and transfer data were prospectively collected in a relational database (Fertilab, Italy). Continuous data are presented as mean with standard deviation and range. Categorical variables are presented as absolute, percentage frequency, and 95% CI. Fisher’s exact test or chi-square and t tests were used to assess differences between categorical and continuous variables, respectively. A p value < 0.05 was considered significant. Univariable and multivariable logistic regression analyses were performed to identify the significant predictors of the possibility of an oocyte to develop to the blastocyst stage (e.g., the media used for embryo culture, the maternal age, the sperm factor classified as previously described [25] in normozoospermic, moderate male factor or oligoasthenoteratozoospermic, and the kind of incubator used). The software R was adopted for statistics and logistic regression analyses.
Results
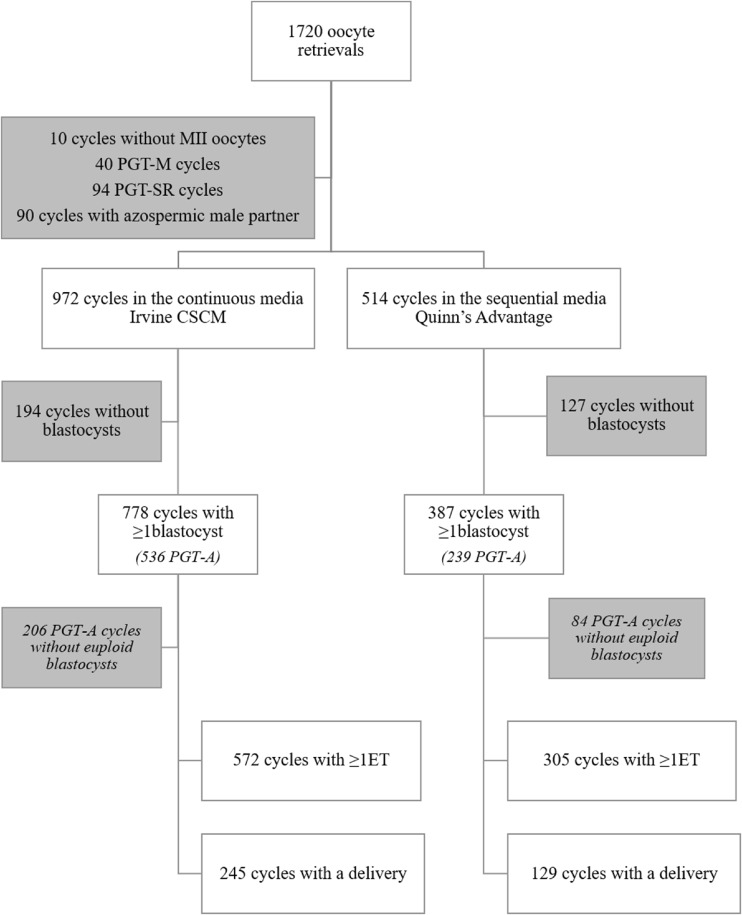
In a 2-year period (September 2013–September 2015), 1486 of the 1720 oocyte retrievals performed were eligible for this study (Fig. 1). In 10 cycles, no MII oocyte was found, 90 cycles were excluded because of azoospermic male partners, and 40 and 94 cycles since PGT-M or PGT-SR were respectively required. Nine hundred seventy-two and 514 cycles with at least 1 MII oocyte were allocated in the Irvine CSCM or Quinn’s Advantage study group, respectively, depending on the day of the week when the retrieval was performed (see Material and methods). The main characteristics of the cycles were similar among the study groups (e.g., the mean maternal age was 38.5 in both arms) (Table 1). In 21.8 and 25.1% of the cycles conducted through Irvine CSCM and Quinn’s Advantage media, respectively, embryo culture was carried out in an undisturbed incubation environment, rather than into the standard (Table 1; p = NS). Six hundred sixty-four and 309 cycles were respectively addressed to PGT-A (Fig. 1).
Fig. 1.
Flowchart of the study. PGT-A, preimplantation genetic testing for aneuploidies; PGT-M, PGT for monogenic diseases; PGT-SR, PGT for structural rearrangements; ET, embryo transfer
Table 1.
