Abstract
Purpose
Cleavage of the zygote during human reproduction is a key event of early embryonic development. The genetic events associated with idiopathic embryonic cleavage failure are not certain. Mutations in the tubulin beta 8 class VIII (TUBB8) gene have been reported to be associated with oocyte maturation, fertilization, and developmental arrest. Here, we aimed to assess the clinical and genetic characteristics of complete cleavage failure in fertilized eggs.
Methods
We have characterized a patient with a 9-year history of primary infertility in a non-consanguineous family from China. The patient presented complete cleavage failure in all two-pronuclear (2PN) fertilized oocytes after 2 cycles of in vitro fertilization (IVF). We performed Sanger sequencing of the TUBB8 gene in the patient, and further bioinformatics analysis to identify pathogenesis of gene.
Results
A novel homozygous mutation, c.322G > A (p.Glu108Lys), was detected, and this change was absent from 179 control subjects. Glutamic acid is highly conserved at this position, and replacement by lysine was predicted to be repelled by the α-tubulin positive region, disrupting the α-β tubulin interaction.
Conclusions
Our findings presented a homozygous mutation of TUBB8 associated with complete cleavage failure in fertilized eggs and provided new data for the genotype-phenotype of TUBB8-related diseases.
Keywords: Cleavage failure, TUBB8, Mutation, 2PN arrest, Infertility
Introduction
The formation and development of early embryos is a complex process and comprises of a series of programmed morphological changes that are regulated by complex molecular pathways. It includes penetration of a mature oocyte (metaphase II [MII] oocyte) by a mature spermatozoa, oocyte activation, prevention of polyspermy, cytoskeletal rearrangements, resumption of meiosis, polar body extrusion, the formation of male and female pronuclei (two-pronuclear [2PN] zygote), pronuclear fusion, and the initiation of early embryonic cell cycles [1, 2]. The key stages are sperm–oocyte fusion and cleavage of the zygote, which involves several signaling pathways, such as Ca2+ signaling [3–5]. As a means of assisted reproduction, the MII oocyte can be activated by intracytoplasmic sperm injection (ICSI), which involves directly injecting a single spermatozoon, allowing complete fertilization and the subsequent cleavage steps [6, 7]. However, there are some case reports of ICSI fertilization failure (MII arrest) and cleavage failure (2PN arrest) during in vitro fertilization (IVF) [8, 9]. Using artificial oocyte activation (AOA) to overcome the above phenomena has only resulted in partial success [8–10].
A genetic defect in the tubulin beta 8 class VIII (TUBB8, Gene ID: 347688) gene has been shown to be responsible for oocyte maturation, fertilization, and early embryonic developmental arrest [11–14]. This gene encodes a special β-tubulin isotype that is the major constituent of the oocyte and early embryo spindle and only exists in primates [11]. Heterozygous or de novo mutations in the TUBB8 gene are considered to primarily cause metaphase I (MI) arrest through dominant-negative effects, although some arrest during early embryonic development also occurs. However, homozygous or compound heterozygous mutations may result in fertilization failure (MII arrest) and early embryonic developmental arrest [13].
In this study, we present a patient with complete cleavage failure in all 2PN fertilized oocytes and a novel mutation in the TUBB8 gene. Using molecular genetic analysis, this study provides additional evidence for the role of TUBB8 in 2PN arrest and advances our understanding of the genotype-phenotype relationship of TUBB8 mutations. This is the first study to report complete cleavage failure in fertilized eggs for homozygous mutations in TUBB8.
