Abstract
Purpose
This paper reports the use of a novel automatic vitrification device (Sarah, Fertilesafe, Israel) for cryopreservation of oocytes and embryos.
Methods
Mice oocytes (n = 40) and embryos (8 cells, n = 35 and blastocysts, n = 165), bovine embryos (2PN, n = 35), and MII oocytes (n = 84) were vitrified using this automated device. A total of 42 (2 cells) mice embryos, 20 (2PN) bovine embryos, and 150 MII bovine oocytes were used as fresh controls and grown to blastocysts. Upon rewarming, all were assessed for viability, cleavage, blastocyst, and hatching rates.
Results
Ninety-five % (38/40) of the mice MII oocytes regained isotonic volumes and all (100%) the surviving were viable. Rewarmed 8-cell mice embryos had 95% (33/35) blastulation rate and 80% (28/35) hatched. Rewarmed mice blastocysts had 97% survival rate (160/165) and 81% (135/165) hatched. Fresh control mice embryos had 100% (42/42) blastulation and 73% (21/42) hatching rates. Bovine embryos’ survival was 100% with 54% (19/35) cleavage and 9% (3/35) blastulation rate. Fresh control bovine embryos had 65% (13/20) cleavage and 20% (4/20) blastulation rate. Vitrified bovine oocytes had 100% survival (84/84), 73% (61/84) cleavage, and 7% (6/84) blastocysts’ rates; fresh control had 83% (125/150) cleavage and 11% (17/150) blastocysts’ rates.
Conclusion
This novel automatic vitrification device is capable to produce high survival rates of oocytes and embryos. We anticipate that as the demand for vitrification of gametes, embryos, and reproductive tissues increases worldwide, the availability of an automated vitrification device will become indispensable for standardization, simplification, and reproducibility of the entire process.
Keywords: Vitrification, Oocytes, Embryos, Automation, Cryopreservation
Introduction
Over the past 5 years, clinical demand for oocyte vitrification has been steadily increasing, favored by not being considered any longer an experimental procedure [1]. Today, oocyte vitrification is recommended for young women who are postponing their plans for motherhood or who are at risk of losing their reproductive ability because of being treated with cytotoxic drugs for cancer or other medical conditions [2].
Furthermore, recently, many oocyte banks have opened, offering cryopreserved oocytes for donation cycles, and many entrepreneurs or Fortune 500 companies (Google, Facebook, and Apple) have begun offering elective oocyte cryopreservation as a paid benefit for their employees. By surveying the US Society for Assisted Reproductive Technology (SART) database, it is noticeable that most, if not all, clinics reporting to SART offer oocyte cryopreservation [3]. However, what has become also apparent is that pregnancy rates from vitrified/rewarmed oocytes are very variable among ART centers. One of the most important reasons for this is the lack of standardization of vitrification protocols (by some accounts, more than 100 different protocols are available) [4]. Equally important is the lack of proper training of embryologists to the tedious handling of the vitrification process, requiring exact exposure time of oocytes, one at time, to the various vitrification and equilibration solutions.
Vitrification is the solidification of a biological sample without the formation of ice crystals, thus resulting in a glassy amorphous state [5]. The vitrification process resolved two of the main reasons for oocyte damage during slow freezing: membrane chilling injury and the lethal ice crystals formation. The chilling injury of the oocytes is avoided by the fast cooling and warming rates of the process which basically outruns the time needed for membrane phase transition to occur [6]. Ice crystals are avoided due to the high viscosity of the cryoprotectants in the solution and by keeping the biological samples in a small volume (minimal drop size) [7] and a high cooling rate, thus enabling the vitrification process to occur [8].
The first successful vitrification of mice embryos was reported in 1985 [9], by using a mixture of cryoprotectant solutions (CPs) (DMSO, acetamide, and polyethylene glycol) in a relatively high volume inside 0.25-mL straws plunged into liquid nitrogen (LN). However, the results were suboptimal due to the relatively slow cooling and warming rates.
In 1989, the “minimum drop size” method was introduced [7]. The volume that was used for the vitrification was in the range of 0.07 μL (70 nL) and the concentration of the vitrification solution (VS) was about 50% lower than the one used for large-volume vitrification [10]. This breakthrough led over the years to the development of many disposables carriers (open system carriers) that made it easy to vitrify in a minimal volume oocytes and embryos [11].
Currently, vitrification is the method of choice for preserving oocytes and embryos and the results are very satisfactory in capable hands [2, 12]. The existence of many different protocols and types of carriers for vitrification further complicates the reproducibility of results [13]. Additional variables, also key for a successful vitrification program, are (a) the type and concentration of cryoprotectants, (b) temperature and timing of oocyte and embryo exposure, (c) the rates of cooling and the subsequent warming, and (d) whether oocytes or embryos come in direct contact with LN [14, 15]. All these variables make it difficult to standardize the vitrification procedure when performed manually. Having an automated device allowing the precise exposure of oocytes or embryos to the various equilibration solutions (ES) and vitrification solutions (VS), including the final immersion into LN, will overcome all the abovementioned disadvantages.
