Abstract
Measurements of skeletal muscle cross-sectional area, index, and radiation attenuation utilizing clinical computed tomography (CT) scans are used in assessments of sarcopenia, the loss of skeletal muscle mass and function associated with aging. To classify individuals as sarcopenic, sex-specific cutoffs for ‘low’ values are used. Conventionally, cutoffs for skeletal muscle measurements at the level of the third lumbar (L3) vertebra are used, however L3 is not included in several clinical CT protocols. Non-contrast-enhanced CT scans from healthy kidney donor candidates (age 18–40) at Michigan Medicine were utilized. Skeletal muscle area (SMA), index (SMI), and mean attenuation (SMRA) were measured at each vertebral level between the tenth thoracic (T10) and the fifth lumbar (L5) vertebra. Sex-specific means, standard deviations (s.d.), and sarcopenia cutoffs (mean-2 s.d.) at each vertebral level were computed. Associations between vertebral levels were assessed using Pearson correlations and Tukey’s difference test. Classification agreement between different vertebral level cutoffs was assessed using overall accuracy, specificity, and sensitivity. SMA, SMI, and SMRA L3 cutoffs for sarcopenia were 92.2 cm2, 34.4 cm2/m2, and 34.3 HU in females, and 144.3 cm2, 45.4 cm2/m2, and 38.5 HU in males, consistent with previously reported cutoffs. Correlations between all level pairs were statistically significant and high, ranging from 0.65 to 0.95 (SMA), 0.64 to 0.95 (SMI), and 0.63 to 0.95 (SMRA). SMA peaks at L3, supporting its use as the primary site for CT sarcopenia measurements. However, when L3 is not available alternative levels (in order of preference) are L2, L4, L5, L1, T12, T11, and T10. Healthy reference values reported here enable sarcopenia assessment and sex-specific standardization of SMA, SMI, and SMRA in clinical populations, including those whose CT protocols do not include L3.
Introduction
Sarcopenia is an important aspect of malnutrition diagnosis1 and a risk factor for poor clinical outcomes2–10. Consensus recommendations for its diagnosis define sarcopenia as both low muscle function (performance or strength) and low muscle mass, using sex-specific cutoffs for ‘low’ values11–15. Cutoffs set at two standard deviations below the mean of a healthy, young adult population were recommended by the European Working Group on Sarcopenia in Older People (EWGSOP)11. In addition to gait speed for performance and handgrip testing for strength, gold standards for measurement include computed tomography (CT) for muscle mass. For research purposes such measurements are often collected retrospectively and opportunistically; extracting measurements from CT scans that were obtained during the normal course of clinical care adds no additional risk and is convenient. CT measurements of lumbar skeletal muscle area (SMA) have been shown to be strongly correlated with whole body muscle mass in healthy adults16–19, while mean skeletal muscle radiation attenuation (SMRA), a measure of muscle fat content, has been shown to be associated with physical function18,20–22. SMA or height-adjusted SMA (SMI) at the third lumbar vertebra (L3) is prevalent in sarcopenia assessments of low muscle mass related to poor clinical outcomes2–10. In the absence of functional testing data, SMRA could be used as a surrogate; SMA or SMI, combined with SMRA, would enable a complete sarcopenia assessment from CT alone, given the appropriate cutoff values from a healthy reference population.
Reference values from a young, healthy population provide a necessary basis for comparisons between different clinical populations, and any sarcopenia cutoffs derived therefrom. Such cutoffs for low L3 skeletal muscle measurements have been previously reported for US and European cohorts10,23 and for L3 psoas muscle in an Asian cohort9.
Because clinical CT protocols do not always include L3, we previously reported T12 values as an alternative for chest-only CT protocols. However, there is no practical reason why skeletal muscle evaluation should be limited to these two levels. Indeed, Shen et al.17 reported high correlations around L4-L5 suggesting that these would be suitable alternatives to L3. Our primary aim is to define means, standard deviations, and sarcopenia cutoffs in males and females at all vertebral levels from T10 to L5, enabling a broader range of CT protocols to be used in assessments of sarcopenia and facilitating comparisons of clinical populations to a healthy reference.