Materials and methods
Case report
The patient was a 34-year-old woman with a 9-year history of primary infertility with regular menses since menarche at the age of 16. Her basic follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels were normal (6.95 IU/l [normal range 3.85–8.78 IU/l], 6.59 IU/l [normal range 2.12–10.89 IU/l], and 56.00 pg/ml [normal range 27–122 pg/ml], respectively). Her anti-Müllerian hormone (AMH) levels were also normal (4.84 ng/ml [normal range 0.24–11.78 ng/ml]). An ultrasound scan showed a normal uterus and ovaries with a total of 17 antral follicles (2 to 5 mm in diameter). Hysterosalpingography revealed that there was a liquid dark area near the left ovary in the pelvis. Chromosome analysis with traditional G-band karyotyping techniques showed the patient to be 46, XX. Her husband was 39 years old, and his physical examination was normal. Anamnesis revealed no inflammatory or other important andrological events. An ultrasound revealed a normal testis, epididymis, and bilateral spermatic vein. The semen analysis was marginal normal (the total sperm concentration was 37 × 106/ml [lower reference limit, 15 × 106/ml], progressive motility rate (PR) was 31% [lower reference limit, 32%], normal sperm morphology was 4% [lower reference limit, 4%], and sperm acrosin activity was 45.86 μIU/M [normal range 48.20–218.70 μIU/M]). His chromosome karyotype was 46, XY. Subsequently, the patient underwent controlled ovarian hyperstimulation and 2 cycles of ICSI at our hospital, as shown in Table 1.
Table 1.
The description of ART cycles and characteristics of oocytes, fertility, and cleavage
| Characteristic | Cycle 1 | Cycle 2 |
|---|---|---|
| Date | 1/2016 | 6/2016 |
| Protocol | GnRH agonist, long protocol | GnRH antagonists (ganirelix), fixed |
| Gonadotropin | r-FSH | hMG |
| Initiation dose (IU) | 150 | 150 |
| Duration of simulation (d) | 9 (150 IU) + 2 (100 IU) | 9 (150 IU) |
| Total dose of gonadotropin (IU) | 1550 | 1350 |
| On the day of hCG injection | ||
| Serum E2 level(pg/ml) | > 4839 | 4048 |
| Serum Progesterone level(ng/ml) | 1.50 | 0.81 |
| Serum LH level (IU/L) | 1.38 | 1.50 |
| No. of total follicles | 29 | 22 |
| No. of the leading follicles (≥ 18 mm) | 7 | 10 |
| Ovulation triggering (dose) | 250 μg (r-hCG) | 10,000 IU (hCG) + 0.2 mg (GnRH agonist) |
| Time interval between hCG administration and oocyte retrieval (h) | 36 | 36 |
| No. of retrieved oocytes | 17 | 14 |
| No. of MII oocytes | 12 | 10 |
| No. of fertilization oocytes | 7 | 7 |
| No. of normal fertilization oocytes (2PN) | 6 | 7 |
| No. of cleavage embryos | 0 | 0 |
r-FSH (recombinant FSH, Puregon®, Organon, Netherlands); GnRH antagonist, ganirelix (Orgalutran®, Organon, Netherlands); r-hCG (recombinant hCG, Ovidrel®, Merck Serono, Italy); hMG (Menopur®, Ferring, Germany); hCG (Livzon, China); GnRH agonist, Triptorelin acetate (Diphereline, Ipsen Pharma Biotech); OPU, ovum pick up
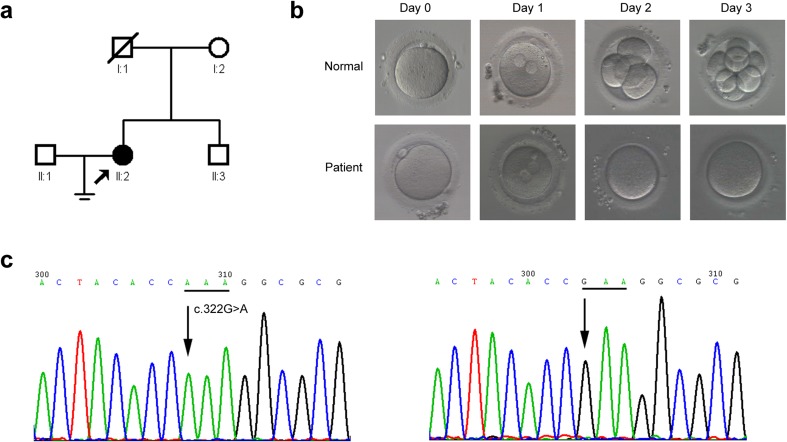
The patient was a child of a non-consanguineous marriage, and her brother was not married (Fig. 1a). A control population of 179 unrelated Chinese volunteers with normal fertility was recruited. Written informed consent was obtained from all subjects. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of our hospital.
Fig. 1.
