Abstract
Purpose
Individual follicle cryopreservation techniques, without hydrogel support, are labor-intensive and a substantial proportion of isolated follicles are lost during handling and after warming. Therefore, the viability and morphology of isolated bovine (as a model for human) pre-antral follicles after vitrification and warming, when encapsulated in alginate beads, were investigated.
Methods
Bovine pre-antral follicles were mechanically isolated and divided into four different groups: (1) culture in 2% alginate beads (3D system) and vitrification in beads using mesh cups (3DVIT), (2) culture in 2% alginate beads (3DCUL), (3) culture in 96-well plates (2D system) and vitrification using High Security Vitrification straws® (2DVIT), (4) culture in a 2D system (2DCUL). The same vitrification and warming protocols were used for embedded (3DVIT) and non-embedded follicles (2DVIT).
Results
No differences were observed in follicle viability between group 2DCUL and 3DCUL. Group 3DVIT showed the lowest viability (45.9%) according to calcein and neutral red staining among all groups. Group 2DVIT displayed the highest viability (87.5%) and largest percentage of follicles with a well-preserved morphology.
Conclusions
Our results show that, using a vitification protocol optimized for non-embedded follicles, 2D culture is more effective in vitrifying isolated follicles. However, embedding in alginate allow to handle follicles more efficiently, i.e., without excessive manipulation and thus less labor-intensive in combination with a reduced loss of follicles during the procedure. Based on the increased work efficiency, but lower viability and higher proportion of follicles showing impaired morphology, we consider it advantageous to optimize the protocol for the vitrification of embedded follicles to increase survival and maintain morphology after vitrification.
Keywords: Pre-antral follicles, Bovine, Alginate, Vitrification, 2D culture system, 3D culture system
Introduction
According to recent data, women under 40 years of age have an estimated 2.5% chance of developing cancer [1]. Fortunately, thanks to constant improvements in diagnosis and cancer treatment [2], up to 90% of woman who are diagnosed with reproductive tract cancer are now long-term survivors [3]. However, as chemo- and radiotherapy often damage ovarian tissue [4, 5], patients are likely to show compromised fertility [6, 7] up to a level where survivors of childhood cancer have an overall reduction of 19% in the likelihood of ever being pregnant [8]. As a consequence, the interest in fertility preservation (FP) strategies in women has been sparked to the extent that it has now become a key medical sub-discipline [9]. Current FP techniques for women comprise ovarian transposition, cryopreservation of embryos and unfertilized oocytes [10–12], and ovarian tissue cryopreservation (OTC) containing pre-antral follicles (PAFs) [9]. Clearly, the most suitable option for a specific patient is based upon different parameters such as the patient’s age and relationship status, the type of cancer, and the time available between diagnosis and the onset of treatment [10].
Although a few dozens of births have already been reported worldwide [13–15], several authors have raised concerns associated with OTC (which is still in the experimental phase), the most important of which being the risk of the reintroduction of malignant cells following autotransplantation of the frozen-thawed tissue [16–18]. On top of that, transplantation often results in extensive follicular loss due to delayed and deficient revascularization [19, 20]. Furthermore, both quantitative and qualitative assessments of the follicle population in ovarian tissue samples are difficult, if not impossible [21–23]. Consequently, the risk of freezing tissue samples devoid of (viable) follicles is significant, creating a false idea for the patient on preserved fertility. On the contrary, cryopreservation of isolated PAFs and subsequent autotransplantation, grafting [24, 25], or future in vitro culture [26] offers interesting new perspectives [27], minimizing the risk of transfer of malignant cells and avoiding banking of ovarian tissue devoid of PAFs. Recently, there is an increasing interest for bovine in vitro models in human studies on assisted reproductive techniques (ARTs) and FP as reviewed by Langbeen et al. [28]. This is due to physiological similarities and the easy access to slaughterhouse ovaries which guarantee unrestricted availability of study specimens such as PAFs [28]. Early pre-antral follicles account for the vast majority of follicles in the ovarian cortex and the in vitro culture of PAFs could be an excellent strategy to produce fertilizable oocytes. However, the development of a culture system that allows the development of a primordial follicle to a mature fertilizable oocyte remains a challenge in larger mammalian species because of the lengthy period of folliculogenesis and the larger size of the follicle [29]. In bovine species, the growth of PAFs was limited to the stage of antrum formation [30]. Production of live offspring with oocytes from in vitro cultured primordial follicles has, so far, only been achieved in mice [31]. Gupta et al. [32] reported the successful production of embryos from in vitro grown pre-antral follicles from buffalos after long-term PAF culture (100 days). Xiao et al. [33] accomplished for the first time the development of human follicles until the antral stage and production of meiotically competent MII oocytes, using a two-step culture method. In vitro growth of pre-antral follicles is a delicate process because the dynamics of the ovarian environment need to be mimicked.
Three-dimensional (3D) culture systems seem to simulate more effectively the physiologic conditions of the ovary than traditional two-dimensional (2D) systems (monolayer, such as multiwell plates) [26]. According to West et al. [34], alginate is a suitable matrix for in vitro culture of isolated follicles due to its gentle gelling properties and biochemical characteristics. Encapsulation of follicles better preserves the follicular morphology which is imperative to maintain communication between the oocyte and the granulosa cells. Alginate encapsulation is not only a valuable tool for in vitro culture of isolated follicles, but also for their cryopreservation. It would protect the cells from direct exposure to cryoprotectants and reduce the impact of cooling and warming. Alginate hydrogels, also known as beads, are already successfully applied for cryopreservation of human isolated follicles [35, 36]. However, the available scientific literature on the possible effects of vitrification on embedded human isolated PAFs is scarce and as far as we know non-existing for bovine isolated PAFs. Encapsulation of follicles is advantageous as one bead can contain several follicles, it simplifies follicle handling and the number of lost follicles during the process can be minimized. To assess the effects of vitrification on follicles after warming, survival during short-term culture is an important parameter, next to the use of different other yet more invasive methods such as calcein staining.
Because of the advantages of 3D systems for vitrification and culture, in the current study, we aimed to (1) assess viability and morphology of follicles after short-term culture in alginate beads using the bovine model, (2) vitrify isolated bovine pre-antral follicles on a time and labor efficient way using alginate beads in mesh cups, (3) assess viability of follicles, that were vitrified either in alginate beads or without encapsulation using High Security Vitrification (HSV) straws®, after warming.