Results
Population Summary
The majority of subjects (494, 67%) eventually donated a kidney, the remainder (241, 33%) had no record of donation (Table 1). There were no significant sex differences in mean age (31.2/30.9 yr, p > 0.53), donor proportion (69.2/64.7%, p > 0.20), mean BMI (26.9/27.6 kg/m2, p > 0.069), or tube current (196.5/199.9 mA, p > 0.25). Males had significantly (p < 0.001) greater height (164.2/179.1 cm) and weight (72.7/88.7 kg).
Table 1.
Cohort demographics and CT parameters split by sex.
| Female | Male | p | |||
|---|---|---|---|---|---|
| n | mean ± s.d. | N | mean ± s.d. | ||
| Age (yr) | 415 | 31.2 ± 6.1 | 320 | 30.9 ± 6.1 | 0.531 |
| Height (cm) | 415 | 164.2 ± 6.9 | 320 | 179.1 ± 7.1 | <0.001 |
| Weight (kg) | 402 | 72.7 ± 15.9 | 308 | 88.7 ± 16.4 | <0.001 |
| BMI (kg/m2) | 402 | 26.9 ± 15.9 | 308 | 27.6 ± 16.4 | 0.069 |
| Underweight < 18.5 | 6 | 1.4% | 1 | 0.3% | |
| Normal (18.5–25) | 171 | 41.2% | 79 | 24.7% | |
| Overweight (25–30) | 102 | 24.6% | 153 | 47.8% | |
| Obese class I (30–35) | 87 | 21.0% | 49 | 15.3% | |
| Obese class II (35–40) | 26 | 6.3% | 24 | 7.5% | |
| Obese class III >= 40 | 10 | 2.4% | 2 | 0.6% | |
| NA | 13 | 3.1% | 12 | 3.8% | |
| Donor | 0.206 | ||||
| Yes | 287 | 69.2% | 207 | 64.7% | |
| No | 128 | 30.8% | 113 | 35.3% | |
| Tube Current (mA) | 415 | 196.5 ± 43.1 | 320 | 199.9 ± 37.9 | 0.253 |
Donor proportion p-value from Chi-squared test, all others from t-test.
Skeletal Muscle Measurements
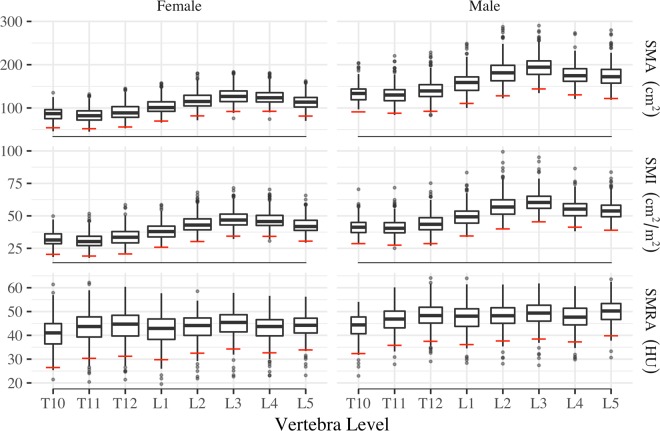
Sex-specific mean, standard deviation (s.d.), and cutoff values for SMA, SMI, and SMRA at all vertebra levels are reported in Table 2. Male SMA, SMI, and SMRA were significantly (p < 0.001) greater than corresponding female means at all levels. Peak and trough mean SMA and SMI values were observed at L3 and T11, respectively, in both females and males (Fig. 1). Mean SMA ranged from 83.5–128 cm2 in females, and 131.7–195.2 cm2 in males.
Table 2.