Oocyte and embryo characteristics and genetic analysis of the family. a Pedigree of the family. The filled circle indicates the cleavage failure patient (II:2). The arrow indicates the proband. Open squares or circles indicate normal family members. b Phenotypes of oocytes and embryos from a normal individual and patient with TUBB8 mutation examined by light microscopy. Upper panel, the morphology of an MII oocyte (on day 0), fertilized oocyte with two pronuclei (arrow, on day 1), four-cell stage (on day 2), and eight-cell stage (on day 3) from a normal individual. Bottom panel, the morphology of an MII oocyte (on day 0), fertilized oocyte with two pronuclei (arrow, on day 1), and cleavage failure (on days 2 and 3) from the patient. Scale bars in B: 50 μm. MII = metaphase II. c Partial forward nucleotide sequences of exon 4 in the TUBB8 gene. Left panel, the arrow points to the homozygous c.322G > A (p.Glu108Lys) mutation in the patient (II:2); the mutated codon is underlined. Right panel, the arrow points to the wild-type c.322G in a control sample; the wild-type codon is underlined
Mutation detection and bioinformatics analysis
Mutation detection in the TUBB8 gene was completed by Sanger sequencing of polymerase chain reaction (PCR) products of all four exons and flanking intronic regions using specific primers in the patient and controls. The mutation was named according to the Human Genome Variation Society (HGVS) standard (http://www.hgvs.org/mutnomen/) with + 1 corresponding to the A of the ATG translation initiation codon of the GenBank cDNA sequence NM_177987.
CLUSTAL X (1.81) [15] was used to compare the human TUBB8 amino acid sequence (Homo sapiens Uniprot ID Q3ZCM7) with six primate species (Pan troglodytes, Pan paniscus, Gorilla gorilla, Papio hamadryas, Papio anubis and Macaca fascicularis). Effects of the sequence variant were predicted using PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), Mutation Taster (http://www.mutationtaster.org/), and SIFT (http://sift.jcvi.org/). And the database of the Exome Aggregation Consortium (ExAC) browser (http://exac.broadinstitute.org/) was used to search the allele frequencies of the variant. Due to the high homology (sequence identity > 90%) with TUBB8, the high-resolution cryo-EM structure of a microtubule (PDB ID: 3JAS) [16] was used to analyze the effect of the mutation. The E108K structure was constructed based on 3JAS by selecting a proper rotamer of lysine using the PyMOL mutagenesis module (www.pymol.org).
Results
Due to years of infertility, ICSI was performed on the patient (II:2) (Fig. 1a). Human chorionic gonadotropin (hCG) was administered 40 h before ICSI was performed. In the first attempt, a total of 17 oocytes were retrieved, of which 12 were in MII and suitable for ICSI. Of these oocytes, only 7 could be fertilized (6 in 2PN, 1 in single pronucleus [1PN], fertilization rate was 58.3%), but none of the zygotes exhibited cleavage. There were no available embryos for transfer. In the second round, 14 oocytes were retrieved. ICSI was performed on 10 oocytes, of which 7 were successfully fertilized (fertilization rate was 70.0%). However, as in the first cycle, the pronuclei of these zygotes disappeared, and no cleavage was observed on days 2 and 3 (Table 1) (Fig. 1b).