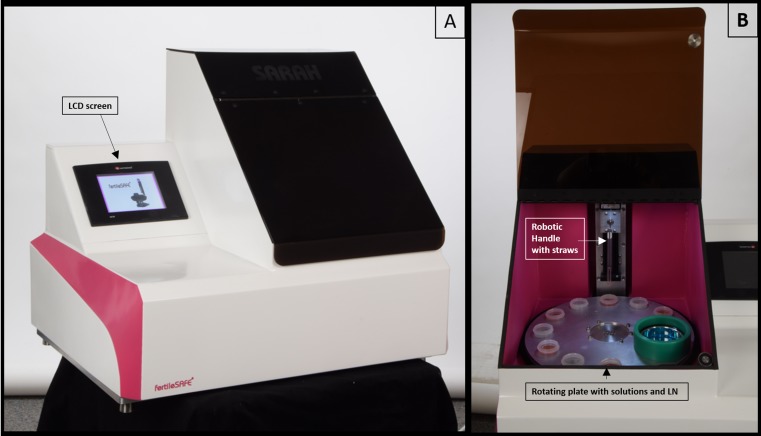
Fertilesafe Ltd. has developed such a device named Sarah® (see Fig. 1) and reported here are the preliminary successful results with mice and bovine oocytes and embryos.
Fig. 1.
Two pictures of the Sarah device. a The closed device with its LCD screen and b the open device showing the rotating metal plate with the solutions and LN container as well as the robotic handle with the straws
Materials and methods
Mice oocytes’ and embryos’ production and culture
Six CBA male mice were bred with 12 BL C57 female mice (Harlan Laboratories, Rehovot, Israel). Mice were kept on a 12-h photoperiod schedule with unlimited water and food supply (Israeli animal ethics authorization no. IL-15-04-119). Six–8 weeks after receiving offspring, each F1 female mouse was injected IP with 5IU PMSG (Sigma, St. Louis, USA). After 48 h, each female was injected IP with 5IU hCG (Sigma, St. Louis, USA). Male mice of proven fertility were then placed together with the superovulated females. Oocytes were recovered from superovulated mice the morning after hCG injection but without mating to males (n = 3). The next morning, the resulting female mice (n = 9) were examined to check for the copulatory plugs. If the plugs existed, the females were then euthanized 24 h later (approximately 36 h post-coitus) and embryos at 2-cell stage were retrieved from the oviducts. The oviducts were dissected in Quinn’s Advantage cleavage media (SAGE, Origio, Malov, Denmark) and the retrieved oocytes/embryos were divided into four groups: The first group (n = 40) was oocytes that underwent vitrification using Sarah (see vitrification procedure below). Upon rewarming, the oocytes were assessed for survival and viability using live/dead CYBR-14/PI fluorescent stains (Molecular Probes, Thermo Fisher Scientific, Waltham, MA, USA). The second group (n = 35) consisted of embryos vitrified at 8-cell stage obtained following 2 days of in vitro culture. The third group (n = 165) included vitrification of embryos at the blastocysts’ stage obtained after 4 days of in vitro culture. The fourth group (n = 42) consisted of fresh embryos (2 cells) used as the control group, cultured in 50-μL drops with Quinn’s Advantage cleavage media (SAGE, Origio, Malov, Denmark) overlaid with mineral oil, incubated at 37.0 °C under 5% CO2 and atmospheric oxygen (Thermo Forma, Series II, Thermo Fisher Scientific, Waltham, MA, USA), and monitored until they reached blastocyst and hatching stages.
In vitro maturation of bovine oocytes, fertilization, and culture
Ovaries were obtained from the local abattoir from primiparous and multiparous Holstein cows. The ovaries were placed in an insulated vessel containing physiological saline (0.9% (w/v) NaCl) (Sigma Aldrich, St. Louis, USA) with penicillin and streptomycin. The ovaries were transferred to the laboratory within 60–90 min after collection and washed with 0.9% (w/v) NaCl at 30–33 °C. Cumulus–oocyte complexes (COCs) were aspirated from follicles sized 3 to 8 mm in diameter using an 18-gauge needle attached to a 10-ml syringe. In vitro production (IVP) of bovine embryos was performed as described by Kalo and Roth [16]. Briefly, cumulus–oocyte complexes (COCs) were washed three times in Hepes–TALP and transferred in groups of 30 to a four-well dish (30 COCs per well). Each well contained 500 μl of oocyte maturation medium (OMM) consisting of TCM-199 and Earle’s salts supplemented with 10% (v/v) heat-inactivated fetal calf serum to prevent COC attachment to the bottom of the well (Promega, Madison, WI, USA), 0.2 mM sodium pyruvate, 50 μg/μl gentamicin, 2.2 g/l sodium bicarbonate, 2000 ng/ml 17-ß estradiol, and 1.32 μg/ml follicle-stimulating hormone isolated from ovine pituitary extract (Ovagen, ICP Bio, Auckland, New Zealand). COCs were incubated in humidified air with 5% CO2 for 22 h at 38.5 °C.