Sex-specific healthy reference values for T10-L5 SMA, SMI, and SMRA.
| VB | Female | Male | p | |||||
|---|---|---|---|---|---|---|---|---|
| n | mean ± s.d. | cutoff | N | mean ± s.d. | cutoff | |||
| SMA (cm2) | T10 | 156 | 86.6 ± 16.0 | 54.6 | 122 | 135.3 ± 22.0 | 91.4 | <0.001 |
| T11 | 336 | 83.5 ± 15.5 | 52.4 | 241 | 131.7 ± 21.8 | 88.1 | <0.001 | |
| T12 | 401 | 91.3 ± 17.6 | 56.1 | 299 | 141.2 ± 24.4 | 92.3 | <0.001 | |
| L1 | 409 | 103.4 ± 16.7 | 70.1 | 315 | 159.2 ± 24.4 | 110.4 | <0.001 | |
| L2 | 411 | 117.7 ± 17.9 | 81.9 | 315 | 183.9 ± 27.8 | 128.2 | <0.001 | |
| L3 | 410 | 128.0 ± 17.9 | 92.2 | 317 | 195.2 ± 25.4 | 144.3 | <0.001 | |
| L4 | 399 | 125.5 ± 16.6 | 92.4 | 305 | 177.1 ± 23.2 | 130.7 | <0.001 | |
| L5 | 295 | 114.9 ± 16.7 | 81.5 | 211 | 176.0 ± 27.0 | 122.0 | <0.001 | |
| SMI (cm2/m2) | T10 | 156 | 32.3 ± 5.9 | 20.4 | 122 | 42.2 ± 6.7 | 28.8 | <0.001 |
| T11 | 336 | 31.0 ± 5.9 | 19.2 | 241 | 41.1 ± 6.8 | 27.6 | <0.001 | |
| T12 | 401 | 34.0 ± 6.6 | 20.8 | 299 | 44.1 ± 7.7 | 28.8 | <0.001 | |
| L1 | 409 | 38.4 ± 6.2 | 25.9 | 315 | 49.7 ± 7.6 | 34.6 | <0.001 | |
| L2 | 411 | 43.7 ± 6.7 | 30.4 | 315 | 57.4 ± 8.7 | 40.1 | <0.001 | |
| L3 | 410 | 47.5 ± 6.6 | 34.4 | 317 | 60.9 ± 7.8 | 45.4 | <0.001 | |
| L4 | 399 | 46.7 ± 6.2 | 34.2 | 305 | 55.3 ± 7.0 | 41.3 | <0.001 | |
| L5 | 295 | 42.8 ± 6.1 | 30.6 | 211 | 54.7 ± 7.9 | 39.0 | <0.001 | |
| SMRA (HU) | T10 | 156 | 40.4 ± 6.9 | 26.5 | 122 | 43.8 ± 5.7 | 32.4 | <0.001 |
| T11 | 336 | 43.4 ± 6.5 | 30.4 | 241 | 46.5 ± 5.3 | 35.8 | <0.001 | |
| T12 | 401 | 44.0 ± 6.4 | 31.3 | 299 | 48.2 ± 5.3 | 37.5 | <0.001 | |
| L1 | 409 | 42.6 ± 6.4 | 29.8 | 315 | 47.5 ± 5.7 | 36.2 | <0.001 | |
| L2 | 411 | 43.4 ± 5.5 | 32.5 | 315 | 48.1 ± 5.2 | 37.7 | <0.001 | |
| L3 | 410 | 44.9 ± 5.3 | 34.3 | 317 | 49.0 ± 5.3 | 38.5 | <0.001 | |
| L4 | 399 | 43.1 ± 5.2 | 32.7 | 305 | 47.6 ± 5.2 | 37.3 | <0.001 | |
| L5 | 295 | 43.9 ± 5.0 | 33.9 | 211 | 49.9 ± 5.0 | 39.8 | <0.001 | |
Figure 1.
Box plots of healthy reference population SMA, SMI, and SMRA values by sex and vertebra. EWGSOP sarcopenia cutoffs (mean − 2 s.d.) shown as horizontal red lines.