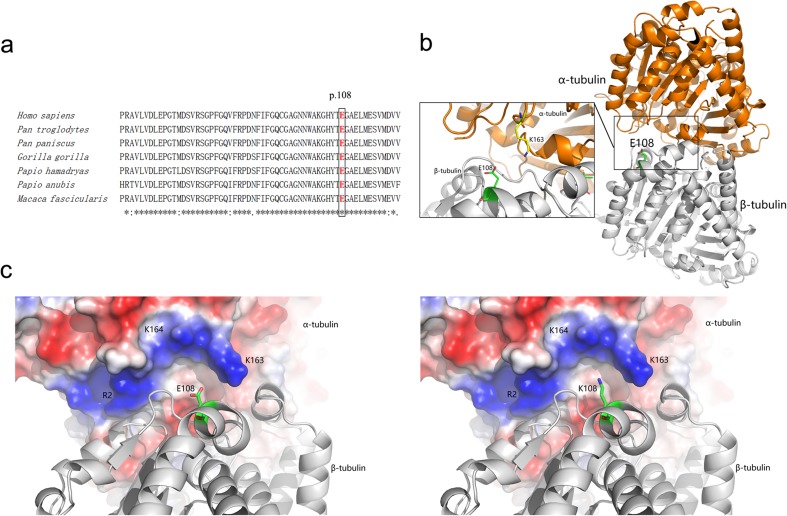
Direct sequencing of both chromosomes in the patient (II:2) (Fig. 1a) revealed a novel homozygous mutation, c.322G > A (p.Glu108Lys), in exon 4 of the TUBB8 gene (Fig. 1c). We further examined 179 healthy control subjects (358 alleles) and found that this variant was absent from our cohort (Fig. 1c), suggesting that it might not be a benign polymorphism. In addition, this variant has been observed at very low frequency in ExAC database (minor allele frequency was 0.0003475 in East Asian). The computational programs SIFT, PolyPhen-2, and Mutation Taster predicted that the effect of this variant is deleterious, probably damaging and disease causing, respectively. Alignment of the TUBB8 protein across seven primate species showed that the glutamic acid at position 108 was highly conserved (Fig. 2a). Based on the microtubule structure of the wild-type and mutant TUBB8, E108 is located at the interface of the α/β tubulin heterodimer and directly contributes to α-β tubulin interactions (Fig. 2b). For the wild-type α/β tubulin heterodimer, negatively charged E108 forms favorable electrostatic interactions with a positive region consisting of α-tubulin R2, K163, and K164 (Fig. 2c). However, once mutated to lysine, the positively charged K108 is repelled by the α-tubulin positive region, thus disrupting the α-β tubulin interaction (Fig. 2c).
Fig. 2.
Bioinformatic analysis of the novel mutation. a Glu108 is conserved in TUBB8. The glutamic acid at the 108th position is highly conserved among Homo sapiens, Pan troglodytes, Pan paniscus, Gorilla gorilla, Papio hamadryas, Papio anubis, and Macaca fascicularis. b The overall structure of an α/β tubulin heterodimer. The α-tubulin and β-tubulin are displayed as orange and gray cartoons, whereas the E108 of β-tubulin and the K163 of α-tubulin are shown as green and yellow sticks, respectively. c The electrostatic potential surface of α-tubulin together with its interaction with wild-type (left) and E108K mutant (right) β-tubulin. The positive- and negative-charged regions of α-tubulin are colored blue and red, respectively. E108 and K108 are shown as cartoon and sticks. Structure display and electrostatic potential surface generation were performed by PyMOL (http://www.pymol.org/)
Discussion
To our knowledge, this is the first report to describe homozygous mutation of the TUBB8 gene in a patient with complete cleavage failure in fertilized eggs. So far, a total of 31 patients with 27 different mutations in TUBB8 have been identified (Table 2). According to this summary, TUBB8-related diseases are associated with the arrested development of human oocytes and early embryos, which had variant phenotypes, including MI arrest, MII arrest (fertilization failure), 2PN arrest (cleavage failure), and early embryonic development arrest (less than four-cell stage). Actually, more than half of these patients (18 cases) were diagnosed with MI arrest, four patients mainly showed early embryonic development arrest, and another eight patients were believed to be mixed arrest (Table 2) [17]. In our study, the patient displayed normal number of MII oocytes and marginal normal fertilization rates in two ICSI cycles which were 58.3 and 70%, respectively (normal range in ICSI 70–80% [18) but complete cleavage failure. However, it did not conform to fertilization failure which was defined as less than 5% fertilization rate [18]. To further exclude slightly decreased fertilization rates, mutation detection of the PLCZ1 gene which was believed to be responsible for fertilization failure [19] was performed in her husband, but no damaging mutations were found (data not shown). Herein, 2PN arrest in this patient also further suggested that oocyte activation and the formation of male and female pronuclei had completed. Cleavage failure might be related to mistakes of the mitotic spindle from oocyte.
Table 2.