Main characteristics of the cycles included in the study
| Continuous media Irvine CSCM |
Sequential media Quinn’s Advantage |
|
|---|---|---|
| IVF cycles, n | 972 | 514 |
| Maternal age, mean ± SD (min-max) | 38.5 ± 3.6 (26–45) | 38.5 ± 3.7 (26–45) |
| Paternal age, mean ± SD (min-max) | 41.4 ± 5.5 (23–70) | 41.4 ± 5.5 (23–61) |
| Obstetric history, n, % | ||
| Never conceived | 686, 70.6 | 362, 70.4 |
| Conceived (either delivery or miscarriage) | 286, 29.4 | 152, 29.6 |
| Main infertility factor, n, % | ||
| Endocrine-ovulatory | 27, 2.8 | 15, 2.9 |
| Endometriosis | 75, 7.7 | 48, 9.3 |
| Tubal factor | 132, 13.6 | 51, 10.0 |
| Idiopathic | 738, 75.9 | 400, 77.8 |
| Ovarian stimulation, n, % | ||
| Antagonist protocol | 960, 98.7 | 504, 98.1 |
| Agonist protocol | 12, 1.3 | 10, 1.9 |
| Sperm factor, n, % | ||
| Normozoospermic | 469, 48.3 | 244, 47.5 |
| MMF | 348, 35.8 | 192, 37.3 |
| OAT | 155, 15.9 | 78, 15.2 |
| Incubation environment, n, % | ||
| Standard | 760, 78.2 | 385, 74.9 |
| Undisturbed | 212, 21.8 | 129, 25.1 |
MMF moderate male factor (1–2 defects), OAT oligoasthenoteratozoospermic
A total of 5841 (mean = 6.0 ± 4.0, range: 1–24) and 3216 (mean = 6.3 ± 4.3, 1–24) MII oocytes were respectively inseminated in Irvine CSCM and Quinn’s Advantage study arm (overall: 9057) (Table 2). The overall fertilization rates were 71.9% (n = 4203/5841, mean = 4.3 ± 3.2, 0–19) and 74.4% (n = 2391/3216, mean = 4.7 ± 3.5, 0–19), significantly higher in the latter (p = 0.01) (Table 2). Although, the mean fertilization rate per cycle was similar in the two groups (71.0% ± 27.8% vs. 72.7% ± 26.5%; p = NS) (Table 2). This translated into 6.7% (n = 65/972) and 6.4% (n = 33/514) of cycles without fertilized oocytes, respectively (p = NS) (Table 2).
Table 2.
Main outcomes of the study
| Continuous media Irvine CSCM |
Sequential media Quinn’s Advantage |
P value | |||
|---|---|---|---|---|---|
| Cycles, n | 972 | 514 | |||
| MII oocytes, n, mean ± SD (min-max) per cycle | 5841, 6.0 ± 4.0 (1–24) | 3216, 6.3 ± 4.3 (1–24) | NS | ||
| Fertilization | |||||
| Mean fertilization rate per cycle ± SD, % (min-max) | 71.0 ± 27.8, (0–100) | 72.7 ± 26.5, (0–100) | NS | ||
| Fertilized oocytes, n, mean ± SD (min-max) per cycle % of MII oocytes (95% CI) |
4203, 4.3 ± 3.2 (0–19) 71.9 (70.8–73.1) |
2391, 4.7 ± 3.5 (0–19) 74.4 (72.8–75.8) |
NS
0.01 |
||
| Cycles without fertilized oocytes, n, % (95% CI) | 65, 6.7 (5.2–8.5) | 33, 6.4 (4.5–9.0) | NS | ||
| Blastulation | |||||
| Mean blastocyst rate per cycle ± SD, % (min-max) | 38.7 ± 29.7 (0–100) | 34.3 ± 29.4 (0–100) | 0.01 | ||
| Blastocysts, n, mean ± SD (min-max) per cycle % of MII oocytes (95% CI) |
2211, 2.3 ± 2.1 (0–13) 37.9 (36.6–39.1) |
1073, 2.1 ± 2.1 (0–11) 33.4 (31.7–35.0) |
NS
< 0.01 |
||
| Cycles without blastocysts, n, % (95% CI) | 194, 20.0 (17.5–22.6) | 127, 24.7 (21.1–28.7) | < 0.01 | ||
| Clinical outcomes | |||||
| Cycle strategy | ICSI | ICSI + PGT-A | ICSI | ICSI + PGT-A | |
| Maternal age, mean ± SD (min-max) | 36.4 ± 4.0 year, 26–45 | 39.4 ± 3.0 year, 28–45 | 36.9 ± 4.0 year, 26–45 | 39.5 ± 3.1 year, 26–45 | |
| ET, n | 384 | 418 | 232 | 201 | |
| Fresh ET, n, % of ETs | 170, 44.3 | / | 109, 47.0 | / | |
| Frozen ET, n, % of ETs | 214, 55.7 | 418, 100 | 123, 53.0 | 201, 100 | |
| Mean number of embryos transferred ± SD | 1.1 ± 0.3 | 1.0 ± 0.2 | 1.2 ± 0.4 | 1.0 ± 0.1 | |
| Total number of blastocysts transferred, n | 430 | 428 | 285 | 203 | |
| SET, n, % of ETs | 338, 88.0 | 408, 97.6 | 181, 78.0 | 199, 99.0 | |
| DET, n, % of ETs | 46, 12.0 | 10, 2.4 | 49, 21.1 | 2, 1.0 | |
| TET, n, % of ETs | / | / | 2, 0.9 | / | |
| Positive pregnancy test, n, % of ETs (95% CI) | 128, 33.3 (26.7–38.3) | 227, 54.3 (49.4–59.1) | 84, 36.2 (30.1–42.8) | 99, 49.3 (42.2–56.3) | |
| BPLs, n, % of positive pregnancy tests (95% CI) | 18, 14.1 (8.8–21.6) | 32, 14.1 (9.9–19.5) | 16, 19.1 (11.6–29.4) | 10, 10.1 (5.2–18.2) | |