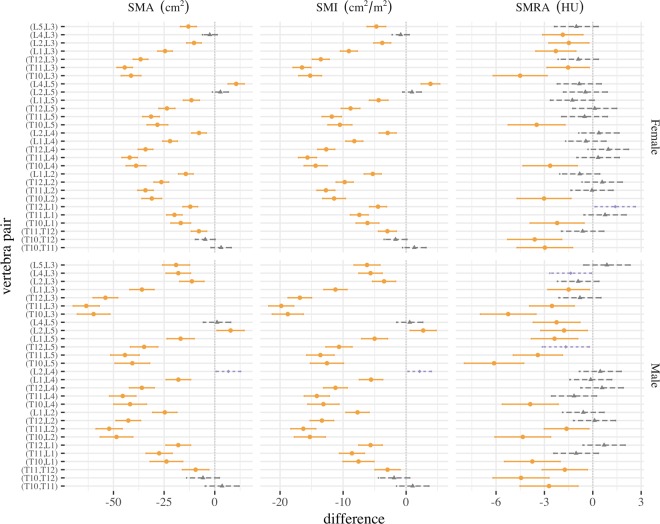
All SMA or SMI mean differences were significantly different (p < 0.01) except for six vertebral pairs: (T10, T11) and (T10, T12) in both sexes, (L2, L5) and (L3, L4) in females, and (L2, L4) and (L4, L5) in males (Supplemental Tables S1 and S2). SMRA mean differences were significantly different for seventeen pairs: (T11, L3), (L1, L3), and all seven T10 vertebral pairs in both sexes, (L2, L3) and (L4, L3) in females, and (T11, T12), (T11, L2), (T11, L5), (L1, L5), (L2, L5), and (L4, L5) in males (Supplemental Table S3). SMRA in all other pairs was not significantly different (p > 0.01) (Fig. 2). The smallest differences in means compared to L3 SMA and SMI were L2, L4, and L5, while for SMRA there was not a clear pattern.
Figure 2.
Tukey mean difference and 95% confidence interval for SMA, SMI, and SMRA at each vertebral level pair, by sex. Differences with p < 0.01 (orange circles), p < 0.05 (purple diamonds), and p > 0.05 (grey triangles) are shown.
Pearson correlations between all levels were statistically significant (p < 0.001) and high, ranging from 0.65 to 0.95 (SMA), 0.64 to 0.95 (SMI), and 0.63 to 0.95 (SMRA) (Supplemental Tables S4–S6). Correlations were highest between adjacent levels and lowest between the farthest apart levels (e.g., T10, L5). The levels that were most highly correlated with L3 SMA were L2 (females: 0.94, males: 0.92), L1 (0.91/0.87), L4 (0.88/0.88), and L5 (0.85/0.84). The same levels were most highly correlated with L3 SMI. L3 SMRA was most highly correlated with L2 (0.94/0.95), L4 (0.92/0.93), L5 (0.87/0.93), and L1 (0.88/0.91).
Overall accuracy of all vertebral level cutoffs (compared against L3) was high, ranging from 95% to 100%. Specificity was also high, ranging from 97% to 100%. However, sensitivity varied widely, ranging from 0% to 100%, partly due to the low number of sarcopenic individuals in the dataset (Table 3).
Table 3.
Accuracy of binary cutoffs by vertebra level and sex using the L3 classifier as reference. (L3 Low = the number of reference cases classified as “low”, e.g., L3 value below the L3 cutoff. Acc. = Overall accuracy. Sens. = sensitivity. Spec. = specificity).