The currently reported phenotypes and genotypes of TUBB8-related diseases
| Patients No. | Mutations | Mutant type (het/homo/com het) | Inheritance patterns (AD-pa, AR, de novo, NA) | Clinical characteristics | Reference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Duration of fertility (years) | Previous IVF/ICSI cycles | Oocyte and fertility feature | Cleavage feature | Diagnosis | |||||||||||||
| Total no. of oocytes retrieved | GV oocyte | MI oocyte | MII (PB1) oocyte | Oocyte with abnormal morphology | Immature oocyte (unknown stage) | Normal fertilized oocyte | Abnormal fertilized oocyte | No. of normal cleavage embryos | Embryos arrested at early stage | |||||||||
| 1 | c.5G > T (p.R2M) | het | AD-pa | 28 | 5 | 1 | 6 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | MI arrest | [13] |
| 2 | c.5G > T (p.R2M) | het | AD-pa | 27 | 3 | 2 | 21 | 0 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | MI arrest | [14] |
| 3 | c.5G > A (p.R2K) | het | AD-pa | 33 | 7 | 2 | 54 | 2 | 52 | 0 | 0 | 0 | 0 | 0 | NA | NA | MI arrest | [11] |
| 4 | c.10A > C (p.I4L) | het | AD-pa | 38 | 15 | 4 | 27 | 2 | 17 | 8 | 0 | 0 | 8 | NA | 0 | 0 | Major in MI arrest, minor in 2PN arrest | [13] |
| 5 | c.35G > A (p.C12Y) | homo | AR | 32 | 7 | 2 | 27 | 0 | 7 | 1 | 0 | 0 | 1 | NA | 0 | 0 | Major in MI arrest, minor in 2PN arrest | [13] |
| 6 | c.80_100del (p.E27_A33del) | homo | AR | 23 | 3 | 3 | 33 | 3 | 19 | 1 | 0 | 10 | 0 | 0 | NA | NA | MI arrest | [12] |
| 7 | c.209C > T (p.P70L) | homo | AR | 33 | 10 | 1 | 25 | 0 | 2 | 20 | 3 | 0 | 2 | NA | 1 | 1 | Major in MII arrest, minor in 2PN arrest and early embryonic development arrest | [13] |
| 8 | c.322G > A (p.E108K) | homo | NA | 39 | 9 | 2 | 31 | 4 | 5 | 22 | 0 | 0 | 14 | 0 | 0 | 0 | 2PN arrest | Our study |
| 9 | c.426_427insG (p.T143Dfs*12) | homo | AR | 42 | 18 | 7 | 19 | 0 | 14 | 1 | 1 | 3 | 0 | 0 | NA | NA | MI arrest | [12] |
| 10 | c.527C > T (p.S176 L) | het | de novo | 34 | 10 | 4 | 43 | 3 | 40 | 0 | 0 | 0 | 0 | 0 | NA | NA | MI arrest | [11] |
| 11 | c.527C > T (p.S176 L) | het | de novo | 29 | 3 | 3 | 32 | 1 | 25 | 0 | 2 | 4 | 0 | 0 | NA | NA | MI arrest | [12] |
| 12 | c.535G > A (p.V179 M) | het | AD-pa | 27 | 5 | 2 | 24 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | MI arrest | [14] |
| 13 | c.535G > A (p.V179 M) | het | AD-pa | 29 | 6 | 3 | 46 | 0 | 46 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | MI arrest | [14] |
| 14 | c.580G > A, c.1245G > A (p.E194K, p.M415I) | com het | de novo | 28 | 2 | 2 | 19 | 0 | 0 | 3 | 0 | 16 | 3 | NA | 3 | 3 | Early embryonic development arrest | [13] |
| 15 | c.613G > A (p.E205K) | het | NA | 29 | 3 | 3 | 38 | 0 | 0 | 7 | 0 | 32 | 3 | NA | 3 | 2 | Early embryonic development arrest | [13] |
| 16 | c.628A > G (p.I210V) | het | NA | 36 | 3 | 2 | 15 | 1 | 1 | 4 | 0 | 9 | 3 | 0 | NA | NA | Early embryonic development arrest | [12] |
| 17 | c.686 T > C (p.V229A) | het | AD-pa | 37 | 8 | 1 | 4 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | NA | NA | MI arrest | [11] |
| 18 | c.686 T > C (p.V229A) | het | AD-pa | 32 | 10 | 2 | 21 | 0 | 21 | 0 | 0 | 0 | 0 | 0 | NA | NA | MI arrest | [11] |
| 19 | c.713C > T (p.T238 M) | het | AD-pa | 29 | 5 | 6 | 30 | 4 | 17 | 6 | 0 | 3 | 0 | 2 | NA | NA | MI arrest, early embryonic development arrest | [12] |