At the end of maturation, COCs were washed three times in Hepes–TALP and divided into two groups: the first group (n = 84) underwent vitrification using the Sarah device followed by rewarming and subsequent fertilization as described hereinafter and the second group (fresh control, n = 150) was immediately used for fertilization and culture as described hereinafter. The (rewarmed or fresh) COCs were transferred in groups of 30 to another four-well dish (30 COCs per well) containing 600 μl of in vitro fertilization (IVF)–TALP and 25 μl PHE (0.5 mM penicillamine, 0.25 mM hypotaurine, and 25 μM epinephrine in 0.9% NaCl) per well. For IVF, COCs were co-incubated for 18 h at 38.5 °C in a humidified atmosphere (5% CO2) with spermatozoa from the same bull prepared by swim-up technique (~ 1 × 106; “Sion,” Hafetz-Haim, Israel). After fertilization, zygotes were denuded of cumulus cells by gentle vortex in Hepes–TALP containing 1000 U/ml hyaluronidase. The experiments using bovine embryos (2PN|) were formed by the third group (n = 35), vitrified using Sarah, stored for 1 week in LN, and then rewarmed (as described below), and the fourth group (fresh control, n = 20). The rewarmed (n = 35) and the fresh control groups (n = 20) were placed in groups of 10 in 25-μl droplets of potassium simplex optimized medium (KSOM) containing 95 mM NaCl, 2.5 mM KCl, 0.35 mM KH2PO4, 0.2 mM MgSO4·7H2O, 0.8% (v/v) sodium lactate, 0.2 mM sodium pyruvate, 0.2 mM D(+)-glucose, 25 mM NaHCO3, 0.01 mM phenol red, 1 mM L-glutamine, and 0.01 mM ethylenediaminetetraacetic acid (EDTA) supplemented with 1.7 mM CaCl2·2H2O, 0.1 mg/ml polyvinyl alcohol, 10 μl/ml essential amino acids and 5 μl/ml non-essential amino acids, 100 U/ml penicillin-G, and 0.1 mg/ml streptomycin. All embryo droplets were overlaid with mineral oil and cultured for 7 days at 38.5 °C in an atmosphere of humidified air with 5% CO2 and 5% O2. Oocyte developmental competence was evaluated as the proportion of oocytes that fertilized, cleaved to 2- to 4-cell-stage embryos 42–44 h post-fertilization, and the proportion of embryos that developed to blastocysts by 7 days post-fertilization.
Mice oocytes’ live/dead fluorescent stains
Rewarmed vitrified oocytes’ survival was based on oolema integrity by propidium iodide (PI) and Hoechst fluorescent stains (Sigma-Aldrich, Israel). For this purpose, oocytes were stained with PI (10 μg/ml) and Hoechst 33342 (10 μg/ml) for 10 min, washed, and then observed under a fluorescent microscope. The dead cells showed red fluorescence (PI-positive) for disruption of cellular membrane and the viable cells showed blue fluorescence without red fluorescence (PI-negative) for the intact cell membrane.
Description of the Sarah® device
The automatic device used to vitrify oocytes and embryos consists of a vertical robotic handle where a special straw-holder, that can load up to six straws, is attached. This robotic arm moves in a vertical plane (up and down), at predetermined time intervals, and by so doing carries the biological samples contained in the straws between different solutions (vitrification and equilibration) arranged into nine cups placed in a temperature-controlled metal carousel plate (see Fig. 1).
The final station on the carousel plate is the one containing LN where the straws are ultimately plunged and the entire cycle of vitrification is considered completed.
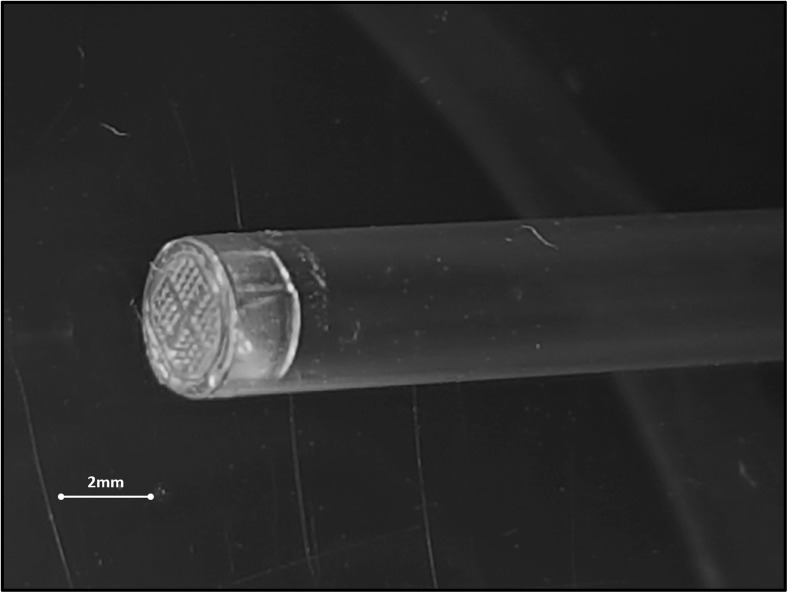
The straws utilized in these experiments are 0.25-ml straws (CBS, L’Aigle, France). Prior to attaching the straws to the holder, the oocytes or embryos are first manually loaded into the straws and then the straws are closed at one extremity by special capsules (50-μm pores) (Fertilesafe Ltd., Nes-Ziona, Israel) (Fig. 2). After the holder is placed on the robotic handle and the protocol has been selected from the touch screen, it is sufficient to press the “ON” button for the vitrification cycle to begin. Once the entire preparatory steps are completed, the holder plunges the straws into a special insulated vessel containing LN or sterile liquid air [17]. The special straw holder is then disconnected from the handle and the straws can be either stored “as it is” or they can be inserted into a 0.3-ml straw heat-sealed and then placed in LN tanks for long-term storage.
