Abstract
Purpose
The aim was to describe the first experience with fertility preservation by cryopreservation of ovarian tissue (OTC) in pre-pubertal girls with galactosemia and further to characterize ovarian follicular morphology and expression of proteins important for ovarian function.
Methods
Retrospectively, follicle density was estimated in ovarian cortical tissues from 6 pre-pubertal girls below the age of 12 years diagnosed with galactosemia and from 31 girls below the age of 18 years who had one ovary removed for fertility preservation for other reasons prior to gonadotoxic treatment. Additionally, expression of 4 glycoproteins important for follicle development were analyzed with immunohistochemistry in two galactosemic ovaries (aged 0.9 and 1.7 years) and compared to normal age-matched controls. The proteins included were: anti-Müllerian hormone (AMH) pro-mature and C-terminal, growth differentiation factor-9 (GDF-9), bone morphogenetic protein 15 (BMP-15), and pregnancy-associated plasma protein A (PAPP-A).
Results
Girls with galactosemia below the age of 5 years presented with morphological normal follicles and follicle densities within the 95% confidence interval (CI) of controls. No follicles were detected in the ovary from an 11.7-year-old girl with galactosemia. Expression of AMH, GDF-9, BMP-15, and PAPP-A appeared similar in follicles from girls with galactosemia and controls.
Conclusions
These findings suggest that young girls with galactosemia maintain follicles in early childhood and fertility cryopreservation may be considered an option in this patient group. The pathophysiology of galactosemia leading to an accelerated follicle loss is unknown and it is currently unknown to what extent transplanted ovarian tissue can sustain fertility in adult life.
Keywords: Galactosemia, Premature ovarian failure, Fertility preservation, Ovarian function, AMH, PAPP-A, GDF-9, BMP-15
Introduction
Galactosemia is an autosomal recessive genetic disorder in the metabolism of galactose caused by impaired activity of galactose-1-phosphate uridyltransferase (GALT). Complete deficiency or severely reduced activity of GALT causes classical galactosemia and affects in its most common form approximately 1:30,000 to 1:50,000 persons [1–3].
Girls and women with classic galactosemia have significantly higher serum concentrations of follicle-stimulating hormone (FSH) and reduced concentrations of anti-Müllerian hormone (AMH) compared to age-matched controls, reflecting a reduced ovarian reserve [4, 5]. It has been estimated that as many as 80–100% of girls diagnosed with classic galactosemia will experience premature ovarian insufficiency (POI) [6–9]. In 47 cases examined, absent or abnormal menstrual cycles or elevated serum FSH concentrations have been found in 81% of females ≥ 15 years, which were considered signs of abnormal ovarian function [6]. In a study of 53 girls with galactosemia, 77% experienced POI at a mean age of 13 years [7], suggesting that follicular depletion is likely to initiate already before puberty.
It has recently been published that almost 30% of women with classic galactosemia and POI who actively attempted to conceive actually succeeded within one year, increasing to almost 50% after 2 years of attempting to become pregnant [10]. The prevalence of spontaneous conception for women with POI of any cause is only 5–10% [11, 12], suggesting that women with galactosemia-induced POI are more likely to become spontaneously pregnant compared to women with other causes of POI. Galactosemic POI may be caused either by an initial reduced pool of primordial follicles or by an accelerated depletion of the primordial follicle pool [13]. Aberrant FSH activity caused by glycosylation has also been suggested to cause follicle development arrest and infertility [14–16]. The three major enzymes important for the conversion of galactose to glucose show high ovarian activity in normal females as compared to the testis in males, which may indicate an increased synthesis of glycosylated proteins and lipids in the ovary compared to the testis. It has been suggested that POI in patients with galactosemia may be due to errors in the synthesis of galactose-containing glycoproteins and glycolipids in the ovary [17].
Cryopreservation of ovarian tissue is a fertility preservation option primarily used for girls and women diagnosed with a cancer requiring gonadotoxic treatment that may eliminate the ovarian pool of follicles and leave the patient menopausal. For pre-pubertal girls and women undergoing urgent gonadotoxic treatment leaving no time for ovarian stimulation and oocyte collection, ovarian tissue preservation is the only option available to safeguard fertility. Today, almost 100 babies worldwide have been born from transplanted frozen/thawed ovarian tissue [18, 19]. The technique may also be considered for pre-pubertal girls with a genetic condition that causes an increased risk of POI including girls with galactosemia and Turner syndrome [8, 14, 16, 20], though general recommendations cannot been given. For galactosemia, this reflects that the speed by which the ovarian pool of follicles becomes depleted varies from patient to patient parallel to the phenotypic spectrum and GALT mutations. Further, new data suggest that spontaneous pregnancy rates may be higher in women with galactosemia than it has previously been suggested [10], which indicates that fertility preservation may only be relevant to consider for a subset of girls with galactosemia. Unfortunately, women with galactosemia who did and did not spontaneously conceive were similar with respect to age, genotype, age at dietary onset, spontaneous/induced menarche and age at menarche, and socio-economic factors [10], and it was not possible to predict patients who may benefit from early fertility preservation.