| Miscarriages, n, % of clinical pregnancies (95% CI) | 28, 25.4 (17.8–34.8) | 28, 14.4 (9.9–20.2) | 14, 23.5 (14.4–35.6) | 9, 10.1 (5.0–18.8) | |
| Deliveries, n, % of ETs (95% CI) | 82, 21.4 (17.4–25.9) | 167, 40.0 (35.2–44.8) | 54, 23.3 (18.1–29.4) | 80, 39.8 (33.0–46.9) | |
| Live births, n, % of blastocysts transferred (95% CI) | 83, 19.3 (15.7–23.4) | 168, 39.3 (34.6–44.1) | 56, 19.6 (15.3–24.8) | 81, 39.9 (33.2–47-0) | |
| Singleton, n, % of deliveries | 81, 98.8 | 166, 99.4 | 52, 96.3 | 79, 98.8 | |
| Twin, n, % of deliveries | 1, 1.2 | 1, 0.6 | 2, 3.7 | 1, 1.2 | |
| Perinatal and obstetrical outcomes | |||||
| Gestational age, mean ± SD (min-max) | 38 ± 1 weeks (29–41) | 38 ± 2 weeks (28–41) | NS | ||
| Birthweight, mean ± SD (min-max) | 3247 ± 509 g (1600–4700) | 3185 ± 631 g (1400–4650) | NS | ||
The statistically-significant differences are highlighted in bold
PGT-A preimplantation genetic testing for aneuploidies, MII metaphase II, ET embryo transfer, SET single ET, DET double ET, TET triple ET, BPL biochemical pregnancy loss, NS not significant
The overall blastocyst rate per MII oocyte was significantly higher (p < 0.01) when embryo culture was conducted through Irvine CSCM instead of Quinn’s Advantage (n = 2211/5841, 37.9% vs. n = 1073/3216, 33.4%) (Table 1). Similarly, the mean blastocyst rate per MII oocyte per cycle was significantly higher (p = 0.01) in the former (38.7% ± 29.7% vs. 34.3% ± 29.4%) (Table 2). Specifically, 2211 (mean = 2.3 ± 2.1, 0–13) and 1073 (mean = 2.1 ± 2.1, 0–11) blastocysts were respectively obtained in the two groups, and the rate of cycles without blastocysts was significantly lower (p < 0.01) when embryo culture was conducted through Irvine CSCM (n = 194/972, 20.0% vs. n = 127/514, 24.7%) (Fig. 1, Table 2). Interestingly, the blastocysts obtained after embryo culture conducted through Irvine CSCM also showed a better morphological quality (excellent: n = 1262/2211, 57.1% vs. n = 563/1073, 52.5%; p = 0.01) (Supplemental Fig. 1A), and the embryos were faster in reaching such stage of preimplantation development (day5: n = 1260/2211, 57.0% vs. n = 482/1073, 44.9%; p < 0.01) (Supplemental Fig. 1B) with respect to the ones cultured through Quinn’s Advantage media.
A sub-analysis of the primary outcome was performed based on the incubation system adopted. The results were confirmed for the cycles conducted in a standard incubation environment. Specifically, the rate of cycles without blastocyst produced was significantly lower in the Irvine CSCM group (n = 167/760, 22.0% vs. n = 106/385, 27.5%; p = 0.04), because of a higher blastocyst rate per inseminated MII oocyte (overall: n = 1715/4569, 37.5% vs. n = 771/2403, 32.1%, p < 0.01; mean per cycle: 39.0% ± 30.4% vs. 33.6 ± 30.4, p < 0.01). Conversely, when accounting only cycles conducted in an undisturbed incubation system, the differences in the two study groups did not reach statistical significance (rate of cycles without blastocyst: n = 28/212, 13.2% vs. n = 21/129, 16.3%, p = NS; overall blastocyst rate per MII oocyte: n = 496/1272, 39.0% vs. n = 302/813, 37.2%, p = NS; mean blastocyst rate per cycle: 36.6% ± 26.9% vs. 35.1 ± 26.5) (Supplemental Table 1).
In 536 (n = 536/664, 80.7%) and 239 PGT-A (n = 239/309, 77.4%; p = NS) cycles conducted through Irvine CSCM and Quinn’s Advantage media, respectively, at least 1 blastocyst was produced (Supplemental Table 2). The overall euploidy rate in the two study groups was similar if calculated upon the number of biopsied blastocysts (n = 654/1541, 42.4% vs. n = 295/650, 45.4%; p = NS), but significantly higher in the Irvine CSCM group if calculated upon the number of inseminated MII oocytes (n = 654/4232, 15.5% vs. n = 295/2161, 9.2%; p < 0.01) (Supplemental Table 2). Instead, the mean euploid blastocyst rate per cycle was similar in the two groups (14.7% ± 20.4% vs. 13.3% ± 19.1%; p = NS), as well as the rate of cycles with at least one euploid blastocyst (n = 330/664, 49.7% vs. n = 155/309, 50.2%; p = NS) (Supplemental Table 2).