Materials and methods
Collection of ovaries, ovarian follicle isolation, and culture
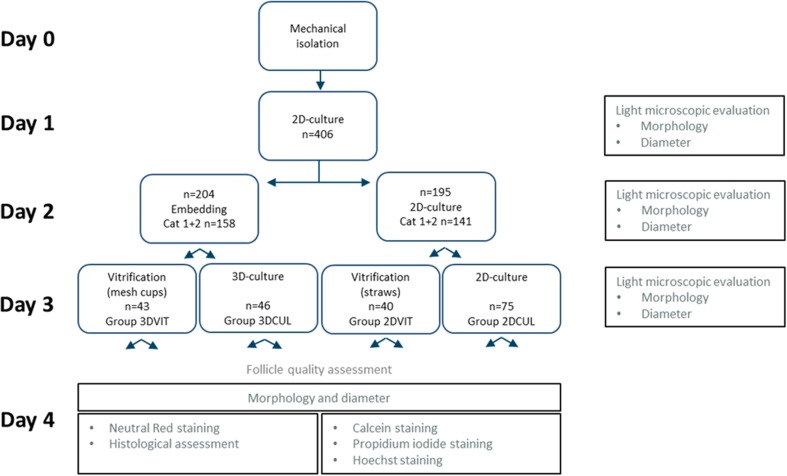
As described earlier [37], adult bovine ovaries were collected upon slaughter and transported in warm physiologic saline (0.9% NaCl, Braun) to the laboratory within 3 h at 25 °C. Following removal of the adnexa, the ovaries were washed in warm (38.5 °C) physiologic solution supplemented with kanamycin (0.25%) and rinsed in alcohol (70%). Active ovaries were selected, free of abundant antral follicles, corpora lutea or scar tissue. The ovarian cortex was cut into pieces of approximately 1 mm3, using a scalpel. The pieces of ovarian cortex were transferred to isolation medium: M199, supplemented with hepes (0.04 M), gentamycin (50 μL/ml), bovine serum albumine (10 mg/ml), polyvinylpyrrolidone (4 mg/ml) and filtered through a 0.2-μm filter. Ovarian cortex tissue was mixed and dispersed using an Ultra Turrax T18 Basic device (IKA®, VWR, Leuven, Belgium) with a larger plastic dispersing tool (IKA®, S18D-14G-KS) for 2 min and with a smaller one (IKA®, S18D-10G-KS) for 1 min. The resulting follicle suspension was subsequently filtered through a 100-μm, a 70-μm filter (BD Falcon®, Corning, NY, USA) and a 20-μm nylon filter (Millipore®, Cork, Ireland). Early PAFs were recovered from the 20 μm mesh filter by rinsing with isolation medium. Follicles were visualized using standard inverted light microscopy (Olympus, Aartselaar, Belgium). PAFs with an oocyte surrounded by one layer of cuboidal granulosa cells and intact basal membrane were selected [38]. They were individually transferred and cultured in 70 μl culture medium in 96-well plates (Greiner Bio-One, Germany) at 38.5 °C and 5% CO2. The culture medium consisted of equal parts DMEM and Ham’s F12 nutrient supplemented with penicillin G (240 U/ml) and streptomycin (240 μg/ml), fungizone (5 μg/ml), fetal calf serum (2.3(v/v)%) and newborn calf serum (2.3(v/v)%), bovine serum albumin (0.75(w/v)%), insulin (0.01 mg/ml), transferrin (0.55 μg/ml) and selenium (6.7 ng/ml). Two plates were used per isolation. Per plate, 30 wells were used for culture. Plates were filled alternately per row to minimize exposure time of follicles to light. The experimental set-up is described below and summarized in a flow chart (Fig. 2). To evaluate follicles, the connection between the oocyte and the surrounding granulosa cells and the integrity of the basal membrane were microscopically evaluated as reported by [37] (Fig. 1). Category 1 contains follicles with both an intact basal membrane and an intact connection between the oocyte and surrounding granulosa cells. Category 2 contains follicles with an intact connection between the oocyte and the surrounding granulosa cells but shows signs of a disrupted basal membrane. Category 3 holds the follicles with a disrupted basal membrane and a disrupted connection between the oocyte and granulosa cells. During follicle isolation (day 0), selection for embedding (day 2) and vitrification (day 3), only category 1 and 2 follicles were selected. Category 3 follicles were not further used.
Fig. 2.
Experimental design (divided over 6 replicates). On each evaluation day (1, 2, 3, 4), morphology was assessed and follicle diameter measured. On day 4, half of the follicles from each group was stained with neutral red (NR) for viability assessment and fixated for histological assessment. The other half was used for calcein, propidium iodide and Hoechst staining. Grey text describes follicle quality assessment methods
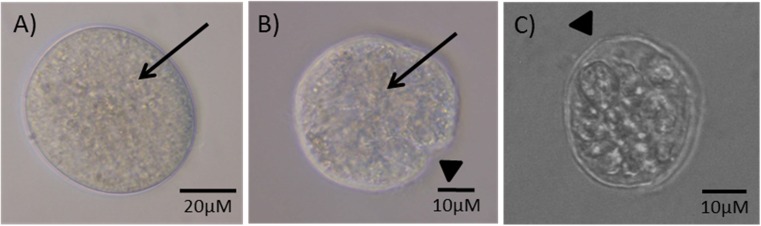
Fig. 1.
Follicle morphological categories. a A category 1 follicle at day 7 of in vitro culture showing an intact basal membrane and connection between the oocyte and surrounding granulosa cells. b A category 2 follicle at day 4 of in vitro culture showing a tight connection between the oocyte and surrounding granulosa cells but a disrupted follicle basal membrane. c A category 3 follicle at day 4 of in vitro culture showing a disrupted basal membrane and a disrupted connection between the oocyte and surrounding granulosa cells. Arrows indicate a tight connection between the oocyte and granulosa cells and the arrow heads indicate disruptions in the basal membrane
On day 0, primary follicles were selected and 60 wells from two 96-well plates were filled. On day 2, follicles were divided into two groups within which follicle categories 1 and 2 were represented on an equal basis. Half of all category 1 and 2 follicles were embedded in alginate beads, the other half stayed in culture in the corresponding 96-well plate. On day 3, the two groups were each splitted into two groups in which follicle categories 1 and 2 were again represented on an equal basis. Half of the embedded category 1 and 2 follicles were vitrified using mesh cups. Half of the category 1 and 2 follicles cultured in a 96-well plate were vitrified using HSV straws®.
At each day of evaluation, follicles were assigned to three categories. Follicles were not allocated to a category, when the basement membrane or connection between oocyte and granulosa cells could not be fully defined, for example when a follicle was located to the side of a well. In addition, follicle diameter was determined at each evaluation day. Two perpendicular measures were recorded for each follicle, and the average of the two values was reported as follicular diameter (μm). When the diameter of a follicle could not be measured (when a follicle was located to the side of a well and the basal membrane could not be fully defined), it was reevaluated the next day. At day 4, all follicles were used to evaluate follicle quality by morphology, diameter, neutral red staining, calcein, propidium iodide and Hoechst staining and histological assessment. For practical reasons and time constraints to minimally expose follicles to light and low temperatures, follicles were not followed up individually but as a group. Every 48 h, half of the medium in each well was refreshed. Follicles from two 96-well plates were alternately assessed per row to minimize exposure time of follicles to light (Fig. 2).
Calcium alginate embedding
Alginate bead size
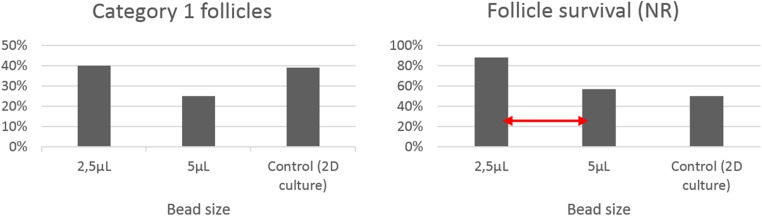
To determine the optimal bead size, in preliminary experiments, PAFs were embedded in 2.5 or in 5 μL alginate beads. At day 2 of culture, category 1 and 2 follicles were selected for embedding. A 2.0% (w/v) solution of sodium alginate (Sigma-Aldrich) in DPBS was prepared and filtered through a 0.2-μm filter. Follicles were transferred to a 20-μL droplet of sodium alginate solution at 38.5 °C. On average, two follicles in 2.5 μL (n = 47) or 5 μL (n = 51) alginate solution were released in a small beaker containing CaCl2 (0.1 M) to form beads at 38.5 °C (Fig. 3). After 3 min, the beads were removed with a small spoon and washed in culture medium. Alginate beads were incubated individually in a 24-well plate (38.5 °C, 5% CO2). To each well, 500 μL culture medium was added and 10 ml distilled water was added to the plate outside the wells. To assess survivability, 24 h post embedding, follicle morphology and follicle survival using NR staining were determined. As follicles embedded in smaller (2.5 μL) beads resulted in a higher viability and higher rate of category 1 follicles, in the current experiment follicles were embedded in 2.5 μL alginate beads.