Fig. 2.
A picture of the special capsule attached to a 0.25-ml straw
Details of the vitrification and warming process with Sarah
Mice oocytes (n = 40) or day 2 (8-cell stage) embryos (n = 35) or day 4 (blastocysts) (n = 165) were automatically vitrified. Up to 5 oocytes/embryos were first loaded into 0.25-ml straws (CBS, L’Aigle, France) end-closed by special capsules (50-μm pores) (Fertilesafe, Nes-Ziona, Israel). The straws were then connected to the holder placed vertically in the robotic arm of the Sarah device. The device was then turned ON and the robotic handle plunged the straws in and out of cups containing the various solutions. For the mice experiments, the samples were exposed to 3 equilibration solutions: 30, 60, and 100%, 3 min each, followed by one vitrification solution, 100% for 1 min. For the bovine experiments, the samples were exposed to 6 different equilibration solutions: 10, 20, 40, 60, 80, and 100% ES (100% ES for mice and bovine was composed of 7.5% DMSO +7.5% EG + 20%FCS in TCM-199) (90 s in each tube) followed by 30 s in 75 and 100% VS (100% VS for mice and bovine was composed of 18% DMSO + 18% EG + 0.5 M Trehalose + 20% FCS in TCM-199). Of note, after the 100%VS and prior to immersion into LN, there is a step where the straws are inserted into a cup containing an absorbing paper to remove the VS and achieve the lowest volume possible. All solutions were kept at 24 °C. The vitrification cycle was completed when the straws were plunged directly into LN. At this point, the straws were disconnected from the holder and placed in LN tanks for storage (Fig. 3).
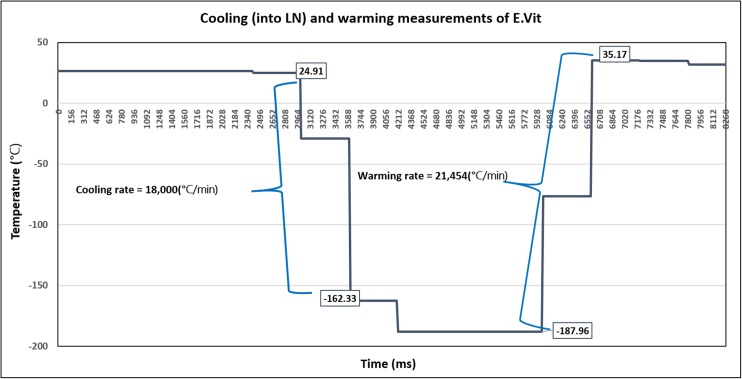
Fig. 3.
A graph of the cooling and warming rates measure using a thermocouple connected to the straw next to the capsule. The X axis is the time in milliseconds and the Y axis is the temperature in Celsius. The cooling and warming rates were calculated according to following equation: C.R. = [ΔT°C/Δt (ms)] × 1000 × 60 = °C/min
The warming procedure consisted of first plunging the straws into 100% warming solution (WS) at 37 °C for 5 s and then immediately placing the straws in the vertical robotic arm of the Sarah device (as seen in Fig. 1). In a reverse process than used for vitrification, for the rewarming, the straws are automatically plunged into temperature-controlled 5-ml tubes containing 100, 50, 25, and 12.5% WS (WS = 1 M sucrose in TCM-199 + 20% FCS) kept at room temperature for 2.5 min each, before arrival into the final holding medium station. Now, the oocytes and embryos were either evaluated or continued to culture and/or insemination as described above in the “Materials and methods” section.
Cooling and warming rates’ measurements
Cooling and warming rates were measured using a T-type thermometer connected to the straw at the point of insertion of the capsule. The temperature was recorded with a data logger (Amemo 2290-4, MRC Ltd. Holon, Israel). The cooling rate was measured when the straws were immersed into the LN after being exposed to the VS. Warming rates were measured when the straws were removed from LN and inserted into a vial containing 1 M sucrose solution warmed to 37 °C.
Statistical analysis
Statistical analysis was performed using chi-square test for contingency tables (www. Socscistatistic.com on line calculator software) and statistical difference was set to be below 0.05. Power analysis was calculated for each comparison, assuming an effect size of 0.5 and alpha of 0.05.