| VB | Female | Male | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | L3 Low | Acc. | Sens. | Spec. | N | L3 Low | Acc. | Sens. | Spec. | ||
| SMA | T10 | 156 | 2 | 98.7 | 50.0 | 99.4 | 122 | 0 | 100.0 | NA | 100.0 |
| T11 | 335 | 4 | 99.4 | 50.0 | 100.0 | 241 | 1 | 99.2 | 100.0 | 99.2 | |
| T12 | 400 | 6 | 98.8 | 33.3 | 99.7 | 299 | 3 | 99.3 | 66.7 | 99.7 | |
| L1 | 408 | 6 | 99.3 | 66.7 | 99.8 | 315 | 4 | 99.4 | 75.0 | 99.7 | |
| L2 | 410 | 6 | 99.8 | 83.3 | 100.0 | 315 | 4 | 99.7 | 75.0 | 100.0 | |
| L4 | 398 | 5 | 98.0 | 20.0 | 99.0 | 305 | 4 | 98.7 | 25.0 | 99.7 | |
| L5 | 294 | 5 | 98.6 | 20.0 | 100.0 | 211 | 3 | 100.0 | 100.0 | 100.0 | |
| SMI | T10 | 156 | 2 | 99.4 | 100.0 | 99.4 | 122 | 0 | 100.0 | NA | 100.0 |
| T11 | 335 | 3 | 99.1 | 33.3 | 99.7 | 241 | 1 | 98.8 | 0.0 | 99.2 | |
| T12 | 400 | 3 | 99.2 | 0.0 | 100.0 | 299 | 1 | 99.0 | 0.0 | 99.3 | |
| L1 | 408 | 3 | 99.3 | 33.3 | 99.8 | 315 | 1 | 98.7 | 0.0 | 99.0 | |
| L2 | 410 | 3 | 99.5 | 33.3 | 100.0 | 315 | 1 | 99.7 | 0.0 | 100.0 | |
| L4 | 398 | 3 | 99.5 | 66.7 | 99.7 | 305 | 1 | 99.0 | 0.0 | 99.3 | |
| L5 | 294 | 3 | 99.3 | 66.7 | 99.7 | 211 | 0 | 99.5 | NA | 99.5 | |
| SMRA | T10 | 156 | 9 | 95.5 | 33.3 | 99.3 | 122 | 6 | 97.5 | 66.7 | 99.1 |
| T11 | 335 | 12 | 98.5 | 75.0 | 99.4 | 241 | 8 | 95.0 | 37.5 | 97.0 | |
| T12 | 400 | 12 | 98.5 | 83.3 | 99.0 | 299 | 8 | 98.7 | 75.0 | 99.3 | |
| L1 | 408 | 12 | 99.0 | 75.0 | 99.7 | 315 | 8 | 97.8 | 62.5 | 98.7 | |
| L2 | 410 | 12 | 99.0 | 75.0 | 99.7 | 315 | 8 | 99.4 | 87.5 | 99.7 | |
| L4 | 398 | 12 | 98.2 | 75.0 | 99.0 | 305 | 9 | 98.0 | 55.6 | 99.3 | |
| L5 | 294 | 9 | 98.0 | 66.7 | 98.9 | 211 | 6 | 98.6 | 66.7 | 99.5 | |
Discussion
Torso CT protocols appropriate for measuring skeletal muscle generally fall into three categories: chest (T1-to-L1), abdomen (T10-to-L4), and pelvis (L4-to-L5). Measurements of skeletal muscle for the identification of sarcopenia are typically taken at the level of the third lumbar vertebra. Our results demonstrate that L3 measurements are significantly different from other vertebral levels. Given these limitations, L3 sarcopenia cutoffs only apply to patients who receive abdominal CT imaging, excluding those who receive imaging of the chest only (e.g., lung cancer screening) and of the pelvis without abdomen (e.g., pelvis or hip fracture). The values reported here expand sarcopenia evaluations to these chest and pelvis protocols to enable wider clinical applications. We had previously reported L3 means/cutoffs in females as 126.8/91.2 (SMA) and 47.0/34.0 (SMI), and in males as 190.9/141.7 (SMA) and 59.7/44.6 (SMI); and T12 means/cutoffs in females as 90.8/55.9 (SMA) and 33.7/20.6 (SMI), and in males as 138.6/91.5 (SMA) and 43.3/28.7 (SMI)23. The updated mean values presented here were neither statistically nor clinically significantly different from the previous values, and the cutoffs were within 2% of previous. Werf et al. (p.292)10 reported 5th percentile cutoff values for L3 SMA in a healthy European Caucasian population (age 20–40) as ‘44.7 cm2/m2 in men and 33.0 cm2/m2 in women’, within 2% (males) and 4% (females) of our reported values. Cutoffs reported for age 20–82 are not comparable due to the inclusion of those over age 40.