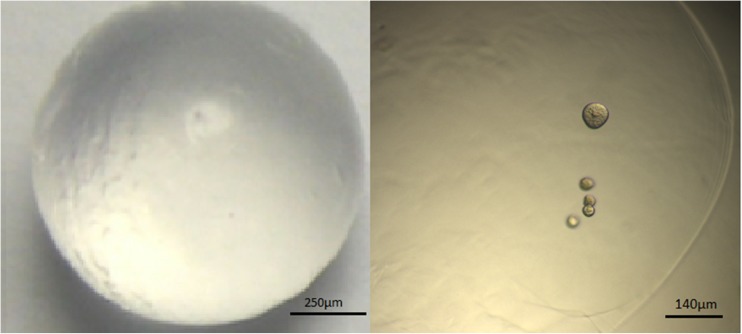
Fig. 3.
Left: macroscopic image of an alginate bead. Right: light microscopic image of five encapsulated follicles in an alginate bead
Follicle embedding in alginate beads
At day 2 of culture, category 1 and 2 follicles were selected for embedding. Follicles were embedded in 2.5 μL alginate beads as described above.
Freezing and thawing
Encapsulated follicles using mesh cups
Embedded follicles were given 24 h to recover from the embedding procedures and equilibrate within the alginate bead. One day post embedding (day 3), half of all embedded follicles were vitrified (group 3DVIT). The other follicles stayed in 3D culture (group 3DCUL). Beads were vitrified using mesh cups [36]. Pieces of metal mesh (mesh size 20 μm; Shijiazhuang Qunkun Metal Products Co., Ltd., Hebei, China) were molded into a cup shape and sterilized (Fig. 4a). Two beads were transferred to a cup immersed in culture medium in a 35-mm petri dish. Cups with beads were transferred with tweezers to equilibration solution consisting of 7.5% DMSO and 7.5% EG in M199 with 10 mg/ml BSA for 2 min at room temperature twice (Fig. 4b). Subsequently, cups were transferred to vitrification solution (15% DMSO, 15% EG, 0.5 M sucrose) at room temperature for 30 s and directly plunged into LN2. To eliminate the media from the metal mesh, the mesh was placed on an absorbent paper towel after each step. The vitrification procedure was carried out at room temperature.
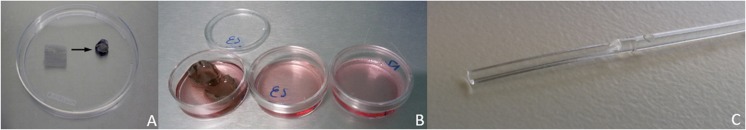
Fig. 4.
a A piece of stainless steel mesh is molded into a cup shape. b Cups with alginate beads are placed in a petri dish with cryoprotectants, cups are transferred with tweezers between different media. Cups can also serve as cryocontainer. c High Security Vitrification straw® with on the left hand side the gutter of the capillary rod where the follicles are deposited
Follicles were cryopreserved for 2 h in liquid nitrogen and subsequently warmed. After plunging the cups as fast as possible in thawing solution (1 M sucrose in M199 with 10 mg/ml BSA) for 1 min at 38.5 °C, cups were transferred to dilution solution (0.5 M sucrose) for 4 min at room temperature (RT). The beads were washed twice in culture medium for 4 min. After removing beads from the cups with a spatula and tweezers, they were cultured individually in a 96-well plate (38.5 °C, 5% CO2).
Isolated follicles using HSV straws®
At day 3 of culture, half of all non-embedded follicles, classified as categories 1 and 2, were selected for vitrification using HSV Straws® (HSV Kit, Groupe I.M.V. Technologies, Clemenceau, France) (group 2DVIT). The other follicles stayed in 2D culture (group 2DCUL). Follicles were transferred using a Stripper® (Origio). After placement of a follicle in a drop of culture medium on a 90-mm petri dish, the drop was merged into the first drop of equilibration solution (7.5% DMSO, 7.5% EG) for 2 min. Then, the follicle was transferred to a second drop of equilibration solution for 2 min and subsequently transferred to a drop of vitrification solution (15% DMSO, 15% EG, 0.5 M sucrose) for 30 s. Follicles were loaded on the gutter of a capillary rod (Fig. 4c) that was placed into a straw and quickly plunged into LN2.
Follicles were thawed the same day. After merging the capillary rod in a drop of thawing solution (1 M sucrose) for 1 min at 38.5 °C, follicles were transferred to a drop of dilution solution (0.5 M sucrose) for 4 min at room temperature. The follicles were washed twice in culture medium. Follicles were cultured individually in a 96-well plate (38.5 °C, 5% CO2).
Follicle quality assessment
Neutral red (NR) staining
To assess follicle survival and immediate viability, follicles were stained with the non-toxic viability indicator Neutral Red [39]. Five microliters neutral red solution (3.3 g/L, Sigma) was added to each well. A follicle was considered to be positively stained when both the oocyte and at least ¾ of the granulosa cells colored red. When the dye is incorporated in the lysosomes of the follicles, they are considered metabolically active and thus viable. Following 20 min of incubation (38.5 °C, 5% CO2), follicle staining was evaluated [39].
Follicle mounting and histological assessment
Morphological integrity of the oocyte, the granulosa cells and the basement membrane were investigated by histological examination. Follicles in alginate beads were washed in 0.1 M phosphate buffer (PB) and fixed at 4 °C for 4 h in 4-well dishes in a solution containing 4% formaldehyde. Follicles were washed and stored in PB until further use. Non-embedded follicles were fixed in 4% formaldehyde for 4 h in 4-well dishes. The follicles were washed and stored in PB until further use.
As stated previously [37] fixed follicles were placed on a glass slide. Thirty microliters of 80 °C pre-heated Histogel® (Thermofisher, Breda, The Netherlands) was added to the follicles. The mixture was subsequently cooled down at room temperature for 3 min and the Histogel® was cut into a small cube and stored in PB in the fridge. The resulting Histogel® containing the follicles was dehydrated through increasing ethanol solutions and embedded in paraffin. Paraffin blocks were cut in 5 μm thick slices using a microtome and stained with hematoxylin and eosin. All sections were examined and the follicles morphologically evaluated. Follicle morphology was considered to be normal when they contained an oocyte with regular shape and uniform cytoplasm, and organized layers of granulosa cells. Follicles were considered degenerated when the oocyte exhibited a pyknotic nucleus and/or ooplasm shrinkage, granulosa cell layers were disorganized or detached from the basement membrane or when the basement membrane showed irregularities.
Follicle staining with calcein blue AM, propidium iodide and Hoechst 33342
Calcein is a substrate to determine the enzymatic activity and cell-membrane integrity. In living cells non-polar, non-fluorescent cell-permeant calcein acetomethylester (AM) enters the cells and is hydrolyzed by intracellular esterases. It produces a polar, fluorescent molecule, calcein, which is retained in the cytoplasm for several hours and generates intense and uniform blue fluorescence [40]. Dead and dying cells stain with propidium iodide. One microliter calcein blue AM (1 mM, Molecular probes) and 1 μL propidium iodide solution (1 mg/ml, Sigma) were added to 70 μL culture medium. Follicles were incubated for 20 min (38.5 °C, 5%CO2). Follicles were observed under an inverted fluorescence microscope (Olympus IX71) equipped with a DAPI and a TRITC filter. Blue fluorescence was visualized in live cells and red fluorescence in dead cells. Two hours later, 1 μL Hoechst 33342 (10 mg/ml, Invitrogen) was added to the culture medium to determine the total cell number. After 20 min incubation, Hoechst positive nuclei were counted.