Results
Upon rewarming, oocyte survival was evaluated by their ability to return into the isotonic volume while viability was assessed by live/dead fluorescent stains. Rewarmed 8-cell and blastocyst-stage embryos were placed in culture and their viability was evaluated by the rate of resumption of their development and by assessing blastocyst and hatching rates. Table 1 described the experimental and the control groups. Table 2 summarized the mice overall results showing that 95% (38/40) of the MII oocytes (Fig. 4a, b) regained isotonic volumes and all (38/38 (100%)) of the surviving were viable according to the live/dead stains. Rewarmed 8-cell stage mice embryos had 94% (33/35) survival and blastulation rate (by day 4) and 80% (28/35) hatching rate (by day 5). Rewarmed mice blastocysts had 97% (160/165) survival and 81% (135/165) hatching rates (by day 5) (Fig. 4c, d). The fresh control mice embryos had a lower hatching rate of 76% (32/42). There were no statistical differences between the rewarmed mice oocytes and embryos and the fresh controls.
Table 1.
A summary of mice and bovine (oocytes and embryos) in the experimental and control groups
| Mice experimental groups | |||
| Oocytes vitrified | Embryos | ||
| Fresh control (2 cell) | 8 cells vitrified | Blastocysts vitrified | |
| 40 | 42 | 35 | 165 |
| Bovine experimental groups | |||
| Oocytes | Zygotes (2PN) | ||
| Fresh | Vitrified | Fresh | Vitrified |
| 150 | 84 | 20 | 35 |
Table 2.
Mice oocytes’ survival and mice embryos survival and development after vitrification with the Sarah device (p, NS)
| Oocytes | 8-cell embryo | Blastocysts | Control (fresh Embryos) | |
|---|---|---|---|---|
| Number | 40 | 35 | 165 | 42 |
| Survival (%) | 38/40 (95) | 33/35 (94) | 160/165 (97) | – |
| Viability (%) | 38/38 (100) | – | – | – |
| Blastocysts (%) | – | 33/35 (94) | – | 42/42 (100) |
| Hatching (%) | – | 28/35 (80) | 135/165 (81) | 32/42 (76) |
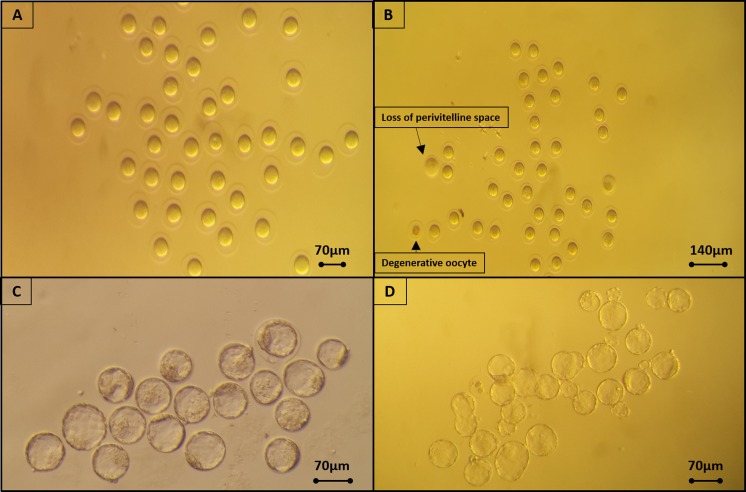
Fig. 4.
a Mice oocytes prior to vitrification using Sarah. b Oocytes after warming, revealing two damaged oocytes (pointed by arrows). c Fresh mice blastocysts and d blastocysts that were vitrified using Sarah
Table 3 summarizes the overall bovine results (Fig. 5) showing that zygote survival was 100% (35/35); following in vitro culture, 54% (19/35) cleaved and 9% (3/35) reached the blastocyst stage. These results were lower than the control cleavage and blastocyst rates which were 65% (13/20) and 20% (4/20), respectively (Table 3). Nevertheless, there were no statistical differences between the rewarmed bovine oocytes and embryos and the fresh controls.
Table 3.