| 20 | c.763G > A (p.V255 M) | het | de novo | 33 | 7 | 2 | 29 | 0 | 25 | 0 | 2 | 2 | 0 | 0 | NA | NA | MI arrest | [12] |
| 21 | c.784C > T (p.R262W) | het | de novo | 34 | 3 | 2 | 36 | 0 | 32 | 0 | 0 | 0 | 0 | 4 | NA | NA | MI arrest | [12] |
| 22 | c.785G > A (p.R262Q) | het | de novo | 37 | 10 | 2 | 12 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | NA | NA | MI arrest | [11] |
| 23 | c.853A > C (p.T285P) | het | NA | 32 | 10 | 4 | 11 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | NA | NA | MI arrest | [12] |
| 24 | c.900G > A (p.M300I) | het | AD-pa | 26 | 6 | 2 | 26 | 0 | 26 | 0 | 0 | 0 | 0 | 0 | NA | NA | MI arrest | [11] |
| 25 | c.990G > A (p.M300I) | het | AD-pa | 32 | 10 | 2 | 6 | 0 | 4 | 2 | 0 | 0 | 2 | NA | 2 | 2 | MI arrest, early embryonic development arrest | [13] |
| 26 | c.990G > A (p.M300I) | het | AD-pa | 30 | 10 | 2 | 20 | 0 | 15 | 5 | 0 | 0 | 5 | NA | 5 | 5 | MI arrest, early embryonic development arrest | [13] |
| 27 | c.1043A > G (p.N348S) | het | NA | 34 | 7 | 4 | 55 | 0 | 5 | 0 | 0 | 42 | 8 | 0 | NA | NA | Early embryonic development arrest | [12] |
| 28 | c.1057G > A (p.V353I) | het | NA | 44 | 23 | 3 | 14 | 7 | 11 | 4 | 1 | 4 | 4 | NA | 4 | 4 | Major in MII arrest, minor in early embryonic development arrest | [13] |
| 29 | c.1088 T > C (p.M363 T) | het | AD-pa | 25 | 4 | 3 | 18 | 1 | 17 | 0 | 0 | 0 | 0 | 0 | NA | NA | MI arrest | [11] |
| 30 | c.1249G > A (p.D417N) | het | AD-pa | 37 | 9 | 5 | 37 | 7 | 30 | 0 | 0 | 0 | 0 | 0 | NA | NA | MI arrest | [11] |
| 31 | exon1–4 deletion | homo | AR | 30 | 6 | 2 | 24 | 0 | 12 | 3 | 1 | 9 | 3 | NA | 1 | 1 | Major in MI arrest, minor in 2PN arrest and early embryonic development arrest | [13] |
het heterozygous mutation, homo homozygote mutation, com het compound heterozygote mutation, AD-pa autosomal dominant inheritance-paternal origin, AR autosomal recessive inheritance, IVF in vitro fertilization, ICSI intracytoplasmic sperm injection, GV germinal vesicle, MI metaphase I, MII metaphase II, PB1 the first polar body, NA data not available or without experimental data
TUBB8 was the first gene found to be associated with the arrest of human oocyte maturation. It encodes a β-tubulin isotype, uniquely expressing at different stages of human oocyte development and early embryos. β-tubulin combines with α-tubulin to form a heterodimer, which has an important role in spindle assembly [11]. TUBB8 is uniquely and abundantly expressed in human oocytes (germinal vesicle [GV], MI, MII) and during early embryonic development [11]. Mutant tubulin proteins may influence meiosis [11] and mitosis, resulting in failures of first or second polar body extrusion or zygote cleavage, manifesting as MI arrest, MII arrest, or 2PN arrest, respectively. Our study identified a novel missense mutation, c.322G > A (p.Glu108Lys) in a patient with primary infertility due to cleavage failure. This mutation is located at the interface of the α/β tubulin heterodimer and is predicted to directly disrupt α-β tubulin interactions (Fig. 2b) due to a change in charge (Fig. 2c). And according to the 2015 ACMG Standards and Guidelines [20], this novel mutation could be classified as likely pathogenic. There are three known inheritance patterns of TUBB8-related diseases, including AD-pa (autosomal dominant inheritance-paternal origin), AR (autosomal recessive