Abstract
Purpose
Mammalian oogenesis and folliculogenesis share a dynamic connection that is critical for gamete development. For maintenance of quiescence or follicular activation, follicles must respond to soluble signals (growth factors and hormones) and physical stresses, including mechanical forces and osmotic shifts. Likewise, mechanical processes are involved in cortical tension and cell polarity in oocytes. Our objective was to examine the contribution and influence of biomechanical signaling in female mammalian gametogenesis.
Methods
We performed a systematic review to assess and summarize the effects of mechanical signaling and mechanotransduction in oocyte maturation and folliculogenesis and to explore possible clinical applications. The review identified 2568 publications of which 122 met the inclusion criteria.
Results
The integration of mechanical and cell signaling pathways in gametogenesis is complex. Follicular activation or quiescence are influenced by mechanical signaling through the Hippo and Akt pathways involving the yes-associated protein (YAP), transcriptional coactivator with PDZ-binding motif (TAZ), phosphatase and tensin homolog deleted from chromosome 10 (PTEN) gene, the mammalian target of rapamycin (mTOR), and forkhead box O3 (FOXO3) gene.
Conclusions
There is overwhelming evidence that mechanical signaling plays a crucial role in development of the ovary, follicle, and oocyte throughout gametogenesis. Emerging data suggest the complexities of mechanotransduction and the biomechanics of oocytes and follicles are integral to understanding of primary ovarian insufficiency, ovarian aging, polycystic ovary syndrome, and applications of fertility preservation.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1180-y) contains supplementary material, which is available to authorized users.
Keywords: Mechanical signaling, Folliculogenesis, Ovarian biomechanics, Mechanotransduction, Oocyte maturation
Introduction
The key to the propagation of the human species comes in a small package, the ovarian follicle. The number of primordial follicles is established prior to birth. The steady, metered depletion of follicles throughout a female’s lifetime culminates in reproductive senescence and clinical menopause. Primordial follicle activation is the process by which a follicle is irreversibly recruited to undergo maturation and either completes this process culminating in ovulation, or more commonly undergoes atresia [1, 2]. Thus, among the most prominent feature of a primordial follicle is its ability to remain dormant for years and then, in response to yet ill-defined cues, be triggered from quiescence to promote maintenance of female fertility.
A growing body of evidence implicates biomechanical forces as critical to the establishment and maintenance of the primordial follicle pool. How active and/or passive interactions of extracellular and intracellular forces direct cell behaviors is an area of broad interest. The process through which mechanical stimuli are translated into biochemical signals effecting gene expression or cellular signaling is known as mechanical signaling or mechanotransduction. It is becoming increasingly clear that mechanical signaling plays an essential role throughout the dynamic lifespan of the ovarian follicle including oocyte storage, activation, growth, meiotic maturation, and ultimately ovulation. Multiple mediators of mechanotransduction have been identified in various species (i.e., Homo sapiens, Drosophila, Caenorhabditis elegans) including intercellular junctions, nuclear scaffolding proteins, cellular polarity, extracellular matrix (ECM) proteins and their linkage to receptors at the plasma membrane, cytoskeletal proteins, and cellular membrane ion channels [3–5]. Thus, the bidirectional interplay of forces between cells and their microenvironment influences gene expression and ultimately cellular behavior through the process of dynamic reciprocity [6].
Our objective was to review current evidence relating to the contribution of biomechanics and mechanical signaling during female mammalian gametogenesis. Specifically, we assessed current understanding of the biomechanics of ovarian function and the influence of ovarian rigidity on the follicle with respect to the establishment and maintenance of the primordial follicle endowment. Next, we considered follicular activation, highlighting the prominent role of mechanical signaling, particularly in the Hippo pathway. We then traced ovarian follicle development through the milestone processes of maturation and ovulation, incorporating the simultaneous mechanics of the oocyte including oogenesis and cytokinesis. These topics highlight the indispensable role that mechanotransduction plays throughout each phase of folliculogenesis and oogenesis. Finally, we discussed the clinical applications underlying ovarian and oocyte biomechanics.
Methods
A systematic review was performed according to the PRISMA guidelines [7]. We conducted a search on January 19, 2018, using PubMed and the Embase database (January 1998–January 2018). Key terms were determined and utilized in the search (Supplementary Table 1). Only English-language, mammalian-based, and ovarian publications were included. The size and quality of articles reviewed varied depending on the specific subject matter. We included literature reviews, expert opinion, and prospective studies. No case reports, case series, or retrospective studies were identified. All publications meeting inclusion criteria were assessed for quality by three authors (J.S.S., R.S., K.C.V.). From our search strategy, titles of publications and abstracts were reviewed and if deemed pertinent to our topic and meeting the inclusion criteria, the full-length articles were reviewed. Additional publications came from reference lists or by the listed authors. Six additional articles that were pertinent were included outside of the systematic search. Any discrepancies were discussed with the senior author. This systematic review was exempt from institutional review board approval given the research design.
Results
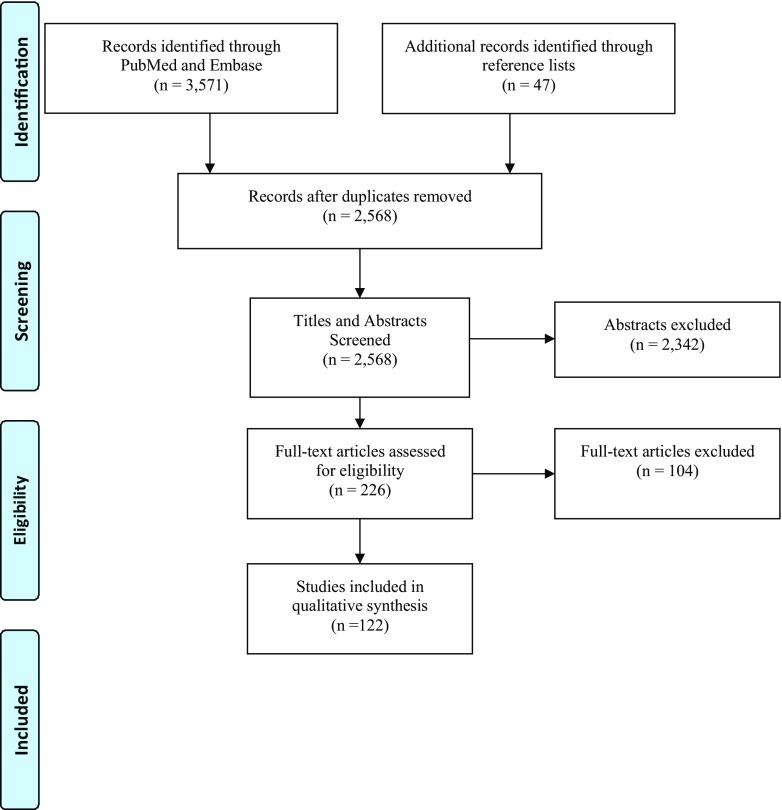
In this systematic review, 2568 publications were identified after duplications were removed (Fig. 1). There were 122 publications determined to be pertinent to the subject matter. We summarized the publications by these categories: biomechanics of the ovary, mechanical signaling in ovarian follicular activation, follicle maturation, mechanics of cytokinesis/cellular polarity/oocyte maturation, and clinical relevance to ovarian biomechanics.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analysis) diagram of search results and article flow
The biomechanical ovary
From a simplified structural viewpoint, the ovary may be divided into three zones: the epithelium, the cortex, and the medulla. Ovarian follicles housed within the dynamic architecture of the ovary are the functional units of the female reproductive biology. Not only are ovarian follicles essential for supporting oogenesis but they are also essential for the production of hormones to support female secondary sexual characteristics and early pregnancy. Each follicle is composed of a single oocyte surrounded by supportive layers of granulosa cells and theca cells that invest and nurture the growing oocyte as the follicle matures.
The ovary is a dynamic, mechanically responsive structure. The collagen-rich cortex is more rigid compared to the inner medullary region [8, 9]. The follicles are systematically distributed along this collagen gradient with the majority of primordial follicles situated in the outermost stiff cortical region, whereas the larger, developing pre-antral follicles are more commonly found in the less rigid medullary region [9]. The gradient of ovarian rigidity appears critical for oocyte storage, increases follicle survival, and supports proper folliculogenesis [9].
Investigators studied primate folliculogenesis by testing various concentrations of alginate within a follicle support structure in vitro and concluded primordial follicles require a more rigid environment (i.e., 2% alginate) to maintain proper follicular architecture and growth [9]. Culturing early stage follicles in comparison to secondary follicles is challenging because early stage follicles have a higher propensity to lose their critical physical association between the oocyte and the granulosa cells in humans [10]. Conversely, a separate group of investigators has shown in a murine model that advanced stage follicles require a less rigid environment (i.e., 0.25% vs. 1.5% alginate) to produce larger, more hormonally productive follicles that result in higher quality gametes [11, 12]. This same study also demonstrated that the follicles in the less rigid environment were more likely to extrude the first polar body and had higher fertilization rates [11, 12]. These in vitro studies support the concept that the structural gradient of ovarian rigidity can both limit follicle expansion to maintain dormancy and conversely facilitate follicle activation, maturation, and hormone production in primates and mice.