Bovine zygotes’ and MII oocytes’ development after vitrification with the Sarah system compared to the fresh controls. (p, NS)
| Bovine results | % cleavage | % blastocysts |
|---|---|---|
| Vitrified zygotes | 54% (19/35) | 9% (3/35) |
| Fresh zygotes | 65% (13/20) | 20% (4/20) |
| Vitrified oocytes | 73% (61/84) | 7% (6/84) |
| Fresh oocytes | 83% (125/150) | 11% (17/150 |
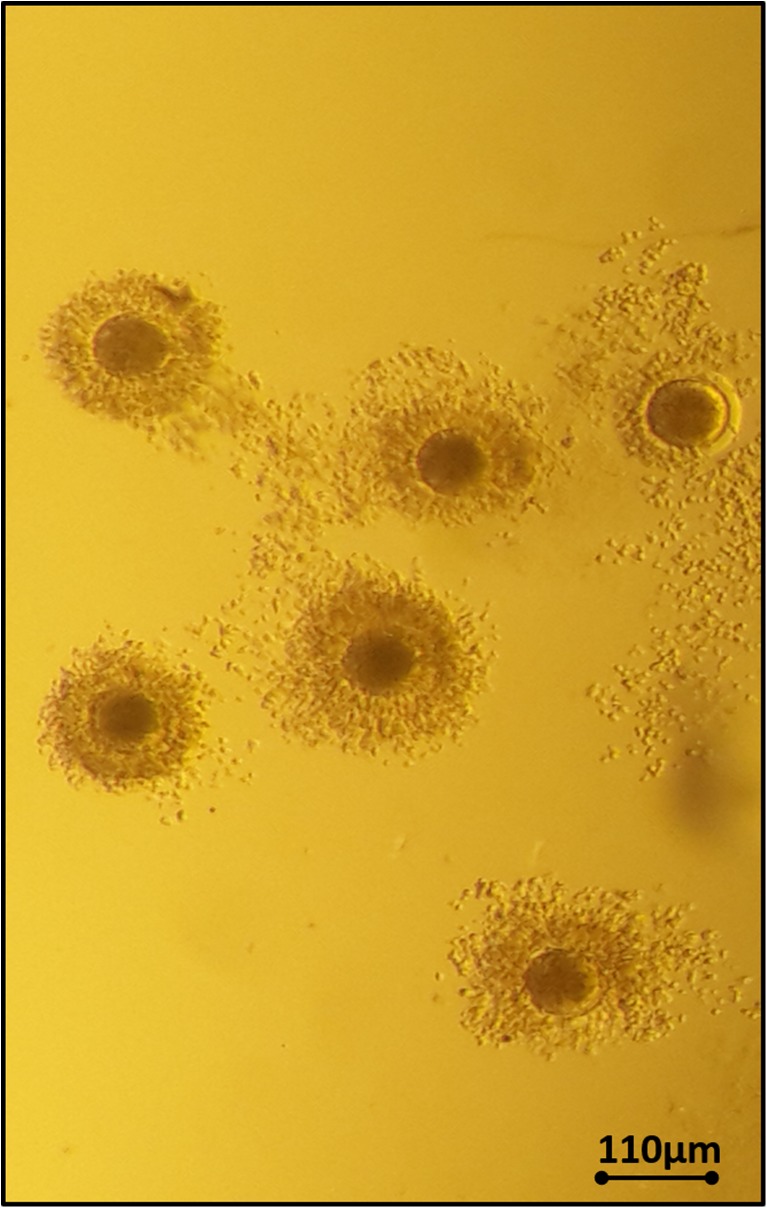
Fig. 5.
Bovine oocytes after vitrification and warming using Sarah
MII bovine oocytes vitrified with Sarah resulted in 100% survival (84/84), 73% (61/84) cleavage rate, and 7% (6/84) developed into blastocysts. The fresh control had 83% (125/150) cleavage rate and 11% (17/150) developed into blastocysts (Table 3).
Cooling and warming rates were 18,000 and 21,454 °C/min, respectively (see Fig. 3).
Post hoc power analysis showed 99% chance of detecting differences at alpha = 0.05 for all the comparisons.
Conclusions
In the last decade, vitrification has replaced the slow freezing technology, providing high oocyte survival rates and subsequent high developmental competence as published by leading clinics in the field [18–23]. These successful outcomes have led to the replacement of slow freezing with vitrification also for embryos at different development stages [24–26] and for ovarian slices [27–29]. However, the efficiency, consistency, reliability, and safety of vitrification need to be improved and only by advancing to an automated vitrification system this can be guaranteed.
In this work, we reported data on full automation of the vitrification procedure, even to include the plunging into LN, with mice oocytes and embryos and bovine oocytes and embryos. The most important features of an automated vitrification system should be the ability to produce high survival and viability of both oocytes and embryos by generating high cooling and warming rates with the reduction of the sample drop volume to less than 0.1 μl and by exposing the cells to the increasing and decreasing concentrations of the cryoprotectant solutions by using many steps to avoid the osmotic damages [30]. Furthermore, an ideal device should enable time and temperature control and reduce the vitrification working time required per patient. Disposable containers should be easy to label and easy to store in LN tanks without making changes in storage space. These features are all present in the Sarah device reported here.
Currently, there is no methodology able to fully automate the vitrification process. Few publications have described solutions for automated vitrification; however, the only commercially available semi-automated vitrification machine is the one marketed by EMD Serono [13]. Here, we reported a simplified methodology that uses commercially available straws and is almost completely operator independent since it includes also the immersion into LN thus providing flexibility to embryologists since the biological samples are vitrified by the device and kept in LN until placed in long-term storage. The Sarah system allows for the automated control of each of its steps including time and temperature. The Sarah device can operate up to 6 straws, and since each straw can load 5 oocytes (or embryos), a total of 30 oocytes/embryos can be vitrified simultaneously, thus shortening the time required to complete the entire task. This is an important feature, particularly for busy units. But the most important characteristic of the presented device here is the use of a very small capsule connected at the extremity of the straw which allows the use of the minimal volume size of the samples. The minimal drop size is the most critical feature for a successful vitrification allowing the high cooling and warming rates of over 20,000 °C/min. It was shown that human oocytes and bovine early-stage embryos suffer from chilling sensitivity in both membrane [7, 31] and on the meiotic spindle [32] and that to minimize or avoid similar damages in bovine oocytes, rapid cooling and warming rates were necessary to out run and avoid the chilling injuries [33, 34]. Although results have improved for vitrification of bovine oocytes and embryos, they remain lower than fresh controls due to their known chilling sensitivity [34–36 -3]. Our results with fertilized oocytes (2PN embryos) are comparable with results obtained with other vitrification systems such as OPS and cryotop [35] or when vitrification was done with long or short equilibrations [36] which consistently show lower blastulation rates than the fresh controls. An important reason of why slow vitrification [5, 7] and slow freezing did not work for human oocytes is the chilling injury and not the ice formation for a cell (the oocyte) of large volume. In fact, MII oocyte and 2PN embryo share the same cell volume and probably the same water content and therefore the probability of intracellular nucleation and crystallization is similar. However, while MII oocytes are sensitive to slow freezing, 2PN embryos do survive very well slow freezing [37, 38] indicating that the main reason for the differences between the two is their chilling sensitivity.