The logistic regression analyses were conducted upon the possibility of an inseminated MII oocyte to develop to the blastocyst stage (Supplemental Table 3). Both maternal age and sperm factor showed a significant negative correlation with this outcome from univariable and multivariable analyses (p < 0.01). The kind of incubation strategy significantly correlated with the possibility of a MII oocyte to develop to the blastocyst stage in favor of the undisturbed culture system (OR = 1.22, 95% CI 1.10–1.36, p < 0.01). Finally, also the use of Irvine CSCM positively correlated with its possibility to develop to the blastocyst stage with respect to Quinn’s Advantage media (OR = 1.23, 95% CI 1.12–1.34, p < 0.01).
The clinical outcomes in the two study groups divided per ICSI and ICSI+PGT-A cycles are shown in Table 2. No significant difference was reported. For instance, the delivery rate per ET was 21.4% (n = 82/384) in Irvine CSCM ICSI group and 23.3% (n = 54/232; p = NS) in Quinn’s Advantage ICSI group. Similarly, delivery rate per ET was 40.0% (n = 167/418) in Irvine CSCM ICSI+PGT-A group and 39.8% (n = 80/201; p = NS) in Quinn’s cleavage-blastocyst ICSI+PGT-A group. The live birth rates per ET of the blastocysts transferred after Irvine CSCM and Quinn’s Advantage culture with or without PGT-A were also similar from both the analyses: namely 19.3% (n = 83/430) vs. 19.6% (n = 56/285; p = NS) for untested blastocysts, and 39.3% (n = 168/428) vs. 39.9% (n = 81/203; p = NS) for euploid blastocysts.
Finally, we aimed at assessing the efficacy derived from each culture media used. Nine hundred three cycles conducted through Irvine CSCM out of 972 (92.9%) and 475 cycles conducted through Quinn’s Advantage out of 514 (92.4%) were respectively completed by September 2017. The cumulative delivery rate per completed cycle was similar between Irvine CSCM and Quinn’s Advantage culture media (27.0%, n = 244/903 vs. 27.2%, n = 129/475; p = NS).
The distribution of the deliveries along the two groups according to gestational age (pre- or full-term) was similar (Supplemental Fig. 2A). Specifically, the pregnancies lasted on average 38 ± 1 and 38 ± 2 weeks in the Irvine CSCM and Quinn’s Advantage study groups, respectively (p = NS) (Table 2; Supplemental Fig. 2B). The distribution of the newborns along the three classes according to birthweight (low, normal, or large) was also similar (Supplemental Fig. 2C). Specifically, the newborns on average weighed 3247 ± 509 and 3185 ± 631 g in the Irvine CSCM and Quinn’s Advantage study groups, respectively (p = NS) (Table 2; Supplemental Fig. 2D).
Discussion
In this prospective study, we assessed the differences between a single-step media (here Irvine CSCM) and a sequential one (here Quinn’s Advantage). We set the main outcomes as embryological from both an oocyte-based and cycle-based perspective, but also monitored clinical outcomes after ET and the birthweight and gestational age after each delivery. Some of the cycles were conducted in an undisturbed time-lapse incubator (ca. 23%), thus providing us with the possibility to perform an interesting sub-analysis of the primary outcome of the study. Furthermore, in most of the cycles with blastocyst(s) produced, PGT-A was conducted to define an euploid chromosomal constitution (ca. 65%). This allowed us to assess the euploidy rate among the two groups, as well as to avoid the bias of aneuploidies upon the investigation of embryo reproductive competence after either single-step or sequential embryo culture.
The sample size analysis conducted upon the primary outcome of the study, namely the blastocyst rate per inseminated MII oocyte, highlighted that we required 8562 MII oocytes (5708 in the Irvine CSCM and 2854 in the Quinn’s cleavage-blastocyst arm, respectively) from 1427 cycles (951 and 476, respectively) to achieve a 95 and 80% power (α = 0.05) in detecting down to 4% of difference among the study groups from an oocyte- and a cycle-based perspective, respectively. After the first 2 years of this study, the required sample size was outnumbered (9057 MII oocytes included from 1486 cycles), thus providing us with a satisfactory statistical power. To our knowledge, this study involves the largest number of blastulation events analyzed to date in a single trial, which compares continuous and sequential embryo culture.
The quasi-randomization provided an equal distribution of the cycles in the study arms, as attested for instance by the same mean maternal and paternal age (38.5 and 41.4, respectively). Importantly, also the distribution of the cycles according to the sperm factor (normozoospermic, moderate male factor, or oligoasthenoteratozoospermic) was homogenous, thus limiting the potential uneven impact on both fertilization and blastocyst development we reported previously [25]. Nonetheless, the IVF cycles conducted by azoospermic male patients were excluded from this study, and the sperm factor was included as a confounding factor in the logistic regression analysis.