The aim of this study was to present our initial experience with ovarian tissue cryopreservation (OTC), fertility preservation, and ovarian characterization in the largest series published until now in girls with galactosemia albeit in a very small group of young girls of only 6.
Materials and methods
Patients
A total of 6 pre-pubertal girls below the age of 12 (mean age 3.8 years ± 1.7 (± SEM); range, 0.4–11.7 years) diagnosed with galactosemia had one entire ovary surgically removed by laparoscopy and OTC for fertility preservation at the Laboratory of Reproductive Biology, Rigshospitalet, Denmark. The control group consisted of 31 girls below the age of 18 years (mean age 13.5 years ± 0.7 (± SEM); range, 1.5–17.9 years) diagnosed with a malignant disease, who had one ovary removed for OTC and fertility preservation prior to gonadotoxic treatment [21]. Controls underwent an identical surgical procedure and cryopreservation program at the same department as the girls with galactosemia.
All patients were retrospectively included. No surgical complications were reported in connection with oophorectomy. Additionally, two control ovaries from the archive of the Laboratory of Reproductive Laboratory were included for IHC staining.
Cryopreservation of ovarian tissue
Ovarian cortex was isolated, cut into small pieces (Table 1), and processed for cryopreservation as previously described [22–24]. One small randomly chosen piece of cortex was fixated in Bouin’s solution for histology.
Table 1.
Genotype, age, follicle density, no., and size of the ovarian cortex pieces preserved from 6 girls with galactosemia
| Genotype | Age (years) | Follicle density (follicles/mm3) | No. of cortex pieces cryopreserved | Size of cortex pieces cryopreserved (mm) |
|---|---|---|---|---|
| p.Q188R | 0.3 | 2521 | 8 | 2 × 3 |
| p.Q188R | 0.9 | 1444 | 13 | 3 × 4 |
| p.S236I | 1.7 | 1041 | 4 | 5 × 5 |
| p.Q188R | 3.9 | 631 | 7 | 2 × 2 |
| p.Q188R and p.R333Q | 4.5 | 17 | 4 | 3 × 3 |
| N/Aa | 11.7 | 0 | 3 | 2 × 2 |
aClinical classic appearance
Follicle density
Tissue was cut in serial sections of 30 or 5 μm for density measurement. The follicle sampling was conducted on a Zeiss Axiophot upright microscope. The area of the sections was measured on an Olympus BH-2 microscope with VIS version 4.6.1779 software (Visiopharm, Hoersholm, Denmark). The follicular density was in 30-μm sections estimated using the mathematical model described by Schmidt and colleagues [25]. In brief, this model was based on the fraction of sections measured, the height and area of the sections, the mean primordial follicular diameter, and a correction factor (α) to account for the possibility to count the same follicle more than once. Since the mean diameter of a primordial follicle is 44 μm [26] and the sections were 30 μm, there was a possibility for counting the same follicle two or three times [25]. In two cases, the density was measured in 5-μm serial sections as described by McLaughlin and colleagues [27]. In brief, follicles were only counted when the nucleolus was observed and the total number of follicles divided by the volume of tissue analyzed. Despite these methodological differences, the two analyses showed good agreement [27].
Immunohistochemistry
The tissue was cut in serial sections of 5 μm and prepared for histology using standard methods. Antigen was retrieved in citrate buffer, incubated with monoclonal mouse primary antibodies overnight at 4 °C, washed in phosphate-buffered saline with Tween20® (PBST), incubated with polyclonal mouse-anti-mouse secondary antibody (Dako, Glostrup, Denmark, Cat. no: P0260), visualized with DAB+ Novolink™ detection system (Leica Biosystems, Nussloch, Germany, cat. no.: RE7230-K), and counterstained with Meyer’s hematoxylin (Amplicon, Odense, Denmark). AMH, produced by the granulosa cells of developing pre-antral and antral follicles and is increasingly being used to assess the ovarian function, was used [28, 29]. Two antibodies against AMH, one detecting the pro-mature form of AMH (Ansh Lab, Texas, US, 1.7 μg/ml) and one detecting the active C-terminal of AMH (Ansh Lab, 7.3 μg/ml) [30], were used. Growth differentiation factor-9 (GDF-9) and bone morphogenetic protein 15 (BMP-15) expressed by the oocyte and essential for follicular development beyond the primary follicle stage [31, 32] was used (Ansh Lab, 5.5 and 6.8 μg/ml, respectively). Pregnancy-associated plasma protein A (PAPP-A) suggested to be involved in the regulation of the steroidogenesis in human antral follicles [33] was used (PACI-D8-mlgG2a, 7.0 μg/ml).
Ethical considerations
In Denmark, OTC is by law offered exclusively to girls and women who are at risk of iatrogen-induced follicle loss or have a genetic disease that poses a risk of destroying the ovarian function. Based on patient age, general health conditions, the risk of inducing or achieving POI, and other factors, the doctor decides whether OTC is offered or not. The procedure is performed in the public health care system and is free of costs for the patient in all aspects including OTC, storage, transplantation, and potential-assisted reproduction procedures.