The successful utilization of artificial microenvironments to mimic the ovarian rigidity gradient and hormonal milieu, as well as to support various stages of follicular development represents a challenging endeavor given the alterations in ovarian composition needed to maintain primordial follicle quiescence, versus supporting growth and maturity of antral follicles. Nevertheless, the use of these artificial ovarian models highlights the critical role of ovarian biomechanics on folliculogenesis [13]. To simulate the dynamic cell-responsive mechanical properties of the murine ovary, fibrin-alginate interpenetrating networks (FA-IPN) hydrogels were developed that temporally mimic the migration of a maturing follicle down the rigidity gradient of the ovary [14]. Within the FA-IPN hydrogels, cellular production of plasminogen slowly degraded fibrin within the matrix leaving behind the alginate structure, thereby gradually decreasing the rigidity of the microenvironment [14]. With the advent of dynamic in vitro models of ovarian biomechanics, the critical importance of mechanical regulation of folliculogenesis becomes self-evident [15].
The role of mechanical signaling in ovarian follicular activation
The pool of ovarian primordial follicles established at birth serves as the reserve for the female reproductive life span, often being maintained quiescent for decades. Of the thousands of follicles present at birth, only a few primordial follicles develop to an advanced follicular stage. Advances in the understanding of the mechanisms governing the maintenance of dormancy and survival of primordial follicles have only recently been achieved and are largely due to new investigations into the role of ovarian mechanical signaling. Follicular activation is understood to be an irreversible process in which a primordial follicle is triggered to exit dormancy and proceed along the maturation pathway. Once initiated, folliculogenesis and oogenesis proceed in a coordinated fashion.
Activation of the primordial follicle to an early secondary follicle occurs independent of pituitary secretion of follicle-stimulating hormone (FSH) [16]. Support for this tenet includes an FSH receptor knock-out mouse model where follicular development was demonstrated to proceed until arrest at the pre-antral stage [17]. Thereafter, FSH is necessary to progress beyond the antral follicle to the pre-ovulatory and, finally, ovulatory follicle.
Ovarian follicles are initially or cyclically recruited [18]. The initial recruitment is believed to be a continuous process with intraovarian mechanical and other unknown factors governing the transition from primordial follicle [18]. As described earlier, ovarian rigidity appears to play a substantial role in both follicular quiescence and activation. Cyclic recruitment begins after puberty with an elevated FSH during the follicular phase of the menstrual cycle. The rising FSH rescues a cohort of antral follicles from atresia [18]. One or more of the rescued follicles will be selected to proceed through the final maturation stages and eventually ovulate.
The Hippo and Akt (protein kinase B) signaling pathways have been identified as two major regulators of primordial follicle activation during initial recruitment (Fig. 2). As shown in a murine model, the Hippo signaling pathway is known for its role in conserving optimal organ size via growth inhibition [19, 20]. Through a complex kinase cascade which is mechanoresponsive, mediators of the Hippo pathway phosphorylate and inactivate yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) to maintain the basal state and inhibit organ growth [19, 21]. Rho GTPase and Rho-associated protein kinase (ROCK) have been shown to regulate YAP/TAZ through a mechanotransduction pathway in mice [22, 23].
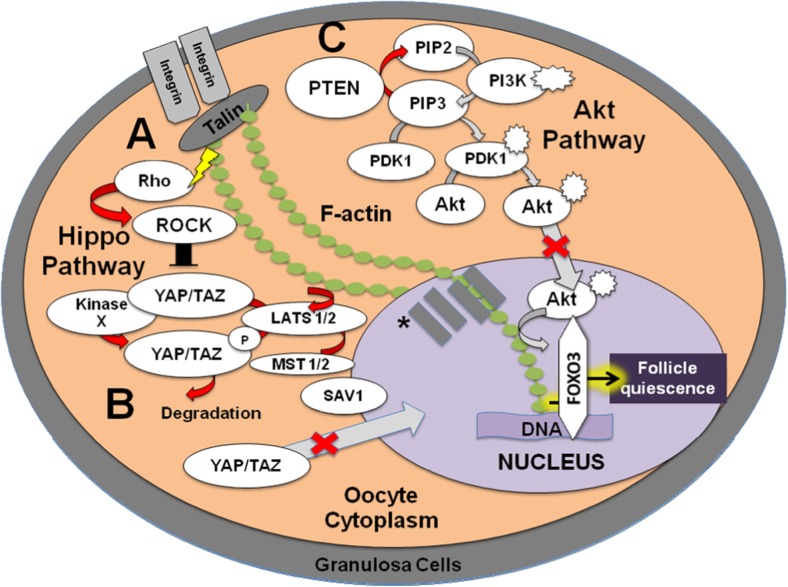
Fig. 2.
Maintenance of a quiescent primordial follicle is regulated by Hippo and Akt signaling. The Hippo signaling pathway inhibits primordial follicle activation. Cells probe the rigidity of their microenvironment and respond through central regulators of intracellular contractility (e.g., Rho GTPase and Rho-associated protein kinase (ROCK)). (A) An increase in internal stress from environmental rigidity (lightning bolt) is likely mechanically mediated through actin by negative growth factors (i.e., macrophage stimulating (MST1/2), salvador 1 (SAV1), and large tumor-suppressor homolog (LATS1/2)) that signals to the cell via Rho GTPase and ROCK phosphorylation of yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), (B) inhibiting it from crossing to the nucleus to bind to promoter regions on the DNA to promote transcription of growth stimulators. Thus, the Hippo signaling pathway appears to utilize internal mechanical stress and associated mechanical signaling to maintain the follicle in a quiescent state and inhibit follicular activation. (C) Phosphatase and tensin homolog deleted from chromosome 10 (PTEN) dephosphorylates activated phosphatidylinositol-3,4,5-triphosphate (PIP3) to phosphatidylinositol-4,5-bisphosphate (PIP2) preventing follicular activation by counteracting the Akt (protein kinase B) pathway and allowing forkhead box O3 (FOXO3) transcription factor, an essential regulator of follicular quiescence, to maintain the latency period of the follicle. The red arrows indicate pathways that are likely active to maintain follicle latency while gray arrows signify non-active steps. PI3K, phosphatidylinositol 3-kinase; PDK1, 3-phosphoinositide-dependent protein kinase-1 *SUN1/2, transmembrane proteins; Nesprin, nuclear envelope spectrin repeat protein.
Internal or external forces can trigger a mechanical signaling pathway that regulates cellular behavior. As human cells sense the rigidity of their microenvironment, they respond through central regulators of intracellular contractility (e.g., Rho GTPase) and effector proteins (e.g., ROCK) [24]. An increase in internal mechanical stress likely results in the expression of negative growth factors (i.e., macrophage stimulating [MST1/2], salvador 1 [SAV1], and the large tumor-suppressor homolog [LATS1/2]) through the phosphorylation and consequent inhibition of YAP/TAZ translocation from the cytoplasm to the nucleus by Rho GTPase and ROCK in humans and mice [25–27]. Nuclear localization of YAP is necessary for the transcription of growth factors (i.e., cysteine-rich protein 61 (CYR61/CCN1), connective tissue growth factor (CTGF/CCN2), and nephroblastoma overexpressed (NOV/CCN3)) [28] and baculoviral inhibitors of apoptosis repeat containing (BIRC) proteins [25]. Thus, inhibition of follicular growth and maintenance of follicular quiescence through the Hippo signaling pathway can be achieved through high internal mechanical stress as a cellular response to a rigid external environment in mice and humans.
Conversely, application of ovarian tissue fragmentation altering ovarian mechanical forces has been demonstrated to disrupt the Hippo signaling pathway leading to primordial follicle activation [19, 25]. Kawamura et al. demonstrated that ovarian fragmentation leads to increased actin polymerization, decreased phosphorylated YAP levels with subsequent increased nuclear localization of YAP, and enhanced expression of CCN growth factors and BIRC proteins [19] (Fig. 3). Disruption of Hippo signaling was characterized by polymerization of globular actin (G-actin) to filamentous actin (F-actin) [29] as seen in actin polymerization-enhancing drugs (i.e., Jasplakinolide (JASP) and sphingosine-1-phosphate (S1P)) resulting in increased CCN2 levels, increased ovarian graft weights, and increased number of late secondary follicles in mice and humans [27]. The polymerization of G-actin to F-actin is essential for cell shape preservation, adhesion, and locomotion [27]. Actin polymerization could link biophysical changes, such as variations in intercellular tension of an organ after physical damage, to the suppression of Hippo signaling which increased nuclear YAP concentration leading to follicle growth [27] (Fig. 4). Notably, fewer primordial and more secondary/antral follicles were found in the fragmented ovaries when compared to intact ovaries [19]. Tissue-level checkpoints help control cell growth by YAP/TAZ regulation with the F-actin-capping and severing proteins (e.g., CapZ, Cofilin, and Gelsolin) by limiting activity in human cells with low mechanical stress [30]. These data show that manipulation of the mechanical force on the follicle through ovarian fragmentation and disruption of the Hippo pathway can result in follicular activation and reinforces the notion that mechanical signaling plays a key role in the initiation and progression of folliculogenesis.
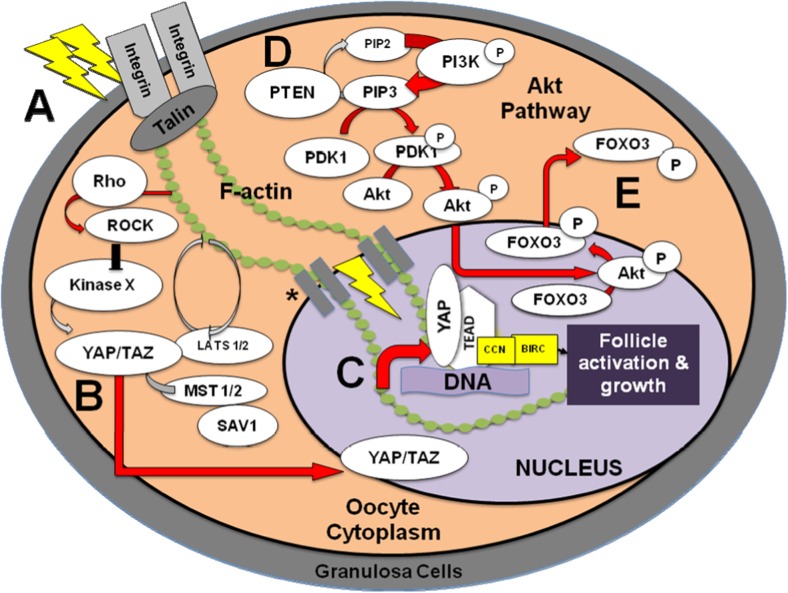
Fig. 3.