Finally, another important feature of Sarah is the possibility of standardizing also the rewarming process, by using the same device.
A limitation of this study is that although these preliminary results in animal models are successful, they need to be replicated in clinical human IVF settings.
In summary, a key element for oocyte survival is a high cooling and warming rate which is achieved by using small-volume and a small-size carrier for the cells. Both these important requirements are present in the Sarah automatic vitrification device. This device has simplified the vitrification process due to two main advantages: (1) the embryos/oocytes are within the same straw during the entire vitrification cycle (up to 6 oocytes or embryos can be loaded in each straw), from the initial bathing in ES medium until the final step being the plunging into LN or LN slush; (2) The movements between the different solutions are done in an automated and precisely timed manner. This means that the time the oocytes or embryos are exposed to each solution is pre-determined and very accurate. These two advantages eliminate the need for searching the embryos/oocytes, allowing the simultaneous transfer of multiple oocytes or embryos between the various solutions and standardize the exposure time to each of the vitrification steps resulting in a consistent reproducibility of the process and results. It is anticipated that this new device by maintaining all the important features needed for successful vitrification such as rapid cooling and warming, small volume, small carrier, and relatively low concentrations of CPs and controlled temperature and time for each step would be a breakthrough for simplifying and standardizing the vitrification process worldwide.
Contributor Information
Amir Arav, Phone: +972-88670118, Email: Fertilesafe@gmail.com.
Zvika Roth, Email: roth@agri.huji.ac.il.
Paolo Emanuele Levi-Setti, Email: paolo.levi_setti@humanitas.it.
Milton Leong, Email: milton.leong@gmail.com.
Pasquale Patrizio, Email: pasquale.patrizio@yale.edu.
References
- 1.American Society for Reproductive Medicine Mature oocyte cryopreservation: a guideline. Practice committees of American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Cobo A, Garcia-Velasco JA, Domingo J, Remohi J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil Steril. 2013;99:1485–1495. doi: 10.1016/j.fertnstert.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Rudick B, Opper N, Paulson R, Bendikson K, Chung K. The status of oocyte cryopreservation in the United States. Fertil Steril. 2010;94:2642–2646. doi: 10.1016/j.fertnstert.2010.04.079. [DOI] [PubMed] [Google Scholar]
- 4.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arav A. Cryopreservation of oocytes and embryos. Theriogenology. 2014;81(1):96–102. doi: 10.1016/j.theriogenology.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Mazur P, Schneider U, Mahowald AP. Characteristics and kinetics of subzero chilling injury in Drosophila embryos. Cryobiology. 1992;29(1):39–68. doi: 10.1016/0011-2240(92)90005-M. [DOI] [PubMed] [Google Scholar]
- 7.Arav A. Cryopreservation of oocytes and embryos, DVM thesis 1989. [DOI] [PubMed]
- 8.Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141(1):1–19. doi: 10.1530/REP-10-0236. [DOI] [PubMed] [Google Scholar]
- 9.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313(6003):573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 10.Arav A. Vitrification of oocytes and embryos. In: Lauria A, Gandolfi F, editors. Embryonic development and manipulation in animal reproduction. Portland press; 1992: 255–264.