All patients below the age of 18 years were represented by their parents or guardians who were counseled by a doctor and who received information about the procedure including the current results and the outlook for providing fertility in the future. The parents were informed about their daughters’ personal risk and the fertility preservation options—for pre-pubertal, OTC is the only available treatment, and that this procedure in young girls is experimental and there will be no guarantee for subsequent restoration of fertility. It was the parents or guardians who decided whether they want to accept the offer for OTC. In this group of galactosemic patients, OTC at an early age was recommended due to the rapid follicle decline associated with the disease, the speed of which for individual patients is currently unknown. As all participating patients were underage, their parents gave their informed consent on behalf of their daughters to participate according to Danish legislation, and this study was approved by the Scientific Ethical Committee for the Capital Region (KF (01) 170/99).
Statistic model
Microsoft Excel version 14.6.1 was used to analyze the data. Data on follicle densities in girls below the age of 18 years who have received no medical treatment before oophorectomy has previously been compared to the densities in girls who had received chemotherapy before ovariectomy, and has been published by El Issaoui and colleagues [21]. The quadratic model presented by El Issaoui and colleagues [21] was useful for comparing the treated and untreated groups for that study, but is not useful for age-related normative predictions due to residuals being non-normally distributed. The same data was used to derive a more suitable model: log10(density + 1) = a + bx^(0.5)ln(x) with parameter values a = 3.067 (95% confidence interval (CI) 2.30–3.83) and b = − 0.13 (95% CI − 0.21 to − 0.05). This model has more normally distributed residuals (84% of a perfect Gaussian distribution).
Results
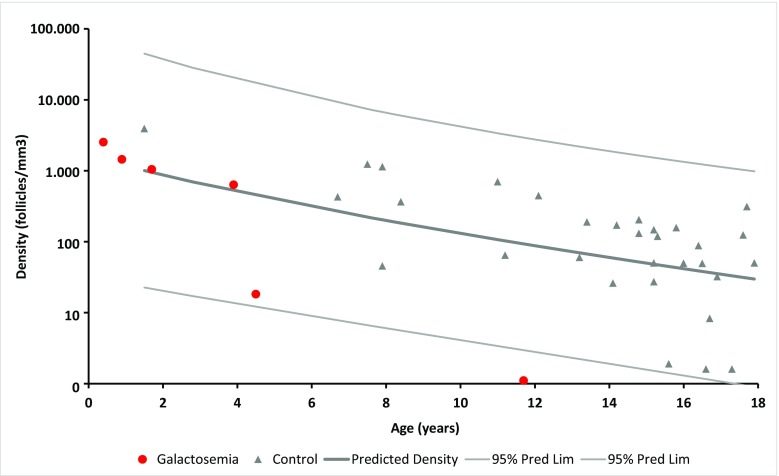
All 5 girls with galactosemia below the age of 5 years presented with a mean follicle density ± SEM of 1130 ± 420 follicles per cubic millimeter, which were within the 95% CI of the control group (Fig. 1). The mean follicle density ± SEM in the control group was 333 ± 131. In contrast, no follicles were detected in the ovarian biopsy obtained from the 11.7-year-old girl. The exact follicle densities together with the number and size of the cryopreserved ovarian cortex pieces and patient genotype are shown in Table 1. All follicles were primordial follicles.