Activation of a primordial follicle is regulated by Hippo and Akt signaling. (A) Fragmentation force is likely transmitted through actin to activate the Hippo signaling pathway via Rho GTPase and Rho-associated protein kinase (ROCK). (B) This leads to a dephosphorylated yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) which enables entry into the nucleus. (C) Simultaneously, the Akt (protein kinase B) pathway is also activated by the phosphorylation of phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3) that subsequently leads to Akt phosphorylation allowing it to enter the nucleus. (D) Upon entry, the forkhead box O3 (FOXO3) transcription factor, an essential mediator of follicle dormancy, is phosphorylated at the Akt site causing nuclear export into the cytoplasm. (E) The nuclear YAP/TAZ binds the transcriptional enhancer associate domain (TEAD) allowing for expression of growth factors (i.e., cysteine-rich protein 61 (CYR61/CCN1), connective tissue growth factor (CTGF/CCN2), and nephroblastoma overexpressed (NOV/CCN3)) and baculoviral inhibitors of apoptosis repeat containing (BIRC) proteins. CCN and BIRC are growth factors that lead to follicular activation and cell growth. The red arrows indicate pathways that are likely active to activate the follicle while gray arrows signify non-active steps. PI3K, phosphatidylinositol 3-kinase; PDK1, 3-phosphoinositide-dependent protein kinase-1; MST1/2, macrophage stimulating; SAV1, salvador 1; LATS1/2, large tumor-suppressor homolog
*SUN1/2, transmembrane proteins; Nesprin, nuclear envelope spectrin repeat protein.
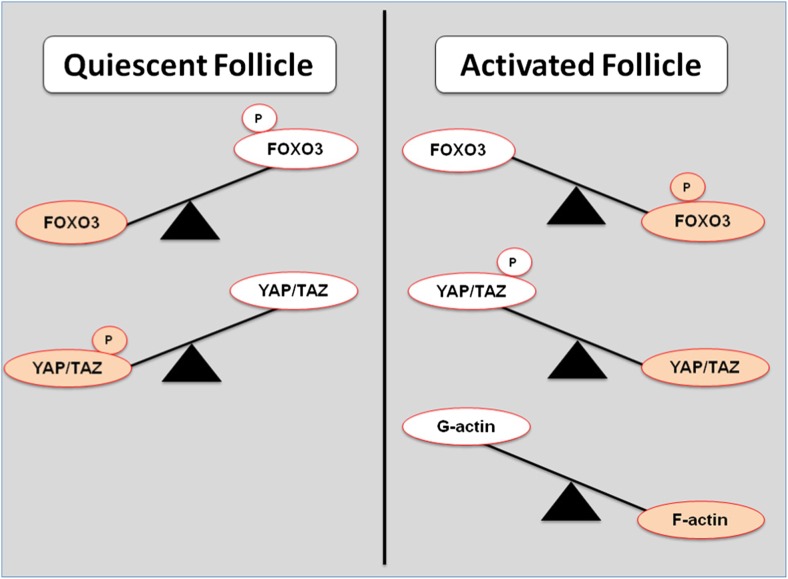
Fig. 4.
Different concentrations of key effectors of the Hippo and Akt pathways. In the quiescent state, the Akt (protein kinase B) pathway can keep forkhead box O3 (FOXO3) unphosphorylated preventing follicular activation. In the Hippo pathway, yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are phosphorylated keeping them from relocating to the nucleus thereby preventing activation. In contrast, in an activated follicle, there will be a higher concentration of phosphorylated FOXO3 causing it to leave the nucleus and enabling follicular activation. In addition, more YAP/TAZ will be in an unphosphorylated state. When the follicle senses mechanical disruption, globular actin (G-actin) is polymerized to filamentous actin (F-actin) which is also associated with follicular activation
The second major signaling pathway known to be involved in follicular activation is Akt. The Akt pathway mediates primordial follicle activation via two main targets: phosphatidylinositol 3-kinase (PI3K) and the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) gene. The PI3K pathway regulates cellular proliferation, growth, and survival in the human ovary/throughout the body while the PTEN protein negatively regulates PI3K [31, 32]. It is possible that ligands in oocytes bind to tyrosine kinase receptors to activate PI3K downstream [33]. Once PI3K is activated, it can phosphorylate and activate phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-triphosphate (PIP3) which activates Akt [20] (Fig. 3). The activated Akt suppresses the forkhead box O3 (FOXO3) transcription factor, an essential regulator of follicular quiescence [34]. A phospho-specific antibody that detects FOXO3 phosphorylated (Thr32) at its Akt phosphorylation site was found exclusively in the cytoplasm in a murine model [1]. This suggests that the Akt pathway in humans and mice has an important role in regulating the location of FOXO3 and activating the primordial follicle [20, 34]. Similarly, when the stem cell factor Kit receptor binding occurs in murine models, it is suggested that there is an increased proportion of cytoplasmic as compared to active nuclear Foxo3 causing primordial follicle activation [35–40]. Supporting this interpretation, a knock-out mouse model of Foxo3 expression resulted in global follicular activation, leading to premature oocyte depletion and secondary infertility [41]. One study pertaining to gastric carcinoma also implicates Rho GTPase in the inhibition of the Akt/PI3K pathway suppressing tumor growth and invasiveness [42]. As previously mentioned, Rho GTPase is a mediator of mechanotransduction in ovarian follicles through the Hippo pathway leading to inhibition of YAP and follicle growth inhibition. Overall, these data suggest a link between two well-known pathways of folliculogenesis and imply a common critical regulation of follicle activation through mechanical signaling in mammals.
While the Akt pathway is a major signaling pathway involved in activation, negative regulation of this pathway by PTEN is believed to be critical to maintenance of follicular dormancy in humans [43]. PTEN dephosphorylates activated PIP3 to PIP2 which prevents follicular activation by suppressing the Foxo3 gene in mice [20]. The initial recruitment of primordial follicles has been shown to be tightly regulated by PI3K/PTEN signaling in mice [44]. If Pten or the 3-phosphoinositide-dependent protein kinase-1 (Pdk1) gene is absent or deleted, the entire pool of primordial follicles will become activated in mice [45, 46]. Furthermore, in more developed follicles, it has been shown that the effect of a Pten deletion has no effect on the primordial follicle pool, follicular development, ovulation, or overall fertility in mice suggesting that PTEN/PI3K is stage-specific [47]. Considering that ovarian architecture differentially affects ovarian follicles at various stages of development and that PI3K/PTEN signaling is mediated by mechanical signals via Rho GTPase, it is likely that mechanical signaling underlies the stage-specific function of PI3K/PTEN activation of ovarian follicles.
Mammalian target of rapamycin (mTOR) signaling, a regulator of cell growth and proliferation, is also a known mediator of primordial follicle activation via the Akt pathway in mice [20, 48, 49]. Activated mTOR works upstream of Akt and promotes Akt phosphorylation leading to suppression of Foxo3 nuclear transcription and follicle activation. However, tuberous sclerosis complex 1 or 2 (TSC) negatively regulates mTOR complex 1 (mTORC1) and with an oocyte-specific deletion of TSC1 or TSC2, this will promote activation of all primordial follicles leading to premature ovarian failure in mice [50]. In addition, when 5′ adenosine monophosphate-activated protein kinase (AMPK) is inhibited, this will inactivate TSC2 and promote phosphorylation of mTOR in mice [51]. This impacts the Akt pathway by causing localization of YAP to the nucleus which promotes CTGF expression and subsequent follicular activation [51]. Also, the mitogen-activated protein kinase 3 and 1 (MAPK3/1) signaling in mice has an important role in follicle activation by activating mTOR [52]. Treating mice ovaries simultaneously with both mTOR and Akt signaling activators showed a significant increase in the number of antral/pre-ovulatory follicles when compared to just Akt activators [48]. In contrast, the mTOR inhibitor, rapamycin, can suppress the Akt activation to aid in follicular latency [48, 53]. It was also demonstrated that rapamycin treatment in a rat model caused a twofold increase in the number of primordial follicles likely due to suppression of follicular atresia with no significant difference in primary or secondary follicular count [54]. Of note, rapamycin-treated rats had irregular estrous cycles and were unable to be impregnated likely due to decreased estrogen levels from having less antral follicles [54]. Another mouse study showed liver kinase b1 (Lkb1) was critical in maintaining a primordial follicle pool [55]. If this gene is absent, the entire primordial follicular pool will be depleted because the mTOR signaling pathway is enhanced without a change in Akt activity and reduced AMPK activity [55]. Another gene, Lhx8 (LIM Homeobox 8), has been shown in a knock-out mouse model to indirectly interact with the Akt/PI3K/mTOR pathway to partially suppress primordial follicular activation [56]. The resultant interactions of mechanical signaling with mTOR, TSC1/2, rapamycin, AMPK, MAPK3/1, Lkb1, Lhx8, and the Akt pathway provide evidence of influence with follicle activation or quiescence. However, the link between mechanical signaling and these known pathways of follicular activation has not been fully elucidated. Further research is needed in this area as mechanical signaling seems to be an important regulator of follicular activation utilizing the other previously discussed pathways.