inheritance), and de novo mutations (Table 2). Only five patients were identified to have the autosomal recessive inheritance pattern for the TUBB8 gene (Table 2). Based on these previous studies [12, 13], it seemed that homozygous and compound heterozygous missense mutations would not prevent the MI oocyte from extruding the first polar body completely. Strikingly, however, our patient with the homozygous TUBB8 mutation showed a satisfactory number of MII oocytes that could be fertilized, but the cleavage rate was unsatisfactory. Due to no genetic data available for the deceased father and the out-of-town mother, we speculated that the homozygous p.Glu108Lys mutation might affect female fertility due to a haploinsufficiency effect, similar to other homozygote reports [13]. As far as we know, microtubules were condensed around the mitotic spindle of the prometaphase; α-tubulin gradually gathered to form a network structure [13]. It indicated that microtubule defects caused by this mutation could result in irregular arrangements of the spindles and obstruct the first mitotic division after fertility in human early embryo [21, 22]. Meanwhile, due to lack of the observation of morphokinetics of embryo growth by time-lapse monitoring, it was not excluded that irregular arrangements of the spindles had caused incomplete separation of blastomeres and type II reverse cleavage during the first mitotic division [23]. As shown in Table 2, the different mutations in the TUBB8-related diseases resulted in the diversity of oocyte and embryonic development arrest, which indicated that different kinds of defective spindle microtubules might impair multiple pathways up/downstream required for oocyte maturation, fertilization, cleavage, and embryonic development.
Some previous studies indicated that calcium could participate in early embryonic mitotic division [24–27]. And cleavage failure was reported in four other cases, for which artificial oocyte activation (AOA, A23187 Ca2+ ionophore) was able to overcome the problem successfully [8]. This technique would be likely to work well on our patient, since modestly elevated Ca2+ promoted the breakdown of spindle microtubules and by the mechanism modulated chromosome move to the spindle poles [24]. Unfortunately, due to economic factors, our patient did not try another ART cycle with AOA. So it is not clear whether AOA could overcome the cleavage failure in our patient with TUBB8 gene mutation.
In conclusion, we report a novel mutation in the TUBB8 gene in a cleavage failure patient. This mutation demonstrated that the TUBB8 protein has an important function in zygote cleavage. This case demonstrates the need to be aware of genetic factors in the arrest of meiosis and mitosis in human oocytes, zygotes, and early embryos, especially in extreme cases of repeat failure in ART. This case also illustrates the importance of screening for mutations in the TUBB8 gene in patients undergoing IVF.
Acknowledgements
The authors thank all patients and control subjects for their participation in this study. This work was partially supported by the Fundamental Research Funds for the Central Universities (no. 16ykjc15), the Natural Science Foundation of Guangdong Province (no. 2014A030310158), and the Natural Science Foundation of Guangdong Province (no. 2015A030313086).