Fig. 1.
Follicular density (follicles/mm3) in ovarian cortex in 6 girls with galactosemia (circles) and in normal control ovaries (triangles). The lines illustrate the predicted follicular density and the 95% confidence intervals (CI). Girls with galactosemia below the age of 5 years have follicle densities similar to controls, whereas the girls with galactosemia aged 11.7 years had no follicles
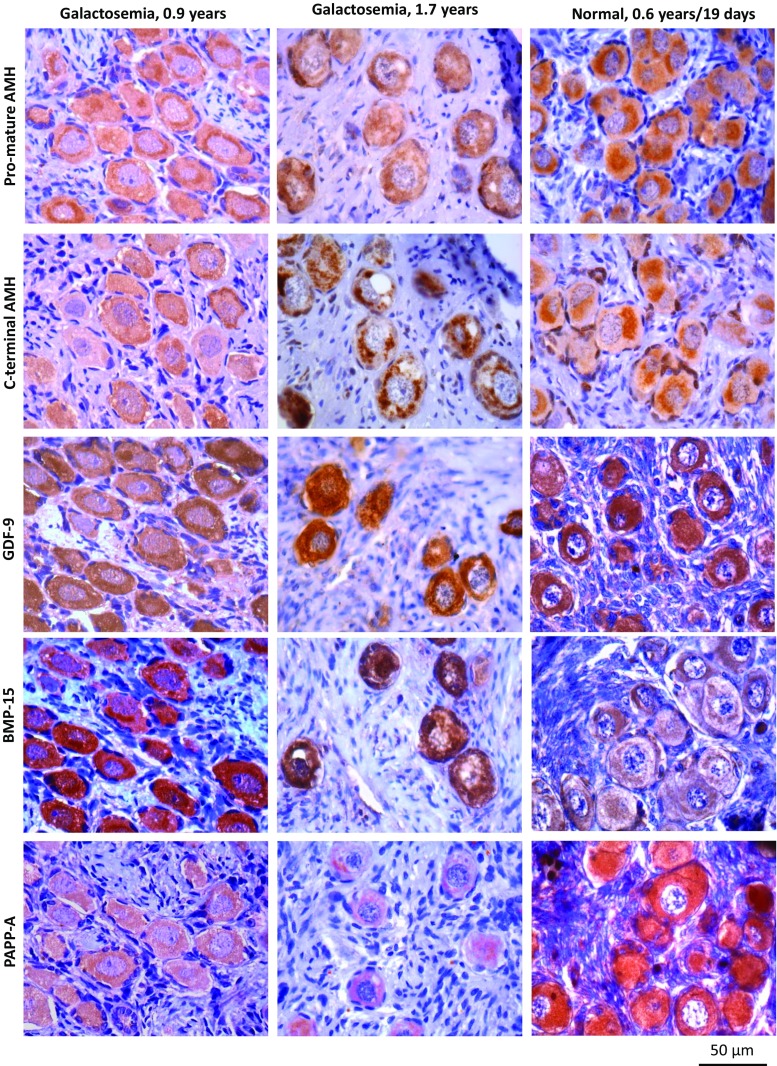
The glycoproteins important for follicular health are the following: pro-mature AMH, C-terminal AMH, GDF-9, BMP-15, and PAPP-A were present in follicles from 2 girls with galactosemia aged 0.9 and 1.7 years and in age-matched normal control ovaries. Due to limited material, controls were performed in normal ovaries of two girls aged 0.6 years and 19 days (Fig. 2).
Fig. 2.
Immunohistochemical detection of pro-mature and C-terminal AMH, GDF-9, BMP-15, and PAPP-A in ovarian cortex from two girls with galactosemia (aged 0.9 and 1.7 years old), together with controls (0.6 years and 19 days old). The evaluated proteins important for follicular health were detected both in follicles with galactosemia and in normal controls
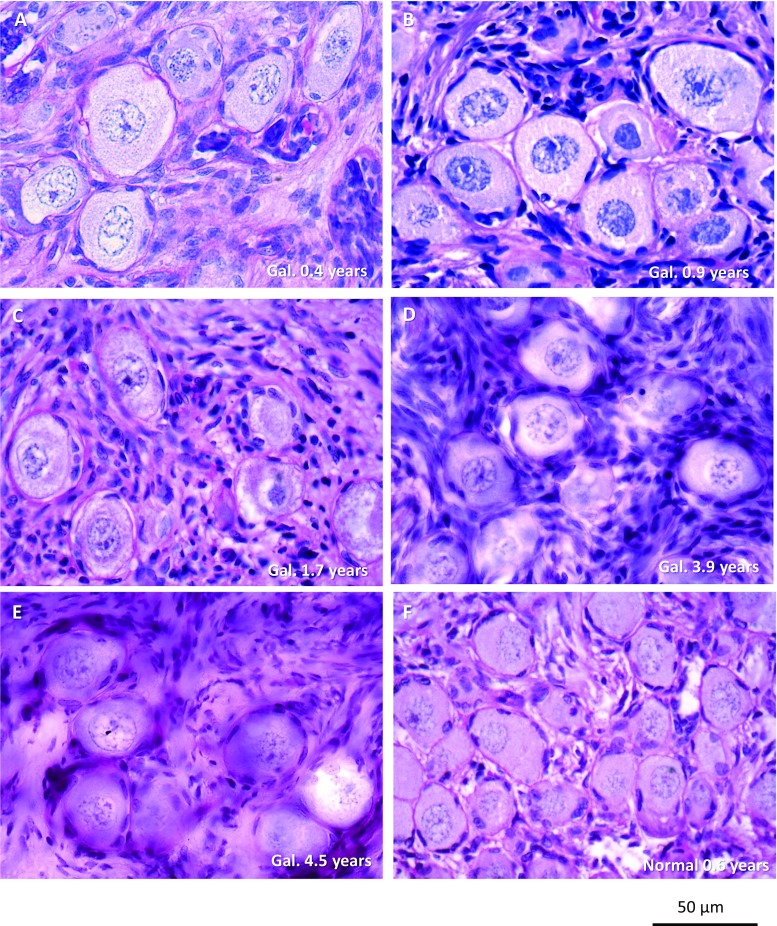
Histological sections of the ovarian cortex from girls with galactosemia containing follicles showed morphological normal primordial follicles, though no growing follicles were observed (Fig. 3). A fraction of the follicles in the 1.7-year-old ovary appeared to have lost the oocyte nucleus and be in a process of atresia.
Fig. 3.
Ovarian cortex from 5 girls below the age of 5 years with galactosemia had morphological normal primordial follicles, though no growing follicles were observed (A–E). A fraction of the follicles in (C) appeared to have lost the oocyte nucleus and be in a process of atresia. An age-matched control ovary (0.6 years old) had morphologically normal follicles (F)
Discussion
This is to our knowledge the first study to evaluate the possibility of OTC and fertility preservation in young girls with the rare genetic disease galactosemia. Further, follicular density and immunohistochemical expression of oocyte and granulosa cell-expressed proteins were evaluated. The study only includes a total of 6 patients with galactosemia and is obviously limited in size but is nonetheless the largest and only study to evaluate the ovarian fertility potential.