Follicle maturation: the dynamic progression to ovulation
Follicular maturation leading to ovulation is a dynamic process involving the interplay of multiple factors. The primordial follicle consists of an immature oocyte arrested in prophase I surrounded by a flat layer of pre-granulosa cells. The granulosa cells of the primordial follicle become more cuboidal in shape and the zona pellucida starts to encapsulate the oocyte while maintaining direct contact with the granulosa cell via the transzonal processes which originates from the granulosa cell [57]. Once the oocyte is entirely surrounded by cuboidal granulosa cells, it becomes a primary follicle [58]. These primary follicles begin developing FSH receptors, but are believed to not respond to gonadotropins until the antral phase of follicular development [16]. Activin, a protein complex with an intraovarian role, supports folliculogenesis with a polarization of adhesion events by maintaining oocyte to granulosa interactions, increasing granulosa cell adhesion to the basement membrane, and retention of adhesions to the zona pellucida [59, 60]. In addition, activin has been shown to upregulate lysyl oxidase (LOX), an important enzyme involved in the ECM formation and tissue remodeling, via the activin/transforming growth factor-β (TGF-β) type 1 receptor-mediated pathway in humans [61]. LOX activity is impacted when there is an increase of CTGF expression causing the growth differentiation factor 8 (GDF8) induced increase of LOX expression in humans [62]. Also, when TGF-β downregulates microRNA-29a (MIR29A), LOX activity is increased in humans [63]. Yang et al. suggest that there are additional microRNAs expressed during primordial follicle recruitment that may regulate follicular activation and quiescence in mice [64]. At this early stage, the follicles are influenced by paracrine and other intraovarian factors including ovarian biomechanics as demonstrated by in vitro ovarian cultures of early follicles and the need for rigidity to maintain the close association between the oocyte and granulosa cells [10, 65, 66].
As the primary follicle transforms into a secondary follicle, more layers of granulosa cells begin to circumscribe the oocyte and theca cells are recruited to surround the basal lamina, the outermost layer of the follicle. This may cause granulosa cell inducted polarity that could provide directional secretion/uptake of key molecules [67]. The theca cells differentiate into theca interna and externa. The basal lamina composition changes with folliculogenesis having varying proportions of isoform type IV collagen and laminin [68, 69]. The stiffness from the combination of basal lamina and tightly packed theca cells provides mechanical constraint forcing an inward division [58]. The formation of the fluid filled antrum requires movement of the granulosa cells relative to one another and the aquaporins present in the granulosa cells assist in water transport into the follicle [70]. Formation of the antrum marks the transition to the antral follicle. By this stage of development, follicular growth is dependent on FSH stimulation. The majority of follicles will undergo atresia at this phase in maturation. Within the small group of antral follicles that become pre-ovulatory follicles, a dominant follicle is selected which deprives the remaining antral follicles of FSH by generating estrogen and inhibin to suppress pituitary secretion of FSH [18]. This dynamic maturation process culminates in expulsion of the oocyte from the dominant follicle during ovulation. Simultaneously, the primary oocytes resume meiosis and complete meiosis I, entering meiosis II which is not completed until fertilization of the oocyte. C-type natriuretic peptide (CNP), secreted by granulosa cells of secondary and antral follicles, inhibits meiotic resumption of oocytes and prevents their premature maturation prior to the luteinizing hormone (LH) surge [71]. Following the LH surge, CNP levels are negatively regulated allowing meiosis progression [71].
As the developing follicle matures and acquires gonadotropin receptors, the follicle is progressively displaced toward the less rigid ovarian medulla. Different ovarian rigidity zones may modulate the follicle’s response to hormones leading to a cascade of cellular processes [16]. Follicles grown in stiffer and denser in vitro follicular cultures secrete higher levels of androgen and progesterone and relatively lower levels of estrogen [12]. The differential response to sex steroids may be mediated through mechanically responsive signaling by A-kinase anchor protein 13 (AKAP13) in humans [72]. AKAP13 can modulate sex steroid receptor activity and has been shown to effect intracellular osmolarity through stress-stimulated expression of nuclear factor of activated T-cells 5 (NFAT5) in lymphocytes [72]. These data reinforce the interplay between stiffness of the ovarian environment, cellular osmolarity, and hormonal secretion through mechanical signaling.
Mechanical signaling works in conjunction with multiple local paracrine and autocrine signaling pathways to aid in early follicular maturation. Several factors stimulate early follicles to mature and differentiate including platelet-derived growth factor (PDGF), bone morphogenic protein (BMP) 4/6/7/15, anti-Mullerian hormone (AMH), kit ligand, basic fibroblast growth factor (bFGF), growth differentiation factor 9 (GDF9), activin, and inhibin [16, 73, 74]. Insulin growth factor (IGF) promotes growth of the theca and granulosa cells and further enables gonadotropins to stimulate steroidogenesis in humans and mice [75]. IGF is secreted selectively by healthy follicles and has been shown to be regulated through the Akt and extracellular signal kinase (ERK) pathways in bovine models [76, 77]. Not only are the Akt and ERK pathways involved in mechanotransduction from the extracellular environment but they are also implicated as downstream effectors of the stimulatory action of FSH [76]. Sufficient exposure to FSH is crucial for antral follicles to reach the pre-ovulatory stage and escape atresia [75]. FSH provides a survival signal to the granulosa cells as partially regulated by the B-cell lymphoma 2 (BCL2) family of proteins via the previously mentioned PI3K pathway which promotes follicular growth [75].
Continuous remodeling of the ECM, a decrease in tensile strength in the apical follicle wall, and an increase in intrafollicular pressure is necessary for ovulation to occur as seen in rats [78]. These changes in tensile strength and intrafollicular pressure are generated by the action of smooth muscle cells and by osmotic fluid shifts. Regulation of the intracellular osmotic pressure is accomplished by a glycine transporter (GLYT1) encoded by the solute carrier family 6, member 9 (Slc6a9) gene in mice [79]. The oocytes are able to regulate their cell volume by releasing adhesions between the zona pellucida and the oocyte by a sharp increase in GLYT1 activity [79].
The LH surge that precedes ovulation induces follicular cells to synthesize and secrete multiple proteolytic enzymes including matrix metalloproteinases (MMPs) and plasmin that degrade the surrounding ECM [6, 80, 81]. The tissue factor pathway inhibitor 2 (TFPI2) helps to regulate granulosa cell plasmin activity aiding in the ECM remodeling in humans and rats [82]. The fragmented ECM releases ECM-bound ligands, including tumor necrosis factor-alpha (TNFα), which promotes collagenases and apoptosis of apical superficial epithelial cells in the pre-ovulatory follicle seen in many mammalian species [83–85]. TNFα, an obligate intermediate of the ovulatory ovarian rupture process, is controlled by increased granulosa cell expression of tissue inhibitor of MMPs (TIMP1) and α2-macroglobulins [83]. Proteolytic activity at the apical region of the follicle results in structural weakening due to less apical smooth cells and degradation of ECM. The resulting structural defect at the ovarian cortex and apical follicle will become the site of oocyte expulsion [86].
Endothelin 2 (Edn2) is a mechanotransduction gene proposed to be the driving force of follicular rupture and ovulation by transiently being expressed by granulosa cells in mice [86]. EDN2 production is confined to the granulosa cells while the critical smooth muscle layer is physically separated in the theca externa. With the assistance of proteolytic enzymes to weaken the ECM and follicle wall, EDN2 is then able to diffuse into the theca externa and bind smooth muscle leading to the contractile activity necessary for follicular rupture [86]. Contraction of smooth muscle causes an increase in intrafollicular force and causes a rupture at the weakest point of the follicle (i.e., the apex) [86]. EDN2 acts upon the smooth muscle cells in rats by binding endothelin receptor subtype A (EDNRA) which causes a contractile response to facilitate oocyte release and enable transportation of the cumulus–oocyte complex within the oviduct [87, 88]. Similarly, bradykinin and PGF-2α may play a role in increasing the intrafollicular pressure [8, 78]. Once the oocyte is expelled, the corpus luteum is developed by the invasion of the theca cell blood capillaries into the granulosa layer [89]. Corpus luteum regression occurs by an apoptotic mechanism; a fibrous degradation occurs with collagen synthesis forming a corpus albicans [89]. Ovulation is a prime example of dynamic reciprocity where the inciting hormonal event (i.e., LH surge) leads to a coordinated exchange between soluble mediators, proteolytic enzymes, osmotic fluid shifts, ECM remodeling, and alteration of mechanical forces ultimately leading to expulsion of the oocyte [6].
Mechanics of cytokinesis, cellular polarity, and oocyte maturation
There are considerable mechanical challenges involved in the process of female meiosis. Proper mechanotransduction is necessary to produce euploid embryos. Cytokinesis is the physical process of cellular division that results in two daughter cells. As shown in mice, cytokinesis is the result of an intricate interplay between three linked elements: biomechanical signals, cellular mechanics, and cell shape in mice [90]. Cortical tension of the oocyte, the force of the cortex and plasma membrane that minimizes the surface area to volume ratio, is essential for cellular mechanics and reflects the net sum of all intrinsic and extrinsic stressors on the cell [90]. Morphologically, meiosis differs from mitosis. Mammalian meiosis of the oocyte is an asymmetric cell division with polar body emissions while mitosis is the splitting into two equal daughter cells as seen in somatic cells. Oocytes undergo two rounds of meiosis. Meiosis I begins prior to birth and then arrests at prophase I until the follicle becomes cyclically recruited starting at menarche. The primary oocyte is diploid and undergoes a reduction division during meiosis I. Concurrent to follicular activation, the primary oocyte is stimulated to resume meiosis I and emit the first polar body. A secondary oocyte is formed with the expulsion of the first polar body, but the oocyte will not complete meiosis II and emit the second polar body until after fertilization.