Compliance with ethical standards
Ethics approval and consent to participate
The study protocol and all subjects who participated in this study were approved by the Institutional Review Board of our institute in which informed consent was obtained from all patients prior to participation in accordance with institutional and national guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Anifandis G, Messini C, Dafopoulos K, Sotiriou S, Messinis I. Molecular and cellular mechanisms of sperm-oocyte interactions opinions relative to in vitro fertilization (IVF) Int J Mol Sci. 2014;15(7):12972–12997. doi: 10.3390/ijms150712972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016;22(1):23–47. doi: 10.1093/humupd/dmv040. [DOI] [PubMed] [Google Scholar]
- 3.Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol. 2008;52(5–6):585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- 4.Iwao Y. Egg activation in physiological polyspermy. Reproduction. 2012;144(1):11–22. doi: 10.1530/REP-12-0104. [DOI] [PubMed] [Google Scholar]
- 5.Kashir J, Nomikos M, Lai FA, Swann K. Sperm-induced Ca2+ release during egg activation in mammals. Biochem Biophys Res Commun. 2014;450(3):1204–1211. doi: 10.1016/j.bbrc.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 6.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 7.Ebner T, Moser M, Sommergruber M, Jesacher K, Tews G. Complete oocyte activation failure after ICSI can be overcome by a modified injection technique. Hum Reprod. 2004;19(8):1837–1841. doi: 10.1093/humrep/deh325. [DOI] [PubMed] [Google Scholar]
- 8.Darwish E, Magdi Y. A preliminary report of successful cleavage after calcium ionophore activation at ICSI in cases with previous arrest at the pronuclear stage. Reprod BioMed Online. 2015;31(6):799–804. doi: 10.1016/j.rbmo.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforaki D, Vanden Meerschaut F, de Roo C, Lu Y, Ferrer-Buitrago M, de Sutter P, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105(3):798–806.e2. doi: 10.1016/j.fertnstert.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod BioMed Online. 2014;28(5):560–571. doi: 10.1016/j.rbmo.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML, Jr, Cowan NJ, Wang L. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374(3):223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, Xu Y, Chen B, Qu R, Sun Z, Sun X, Jin L, He L, Kuang Y, Cowan NJ, Wang L. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53(10):662–671. doi: 10.1136/jmedgenet-2016-103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Kuang Y, Sang Q, Wang L. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod. 2017;32(2):457–464. doi: 10.1093/humrep/dew322. [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Tong X, Luo L, Zheng S, Jin R, Fu Y, Zhou G, Li D, Liu Y. Mutation analysis of the TUBB8 gene in nine infertile women with oocyte maturation arrest. Reprod BioMed Online. 2017;35(3):305–310. doi: 10.1016/j.rbmo.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang R, Alushin GM, Brown A, Nogales E. Mechanistic origin of microtubule dynamic instability and its modulation by EB proteins. Cell. 2015;162(4):849–859. doi: 10.1016/j.cell.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beall S, Brenner C, Segars J. Oocyte maturation failure: a syndrome of bad eggs. Fertil Steril. 2010;94(7):2507–2513. doi: 10.1016/j.fertnstert.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI) Cell Calcium. 2014;55(1):24–37. doi: 10.1016/j.ceca.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escoffier J, Lee HC, Yassine S, Zouari R, Martinez G, Karaouzène T, Coutton C, Kherraf ZE, Halouani L, Triki C, Nef S, Thierry-Mieg N, Savinov SN, Fissore R, Ray PF, Arnoult C. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum Mol Genet. 2016;25(5):878–891. doi: 10.1093/hmg/ddv617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asch R, Simerly C, Ord T, Ord VA, Schatten G. The stages at which human fertilization arrests: microtubule and chromosome configurations in inseminated oocytes which failed to complete fertilization and development in humans. Mol Hum Reprod. 1995;10(7):1897–1906. doi: 10.1093/oxfordjournals.humrep.a136204. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Chen C, Cui P, Liao Y, Yao L, Zhang Y, Rui R, Ju S. Plk1 inhibition leads to a failure of mitotic division during the first mitotic division in pig embryos. J Assist Reprod Genet. 2017;34(3):399–407. doi: 10.1007/s10815-016-0864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Chapple V, Roberts P, Matson P. Prevalence, consequence, and significance of reverse cleavage by human embryos viewed with the use of the Embryoscope time-lapse video system. Fertil Steril. 2014;102(5):1295–1300. doi: 10.1016/j.fertnstert.2014.07.1235. [DOI] [PubMed] [Google Scholar]
- 24.Hepler PK. The role of calcium in cell division. Cell Calcium. 1994;16(4):322–330. doi: 10.1016/0143-4160(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 25.Kono T, Jones KT, Bos-Mikich A, Whittingham DG, Carroll J. A cell cycle-associated change in Ca2+ releasing activity leads to the generation of Ca2+ transients in mouse embryos during the first mitotic division. J Cell Biol. 1996;132(5):915–923. doi: 10.1083/jcb.132.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson CA, Arkin AP, Ross J. An endogenous calcium oscillator may control early embryonic division. Proc Natl Acad Sci U S A. 1997;94(4):1194–1199. doi: 10.1073/pnas.94.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesarik J. Calcium signaling in human preimplantation development: a review. J Assist Reprod Genet. 1999;16(4):216–220. doi: 10.1023/A:1020321024973. [DOI] [PMC free article] [PubMed] [Google Scholar]