An overall lower fertilization rate per inseminated MII oocyte with Irvine CSCM was observed (71.9 vs. 74.4%; p = 0.01). However, this difference did not result into a higher rate of cycles without fertilized oocytes (6.7 vs. 6.4%; n = NS) and the mean fertilization rate per cycle was similar in the two groups (71.8% ± 27.8% vs. 72.7% ± 26.5%; p = NS). Therefore, we assume that the difference in the fertilization rate per MII oocyte may be due to a cohort effect, namely some sub-population of cycles with either better or worse results among the two study arms. Indeed, to limit this putative cohort effect on all outcomes under investigation, the mean results per cycle have been always reported.
The most important difference between the two embryo culture systems was observed in terms of blastocyst rate per inseminated MII oocyte from both egg- and cycle-based perspectives. Specifically, blastocyst rate per inseminated oocyte was 37.9% with Irvine CSCM and 33.4% with Quinn’s Advantage media (p < 0.01), a difference confirmed also in terms of mean blastocyst rate per cycle (38.7% ± 29.7% vs. 34.3% ± 29.4%; p = 0.01). Indeed, the rate of cycles where no blastocysts were produced was significantly lower in the Irvine CSCM group (20.0 vs. 24.7%; p < 0.01).
The use of both a standard and an undisturbed incubation in this study allowed us to observe the influence of the two media in different conditions. Interestingly, the culture environment exacerbated the difference between the two study groups. If we are here underpowered to draw conclusions when dealing with the undisturbed incubation system, on the other hand, it is evident that the negative effect of sequential media increases in standard conditions (post hoc power = 99.5%). This may be due to a lower tolerance of the embryo to environmental and physical stress during the manipulation required for the daily check of morphology conducted in the sequential media. Possibly, the way embryo culture is conducted is more important than the culture strategy itself or than the composition of the chosen culture media in terms of effect upon the embryological outcomes.
In this study, we did not perform any changeover of the culture drop in the continuous embryo culture approach. This may indeed represent a procedural difference with respect to the sequential culture approach. Costa-Borges in 2016 conducted an interesting analysis investigating this issue through a time-lapse-based incubation system [26]. Specifically, in a prospective cohort study on sibling donor oocytes cultured through a continuous approach, the authors reported similar results with or without refresh of the media in terms of embryo morphokinetic, euploidy rates, clinical, and perinatal outcomes. Furthermore, the meta-analysis by Sfontouris and colleagues reported comparable outcomes through single-step media, either with or without refresh in day 3 [12]. Yet, other studies are necessary to clarify the meaning of autocrine and paracrine factors released by the embryo and possibly removed by the refresh of the media.
The univariable and multivariable logistic regression analyses conducted upon the possibility of each MII oocyte to develop to the blastocyst stage highlighted that maternal age, sperm factor, and kind of incubation system and of culture media significantly correlate with the outcome. Of note, the positive effect of an undisturbed culture system upon blastocyst formation was even higher (OR = 1.22; p < 0.01) and the improvement due to the use of Irvine CSCM did not vary (OR = 1.23; p < 0.01) after correction. Continuous embryo culture in a time-lapse undisturbed system seems then the safest strategy. Possibly, by extensively reducing the need for embryo manipulation, it also limits the related environmental fluctuations (in temperature, humidity, gas, and hence pH) and risks for embryo damage or loss [27–29]. Moreover, costs and time-consuming labor are associated with the daily check of embryo culture and media changeover in day 3, matters which should not be disregarded in the definition of the ideal management of an IVF lab.
Clearly, here we tested only one among the several commercially available media per each embryo culture approach. However, the overall data from the meta-analysis by Sfontouris and colleagues support our results [12]. Moreover, two different commercially available single-step culture media were recently compared in a prospective RCT on sibling oocytes from good prognosis patients [30]. The outcomes, among which also blastocyst formation was accounted, were similar in the two study groups, thus suggesting that the chosen brand of media for embryo culture may be less (or not) relevant in affecting embryos’ developmental competence when compared to the chosen approach.
The morphological quality of the blastocysts obtained after Irvine CSCM-based embryo culture was different than after Quinn’s Advantage, with a higher rate of excellent and a lower rate of good and average quality groups. Similarly, the embryos were faster in reaching full blastulation with a higher rate of day 5 and lower rate of day 6 and day 7 blastocysts. However, these differences did not involve statistically significant variance in terms of either euploidy rate per blastocyst in the PGT-A cycles conducted or live birth rate, a finding that confirms a previous report from our group [19].