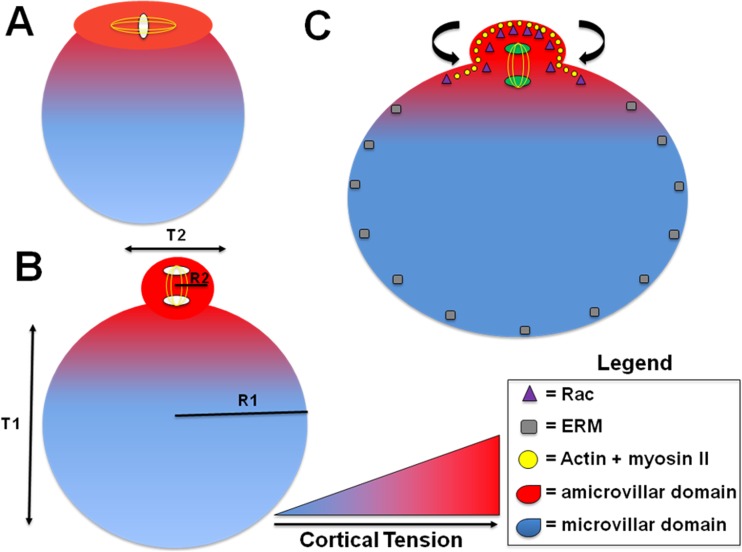
The cortical pressure differential between the oocyte and the polar body disfavors emergence of the polar body; however, establishing a mechanical polarity creates a microdomain, a specialized area of the cell membrane, for sequestration of the metaphase II spindle [91] (Fig. 5). This domain includes actin, myosin II, and ezrin–radixin–moesin (ERM) proteins [91]. As shown in a murine model, they establish a cellular polarity [92] in which the amicrovillar domain (i.e., meiotic MII spindle/polar body) has 2.5 times the cortical tension compared to the microvillar domain (i.e., sperm–egg fusion) [91]. Myosin II, regulated by the activation of myosin light chain 2 (MLC2), may create an enhanced binding to actin under tension, in the amicrovillar domain that generates increased cortical tension to aid the polar body expulsion [90, 91, 93, 94]. It is important to note that myosin-II is sensitive to mechanical stress and accumulates in areas of high stress to localize at the cleavage furrow [90, 91]. It has been shown in mice that there is a difference in effective tension between amicrovillar domains of ovulated oocytes and in vitro matured oocytes as well as spindle morphology and size likely due to gamma-tubulin distribution [95–97]. These spindle characteristics may be associated with the differences in amicrovillar tension impacting the mechanics [90, 91]. The asymmetric forces on the chromosome–spindle complex is a critical step in initiating oocyte polarization in mice [98]. Hooke’s Law is a principle of physics describing the force required to cleave a cell must be proportional to the cortical tension or membrane stiffness [99]. Thus, these instruments of mechanical polarity pull the polar body from the rest of the oocyte and allow it to overcome the inward pressure of the polar body.
Fig. 5.
Cortical tension and polarity facilitators of meiosis for polar body emission. (A) Asymmetric division during cytokinesis of mammalian female meiosis requires a gradient of cortical tension to eject the first polar body. (B) The radius of an oocyte (R1) in comparison to the radius of the polar body (R2) demonstrates an asymmetric division. Hooke’s Law describes the cleavage force is proportional to the surface tension or membrane stiffness. The cortical tension of the oocyte (T1) and the polar body (T2) demonstrates the creation of a microdomain, a specialized area of the cell membrane, for sequestration and completion of meiosis I. (C) The mechanical polarity is contributed by actin and myosin II, which are mechanically sensitive and congregate in areas of high cortical tension (i.e., amicrovillar domain) and by the ezrin–radixin–moesin (ERM) proteins in areas of lower cortical tension (i.e., microvillar domain). Rac, a Rho GTPase, accumulates by polarization in the cortex overlying the spindle, which creates spindle stability and facilitates the release of the polar body. These all contribute to creating a tension gradient allowing for a contraction on the polar body to pull away and overcome the inward forces
Actin polymers interacting with other proteins within the cell are essential for migration and may contribute to the development of oocyte cortical tension [100, 101]. Actin-related protein (ARP2/3), profilin, and cofilin are regulated by adenosine diphosphate ribosylation factor 6 (ARF6), and all aid in spindle migration in a vesicle-based mechanism [102]. Studies in murine and bovine models have suggested that ROCK regulates cofilin, an actin-binding protein, which aids in polar body extrusion by reorganizing and severing actin filaments [103, 104]. Also, formin-like 1 (FMNL1), dynamin-2, non-muscle tropomyosin (TPM3), and formin homology domain protein 1 (FHOD1) may all assist in polar body expulsion by affecting actin dynamics and spindle migration [105–108]. Rac, one of the main Rho GTPase expressed in mouse oocytes, has been shown to help regulate the release of the polar body by anchoring to the oocyte cortex and stabilizing the spindle [109]. Another protein, 14-3-3, has an important role in oocyte mechanics as seen in non-mammalian studies [110]. Consequently, the concepts of mechanics, mechanosensing, and intracellular forces are necessary for a cortical tension gradient and proper polar body formation and extrusion.
During cytokinesis, chromosomal polarity is essential for the generation of competent oocytes and ultimately euploid embryos. In mouse studies, nuclear mitotic apparatus proteins (NuMAs) are implicated in anchoring microtubules at the poles leading to proper organization of the polar domain of spindles [111]. Similarly, the Ras dexamethasone-induced protein (Rasd1) gene has been shown to be vital in maintaining normal spindle formation and chromosomal alignment [112]. In NuMA or Rasd1 deficient murine oocytes, impaired organization of microtubules leads to an altered microtubule organizing center and a disconnect between the microtubules and the meiosis I spindle poles [111, 112]. This creates a highly disorganized early spindle assembly leading to chromosomally abnormal oocytes [111]. Therefore, if the intracellular biomechanics are aberrant and do not create appropriate cellular polarity, chromosomally abnormal oocytes can result.
Clinical relevance of reproductive biomechanics
Polycystic ovary syndrome (PCOS) is a disease phenotype characterized by polycystic ovaries, biochemical or clinical signs of androgen excess, and anovulation. The densely collagenized thickened ovarian cortex likely creates a biomechanically non-permissive environment that may alter mechanical signaling within the ovary [16, 113]. Altered ovarian mechanotransduction may cause multiple early antral follicles. The rigid cortical environment may be related to defects in actin polymerization, leading to higher F-actin content and/or abnormal biosynthesis of intracellular matrix protein [71]. The increased tensile strength could lead to disruption of Hippo signaling and may cause an increase in nuclear localization of YAP, leading to increased secretion of growth factors and stimulation of primordial follicular activation and maturation [71]. Ovarian drilling in PCOS ovaries has been shown to promote actin polymerization and disrupt Hippo signaling which can cause follicle growth [19]. Therefore, treating these patients with CCN growth factors or local administration of actin polymerization drugs may counteract these effects on the ovary and provide potential treatments for PCOS patients [19]. Urbanek et al. identified a PCOS susceptibility gene in women, fibrillin-3 (FBN3), which encodes a protein involved in the ECM and is a regulator of the TGF-β family [114–116]. FBN3 is found in the stromal component of fetal ovaries and is highly expressed during stromal expansion and folliculogenesis in humans [114]. The exact role of FBN3 in PCOS remains to be elucidated.
Another key diagnostic criterion of PCOS, androgen excess, may be facilitated by the stiff biomechanical environment [12]. Usually the follicles are small, accumulate in the cortex, and secrete high levels of androgens [16]. In a murine model, follicles cultured in vitro in stiffer and denser alginate substrates secreted higher levels of androgens and progesterone relative to estrogen [12]. Furthermore, anovulation may occur due to the relative inability to degrade the ECM either from a lack of proteolytic enzymes (e.g., MMPs) or excessive proteolysis inhibitors (e.g., plasminogen activator inhibitor-1 and TFPI2) [8, 82, 117, 118]. Thus, androgen excess and anovulation, hallmarks of PCOS, may be influenced by an altered biomechanical state in the ovary that could contribute to a circular feedback loop with subsequent altered mechanical signaling causing excessive cortical rigidity.
A clearer understanding of oocyte biomechanical properties can potentially help streamline assisted reproductive technologies (ART). An emerging technique of microfluidic oocyte analysis could have the potential to optimize oocyte culture and provide biomechanical cellular data that have an impact on oocyte selection [119, 120]. Applying biomechanical inputs to an oocyte during maturation and development could allow for fertilization of higher quality oocytes [121, 122]. Another technique that highlights how oocyte stiffness may affect reproductive outcome is atomic force spectroscopy (AFS). AFS used with human oocytes can discern the varying mechanical features of the outer zona pellucida from one oocyte to the next [123]. The patients who achieved pregnancy had sibling oocytes (non-fertilized) analyzed by AFS showing a higher stiffness profile more representative of the immature oocytes versus the non-fertilized oocytes [123]. Another hypothesis is that mechanical properties measured by AFS could potentially find an optimal stiffness range in which oocytes could be identified to have increased potential for pregnancy, but further investigation is needed with regard to intracytoplasmic sperm injection outcomes [123]. These are examples of techniques that may assess oocyte biomechanical properties and structural competence with the potential for improved clinical outcomes [119].