- 11.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Levi-Setti PE, Patrizio P, Scaravelli G. Evolution of human oocyte cryopreservation: slow freezing versus vitrification. Curr Opin Endocrinol Diabetes Obes. 2016;23(6):445–450. doi: 10.1097/MED.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 13.Roy TK, Brandi S, Tappe NM, Bradley CK, Vom E, Henderson C, Lewis C, Battista K, Hobbs B, Hobbs S, Syer J, Lanyon SR, Dopheide SM, Peura TT, McArthur SJ, Bowman MC, Stojanov T. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum Reprod. 2014;29(11):2431–2438. doi: 10.1093/humrep/deu214. [DOI] [PubMed] [Google Scholar]
- 14.Alpha Scientists in Reproductive Medicine The alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reprod BioMed Online. 2012;25:146–167. doi: 10.1016/j.rbmo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Kader AA, Choi A, Orief Y, Agarwal A. Factors affecting the outcome of human blastocyst vitrification. Reprod Biol Endocrinol. 2009;7:99. doi: 10.1186/1477-7827-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalo D, Roth Z. Effects of mono(2-ethylhexyl)phthalate on cytoplasmic maturation of oocytes—the bovine model. Reprod Toxicol. 2015;53:141–151. doi: 10.1016/j.reprotox.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Arav A, Natan Y, Levi-Setti PE, Menduni F, Patrizio P. New methods for cooling and storing oocytes and embryos in a clean environment of −196°C. Reprod BioMed Online. 2016;33(1):71–78. doi: 10.1016/j.rbmo.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Cobo A, García-Velasco JA. Why all women should freeze their eggs. Curr Opin Obstet Gynecol. 2016;28(3):206–210. doi: 10.1097/GCO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 19.Cobo A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105(3):755–764.e8. doi: 10.1016/j.fertnstert.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Cobo A, Garrido N, Pellicer A, Remohí J. Six years’ experience in ovum donation using vitrified oocytes: report of cumulative outcomes, impact of storage time, and development of a predictive model for oocyte survival rate. Fertil Steril. 2015;104(6):1426–1434. doi: 10.1016/j.fertnstert.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Nagy ZP, Nel-Themaat L, Chang CC, Shapiro DB, Berna DP. Cryopreservation of eggs. Methods Mol Biol. 2014;1154:439–454. doi: 10.1007/978-1-4939-0659-8_20. [DOI] [PubMed] [Google Scholar]
- 22.Cobo A, Vajta G, Remohí J. Vitrification of human mature oocytes in clinical practice. Reprod BioMed Online. 2009;19(Suppl 4):4385. [PubMed] [Google Scholar]
- 23.Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Kort HI, Vajta G. The efficacy and safety of human oocyte vitrification. Semin Reprod Med. 2009;27(6):450–455. doi: 10.1055/s-0029-1241054. [DOI] [PubMed] [Google Scholar]
- 24.Liebermann J. Chapter 11 human embryo Vitrification. Methods Mol Biol. 2017;1568:141–159. doi: 10.1007/978-1-4939-6828-2_11. [DOI] [PubMed] [Google Scholar]
- 25.Fasano G, Fontenelle N, Vannin AS, Biramane J, Devreker F, Englert Y, Delbaere A. A randomized controlled trial comparing two vitrification methods versus slow-freezing for cryopreservation of human cleavage stage embryos. J Assist Reprod Genet. 2014;31(2):241–247. doi: 10.1007/s10815-013-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):186–193. doi: 10.1016/j.fertnstert.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep. 2017;7(1):8538. doi: 10.1038/s41598-017-09005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016;33(12):1595–1603. doi: 10.1007/s10815-016-0814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silber S. Chapter 13 human ovarian tissue vitrification. Methods Mol Biol. 2017;1568:177–194. doi: 10.1007/978-1-4939-6828-2_13. [DOI] [PubMed] [Google Scholar]
- 30.Arav A, Shehu D, Mattioli M. Osmotic and cytotoxic study of vitrification of immature bovine oocyte. J Reprod Fert. 1993;99:353–358. doi: 10.1530/jrf.0.0990353. [DOI] [PubMed] [Google Scholar]
- 31.Ghetler Y, Yavin S, Shalgi R, Arav A. The effect of chilling on membrane lipid phase transition in human oocytes and zygotes. Hum Reprod. 2005;20(12):3385–3389. doi: 10.1093/humrep/dei236. [DOI] [PubMed] [Google Scholar]
- 32.Zenzes MT, Bielecki R, Casper RF, Leibo SP. Effects of chilling to 0 degrees C on the morphology of meiotic spindles in human metaphase II oocytes. Fertil Steril. 2001;75(4):769–777. doi: 10.1016/S0015-0282(00)01800-8. [DOI] [PubMed] [Google Scholar]
- 33.Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology. 2007;67(1):81–89. doi: 10.1016/j.theriogenology.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Martino A, Pollard JW, Leibo SP. Effect of chilling bovine oocytes on their developmental competence. Mol Reprod Dev. 1996;45(4):503–512. doi: 10.1002/(SICI)1098-2795(199612)45:4<503::AID-MRD13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 35.Morató R, Izquierdo D, Paramio MT, Mogas T. Cryotops versus open-pulled straws (OPS) as carriers for the cryopreservation of bovine oocytes: effects on spindle and chromosome configuration and embryo development. Cryobiology. 2008;57(2):137–141. doi: 10.1016/j.cryobiol.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Do VH, Walton S, Catt S, Taylor-Robinson AW. A comparative analysis of the efficacy of three cryopreservation protocols on the survival of in vitro-derived cattle embryos at pronuclear and blastocyst stages. Cryobiology. 2017;77:58–63. doi: 10.1016/j.cryobiol.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Massip A, Van der Zwalmen P, Leroy F. Effect of stage of development on survival of mouse embryos frozen–thawed rapidly. Cryobiology. 1984;21:574–577. doi: 10.1016/0011-2240(84)90057-9. [DOI] [PubMed] [Google Scholar]
- 38.Camus M, Van Den Abbeel E, Van Waesberghe L, Wisanto A, Devroey P, Van Steirteghem A. Human embryo viability after freezing with dimethylsulfoxide as a cryoprotectant. Fertil Steril. 1989;51(3):460–465. doi: 10.1016/S0015-0282(16)60554-X. [DOI] [PubMed] [Google Scholar]