Statistical analysis
Different statistical models were used to assess the difference in follicle diameter, number of dead cells, total cell number, viability, follicle categorization and follicle morphology between treatment groups. The procedure to isolate the follicles from the ovaries, as described in section 2.1, was carried out six times. To account for possible batch effects, the replicate number was entered as a random effect in the subsequent statistical analyses. Treatment was entered as a fixed effect. Interaction between the treatment and the batch was also entered, but if this latter term was not significant, it was omitted from the model.
Within each batch (in total 6), follicles were derived from 10 ovaries, that originated from 10 different cows. The ovaries were selected as described in section 2.1. Within each batch, follicle isolation was carried out as outlined in paragraph 2.1 on all 10 ovaries together. After the isolation procedure, approximately 60 intact follicles were selected for culture. No separate follicle isolations were performed for each separate ovary. As a consequence, there is no information which follicle originated from which ovary. Therefore, all follicles within one replicate are considered independent. All follicles were considered as statistical units.
Dependent continuous variables were follicle diameter, number of dead cells and total cell number. Kolmogorov-Smirnov test was used to check whether the dependent variables were normally distributed. Dependent categorical variables were viability (binary, viable vs. non-viable), follicle categorization (binary, category 1 vs. category 2) and follicle morphology (binary, normal vs. degenerated). Independent categorical variable was treatment group (categorical: 3DVIT, 3DCUL, 2DVIT, 2DCUL).
Potential differences in follicle diameter, number of dead cells and total cell number between treatment groups were analyzed using analysis of variance (ANOVA) with Scheffé’s method for post hoc comparison. Prior to ANOVA, data were analyzed for normal distribution and homogeneity of variance by performing Kolmonogrov-Smirnov and Levene’s test, respectively. Potential differences in viability, follicle categorization and follicle morphology between treatment groups were analyzed using binary logistic regression.
Differences between treatment groups were considered to be significant when P value < 0.05 [41]. Data are presented as means ± S.E.M. All statistical analyses were performed using IBM SPSS version 24® (New York, USA).
Results
Alginate bead size
One day post embedding, a total of 152 follicles were assessed for microscopic morphology and NR staining (n = 47, n = 51, n = 54 for 2.5 μL beads group, for 5 μL beads group, for control group, respectively). The percentage of category 1 follicles on the total number of follicles was 40%, 25%, 39% for 2.5 μL beads, 5 μL beads or the control group, respectively. Follicles staining positive for the non-invasive dye NR were considered viable. According to NR staining, viability differed significantly between the group follicles embedded in 2.5 μL beads and in 5 μL beads (P = 0.024) (Fig. 5).
Fig. 5.
Left: Percentage category 1 follicles on total number of follicles (categories 1, 2 and 3) 24 h post embedding in 2.5 or 5 μL alginate beads or in the control group (2D culture). Right: follicle survival, 24 h post embedding, assessed after NR staining in 2.5 or 5 μL alginate beads or in the control group (2D culture). Red arrow indicates a significant difference between groups
Follicle quality assessment
Follicle gross morphology
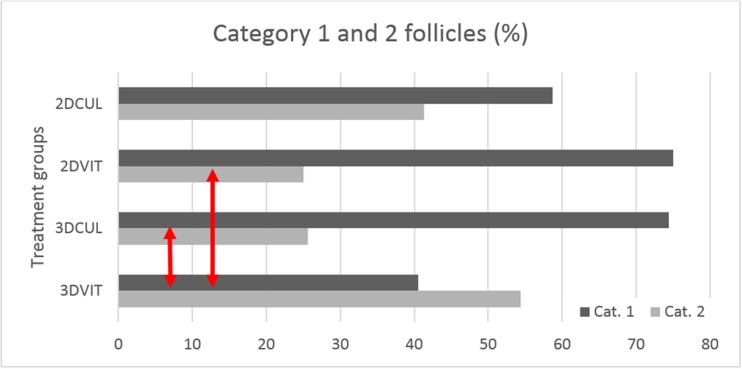
A total of 386 PAFs were evaluated for microscopic morphology on day 1, 406 on day 2, 253 on day 3 and 263 on day 4; divided over six replicates. At day 1, most follicles (50.4%) were allocated to category 2 (23.5% to category 1, 20.5% to category 3, 5.6% remained undetermined). Moreover at day 2, a lower percentage of follicles was allocated to category 2, while more follicles were allocated to category 1 (29.4% category 1, 43.4% category 2, 24.6% category 3, 2.7% remained undetermined). From day 2 onwards, category 1 and 2 follicles developed until the secondary stage, the outline of the basal membrane was more pronounced and morphological distinction between granulosa cells could no longer be made. Ultimately after warming at day 4, group 3DVIT was the only group showing less category 1 than category 2 follicles. Further at day 4, the percentage of category 1 follicles on the total number of category 1 and 2 follicles was 40.5, 74.4, 75, and 58.7% for group 3DVIT, 3DCUL, 2DVIT, and 2DCUL, respectively. The numbers of category 1 and 2 follicles were significantly different between groups 3DVIT and 2DVIT (P = 0.003) and groups 3DVIT and 3DCUL (P = 0.003). Differences were not significant between groups 2DVIT and 2DCUL (P = 0.085) and between groups 3DCUL and 2DCUL (P = 0.088) (Fig. 6).
Fig. 6.
Percentage category 1 and 2 follicles per treatment group at day 4. Group 3DVIT was the only group showing less category 1 than category 2 follicles. Red arrows indicate a significant difference between groups
Follicle diameter
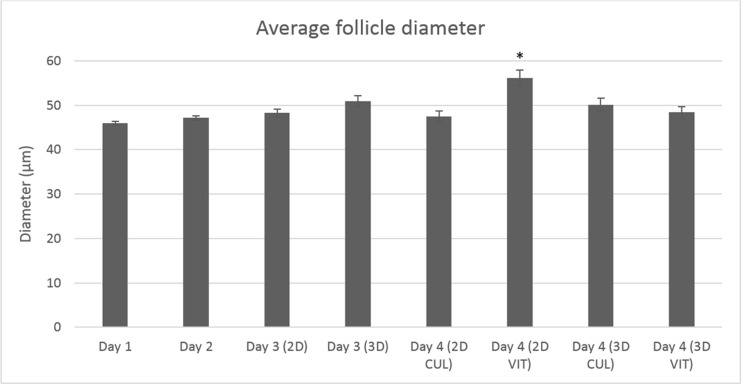
The follicle diameter of PAFs was measured at day 1 (n = 398), day 2 (n = 400), day 3 (n = 251) and day 4 (n = 260) (divided over 6 replicates). The mean follicle diameter ± S.E.M. for category 1 and 2 follicles taken together was 45.92 ± 0.42 at day 1 and 47.14 ± 0.47 at day 2. At day 3 before vitrification, the mean diameter ± S.E.M. for group 3DCUL was 50.48 ± 1.21 and 49.77 ± 0.84 for group 2DCUL. At day 4, the mean follicle diameter ± S.E.M. was 48.61 ± 1.36 for group 3DVIT, 50.45 ± 1.48 for group 3DCUL, 56.10 ± 1.81 for group 2DVIT and 50.34 ± 1.24 for group 2DCUL (Fig. 7). The difference was significant between groups 3DVIT and 2DVIT (P < 0.001), groups 3DCUL and 2DVIT (P = 0.008), and groups 2DVIT and 2DCUL (P < 0.001).
Fig. 7.