In addition, a further understanding of follicular activation might help develop improved fertility-sparing treatments for patients with primary ovarian insufficiency (POI) or patients scheduled for gonadotoxic therapies. POI occurs from early depletion or inhibited activation of existing primordial follicles [20, 124]. Ovarian tissue transplantation for fertility restoration is an existing treatment option that is inefficient with an estimated primordial follicle loss of 50–90% from ischemia and associated issues [20]. A relatively new treatment for POI, currently under investigation, is in vitro activation (IVA), in which primordial follicles within the ovarian cortical tissue are harvested, activated via mechanical disruption in vitro, autotransplanted, and stimulated to develop into pre-antral follicles [20, 124, 125]. Similarly, AMH levels were able to be restored in cryopreserved ovarian tissue from cancer patients who had undergone gonadotoxic therapies [126]. Consequently, IVA overcomes the inefficiency of prior techniques and substantially increases the yield of mature oocytes from cryopreserved tissues previously composed of only immature follicles [20]. Using this process, a few cases of successful pregnancies have been reported in the literature [127, 128]. Thus, understanding the interplay between the Akt pathway and mechanical signaling are critical for follicular activation, and this understanding has been applied to a treatment that may preserve female fertility.
This systematic review focused on oocyte and follicular mechanical signaling and the impact of mechanics at each stage of oocyte and follicle development. Limitations to our study may include selection bias. To minimize the risk, three authors reviewed the publications. Some publications included were literature reviews or expert opinion that could also lead to bias. However, given that advances in knowledge on this topic are still evolving, the number of publications relevant to this topic was limited.
In conclusion, biomechanics and mechanical signaling within the ovary, follicle, and oocyte are implicated throughout the course of folliculogenesis: from primordial follicle storage to activation, follicular maturation to ovulation, and oogenesis to a euploid embryo. Follicular survival has profound implications for female fertility and reproductive potential for the individual and the human species. The mechanical forces imposed and interpreted by cellular components of the follicle through mechanosensing result in alterations in gene expression that dictate the balance between follicular activation and quiescence. Disruption of ovarian biomechanics by pathologic alterations in these physiological cellular forces can lead to ovarian disease phenotypes (i.e., PCOS) or be adapted for fertility preservation therapies (i.e., IVA). Future studies promoting further understanding of ovarian biomechanics are of paramount importance and may have substantial clinical implications.
Electronic supplementary material
(DOCX 14 kb)
Acknowledgements
We would like to thank Janice P. Evans, PhD for her suggestions and edits of the manuscript and Jamie Blanck, MLIS, MPA, AHIP for her assistance with the systematic review query.
Funding
In part, by the Howard W. and Georgeanna Seegar Jones Endowment. C.O. was supported by the intramural research program of NICHD, ZIE HD-008737-14.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Jaimin S. Shah and Reem Sabouni contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1180-y) contains supplementary material, which is available to authorized users.
References
- 1.John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RA, McLaughlin M, Wallace WHB, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29:97–106. doi: 10.1093/humrep/det388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:1230–1232. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 4.Heisenberg CP, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Albertini DF, Barrett SL. The developmental origins of mammalian oocyte polarity. Semin Cell Dev Biol. 2004;15:599–606. doi: 10.1016/j.semcdb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Thorne JT, Segal TR, Chang S, Jorge S, Segars JH, Leppert PC. Dynamic reciprocity between cells and their microenvironment in reproduction. Biol Reprod. 2015;92:1–10. doi: 10.1095/biolreprod.114.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorge S, Chang S, Barzilai JJ, Leppert P, Segars JH. Mechanical signaling in reproductive tissues: mechanisms and importance. Reprod Sci. 2014;21:1093–1107. doi: 10.1177/1933719114542023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27:1801–1810. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng. 2014;16:29–52. doi: 10.1146/annurev-bioeng-071813-105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 12.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007;25:287–299. doi: 10.1055/s-2007-980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials Elsevier Ltd. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith RM, Woodruff TK, Shea LD. Designing follicle–environment interactions with biomaterials. Cancer Treat Res. 2010;156:11–24. doi: 10.1007/978-1-4419-6518-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 18.McGee E, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, Hosoi Y, Yoshioka N, Ishizuka B, Hsueh AJ. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci. 2013;110:17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordeiro CN, Christianson MS, Selter JH, Segars JH. In vitro activation: a possible new frontier for treatment of primary ovarian insufficiency. Reprod Sci. 2016;23:429–438. doi: 10.1177/1933719115625842. [DOI] [PubMed] [Google Scholar]
- 21.Xiang C, Li J, Hu L, Huang J, Luo T, Zhong Z, Zheng Y, Zheng L. Hippo signaling pathway reveals a spatio-temporal correlation with the size of primordial follicle pool in mice. Cell Physiol Biochem. 2015;35:957–968. doi: 10.1159/000369752. [DOI] [PubMed] [Google Scholar]
- 22.Dupont S. Role of YAP/TAZ in cell–matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res Elsevier. 2016;343:42–53. doi: 10.1016/j.yexcr.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 24.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and rho GTPase signaling. J Cell Sci. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawashima I, Kawamura K. Regulation of follicle growth through hormonal factors and mechanical cues mediated by hippo signaling pathway. Syst Biol Reprod Med. 2017:1–9. [DOI] [PubMed]
- 26.Abbassi L, Malki S, Cockburn K, Macaulay A, Robert C, Rossant J, et al. Multiple mechanisms cooperate to constitutively exclude the transcriptional co-activator YAP from the nucleus during murine oogenesis. Biol Reprod. 2016;94:102. doi: 10.1095/biolreprod.115.137968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Feng Y, Jansson L, Sato Y, Deguchi M, Kawamura K, Hsueh AJ. Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the hippo signaling effector YAP. FASEB J. 2015;29:2423–2430. doi: 10.1096/fj.14-267856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 29.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 30.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Cantley LC, Fruman DA, Meyers RE, Cantley LC, Lawlor MA, Alessi DR, et al. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. [DOI] [PubMed]
- 32.Cecconi S, Mauro A, Cellini V, Patacchiola F. The role of Akt signalling in the mammalian ovary. Int J Dev Biol. 2012;56:809–817. doi: 10.1387/ijdb.120146sc. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJW. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci. 2010;107:10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ernst EH, Grøndahl ML, Grund S, Hardy K, Heuck A, Sunde L, Franks S, Andersen CY, Villesen P, Lykke-Hartmann K. Dormancy and activation of human oocytes from primordial and primary follicles: molecular clues to oocyte regulation. Hum Reprod. 2017;32:1684–1700. doi: 10.1093/humrep/dex238. [DOI] [PubMed] [Google Scholar]
- 35.Ezzati MM, Baker MD, Saatcioglu HD, Aloisio GM, Pena CG, Nakada Y, Cuevas I, Carr BR, Castrillon DH. Regulation of FOXO3 subcellular localization by kit ligand in the neonatal mouse ovary. J Assist Reprod Genet. 2015;32:1741–1747. doi: 10.1007/s10815-015-0589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobinoff AP, Sutherland JM, McLaughlin EA. Intracellular signalling during female gametogenesis. Mol Hum Reprod. 2013;19:265–278. doi: 10.1093/molehr/gas065. [DOI] [PubMed] [Google Scholar]
- 37.John GB, Shidler MJ, Besmer P, Castrillon DH. Kit signaling via PI3K promotes ovarian follicle maturation but is dispensable for primordial follicle activation. Dev Biol. 2010;331:292–299. doi: 10.1016/j.ydbio.2009.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones RL, Pepling ME. KIT signaling regulates primordial follicle formation in the neonatal mouse ovary. Dev Biol Elsevier. 2013;382:186–197. doi: 10.1016/j.ydbio.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 39.Saatcioglu HD, Cuevas I, Castrillon DH. Control of oocyte reawakening by kit. PLoS Genet. 2016;12:e1006215. doi: 10.1371/journal.pgen.1006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science (80-. ). 2003;301:215–8. [DOI] [PubMed]