In the PGT-A cycles, the euploidy rate calculated on a per MII oocyte basis was significantly higher with the single-step media than with the sequential one (15.5 vs. 9.2%; p < 0.01). However, this outcome originates from the difference reported in terms of blastocyst rate. In fact, the euploidy rate per biopsied blastocyst was instead comparable in the two arms of the study (42.2 vs. 45.4%; p = NS). Similarly, Werner and colleagues [15], in their paired RCT on sibling 2PN zygotes, did not report any difference in terms of euploidy rate per blastocyst obtained. However, the blastocyst rate per 2PN in their dataset was higher with the sequential culture approach, an evidence in counter-tendency to ours. The main differences between their study and ours are (i) the use of the same culture media for oocyte retrieval, denudation, ICSI, and the first day of culture in the two groups; (ii) the use of two different brands of sequential media; and (iii) the use of a trophectoderm biopsy strategy entailing hatching in day 3 of preimplantation development. All aspects that may have contributed to the different outcome between the studies, and therefore deserve further investigation.
The embryo reproductive competence, namely live birth rate per transferred embryo, was similar in the two study groups among both untested and euploid blastocysts. Similarly, the delivery rate per ET did not vary in the two arms of the study among both ICSI and ICSI+PGT-A cycles. Nonetheless, even if the use of a single-step culture media involved a higher blastocyst rate, the cumulative delivery rate per completed cycle was also similar in the two groups. Possibly the reason is that this last analysis considers only the first delivery obtained per cycle. Hypothetically, increased blastocyst formation rate should translate into a higher number of deliveries and/or of babies born starting from the same cohort of oocytes. However, our study is underpowered to test this hypothesis, which should also involve sub-analyses targeted to specific patient populations.
Since the putative dissimilar effect on the perinatal outcomes after IVF due to different culture media compositions is a yet debated issue in literature, we included 1 year of pregnancy follow-up. Here, we observed similar birthweight with either the continuous or sequential culture approach (n = 388 newborns overall). Our results are in line with the data by Carrasco and colleagues [31] (n = 621 newborns) and De Vos and colleagues [32] (n = 2098 newborns) that reported no difference in the birthweight after IVF ascribable to the culture media adopted. Conversely, Dumoulin and colleagues [33], for instance, found different birthweights in 188 newborns from the comparison of two culture media. An outcome confirmed on 265 babies in a follow-up study of the first 2 years of life after delivery [34]. Similarly, a multicenter RCT resulting in 380 newborns also highlighted a difference in the birthweight according to the media used. However, caution is required towards these data, since the perinatal outcomes are related only to the population of patients who conceived in the two groups. Some confounding factors may indeed have contributed to the variance observed, especially if significantly different results are reported in terms of delivery rate upstream. In general, the birthweights reported should be then considered observational and no longer resulting from the randomization. To conclude, to date, the definition of the effect of culture media on neonatal outcomes due to either different approaches or different brands is still an open task. Future investigations upon this topic have been also recently encouraged by a panel of experts, especially concerned with the culture compositions and their putative consequences upon the phenotype and long-term health of the IVF offspring [35].
Conclusion
This prospective study highlighted that single-step media (Irvine CSCM) provides a higher possibility for an inseminated oocyte to develop to the blastocyst stage during ICSI cycles when compared to sequential media (Quinn’s advantage), especially in a standard incubation environment, from both an oocyte-based and a cycle-based perspective. However, the reproductive competence of the obtained blastocysts, as well as their related obstetrical and perinatal outcomes, was comparable. Indeed, overall, there was no impact on the delivery of at least one live birth, deriving from the use of any of the two approaches for embryo culture. Possibly, future large multicenter studies on targeted patient populations may highlight differences in terms of overall number of deliveries among the two culture systems. However, such studies are yet missing and therefore eagerly needed.
Electronic supplementary material
A) Blastocysts’ morphological quality in the two arms of the study. Blastocyst morphology was assessed as described in [19, 20] (see materials and methods). A statistically-significant higher rate of excellent quality blastocysts, and lower rate of good and average quality ones derived from the culture with single step (Irvine CSCM) with respect to sequential media (Quinn’s Advantage); p = 0.01; B) Day of blastocyst’s full development in the two arms of the study. The embryos cultured with Irvine CSCM were faster in completing their development to blastocyst; p < 0.01. (GIF 34 kb)
A) Distribution of the deliveries in the two classes of gestational age (pre- or full-term) among the two study groups. p = NS; B) Box-plots showing the gestational age in the two arms of the study. p = NS; C) Distribution of the babies born in the three classes of birthweight (low, normal or large) among the two study groups. p = NS; D) Box-plots showing the birthweight in the two arms of the study. p = NS. (GIF 46 kb)
(DOCX 30 kb)
(DOCX 28 kb)
(DOCX 25 kb)
Author’s role
LR, CS, and FMU designed the study. DC analyzed the data and drafted the manuscript. All the authors contributed to the data collection and provided an important contribution for their interpretation and discussion.
Compliance with ethical standards
The Institutional Review Board of the clinic approved the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1195-4) contains supplementary material, which is available to authorized users.