The follicle density in ovarian cortex was evaluated in 6 pre-pubertal girls diagnosed with galactosemia and compared to the density in a group of normal ovaries below the age of 18 years. The control ovaries are older (mean age 13.5 years old) compared to ovaries with galactosemia (mean age 3.8 years old), which is a limitation to the study. All 5 ovaries below the age of 5 years presented with follicle densities within the 95% CI of normal ovaries and with a normal follicular morphology. In contrast, the girl aged 11.7 years presented with no follicles, suggesting that in this cohort follicles are maintained in the first years of life but are at risk of undergoing an accelerated loss in early childhood [6–9].
In two cases, sufficient ovarian tissue for IHC analysis was available. Glycosylated proteins important for normal follicle development were detected in follicles of these two cases and in normal age-matched follicles, suggesting that these young girls may have normal follicles. Using the Santa Cruz anti-AMH antibody, Weenen and colleagues did not observe AMH expression in human primordial follicles [34], which is in contrast to the present findings where AMH was detected in both galactosemic and control ovaries. We used highly specific anti-AMH antibodies provided by Ansh Laboratories and detected AMH in primordial follicles with several AMH-specific antibodies detecting different epitopes. The discrepancy between these studies may be due to the antibodies used. GDF-9 is suggested to be essential for normal folliculogenesis and has in several studies shown to be expressed by the oocyte and the granulosa cells beyond the primary state, but not in primordial follicles [16, 35–37], though gene expression of Gdf-9 has been detected in murine germ cells and primordial follicles already during embryogenesis [38] and in vitro studies have shown that GDF-9 and BMP-15 promote primordial follicle formation in both hamster and human ovary [39, 40]. In the human fetal ovary, both GDF-9 and BMP-15 mRNA have been detected with marked increase in expression across gestation [41] and GDF-9 proteins have been detected in human fetal germ cells from the initiation of oocyte nest breakdown (gestational week 8) to the primordial follicle formation in week 20 [41]. In adult human ovaries, AMH, GDF-9, and BMP-15 mRNA have also been detected in primordial and early primary follicles with increasing expression with follicle size [42] together with GDF-9 and BMP-15 proteins in human primordial follicles [43], supporting the present detection of AMH, GDF-9, and BMP-15 in human primordial follicles. The presence of PAPP-A in human primordial and late primary follicles has been reported [44] and the present finding supports the literature.
Taken together, considering the expression of the four evaluated proteins, there are no obvious differences between normal and galactosemic ovaries, which is supported by the fact that several women with galactosemia actually have conceived spontaneously.
However, this study does not reveal whether these follicles, if subsequently transplanted, will be functional and provide fertility. Further, we cannot state whether these observations are representable for young girls with galactosemia. The present study does not define an age limit beyond which the pool of ovarian follicles becomes too low to consider fertility preservation. However, the pool of follicles appears gradually to decline very early in life and if fertility preservation is considered, it should be performed in early childhood in order to save as large a cohort of primordial follicles as possible.
Previous studies have found significantly lower serum AMH levels in girls and women with galactosemia compared to age-matched controls [4, 5], which are expected since the total number of follicles is reduced. In addition, the number of growing follicles—which especially contribute to the circulating levels of AMH—appears also to be reduced [29]. Low AMH levels in girls with galactosemia are therefore expected. The present study demonstrates the presence of both the pro-mature AMH and the active C-terminal AMH in follicles of two girls with galactosemia, suggesting that in these two cases, AMH is synthesized to some extent.
The present findings confirm and extend the observation by Levy and colleagues who, in a 5-day-old infant with galactosemia who died, found histologically normal ovaries with normal follicle development [45]. Histological studies in adolescents with galactosemia have found a remarkably altered ovarian morphology and reduced or absent follicle number in girls aged 16–17 years [46–48], indicating that the ovarian function may be compromised already at puberty, which corroborates with the observation in the 11.7-year-old girl of the present study.
Despite delayed pubertal development, amenorrhea, and POI in most women with galactosemia, a few manage to conceive—worldwide, around 50 pregnancies have been described [2, 14, 15]. Moreover, a recent study suggests that pregnancy chances in women with galactosemia may be higher than previously reported [10]. These data suggest that almost 50% of women with galactosemia and POI who attempt to conceive actually do succeed within 2 years, though a considerable miscarriage rate (30%) was observed [10]. Women who conceived did not differ from women who could not conceive with respect to patient characteristics [10], which makes it difficult to determine who potentially could benefit from early fertility preservation. However, these findings clarify that galactosemia is not incompatible with caring a pregnancy.