Mean pre-antral follicle growth ± S.E.M. (follicle diameter of category 1 and 2 PAFs at days 1, 2, 3, 4) linked to treatment group. From day 2 onwards, follicles were divided in a 2D and 3D culture group. From day 3 onwards, all four treatment groups were formed. Data are presented as mean. *The diameter of group 2DVIT was significantly different from the three other groups
Neutral red and calcein staining
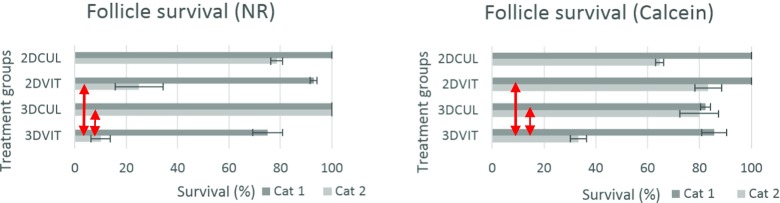
To evaluate follicle survival, PAFs were stained with NR and calcein at day 4. Only category 1 and 2 follicles were taken into account. Half of the follicles in each group were stained with NR (n = 18, n = 19, n = 18, n = 36 for group 3DVIT, 3DCUL, 2DVIT and 2DCUL, respectively) or with calcein (n = 19, n = 24, n = 22, n = 37 for group 3DVIT, 3DCUL, 2DVIT and 2DCUL, respectively). Follicles staining positive for the non-invasive dye NR or calcein were considered viable (Fig. 9). According to NR, viability differed significantly between groups 3DVIT and 2DVIT (P = 0.015), and between groups 3DVIT and 3DCUL (P = 0.002). There was no significant difference between groups 3DCUL and 2DCUL (P = 0.998) and between groups 2DVIT and 2DCUL (P = 0.167) (Fig. 8). According to calcein staining, viability differed significantly between groups 3DVIT and 2DVIT (P = 0.009), and between groups 3DVIT and 3DCUL (P = 0.032). There was no significant difference between groups 3DCUL and 2DCUL (P = 0.846) and between groups 2DVIT and 2DCUL (P = 0.209) (Fig. 8).
Fig. 9.
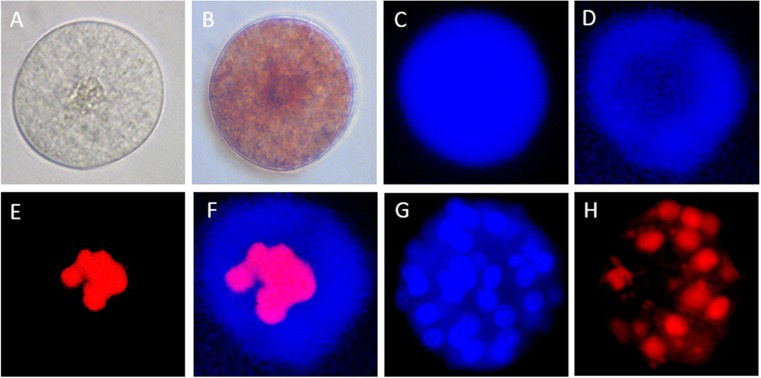
Follicles were stained with neutral red (NR) and calcein-AM to indicate the viability of the follicles, Hoechst was used to assess the total cell number, propidium iodide was used to show nuclei of dead cells. a Light microscopic (LM) image of non-stained follicle. b LM image of follicle staining positive for NR; viable follicle. c Follicle staining positive for calcein; viable follicle. d Follicle of which the oocyte stained negative and the granulosa cells positive for calcein. e Same follicle as in D, nuclei staining positive for propidium iodide. f Merged picture of d and e. g Follicle stained with Hoechst. h Follicle stained with propidium iodide
Fig. 8.
Follicle survival ± S.E.M. of category 1 and 2 follicles at day 4, assessed after NR staining (left) and after calcein staining (right). Error bars represent the S.E.M. between replicates. Red arrows indicate a significant difference between treatment groups
Hoechst and propidium iodide staining
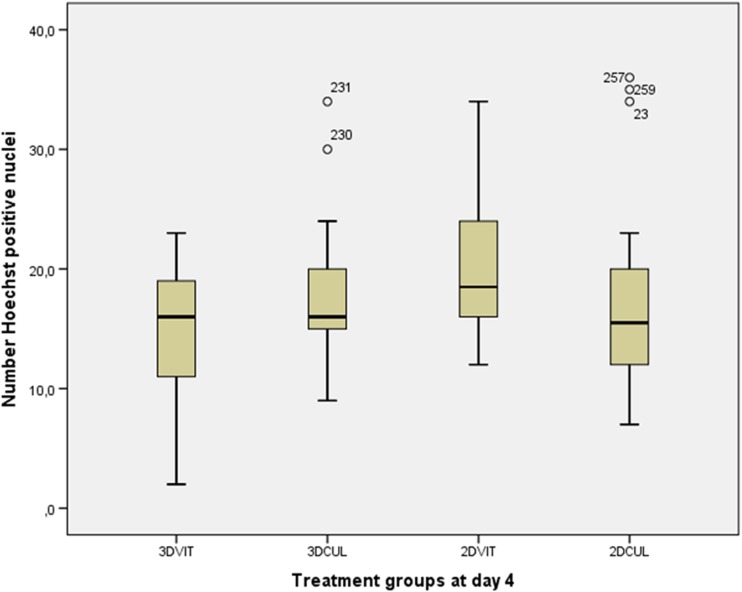
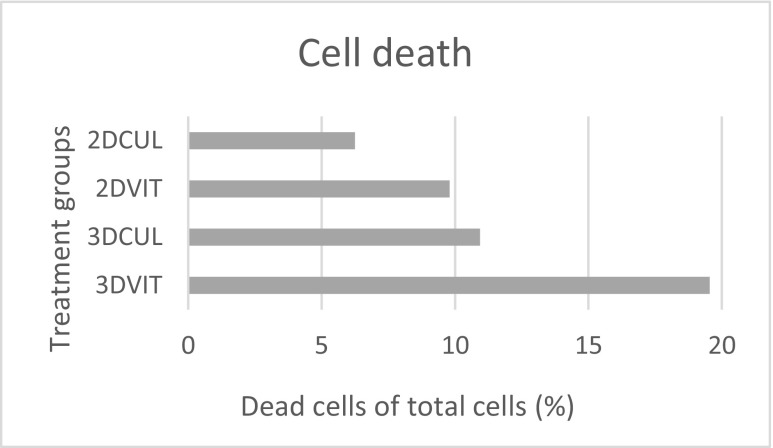
No significant differences in total cell number and total number of dead cells were found between groups using Hoechst and propidium iodide staining (Fig. 9), respectively. The mean total cell number ± S.E.M. was 14.23 ± 1.70, 17.94 ± 1.46, 20.55 ± 1.43 and 16.88 ± 1.26 for group 3DVIT, 3DCUL, 2DVIT and 2DCUL, respectively (Fig. 10). The mean total number of dead cells ± S.E.M. was 5.12 ± 1.56, 2.59 ± 1.10, 3.36 ± 1.57 and 4.36 ± 1.42 for group 3DVIT, 3DCUL, 2DVIT and 2DCUL, respectively. The mean number of dead cells, as a percentage of the total cell number, per follicle per treatment group is shown in Fig. 11.
Fig. 10.
Number of Hoechst positive nuclei in category 1 and 2 follicles in each treatment group at day 4
Fig. 11.