References
- 1.Pool TB. Recent advances in the production of viable human embryos in vitro. Reprod BioMed Online. 2002;4(3):294–302. doi: 10.1016/S1472-6483(10)61820-2. [DOI] [PubMed] [Google Scholar]
- 2.Biggers JD, Summers MC. Choosing a culture medium: making informed choices. Fertil Steril. 2008;90(3):473–483. doi: 10.1016/j.fertnstert.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update. 2014;20(5):617–631. doi: 10.1093/humupd/dmu023. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong S, Arroll N, Cree LM, Jordan V, Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst Rev. 2015;2:CD011320. doi: 10.1002/14651858.CD011320.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Hardarson T, Bungum M, Conaghan J, Meintjes M, Chantilis SJ, Molnar L, Gunnarsson K, Wikland M. Noninferiority, randomized, controlled trial comparing embryo development using media developed for sequential or undisturbed culture in a time-lapse setup. Fertil Steril. 2015;104(6):1452-9 e1-4–14521459.e4. doi: 10.1016/j.fertnstert.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9(6):557–582. doi: 10.1093/humupd/dmg039. [DOI] [PubMed] [Google Scholar]
- 7.Machtinger R, Racowsky C. Culture systems: single step. Methods Mol Biol. 2012;912:199–209. doi: 10.1007/978-1-61779-971-6_12. [DOI] [PubMed] [Google Scholar]
- 8.Quinn P. Culture systems: sequential. Methods Mol Biol. 2012;912:211–230. doi: 10.1007/978-1-61779-971-6_13. [DOI] [PubMed] [Google Scholar]
- 9.Macklon NS, Pieters MH, Hassan MA, Jeucken PH, Eijkemans MJ, Fauser BC. A prospective randomized comparison of sequential versus monoculture systems for in-vitro human blastocyst development. Hum Reprod. 2002;17(10):2700–2705. doi: 10.1093/humrep/17.10.2700. [DOI] [PubMed] [Google Scholar]
- 10.Summers MC, Bird S, Mirzai FM, Thornhill A, Biggers JD. Human preimplantation embryo development in vitro: a morphological assessment of sibling zygotes cultured in a single medium or in sequential media. Hum Fertil (Camb) 2013;16(4):278–285. doi: 10.3109/14647273.2013.806823. [DOI] [PubMed] [Google Scholar]
- 11.Sfontouris IA, Kolibianakis EM, Lainas GT, Petsas GK, Tarlatzis BC, Lainas TG. Blastocyst development in a single medium compared to sequential media: a prospective study with sibling oocytes. Reprod Sci. 2017;24(9):1312–1318. doi: 10.1177/1933719116687653. [DOI] [PubMed] [Google Scholar]
- 12.Sfontouris IA, Martins WP, Nastri CO, Viana IG, Navarro PA, Raine-Fenning N, et al. Blastocyst culture using single versus sequential media in clinical IVF: a systematic review and meta-analysis of randomized controlled trials. J Assist Reprod Genet. 2016;33(10):1261–1272. doi: 10.1007/s10815-016-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieamant F, Petersen CG, Mauri AL, Comar V, Mattila M, Vagnini LD, Renzi A, Petersen B, Ricci J, Oliveira JBA, Baruffi RLR, Franco Júnior JG. Single versus sequential culture medium: which is better at improving ongoing pregnancy rates? A systematic review and meta-analysis. JBRA Assist Reprod. 2017;21(3):240–246. doi: 10.5935/1518-0557.20170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basile N, Morbeck D, Garcia-Velasco J, Bronet F, Meseguer M. Type of culture media does not affect embryo kinetics: a time-lapse analysis of sibling oocytes. Hum Reprod. 2013;28(3):634–641. doi: 10.1093/humrep/des462. [DOI] [PubMed] [Google Scholar]
- 15.Werner MD, Hong KH, Franasiak JM, Forman EJ, Reda CV, Molinaro TA, Upham KM, Scott RT., Jr Sequential versus Monophasic Media Impact Trial (SuMMIT): a paired randomized controlled trial comparing a sequential media system to a monophasic medium. Fertil Steril. 2016;105(5):1215–1221. doi: 10.1016/j.fertnstert.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod. 2017;32(9):1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ubaldi F, Anniballo R, Romano S, Baroni E, Albricci L, Colamaria S, Capalbo A, Sapienza F, Vajta G, Rienzi L. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum Reprod. 2010;25(5):1199–1205. doi: 10.1093/humrep/deq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rienzi L, Ubaldi F, Anniballo R, Cerulo G, Greco E. Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13(4):1014–1019. doi: 10.1093/humrep/13.4.1014. [DOI] [PubMed] [Google Scholar]
- 19.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–1181. doi: 10.1093/humrep/deu033. [DOI] [PubMed] [Google Scholar]
- 20.Gardner DK, Schoolcraft B. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond. Carnforth: Parthenon Press; 1999. pp. 377–388. [Google Scholar]
- 21.Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT., Jr Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril. 2012;97(4):819–824. doi: 10.1016/j.fertnstert.2012.01.115. [DOI] [PubMed] [Google Scholar]