In Denmark, ovarian tissue fertility preservation is offered to girls and women who run the risk of iatrogen-induced POI or have a genetic disease with the risk of POI. Case reports from Denmark and France have demonstrated that puberty can be induced in girls who had OTC before puberty (9 and 10 years of age) [49, 50]. These reports indicate that frozen/thawed transplanted ovarian tissue can work properly and even a small fraction of the cryopreserved tissue is sufficient to induce puberty. In case of a later pregnancy wish, additional cortex pieces can be transplanted. Moreover, a healthy child has just been born in England from pre-pubertal cryopreserved ovarian tissue auto-transplanted in Denmark, demonstrating that fertility can be restored from ovarian tissue preserved before puberty (Mathwes et al. 2018, in press). Irrespective of diagnosis, there are currently no reports on whether OTC at very early ages as the present study (mean age 3.8 years) actually will reactivate and undertake ovarian function including fertility when transplanted. Many of these girls may not need the tissue for fertility purposes before after 20 years of storage or more, why more years need to elapse before these patients may return for transplantation. However, a recent follow-up study evaluating complication in connection with laparoscopical removal and transplantation of ovarian tissue found the procedure to have a low risk of complications with 0.2% complications occurred (3 in 1302 procedures) irrespective of age [51].
Even though cryopreservation is a valid method for fertility preservation [18, 19], it is not known for how long frozen/thawed ovarian tissue will function in women with galactosemia after transplantation. Women with other diagnoses than galactosemia have shown ovarian activity for more than 8 years [52]. There is a risk that the underlying disease will affect the graft longevity and the period of ovarian function following transplantation in patients with galactosemia cannot be estimated. Although the function of the tissue could be short because of the possible negative influence of the metabolic situation in galactosemia, 6–12 months of function may be sufficient to mature follicles, which can then be used in connection with assisted reproduction. If the ovarian tissue survives longer, assisted reproduction may not even be necessary, though most women with ovarian transplants will require assisted reproduction to conceive. Alternatively, in vitro methods to mature oocytes from cortical tissue may be developed and thereby provide mature oocytes to be used for assisted reproduction. However, actual experience with transplantation to galactosemic patients will have to wait for these girls reach a normal childbearing age.
Taken together, these findings suggest that girls with galactosemia may have ovarian follicles in the first years of life; OTC and fertility preservation of ovarian tissue may be considered an experimental treatment option. If ovarian preservation is considered, it is suggested to preserve early in life in order to preserve as large a pool of ovarian follicles as possible, especially because the speed by which the follicle number decline in girls with galactosemia has only just started to be outlined. However, the larger the follicle reserve, the greater the chance of a successful outcome. It is important to note that OTC in this particular group of patients is experimental and the potential benefit of the method is unknown and needs to await actual transplantation of frozen/thawed tissue.
Acknowledgements
We thank Marianne Sguazzino for the excellent technical assistance.
Authors’ role
L.S.M. and C.Y.A. designed and drafted the study. L.S.M. was responsible for the IHC staining and estimating the follicle densities in ovaries from patients with galactosemia. T.W.K. designed the mathematics for follicle density estimation and developed the density prediction model. E.E. and K.T.M. did the initial fertility preservation counseling with the parents and was responsible for the collection of the tissue. A.M.L. is the physician in charge of the girls with galactosemia and obtained their genetic information. The final manuscript was approved by all authors.
Funding information
The Novo Nordisk Foundation and the EU interregional project ReproUnion funded this study. They had no role in the study design, collection and analysis of data, and data interpretation or in writing the report.
Compliance with ethical standards
The study was approved by the Scientific Ethical Committee for the Capital Region (KF (01) 170/99).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Suzuki M, West C, Beutler E. Large-scale molecular screening for galactosemia alleles in a pan-ethnic population. Hum Genet. 2001;109:210–215. doi: 10.1007/s004390100552. [DOI] [PubMed] [Google Scholar]
- 2.Gubbels CS, Land JA, Rubio-Gozalbo ME. Fertility and impact of pregnancies on the mother and child in classic galactosemia. Obstet Gynecol Surv. 2008;63:334–343. doi: 10.1097/OGX.0b013e31816ff6c5. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Gozalbo ME, Gubbels CS, Bakker JA, Menheere PP, Wodzig WK, Land JA. Gonadal function in male and female patients with classic galactosemia. Hum Reprod Update. 2010;16:177–188. doi: 10.1093/humupd/dmp038. [DOI] [PubMed] [Google Scholar]