Mean number of dead cells, as a percentage of the total cell number, per follicle per treatment group for category 1 and 2 follicles at day 4
Histological assessment
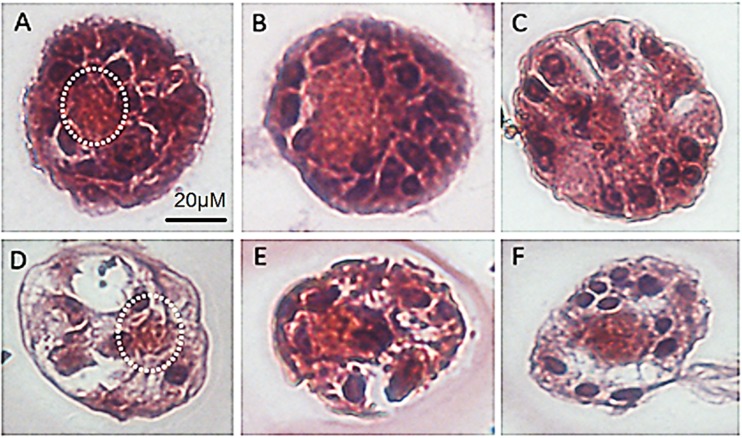
A total of 93 sections of pre-antral follicles were examined (14, 25, 18, 36 in group 3DVIT, 3DCUL, 2DVIT and 2DCUL, respectively). In morphologically normal PAFs, granulosa cells were well-organized in layers surrounding the oocyte and a distinguishable intact basement membrane could be observed (Fig. 12). Degenerated follicles showed a retracted oocyte with or without a pyknotic nucleus. Layers of granulosa cells remained unaltered or became disorganized and were often detached from each other and the basement membrane. Empty spaces or rupture of the basement membrane were also observed. Histological evaluation revealed that the proportion of follicles showing a normal morphology tended to be highest in group 2DVIT (55%) and lowest in group 3DVIT (21%). However, this difference was not statistically significant (P = 0.059).
Fig. 12.
Histological assessment following follicle embedding in Histogel®. a, b, c PAFs from group 2DVIT, 2DVIT and 3DCUL, respectively, showing an oocyte and surrounding granulosa cells. d PAF from group 3DVIT, the oocyte shows signs of degeneration, empty spaces are observed. e PAF from group 2DCUL, basement membrane is ruptured. f PAF from group 2DCUL, empty spaces between oocyte and granulosa cells are visible. The white dotted line indicates the oocyte. PAF: pre-antral follicle
Work efficiency
Follicle encapsulation limited the handling time and beads were easily transferable. The same number of follicles could be vitrified 2 to 3 times faster using beads in mesh cups instead of straws. A mesh cup can contain several beads, and one bead contained 2–3 follicles which allowed the vitrification of many isolated follicles in a short period of time. Mini mesh cups also served as a cryocontainer, so the laborious loading procedure was omitted. The recovery rate of embedded follicles after warming was 86.3%. From the follicles that were vitrified using straws, 56.9% of the follicles were recovered after warming.
Category 1 follicles from group 2DVIT followed a typical shrink-swell curve, the osmotic equilibrium between intracellular and extracellular solutions occurred within 30 s. Category 2 follicles showed less morphological changes, or needed more time to resume their original size, or stayed shrunken. The malformation and small size make these follicles hard to recognize and easy to lose. After thawing, category 2 follicles were often less viable following the vitrification procedure.
Discussion
In this study, isolated bovine PAFs were vitrified on a time and labor efficient way using alginate beads. However, when using the same vitrification protocol, the viability of encapsulated PAFs after vitrification in alginate beads was lower compared to the viability of non-encapsulated PAFs after vitrification when straws were used (45.9 vs. 87.5%). Additionally, we compared 2D and 3D systems for short-term culture of isolated bovine pre-antral follicles. We concluded that isolated bovine PAFs which were short-term cultured in a 3D alginate bead culture system were of the same quality as compared to a 2D culture system, since no significant differences in viability and morphology were seen.
An important advantage of alginate encapsulation is the quick and safe manipulation of follicles. We observed a high loss of follicles when non-encapsulated isolated follicles were cryopreserved individually. The small size and shrinkage of follicles during exposure to equilibration, vitrification and thawing solutions make it difficult to retrieve follicles as also reported by Rodrigues et al. [42] and Camboni et al. [35]. Although it is possible to vitrify several follicles at the same time using straws, we chose to vitrify follicles individually because of the small number of follicles and the high risk of not retrieving them.
Cryopreservation of embedded isolated bovine PAFs has, to the best of our knowledge, never been reported so far. Bian et al. [36] successfully vitrified human PAFs embedded in a 1.5% sodium alginate solution. In preliminary experiments, 1.5 and 2% alginate concentrations were compared to vitrify embedded bovine secondary follicles. As 2% (w/v) sodium alginate solution resulted in highest survival rate of PAFs, it was decided to use a 2% alginate concentration for the experiments. Preliminary experiments revealed that comparisons concerning the bead size showed a significant higher viability of PAFs cultured in beads with a size of 2.5 μL compared to 5 μL. Decreased viability of follicles in larger beads may result from reduced diffusion of nutrients to follicles in the center of the bead. The location of PAFs in the center or in the side of a bead may have an impact on development in larger beads, as nutrients may be in higher concentrations available for follicles located to the side of a bead. However, in our experience, it is not possible to control the position of PAFs in the bead.
We observed that non-embedded category 1 follicles followed a typical shrink-swell curve initiated by volume changes caused by exposure to CPAs [43]. When follicles are exposed to CPAs, they shrink as a consequence of a concentration difference between the extracellular and intracellular spaces. Outflow of water caused by the external hyperosmotic solution is faster than the CPA penetration, indicating that the cells are more permeable to water than to CPAs [44]. When the permeable CPAs move in the cell, the follicle swells again. In our experiments, the osmotic equilibrium between intracellular and extracellular solutions occurred within 30 s for category 1 follicles. However, category 2 follicles did not show a typical shrink-swell curve and were less viable following vitrification.
Survival during short-term culture is an important parameter to assess the viability of follicles, for example after vitrification. When 2D culture systems are used for follicle growth and development, long-term culture periods can cause disruption in follicle architecture due to flattening because granulosa cells break through the basement membrane and attach to the culture dish [26, 45]. This can cause disruption in the cell-cell connection between the oocyte and granulosa cells, which is critical for oocyte growth and cytoplasmic meiotic competence [46]. 3D culture systems are thus essential to ensure survival and further development of PAFs during long-term culture.
During the 4-day culture period in this experiment, or as a previously investigated 10-day [37] culture period, our results showed no follicle attachment to the culture dish, no spreading of granulosa cells or release of the oocyte after in vitro culture of isolated bovine PAFs. However, since we only applied short-term culture periods, these features are likely to occur during longer culture periods. Silva et al. [47] reported follicle adherence to the culture plate after 12 days that resulted in loss of normal morphology by day 18 in goats in a 2D culture system.
Although primordial follicles (56.1%) are more abundant than primary follicles in the bovine ovary (32.3%) [48], we chose to select primary follicles at isolation for in vitro culture. Primary category 1 and 2 follicles developed in 2 days until the secondary stage. Preliminary experiments showed that primordial follicles which did not grow until the secondary stage within 2 days of culture were mostly allocated to category 3 and less viable after short-term in vitro culture. Factors that regulate follicle activation are most likely paracrine, thus surrounding growing follicles would be necessary to enhance the activation of primordial follicles [49]. Furthermore, it was assessed in a study of Yin et al. [50] that secondary medullar follicles survived and grew to the antral stage, while most primordial and primary follicles died during 3D culture. Hornick et al. [51] demonstrated that follicles themselves can exert a beneficial coculture effect, as increased growth and survival were seen when primary follicles were cultured in group. However, in our opinion, the choice for multiple follicle culture or single follicle culture from day 2 onwards did not have an impact on growth and survival, as the culture period was too short (only 2 days). Only category 1 and 2 follicles were selected for the experiments. Category 3 follicles showed signs of ongoing degeneration, as reported in earlier experiments category 3 follicles where they lacked significant growth during 10-day in vitro culture [37].