- 22.Capalbo A, Treff NR, Cimadomo D, Tao X, Upham K, Ubaldi FM, Rienzi L, Scott RT. Comparison of array comparative genomic hybridization and quantitative real-time PCR-based aneuploidy screening of blastocyst biopsies. Eur J Hum Genet. 2015;23(7):901–906. doi: 10.1038/ejhg.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobo A, de los Santos MJ, Castello D, Gamiz P, Campos P, Remohi J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150 warming cycles. Fertil Steril. 2012;98(5):1138–46 e1. doi: 10.1016/j.fertnstert.2012.07.1107. [DOI] [PubMed] [Google Scholar]
- 24.Ubaldi FM, Capalbo A, Colamaria S, Ferrero S, Maggiulli R, Vajta G, Sapienza F, Cimadomo D, Giuliani M, Gravotta E, Vaiarelli A, Rienzi L. Reduction of multiple pregnancies in the advanced maternal age population after implementation of an elective single embryo transfer policy coupled with enhanced embryo selection: pre- and post-intervention study. Hum Reprod. 2015;30(9):2097–2106. doi: 10.1093/humrep/dev159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzilli R, Cimadomo D, Vaiarelli A, Capalbo A, Dovere L, Alviggi E, Dusi L, Foresta C, Lombardo F, Lenzi A, Tournaye H, Alviggi C, Rienzi L, Ubaldi FM. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil Steril. 2017;108:961–972.e3. doi: 10.1016/j.fertnstert.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Costa-Borges N, Belles M, Meseguer M, Galliano D, Ballesteros A, Calderon G. Blastocyst development in single medium with or without renewal on day 3: a prospective cohort study on sibling donor oocytes in a time-lapse incubator. Fertil Steril. 2016;105(3):707–713. doi: 10.1016/j.fertnstert.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Gardner DK. Blastocyst culture: toward single embryo transfers. Hum Fertil (Camb). 2000;3(4):229–237. doi: 10.1080/1464727002000199051. [DOI] [PubMed] [Google Scholar]
- 28.Gardner DK. The impact of physiological oxygen during culture, and vitrification for cryopreservation, on the outcome of extended culture in human IVF. Reprod BioMed Online. 2016;32(2):137–141. doi: 10.1016/j.rbmo.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Gardner DK, Lane M. Alleviation of the ‘2-cell block’ and development to the blastocyst of CF1 mouse embryos: role of amino acids, EDTA and physical parameters. Hum Reprod. 1996;11(12):2703–12. doi: 10.1093/oxfordjournals.humrep.a019195. [DOI] [PubMed] [Google Scholar]
- 30.Sfontouris IA, Kolibianakis EM, Lainas GT, Venetis CA, Petsas GK, Tarlatzis BC, Lainas TG. Blastocyst utilization rates after continuous culture in two commercial single-step media: a prospective randomized study with sibling oocytes. J Assist Reprod Genet. 2017;34(10):1377–1383. doi: 10.1007/s10815-017-0997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrasco B, Boada M, Rodriguez I, Coroleu B, Barri PN, Veiga A. Does culture medium influence offspring birth weight? Fertil Steril. 2013;100(5):1283–1288. doi: 10.1016/j.fertnstert.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 32.De Vos A, Janssens R, Van de Velde H, Haentjens P, Bonduelle M, Tournaye H, et al. The type of culture medium and the duration of in vitro culture do not influence birthweight of ART singletons. Hum Reprod. 2015;30(1):20–27. doi: 10.1093/humrep/deu286. [DOI] [PubMed] [Google Scholar]
- 33.Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod. 2010;25(3):605–612. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- 34.Kleijkers SH, van Montfoort AP, Smits LJ, Viechtbauer W, Roseboom TJ, Nelissen EC, et al. IVF culture medium affects post-natal weight in humans during the first 2 years of life. Hum Reprod. 2014;29(4):661–669. doi: 10.1093/humrep/deu025. [DOI] [PubMed] [Google Scholar]
- 35.Sunde A, Brison D, Dumoulin J, Harper J, Lundin K, Magli MC, van den Abbeel E, Veiga A. Time to take human embryo culture seriously. Hum Reprod. 2016;31(10):2174–2182. doi: 10.1093/humrep/dew157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Blastocysts’ morphological quality in the two arms of the study. Blastocyst morphology was assessed as described in [19, 20] (see materials and methods). A statistically-significant higher rate of excellent quality blastocysts, and lower rate of good and average quality ones derived from the culture with single step (Irvine CSCM) with respect to sequential media (Quinn’s Advantage); p = 0.01; B) Day of blastocyst’s full development in the two arms of the study. The embryos cultured with Irvine CSCM were faster in completing their development to blastocyst; p < 0.01. (GIF 34 kb)
A) Distribution of the deliveries in the two classes of gestational age (pre- or full-term) among the two study groups. p = NS; B) Box-plots showing the gestational age in the two arms of the study. p = NS; C) Distribution of the babies born in the three classes of birthweight (low, normal or large) among the two study groups. p = NS; D) Box-plots showing the birthweight in the two arms of the study. p = NS. (GIF 46 kb)
(DOCX 30 kb)
(DOCX 28 kb)
(DOCX 25 kb)