- 4.Sanders RD, Spencer JB, Epstein MP, Pollak SV, Vardhana PA, Lustbader JW, et al. Biomarkers of ovarian function in girls and women with classic galactosemia. Fertil Steril. 2009;92:344–351. doi: 10.1016/j.fertnstert.2008.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer JB, Badik JR, Ryan EL, Gleason TJ, Broadaway KA, Epstein MP, Fridovich-Keil JL. Modifiers of ovarian function in girls and women with classic galactosemia. J Clin Endocrinol Metab. 2013;98:E1257–E1265. doi: 10.1210/jc.2013-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waggoner DD, Buist NRM, Donnell GN. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis. 1990;13:802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero NV, Singh RH, Manatunga A, Berry GT, Steiner RD, Elsas LJ. Risk factors for premature ovarian failure in females with galactosemia. J Pediatr. 2000;137:833–841. doi: 10.1067/mpd.2000.109148. [DOI] [PubMed] [Google Scholar]
- 8.Fridovich-Keil JL, Gubbels CS, Spencer JB, Sanders RD, Land JA, Rubio-Gozalbo E. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis. 2011;34:357–366. doi: 10.1007/s10545-010-9221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varela-Lema L, Paz-Valinas L, Atienza-Merino G, Zubizarreta-Alberdi R, Villares RV, López-García M. Appropriateness of newborn screening for classic galactosaemia: a systematic review. J Inherit Metab Dis. 2016;39:633–649. doi: 10.1007/s10545-016-9936-y. [DOI] [PubMed] [Google Scholar]
- 10.van Erven B, Berry GT, Cassiman D, Connolly G, Forga M, Gautschi M, Gubbels CS, Hollak CEM, Janssen MC, Knerr I, Labrune P, Langendonk JG, Õunap K, Thijs A, Vos R, Wortmann SB, Rubio-Gozalbo ME. Fertility in adult women with classic galactosemia and primary ovarian insufficiency. Fertil Steril. 2017;108:168–174. doi: 10.1016/j.fertnstert.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Rebar RW. Premature ovarian failure. Obstet Gynecol. 2009;113:1355–1363. doi: 10.1097/AOG.0b013e3181a66843. [DOI] [PubMed] [Google Scholar]
- 12.van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–492. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 13.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakur M, Feldman G, Puscheck EE. Primary ovarian insufficiency in classic galactosemia: current understanding and future research opportunities. J Assist Reprod Genet. 2018;35:3–16. doi: 10.1007/s10815-017-1039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubbels CS, Land JA, Evers JLH, Bierau J, Menheere PPCA, Robben SGF, Rubio-Gozalbo ME. Primary ovarian insufficiency in classic galactosemia: role of FSH dysfunction and timing of the lesion. J Inherit Metab Dis. 2013;36:29–34. doi: 10.1007/s10545-012-9497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forges T, Monnier-Barbarino P, Leheup B, Jouvet P. Pathophysiology of impaired ovarian function in galactosaemia. Hum Reprod Update. 2006;12:573–584. doi: 10.1093/humupd/dml031. [DOI] [PubMed] [Google Scholar]
- 17.Xu YK, Ng WG, Kaufman FR, Lobo RA, Donnell GN. Galactose metabolism in human ovarian tissue. Pediatr Res. 1989;25:151–155. doi: 10.1203/00006450-198902000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325–336. doi: 10.1007/s10815-016-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018;Epub ahead. [DOI] [PMC free article] [PubMed]
- 20.van Erven B, Gubbels CS, van Golde RJ, Dunselman GA, Derhaag JG, de Wert G, Geraedts JP, Bosch AM, Treacy EP, Welt CK, Berry GT, Rubio-Gozalbo M. Fertility preservation in female classic galactosemia patients. Orphanet J Rare Dis. 2013;8:107. doi: 10.1186/1750-1172-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issaoui ME, Giorgione V, Mamsen LS, Rechnitzer C, Birkebaek N, Clausen N, et al. Effect of first line cancer treatment on the ovarian reserve and follicular density in girls under the age of 18 years. Fertil Steril. 2016;6:1757–1762. doi: 10.1016/j.fertnstert.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KLT, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 23.Rosendahl M, Andersen CY, Ernst E, Westergaard LG, Rasmussen PE, Loft A, Andersen AN. Ovarian function after removal of an entire ovary for cryopreservation of pieces of cortex prior to gonadotoxic treatment: a follow-up study. Hum Reprod. 2008;23:2475–2483. doi: 10.1093/humrep/den248. [DOI] [PubMed] [Google Scholar]
- 24.Rosendahl M, Timmermans Wielenga V, Nedergaard L, Kristensen SG, Ernst E, Rasmussen PE, Anderson M, Schmidt KT, Andersen CY. Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertil Steril. 2011;95:2158–2161. doi: 10.1016/j.fertnstert.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt KLT, Byskov AG, Andersen AN, Müller J, Andersen CY. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–1164. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 26.Westergaard CG, Byskov AG, Andersen CY. Morphometric characteristics of the primordial to primary follicle transition in the human ovary in relation to age. Hum Reprod. 2007;22:2225–2231. doi: 10.1093/humrep/dem135. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin M, Kelsey TW, Wallace WHB, Anderson RA, Telfer EE. An externally validated age-related model of mean follicle density in the cortex of the human ovary. J Assist Reprod Genet. 2015;32:1089–1095. doi: 10.1007/s10815-015-0501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–1287. doi: 10.1093/humrep/deq019. [DOI] [PubMed] [Google Scholar]