The highest increase of mean follicle diameter at day 4 was observed in the 2DVIT group (vitrification of non-embedded follicles using straws). An increase in follicle diameter can be induced by an increase of total cell number as a result of cell division, by the change of granulosa cell shape from flattened to cuboidal or by cellular swelling due to follicular membrane damage. This increase in follicle diameter is more likely to occur as a consequence of swelling of granulosa cells caused by osmotic influx after thawing. Although cell swelling during removal of CPAs is likely to be more deleterious than cell shrinkage induced by exposure to CPAs [52], follicles vitrified using HSV straws® showed a high viability (87.5%) according to neutral red and calcein staining. Group 2DVIT showed the highest mean count of total cell number by Hoechst staining. Differences in total cell number were not significantly different between the four groups. Based on the appearance of well-preserved isolated follicles after light microscopic and histological assessment, we can assume that bovine pre-antral follicles can be successfully vitrified using HSV straws without impairing their viability.
As demonstrated by Santos et al. [53], a 24-h in vitro culture period after thawing is essential to evaluate the cryopreservation process. Survival was assessed using neutral red and calcein staining, which resulted in comparable results after statistical analysis. This indicates that both are reliable evaluation tools to assess viability. The reversibility of NR is beneficial when further growth and development need to be assessed or when other follicle assessment methods will be applied [39].
In non-embedded follicles, NR uptake in lysosomes is reversible. However, in embedded follicles NR was not eliminated when subsequently cultured in NR free medium. This demonstrates the ability of NR to spread throughout the alginate bead, but also the inability to diffuse from the bead into a surrounding solution devoid of the substrate. The low molecular weight of NR should not hinder the diffusion into and from the bead because of the high porosity range of alginate beads. Resistance to diffuse into the beads occurs for larger proteins from a molecular weight larger than 2 × 104, diffusion from the bead is not hindered until the molecular weight approaches 3 × 104 [54]. The mechanism behind this process needs still to be elucidated. The vitrification of PAFs using HSV straws resulted in a significantly higher viability (87.5%; group 2DVIT) than the vitrification of encapsulated PAFs using mini mesh cups (45.9%; group 3DVIT). The same CPA concentrations and exposure times were used for vitrification in both groups. These results illustrate that the protocol for the vitrification of encapsulated follicles needs to be adjusted. There was no significant difference in viability between the vitrified follicles using HSV straws (87.5%; group 2DVIT) and those cultured in a 2D culture system without cryopreservation at day 4 (87.7%; group 2DCUL).
Follicles that were vitrified using mini mesh cups (45.9%; group 3DVIT) were significantly less viable than PAFs that were cultured in beads (88.4%; group 3DCUL). These findings were consistent with Sadeghnia et al. [55]. In contrary, Bian et al. [36] showed that human pre-antral follicles encapsulated in alginate could maintain their normal ultra-structure after vitrification. Camboni et al. [35] successfully cryopreserved embedded human primordial/primary follicles using slow freezing.
In conclusion, we studied the effects of encapsulation on follicle viability and morphology after vitrification and short-term culture. No differences were observed between follicle viability and morphology in short-term 2D and 3D culture systems. Vitrification of non-embedded follicles displayed the highest viability and largest percentage of follicles with a well-preserved morphology. Follicles that were vitrified in alginate beads using mesh cups showed the lowest viability (45.9%) according to calcein and neutral red staining among all treatment groups. However, the encapsulation of follicles in beads has important advantages as manipulation is much easier and a lot of time is saved because follicles are vitrified in group. The vitrification protocol was successfully used for non-embedded PAFs; however, further optimization for embedded bovine PAFs is necessary and in our opinion advantageous. A first step in this process could be the use of a longer exposure time to CPAs, considering the increased volume of embedded follicles which may prolong the diffusion time of CPAs [36]. Follicles vitrified using HSV straws® showed a high viability (87.5%) with no significant difference from follicles that were cultured in a 2D culture system. Considering the labor-intensive procedure and low efficiency since many follicles are lost during the vitrification procedure, vitrification in beads may be the method of choice in the future.
Acknowledgements
The authors thank Silke Andries, Els Merckx and Katty Huybrechts for their excellent technical assistance and the local slaughterhouses for their cooperation in sample collection.
Financial support
All (co-)authors state that the funding of this research is provided by the independent Operational Costs of the University of Antwerp.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Statement on the welfare of animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Anniek Bus, Phone: 0032 32652398, Email: Anniek.Bus@uantwerp.be.
Veerle van Hoeck, Email: vvanhoeck@gmail.com.
An Langbeen, Email: An.Langbeen@uantwerp.be.
Jo L. M. R. Leroy, Email: Jo.Leroy@uantwerp.be
Peter E. J. Bols, Email: Peter.Bols@uantwerp.be
References
- 1.Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S, et al. SEER cancer statistics review, 1975–2010. Bethesda, MD. National Cancer Institute. 2013;9
- 2.Detti L, Martin DC, Williams LJ. Applicability of adult techniques for ovarian preservation to childhood cancer patients. J Assist Reprod Genet. 2012;29(9):985–995. doi: 10.1007/s10815-012-9821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Wallberg KA, Oktay K. Options on fertility preservation in female cancer patients. Cancer Treat Rev. 2012;38(5):354–361. doi: 10.1016/j.ctrv.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hyman JH, Tulandi T. Fertility preservation options after gonadotoxic chemotherapy. Clin Med Insights Reprod Health. 2013;7:61–69. doi: 10.4137/CMRH.S10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung W, Hudson MM, Strickland DK, Phipps S, Srivastava DK, Ribeiro RC, Rubnitz JE, Sandlund JT, Kun LE, Bowman LC, Razzouk BI, Mathew P, Shearer P, Evans WE, Pui CH. Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol. 2000;18(18):3273–3279. doi: 10.1200/JCO.2000.18.18.3273. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Schover L, Partridge A, Patrizio P, Wallace W, Hagerty K. American Society of Clinical Oncology: recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 8.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, Donaldson SS, Byrne J, Robison LL. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27(16):2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seli E, Agarwal A. Fertility preservation in females: emerging technologies and clinical applications. first ed. New York: Springer; 2012.