- 42.Sun HW, Tong SL, He J, Wang Q, Zou L, Ma SJ, Tan HY, Luo JF, Wu HX. RhoA and RhoC-siRNA inhibit the proliferation and invasiveness activity of human gastric carcinoma by rho/PI3K/Akt pathway. World J Gastroenterol. 2007;13:3517–3522. doi: 10.3748/wjg.v13.i25.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goto M, Iwase A, Ando H, Kurotsuchi S, Harata T, Kikkawa F. PTEN and Akt expression during growth of human ovarian follicles. J Assist Reprod Genet. 2007;24:541–546. doi: 10.1007/s10815-007-9156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SY, Ebbert K, Cordeiro MH, Romero M, Zhu J, Serna VA, Whelan KA, Woodruff TK, Kurita T. Cell autonomous phosphoinositide 3-kinase activation in oocytes disrupts normal ovarian function through promoting survival and overgrowth of ovarian follicles. Endocrinology. 2015;156:1464–1476. doi: 10.1210/en.2014-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science (80-. ) 2008;319:611–3. [DOI] [PubMed]
- 46.Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- 47.Jagarlamudi K, Liu L, Adhikari D, Reddy P, Idahl A, Ottander U, et al. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One. 2009;4:3–9. doi: 10.1371/journal.pone.0006186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng Y, Kim J, Li XX, Hsueh AJ. Promotion of ovarian follicle growth following mTOR activation: synergistic effects of AKT stimulators. PLoS One. 2015;10:1–9. doi: 10.1371/journal.pone.0117769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong Y, Li F, Lu Y, Cao Y, Gao J, Liu J. Rapamycin-sensitive mTORC1 signaling is involved in physiological primordial follicle activation in mouse ovary. Mol Reprod Dev. 2013;80:1018–1034. doi: 10.1002/mrd.22267. [DOI] [PubMed] [Google Scholar]
- 50.Adhikari D, Zheng W, Shen Y, Gorre N, Hämäläinen T, Cooney AJ, et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2009;19:397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu X, Guo S, Cheng Y, Kim JH, Feng Y, Feng Y. Stimulation of ovarian follicle growth after AMPK inhibition. Reproduction. 2017;153:683–694. doi: 10.1530/REP-16-0577. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Zhang Y, Li J, Zheng N, Xu X, Yang J, Xia G, Zhang M. MAPK3/1 participates in the activation of primordial follicles through mTORC1-KITL signaling. J Cell Physiol. 2018;233:226–237. doi: 10.1002/jcp.25868. [DOI] [PubMed] [Google Scholar]
- 53.Adhikari D, Risal S, Liu K, Shen Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS One. 2013;8:e53810. doi: 10.1371/journal.pone.0053810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene. 2013;523:82–87. doi: 10.1016/j.gene.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 55.Jiang Z-Z, Hu M-W, Ma X-S, Schatten H, Fan H-Y, Wang Z-B, Sun QY. LKB1 acts as a critical gatekeeper of ovarian primordial follicle pool. Oncotarget. 2016;7:5738–5753. doi: 10.18632/oncotarget.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren Y, Suzuki H, Jagarlamudi K, Golnoski K, McGuire M, Lopes R, et al. Lhx8 regulates primordial follicle activation and postnatal folliculogenesis. BMC Biol. 2015;13:1–12. doi: 10.1186/s12915-015-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albertini DF, Combelles CMH, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 58.Da Silva-Buttkus P, Jayasooriya GS, Mora JM, Mobberley M, Ryder TA, Baithun M, et al. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J Cell Sci. 2008;121:3890–3900. doi: 10.1242/jcs.036400. [DOI] [PubMed] [Google Scholar]
- 59.McLaughlin M, Bromfield JJ, Albertini DF, Telfer EE. Activin promotes follicular integrity and oogenesis in cultured pre-antral bovine follicles. Mol Hum Reprod. 2010;16:644–653. doi: 10.1093/molehr/gaq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pangas SA, Rademaker AW, Fishman DA, Woodruff TK. Localization of the activin signal transduction components in normal human ovarian follicles: implications for autocrine and paracrine signaling in the ovary. J Clin Endocrinol Metab. 2002;87:2644–2657. doi: 10.1210/jcem.87.6.8519. [DOI] [PubMed] [Google Scholar]
- 61.Chang HM, Cheng JC, Liu Y, Klausen C, Xu C, Leung PCK. Activin A-induced increase in LOX activity in human granulosa-lutein cells is mediated by CTGF. Reproduction. 2016;152:293–301. doi: 10.1530/REP-16-0254. [DOI] [PubMed] [Google Scholar]
- 62.Chang HM, Fang Y, Liu PP, Cheng JC, Yang X, Leung PC. Connective tissue growth factor mediates growth differentiation factor 8-induced increase of lysyl oxidase activity in human granulosa-lutein cells. MolCell Endocrinol. 2016;434:186–198. doi: 10.1016/j.mce.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Fang Y, Chang HM, Cheng JC, Klausen C, Leung PCK, Yang X. Transforming growth factor-β1 increases lysyl oxidase expression by downregulating MIR29A in human granulose lutein cells. Reproduction. 2016;152:205–213. doi: 10.1530/REP-16-0144. [DOI] [PubMed] [Google Scholar]
- 64.Yang S, Wang S, Luo A, Ding T, Lai Z, Shen W, et al. Expression patterns and regulatory functions of microRNAs during the initiation of primordial follicle development in the neonatal mouse ovary. Biol Reprod. 2013;89:1–11. doi: 10.1095/biolreprod.113.107730. [DOI] [PubMed] [Google Scholar]
- 65.He X, Toth TL. In vitro culture of ovarian follicles from Peromyscus. Semin Cell Dev Biol. 2017;61:140–149. doi: 10.1016/j.semcdb.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zielak-Steciwko AE, Browne JA, McGettigan PA, Gajewska M, Dzieciol M, Szulc T, et al. Expression of microRNAs and their target genes and pathways associated with ovarian follicle development in cattle. Physiol Genomics. 2014;46:735–745. doi: 10.1152/physiolgenomics.00036.2014. [DOI] [PubMed] [Google Scholar]
- 67.Plancha CE, Sanfins A, Rodrigues P, Albertini D. Cell polarity during folliculogenesis and oogenesis. Reprod BioMed Online. 2005;10:478–484. doi: 10.1016/s1472-6483(10)60824-3. [DOI] [PubMed] [Google Scholar]
- 68.Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126:415–424. doi: 10.1530/rep.0.1260415. [DOI] [PubMed] [Google Scholar]
- 69.Bernal AL, Mardon H. Focal adhesion proteins in human granulosa cells. Reprod Technol. 2000;10:29–38. [Google Scholar]
- 70.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 71.Hsueh AJW, Kawamura K, Cheng Y, Fauser BCJM. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kino T, Segars JH, Chrousos GP. The guanine nucleotide exchange factor Brx: a link between osmotic stress, inflammation and organ physiology and pathophysiology. Expert Rev Endocrinol Metab. 2010;5:603–614. doi: 10.1586/eem.10.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142:2554–2563. doi: 10.1242/dev.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bristol SK, Woodruff TK. Follicle-restricted compartmentalization of transforming growth factor beta superfamily ligands in the feline ovary. Biol Reprod. 2004;70:846–859. doi: 10.1095/biolreprod.103.021857. [DOI] [PubMed] [Google Scholar]
- 75.Makker A, Goel MM, Mahdi AA. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: an update. J Mol Endocrinol. 2014;53:R103–R118. doi: 10.1530/JME-14-0220. [DOI] [PubMed] [Google Scholar]
- 76.Ryan KE, Casey SM, Canthy MJ, Crowe MA, Martin F, Evans ACO. Akt and Erk signal transduction pathways are early markers of differentiation in dominant and subordinate ovarian follicles in cattle. Reproduction. 2007;133:617–626. doi: 10.1530/REP-06-0130. [DOI] [PubMed] [Google Scholar]
- 77.Evans AC, Martin F. Kinase pathways in dominant and subordinate ovarian follicles during the first wave of follicular development in sheep. Anim Reprod Sci. 2000;64:221–231. doi: 10.1016/s0378-4320(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 78.Matousek M, Carati C, Gannon B, Brännström M. Novel method for intrafollicular pressure measurements in the rat ovary: increased intrafollicular pressure after hCG stimulation. Reproduction. 2001;121:307–314. doi: 10.1530/rep.0.1210307. [DOI] [PubMed] [Google Scholar]
- 79.Richard S, Tartia AP, Boison D, Baltz JM. Mouse oocytes acquire mechanisms that permit independent cell volume regulation at the end of oogenesis. J Cell Physiol. 2016;232:2436–2446. doi: 10.1002/jcp.25581. [DOI] [PubMed] [Google Scholar]
- 80.Curry TE, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 2006;24:228–241. doi: 10.1055/s-2006-948552. [DOI] [PubMed] [Google Scholar]
- 81.Gubbay O, Guo W, Rae MT, Niven D, Langdon SP, Hillier SG. Inflammation-associated gene expression is altered between normal human ovarian surface epithelial cells and cell lines derived from ovarian adenocarcinomas. Br J Cancer. 2005;92:1927–1933. doi: 10.1038/sj.bjc.6602568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puttabyatappa M, Al-Alem LF, Zakerkish F, Rosewell KL, Brännström M, Curry TE. Induction of tissue factor pathway inhibitor 2 by hCG regulates periovulatory gene expression and plasmin activity. Endocrinology. 2017;158:109–120. doi: 10.1210/en.2016-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murdoch WJ, Gottsch ML. Proteolytic mechanisms in the ovulatory folliculo-luteal transformation. Connect Tissue Res. 2003;44:50–57. [PubMed] [Google Scholar]
- 84.Murdoch WJ. Regulation of collagenolysis and cell death by plasmin within the formative stigma of preovulatory ovine follicles. J Reprod Fertil. 1998;113:331–336. doi: 10.1530/jrf.0.1130331. [DOI] [PubMed] [Google Scholar]
- 85.Murdoch WJ, Colgin DC, Ellis JA. Role of tumor necrosis factor-a in the ovulatory mechanism of ewes. J Anim Sci. 1997;75:1601–1605. doi: 10.2527/1997.7561601x. [DOI] [PubMed] [Google Scholar]
- 86.Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, et al. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–1779. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- 87.Bridges PJ, Jo M, Al Alem L, Na G, Su W, Gong MC, et al. Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype a (EDNRA)-mediated contraction. Reprod Fertil Dev. 2010;22:780–787. doi: 10.1071/RD09194. [DOI] [PubMed] [Google Scholar]