- 29.Jeppesen J, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, et al. Which follicles make the most anti-Müllerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–527. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- 30.Robertson DM, Kumar A, Kalra B, Shah S, Pruysers E, Vanden BH, et al. Detection of serum antimullerian hormone in women approaching menopause using sensitive antimullerian hormone enzyme-linked immunosorbent assays. Menopause. 2014;21:1–10. doi: 10.1097/GME.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 31.McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 32.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 33.Bøtkjær JA, Jeppesen JV, Wissing ML, Kløverpris S, Oxvig C, Mason JI, Borgbo T, Andersen CY. Pregnancy-associated plasma protein A in human ovarian follicles and its association with intrafollicular hormone levels. Fertil Steril. 2015;104:1294–1301. doi: 10.1016/j.fertnstert.2015.07.1152. [DOI] [PubMed] [Google Scholar]
- 34.Weenen C, Laven JSE, von Bergh ARM, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 35.Sidis Y, Fujiwara T, Leykin L, Isaacson K, Toth T, Schneyer AL. Characterization of inhibin/activin subunit, activin receptor, and follistatin messenger ribonucleic acid in human and mouse oocytes: evidence for activin’s paracrine signaling from granulosa cells to oocytes. Biol Reprod. 1998;59:807–812. doi: 10.1095/biolreprod59.4.807. [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Shi F, Blas-Machado U, Yu R, Davis VL, Foster WG, Magoffin DA, Hughes CL. Dietary galactose inhibits GDF-9 mediated follicular development in the rat ovary. Reprod Toxicol. 2006;21:26–33. doi: 10.1016/j.reprotox.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppä L, Louhio H, Tuuri T, Sjöberg J, Bützow R, Hovata O, Dale L, Ritvos O. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84:2744–2750. doi: 10.1210/jcem.84.8.5921. [DOI] [PubMed] [Google Scholar]
- 38.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305(80):1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Roy SK. Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod. 2004;70:577–585. doi: 10.1095/biolreprod.103.023234. [DOI] [PubMed] [Google Scholar]
- 40.Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T, Felz C, Ben-Haroush A, Kravarusic D, Abir R. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011;96:E1246–E1254. doi: 10.1210/jc.2011-0410. [DOI] [PubMed] [Google Scholar]
- 41.Bayne RAL, Kinnell HL, Coutts SM, He J, Childs AJ, Anderson RA. GDF9 is transiently expressed in oocytes before follicle formation in the human fetal ovary and is regulated by a novel NOBOX transcript. PLoS One Libr Sci. 2015;10:e0119819. doi: 10.1371/journal.pone.0119819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kristensen SG, Andersen K, Clement CA, Franks S, Hardy K, Andersen CY. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod. 2014;20:293–308. doi: 10.1093/molehr/gat089. [DOI] [PubMed] [Google Scholar]
- 43.Wei L-N, Huang R, Li L-L, Fang C, Li Y, Liang X-Y. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31:1483–1490. doi: 10.1007/s10815-014-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jepsen MR, Kløverpris S, Bøtkjær JA, Wissing ML, Andersen CY, Oxvig C. The proteolytic activity of pregnancy-associated plasma protein-A is potentially regulated by stanniocalcin-1 and -2 during human ovarian follicle development. Hum Reprod. 2016;31:866–874. doi: 10.1093/humrep/dew013. [DOI] [PubMed] [Google Scholar]
- 45.Levy H, Driscoll S, Porensky R, Wender D. Ovarian failure in galactosemia. N Engl J Med. 1984;310:50. doi: 10.1056/nejm198401053100114. [DOI] [PubMed] [Google Scholar]
- 46.Beauvais P, Guilhaume A. Ovarian insufficiency in congenital galactosemia. Presse Med. 1984;13:2685–2687. [PubMed] [Google Scholar]
- 47.Morrow RJ, Atkinson AB, Carson DJ, Sloan JM, Traub AI. Ovarian failure in a young woman with galactosaemia. Ulster Med J. 1985;54:218–220. [PMC free article] [PubMed] [Google Scholar]
- 48.Kaufman FR, Xu YK, Ng WG, Silva PD, Lobo RA, Donnell GN. Gonadal function and ovarian galactose metabolism in classic galactosemia. Acta Endocrinol. 1989;120:129–133. doi: 10.1530/acta.0.1200129. [DOI] [PubMed] [Google Scholar]
- 49.Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379:588. doi: 10.1016/S0140-6736(11)61781-9. [DOI] [PubMed] [Google Scholar]
- 50.Ernst E, Kjærsgaard M, Birkebæk NH, Clausen N, Andersen CY. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 2013;49(4):911–914. doi: 10.1016/j.ejca.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 51.Beckmann MW, Dittrich R, Lotz L, van der Ven K, van der Ven HH, Liebenthron J, Korell M, Frambach T, Sütterlin M, Schwab R, Seitz S, Müller A, von Wolff M, Häberlin F, Henes M, Winkler-Crepaz K, Krüssel JS, Germeyer A, Toth B. Fertility protection: complications of surgery and results of removal and transplantation of ovarian tissue. Reprod BioMed Online. 2018;36:188–196. doi: 10.1016/j.rbmo.2017.10.109. [DOI] [PubMed] [Google Scholar]
- 52.Jensen AK, Kristensen SG, MacKlon KT, Jeppesen JV, Fedder J, Ernst E, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod. 2015;30:2838–2845. doi: 10.1093/humrep/dev230. [DOI] [PubMed] [Google Scholar]