- 10.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans M-M. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12(5):519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 11.Wallace WHB. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 2011;117(S10):2301–2310. doi: 10.1002/cncr.26045. [DOI] [PubMed] [Google Scholar]
- 12.Revelli A, Molinari E, Salvagno F, Delle Piane L, Dolfin E, Ochetti S. Oocyte cryostorage to preserve fertility in oncological patients. Obstet Gynecol Int. 2012;2012:1–7. doi: 10.1155/2012/525896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43(6):437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 14.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, Smitz J, Dolmans MM. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98(3):720–725. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Jadoul P, Guilmain A, Squifflet J-L, Luyckx M, Votino R, Wyns C, et al. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 454 cases. Hum Reprod. 2017;32(5):1046–1054. doi: 10.1093/humrep/dex040. [DOI] [PubMed] [Google Scholar]
- 16.Dolmans M-M, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116(16):2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 17.Rosendahl M, Andersen MT, Ralfkiær E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril. 2010;94(6):2186–2190. doi: 10.1016/j.fertnstert.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Donnez J, Dolmans M-M. Fertility preservation in women. Nat Rev Endocrinol. 2013;9(12):735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Madrid B, Donnez J, Van Eyck A-S, Veiga-Lopez A, Dolmans M-M, Van Langendonckt A. Chick embryo chorioallantoic membrane (CAM) model: a useful tool to study short-term transplantation of cryopreserved human ovarian tissue. Fertil Steril. 2009;91(1):285–292. doi: 10.1016/j.fertnstert.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Van Eyck A-S, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93(5):1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 21.Shaw J, Cox S-L, Trounson A, Jenkin G. Evaluation of the long-term function of cryopreserved ovarian grafts in the mouse, implications for human applications. Mol Cell Endocrinol. 2000;161(1):103–110. doi: 10.1016/S0303-7207(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 22.Amorim C, Gonçalves P, Figueiredo J. Cryopreservation of oocytes from pre-antral follicles. Hum Reprod Update. 2003;9(2):119–129. doi: 10.1093/humupd/dmg014. [DOI] [PubMed] [Google Scholar]
- 23.Jorssen EP, Langbeen A, Fransen E, Martinez EL, Leroy JL, Bols PE. Monitoring preantral follicle survival and growth in bovine ovarian biopsies by repeated use of neutral red and cultured in vitro under low and high oxygen tension. Theriogenology. 2014;82(3):387–395. doi: 10.1016/j.theriogenology.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Aerts JM, Martinez-Madrid B, Leroy JL, Van Aelst S, Bols PE. Xenotransplantation by injection of a suspension of isolated preantral ovarian follicles and stroma cells under the kidney capsule of nude mice. Fertil Steril. 2010;94(2):708–714. doi: 10.1016/j.fertnstert.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 25.Chiti M, Dolmans M, Orellana R, Soares M, Paulini F, Donnez J, et al. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum Reprod. 2015;31:427–435. doi: 10.1093/humrep/dev299. [DOI] [PubMed] [Google Scholar]
- 26.Green LJ, Shikanov A. In vitro culture methods of preantral follicles. Theriogenology. 2016;86(1):229–238. doi: 10.1016/j.theriogenology.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Vanacker J, Luyckx V, Amorim C, Dolmans M-M, Van Langendonckt A, Donnez J, et al. Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil Steril. 2013;99(5):1363–1368. doi: 10.1016/j.fertnstert.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Langbeen A, Bartholomeus E, Leroy JL, Bols PE. Bovine in vitro reproduction models can contribute to the development of (female) fertility preservation strategies. Theriogenology. 2015;84(4):477–489. doi: 10.1016/j.theriogenology.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Araujo VR, Gastal MO, Wischral A, Figueiredo JR, Gastal EL. Long-term in vitro culture of bovine preantral follicles: effect of base medium and medium replacement methods. Anim Reprod Sci. 2015;161:23–31. doi: 10.1016/j.anireprosci.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62(5):1322–1328. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence 1. Biol Reprod. 2003;68(5):1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 32.Gupta P, Ramesh H, Manjunatha B, Nandi S, Ravindra J. Production of buffalo embryos using oocytes from in vitro grown preantral follicles. Zygote. 2008;16(01):57–63. doi: 10.1017/S096719940700442X. [DOI] [PubMed] [Google Scholar]
- 33.Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323. doi: 10.1038/srep17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology. 2013;67(1):64–69. doi: 10.1016/j.cryobiol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Bian J, Li T, Ding C, Xin W, Zhu B, Zhou C. Vitreous cryopreservation of human preantral follicles encapsulated in alginate beads with mini mesh cups. J Reprod Dev. 2013;59(3):288–295. doi: 10.1262/jrd.2012-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorssen EP, Langbeen A, Marei WF, Fransen E, De porte HF, Leroy JL, et al. Morphologic characterization of isolated bovine early preantral follicles during short-term individual in vitro culture. Theriogenology. 2015;84(2):301–311. doi: 10.1016/j.theriogenology.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Braw-Tal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J Reprod Fertil. 1997;109(1):165–171. doi: 10.1530/jrf.0.1090165. [DOI] [PubMed] [Google Scholar]
- 39.Langbeen A, Jorssen EP, Granata N, Fransen E, Leroy JL, Bols PE. Effects of neutral red assisted viability assessment on the cryotolerance of isolated bovine preantral follicles. J Assist Reprod Genet. 2014;31(12):1727–1736. doi: 10.1007/s10815-014-0340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Clerck LS, Bridts CH, Mertens AM, Moens MM, Stevens WJ. Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. J Immunol Methods. 1994;172(1):115–124. doi: 10.1016/0022-1759(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 41.Dohoo I, Martin S, Stryhn H. Veterinary epidemiological research. Second ed. Charlottetown: VER inc; 2009.
- 42.Rodrigues A, Amorim C, Costa S, Santos R, Lucci C, Nunes J, et al. Cryopreservation and short-term culture of isolated caprine primordial follicles. Small Rumin Res. 2005;56(1):103–111. doi: 10.1016/j.smallrumres.2004.03.007. [DOI] [Google Scholar]
- 43.Amorim CA, Rondina D, Lucci CM, Gonçalves PBD, de Figueiredo JR, Giorgetti A. Permeability of ovine primordial follicles to different cryoprotectants. Fertil Steril. 2006;85:1077–1081. doi: 10.1016/j.fertnstert.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 44.Paynter S, Cooper A, Gregory L, Fuller B, Shaw R. Permeability characteristics of human oocytes in the presence of the cryoprotectant dimethylsulphoxide. Hum Reprod. 1999;14(9):2338–2342. doi: 10.1093/humrep/14.9.2338. [DOI] [PubMed] [Google Scholar]
- 45.Cortvrindt R, Smitz J, Van Steirteghem A. Ovary and ovulation: in-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11(12):2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 46.Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte–granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226(2):167–179. doi: 10.1006/dbio.2000.9863. [DOI] [PubMed] [Google Scholar]
- 47.Silva GM, Rossetto R, Chaves RN, Duarte AB, Araujo VR, Feltrin C, et al. In vitro development of secondary follicles from pre-pubertal and adult goats cultured in two-dimensional or three-dimensional systems. Zygote. 2015;23(4):475–484. doi: 10.1017/S0967199414000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi MH, Oh JH, Kim TM, Han JY, Lim JM. Morphological criteria of bovine ovaries for predicting retrieval efficiency of preantral follicles. Asian australasian journal of animal sciences. 2006;19(12):1711–1715. doi: 10.5713/ajas.2006.1711. [DOI] [Google Scholar]
- 49.Da Silva-Buttkus P, Marcelli G, Franks S, Stark J, Hardy K. Inferring biological mechanisms from spatial analysis: prediction of a local inhibitor in the ovary. Proc Natl Acad Sci. 2009;106(2):456–461. doi: 10.1073/pnas.0810012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin H, Kristensen SG, Jiang H, Rasmussen A, Andersen CY. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture. Hum Reprod. 2016;31(7):1531–1539. doi: 10.1093/humrep/dew049. [DOI] [PubMed] [Google Scholar]
- 51.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145(1):19–32. doi: 10.1530/REP-12-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armitage W, Juss B, Easty D. Differing effects of various cryoprotectants on intercellular junctions of epithelial (MDCK) cells. Cryobiology. 1995;32(1):52–59. doi: 10.1006/cryo.1995.1004. [DOI] [PubMed] [Google Scholar]
- 53.Santos RR, Tharasanit T, Figueiredo JR, Van Haeften T, Van den Hurk R. Preservation of caprine preantral follicle viability after cryopreservation in sucrose and ethylene glycol. Cell Tissue Res. 2006;325(3):523–531. doi: 10.1007/s00441-006-0193-5. [DOI] [PubMed] [Google Scholar]
- 54.Smidsrød O, Skja G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-O. [DOI] [PubMed] [Google Scholar]
- 55.Sadeghnia S, Akhondi MM, Hossein G, Mobini S, Hosseini L, Naderi MM, Boroujeni SB, Sarvari A, Behzadi B, Shirazi A. Development of sheep primordial follicles encapsulated in alginate or in ovarian tissue in fresh and vitrified samples. Cryobiology. 2016;72(2):100–105. doi: 10.1016/j.cryobiol.2016.03.001. [DOI] [PubMed] [Google Scholar]