- 88.Al-Alem L, Bridges PJ, Su W, Gong MC, Iglarz M, Ko C. Endothelin-2 induces oviductal contraction via endothelin receptor subtype a in rats. J Endocrinol. 2007;193:383–391. doi: 10.1677/JOE-07-0089. [DOI] [PubMed] [Google Scholar]
- 89.Rolaki A, Drakakis P, Millingos S, Loutradis D, Makrigiannakis A. Novel trends in follicular development, atresia and corpus luteum regression: a role for apoptosis. Reprod BioMed Online. 2005;11:93–103. doi: 10.1016/s1472-6483(10)61304-1. [DOI] [PubMed] [Google Scholar]
- 90.Evans JP, Robinson DN. The spatial and mechanical challenges of female meiosis. Mol Reprod Dev. 2011;78:769–777. doi: 10.1002/mrd.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Larson SM, Lee HJ, Hung PH, Matthews LM, Robinson DN, Evans JP. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and ezrin–radixin–moesin (ERM) proteins. Mol Biol Cell. 2010;21:3182–3192. doi: 10.1091/mbc.E10-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hosseini SM, Moulavi F, Tanhaie-Vash N, Asgari V, Ghanaei HR, Abedi-Dorche M, Jafarzadeh N, Gourabi H, Shahverdi AH, Dizaj AV, Shirazi A, Nasr-Esfahani MH. The principal forces of oocyte polarity are evolutionary conserved but may not affect the contribution of the first two blastomeres to the blastocyst development in mammals. PLoS One. 2016;11:e0148382. doi: 10.1371/journal.pone.0148382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duan X, Liu J, Zhu CC, Wang QC, Cui XS, Kim NH, Xiong B, Sun SC. RhoA-mediated MLC2 regulates actin dynamics for cytokinesis in meiosis. Cell Cycle. 2016;15:471–477. doi: 10.1080/15384101.2015.1128590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brunet S, Verlhac MH. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update. 2011;17:68–75. doi: 10.1093/humupd/dmq044. [DOI] [PubMed] [Google Scholar]
- 95.Sanfins A, Lee GY, Plancha CE, Overstrom EW, Albertini DF. Distinctions in meiotic spindle structure and assembly during in vitro and in vivo maturation of mouse oocytes. Biol Reprod. 2003;69:2059–2067. doi: 10.1095/biolreprod.103.020537. [DOI] [PubMed] [Google Scholar]
- 96.Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–957. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- 97.Combelles CMH, Albertini DF. Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of gamma-tubulin. Dev Biol. 2001;239:281–294. doi: 10.1006/dbio.2001.0444. [DOI] [PubMed] [Google Scholar]
- 98.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell biol. Nat Publ Group. 2013;14:141–152. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 99.Robinson DN. Cell division: biochemically controlled mechanics. Curr Biol. 2001;11:737–740. doi: 10.1016/s0960-9822(01)00434-1. [DOI] [PubMed] [Google Scholar]
- 100.Kloc M, Ghobrial RM, Borsuk E, Kubiak JZ. Polarity and asymmetry during mouse oogenesis and oocyte maturation. Mouse Dev. 2012:23–44. [DOI] [PubMed]
- 101.Jo Y-J, Jang W-I, Namgoong S, Kim N-H. Actin-capping proteins play essential roles in the asymmetric division of maturing mouse oocytes. J Cell Sci. 2015;128:160–170. doi: 10.1242/jcs.163576. [DOI] [PubMed] [Google Scholar]
- 102.Duan X, Zhang H, Pan M, Zhang Y, Sun S. Vesicular transport protein Arf6 modulates cytoskeleton dynamics for polar body extrusion in mouse oocyte meiosis. BBA Mol Cell Res. 2018;1865:455–462. doi: 10.1016/j.bbamcr.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 103.Duan X, Liu J, Dai X-X, Liu H-L, Cui X-S, Kim N-H, et al. Rho-GTPase effector ROCK phosphorylates cofilin in actin-meditated cytokinesis during mouse oocyte meiosis. Biol Reprod. 2014;90:1–9. doi: 10.1095/biolreprod.113.113522. [DOI] [PubMed] [Google Scholar]
- 104.Lee SR, Xu YN, Jo YJ, Namgoong S, Kim NH. The rho-GTPase effector ROCK regulates meiotic maturation of the bovine oocyte via myosin light chain phosphorylation and cofilin phosphorylation. Mol Reprod Dev. 2015;82:849–858. doi: 10.1002/mrd.22524. [DOI] [PubMed] [Google Scholar]
- 105.Wang F, Zhang L, Duan X, Zhang GL, Wang ZB, Wang Q, Xiong B, Sun SC. RhoA-mediated FMNL1 regulates GM130 for actin assembly and phosphorylates MAPK for spindle formation in mouse oocyte meiosis. Cell Cycle. 2015;14:2835–2843. doi: 10.1080/15384101.2015.1031438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang QC, Liu J, Wang ZB, Zhang Y, Duan X, Cui XS, Kim NH, Sun SC. Dynamin 2 regulates actin-mediated spindle migration in mouse oocytes. Biol Cell. 2014;106:193–202. doi: 10.1111/boc.201400007. [DOI] [PubMed] [Google Scholar]
- 107.Jang WI, Jo YJ, Kim HC, Jia JL, Namgoong S, Kim NH. Non-muscle tropomyosin (Tpm3) is crucial for asymmetric cell division and maintenance of cortical integrity in mouse oocytes. Cell Cycle. 2014;13:2359–2369. doi: 10.4161/cc.29333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pan MH, Wang F, Lu Y, Tang F, Duan X, Zhang Y, Xiong B, Sun SC. FHOD1 regulates cytoplasmic actin-based spindle migration for mouse oocyte asymmetric cell division. J Cell Physiol. 2018;233:2270–2278. doi: 10.1002/jcp.26099. [DOI] [PubMed] [Google Scholar]
- 109.Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–317. doi: 10.1016/j.devcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 110.De S, Marcinkiewicz JL, Vijayaraghavan S, Kline D. Expression of 14-3-3 protein isoforms in mouse oocytes, eggs and ovarian follicular development. BMC Res Notes. 2012;5:57. doi: 10.1186/1756-0500-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kolano A, Brunet S, Silk AD, Cleveland DW, Verlhac M-H. Error-prone mammalian female meiosis from silencing the spindle assembly checkpoint without normal interkinetochore tension. Proc Natl Acad Sci. 2012;109:E1858–E1867. doi: 10.1073/pnas.1204686109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee Y, Kim KH, Yoon H, Lee OH, Kim E, Park M, Jang H, Hong K, Song H, Ko JJ, Lee WS, Lee KA, Chang EM, Choi Y. RASD1 knockdown results in failure of oocyte maturation. Cell Physiol Biochem. 2016;40:1289–1302. doi: 10.1159/000453182. [DOI] [PubMed] [Google Scholar]
- 113.Hughesdon PE. Morphology and morphogenesis of the Stein–Leventhal ovary and of so-called “hyperthecosis.” Obstet Gynecol Surv 1982;37:59–77. [DOI] [PubMed]
- 114.Hatzirodos N, Bayne RA, Irving-Rodgers HF, Hummitzsch K, Sabatier L, Lee S, Bonner W, Gibson MA, Rainey WE, Carr BR, Mason HD, Reinhardt DP, Anderson RA, Rodgers RJ. Linkage of regulators of TGF-activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25:2256–2265. doi: 10.1096/fj.11-181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci U S A. 2007;104:1348–1353. doi: 10.1073/pnas.0607966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007;92:4191–4198. doi: 10.1210/jc.2007-0761. [DOI] [PubMed] [Google Scholar]
- 117.D’Ascenzo S, Giusti I, Millimaggi D, Marci R, Tatone C, Colonna RC, et al. Intrafollicular expression of matrix metalloproteinases and their inhibitors in normally ovulating women compared with patients undergoing in vitro fertilization treatment. Eur J Endocrinol. 2004;151:87–91. doi: 10.1530/eje.0.1510087. [DOI] [PubMed] [Google Scholar]
- 118.Tarkun I, Cantürk Z, Arslan BC, Türemen E, Tarkun P. The plasminogen activator system in young and lean women with polycystic ovary syndrome. Endocr J. 2004;51:467–472. doi: 10.1507/endocrj.51.467. [DOI] [PubMed] [Google Scholar]
- 119.Kort J, Behr B. Biomechanics and developmental potential of oocytes and embryos. Fertil Steril. 2017;108:738–741. doi: 10.1016/j.fertnstert.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 120.Lai D, Takayama S, Smith GD. Recent microfluidic devices for studying gamete and embryo biomechanics. J Biomech Elsevier. 2015;48:1671–1678. doi: 10.1016/j.jbiomech.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 121.Yanez LZ, Camarillo DB. Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies. Mol Hum Reprod. 2017;23:235–247. doi: 10.1093/molehr/gaw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He X. Microfluidic encapsulation of ovarian follicles for 3D culture. Ann Biomed Eng. 2017;45:1676–1684. doi: 10.1007/s10439-017-1823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Andolfi L, Masiero E, Giolo E, Martinelli M, Luppi S, dal Zilio S, Delfino I, Bortul R, Zweyer M, Ricci G, Lazzarino M. Investigating the mechanical properties of zona pellucida of whole human oocytes by atomic force spectroscopy. Integr Biol. 2016;8:886–893. doi: 10.1039/c6ib00044d. [DOI] [PubMed] [Google Scholar]
- 124.Kawashima I, Kawamura K. Disorganization of the germ cell pool leads to primary ovarian insufficiency. Reproduction. 2017;153:R205–R213. doi: 10.1530/REP-17-0015. [DOI] [PubMed] [Google Scholar]
- 125.Yin O, Cayton K, Segars JH. In vitro activation: a dip into the primordial follicle pool? J Clin Endocrinol Metab. 2016;101:3568–3570. doi: 10.1210/jc.2016-2837. [DOI] [PubMed] [Google Scholar]
- 126.Novella-Maestre E, Herraiz S, Rodriguez-Iglesias B, Diaz-Garcia C, Pellicer A. Short-term PTEN inhibition improves in vitro activation of primordial follicles, preserves follicular viability, and restores AMH levels in cryopreserved ovarian tissue from Cancer patients. PLoS One. 2015;10:e0127786. doi: 10.1371/journal.pone.0127786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 128.Zhai J, Yao G, Dong F, Bu Z, Cheng Y, Sato Y, Hu L, Zhang Y, Wang J, Dai S, Li J, Sun J, Hsueh AJ, Kawamura K, Sun Y. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab. 2016;101:4405–4412. doi: 10.1210/jc.2016-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)