Minor variations in reference cutoff values, and the low sensitivity of different vertebral level cutoffs found here, demonstrate a potential difficulty of treating sarcopenia as a binary classification rather than a continuum. Single number cutoffs for defining sarcopenia, while convenient for determining prevalence of sarcopenia in a particular cohort, may not adequately capture individual risk, especially for patients whose measurements are near cutoff values. Muscle mass and function decline with age, which means older, more sarcopenic cohorts may have little practical use for single number cutoffs in statistical models24. However, the sex-specific young adult means and standard deviations we have presented can be easily used to standardize any individual measurement (e.g., SMAtscore = (SMA − mean)/s.d.). Standardization of predictor variables is a standard practice in regression modeling25, and results in muscle measures that are akin to the bone mineral density ‘T-score’26. Standardizing SMA, SMI, or SMRA in this manner has the added benefit of normalizing male and female values to the same scale, controlling for sex differences.
This study has important limitations. Our cohort may not be nationally or globally representative and is not specific to a particular race or ethnicity. Our population-based cutoffs have not been tested against clinical outcomes. We used non-contrast-enhanced CT scans; previous research has shown that IV contrast has a clinically insignificant effect on SMA23,27,28, but a larger, more significant effect on SMRA27,28. Care should be exercised when applying these reference values in IV contrast-enhanced CT images and those using different kVp, convolution kernel, and/or slice thicknesses.
Clinical CT scans obtained in the normal course of patient care can potentially be used for sarcopenia evaluations. However, scans may not include vertebral levels for which sarcopenia cutoffs have been defined (e.g., L3). We updated previously published healthy population reference values for SMA and SMI at T12 and L3, included SMRA, a measure of muscle function, and reported on a wider range of vertebral levels to enable sarcopenia evaluations on a wider range of CT imaging protocols. We calculated female and male mean and standard deviation for each level from T10 to L5, and we followed EWGSOP consensus recommendations to define sex-specific sarcopenia cutoffs as two standard deviations below the mean of a healthy, young adult cohort. Further research is needed to determine the relationship between cutoffs at various vertebral levels and clinical outcomes. While L3 remains the ideal location for skeletal muscle measurement, results indicate that (in order) L2, L4, L5, L1, T12, T11, and T10 are the preferred alternatives when L3 is unavailable.
Methods
Study Cohort
We retrospectively studied persons who underwent CT scans at the University of Michigan as part of evaluation for kidney donation between 1999 and 2011. We have previously studied a subset of these kidney donor candidates as a healthy reference population23.
Patient age, sex, height, and weight were obtained from their medical record proximal to the date of evaluation for kidney donation, and the month and year of the evaluation appointment was recorded29. Candidates were included if they had a CT scan performed as part of evaluation for kidney donation, were deemed healthy enough to donate, had age, sex, and height recorded in their electronic medical record, had non-contrast-enhanced series available, and had a fascia boundary that was fully visible in the display field of view.
Though inconsequential to the current analysis, weight was not retrospectively available for n = 25 (3.4%) subjects. Body mass index (BMI) was computed and categorized into groups according the World Health Organization International Classification standard30. Race, unavailable for 52% of the cohort, was not specifically accounted for in the analysis.
CT imaging was extracted for 1,482 total donor candidates between the ages of 18 and 70. The analysis cohort included n = 735 ‘young adult’ candidates between the age of 18 and 4031–35 scanned using the GE ‘Standard’ reconstruction algorithm at 120 kVp and 5 mm slice thickness in a Discovery or LightSpeed scanner. Tube current was automatically modulated in proportion to body mass.
CT Image Processing
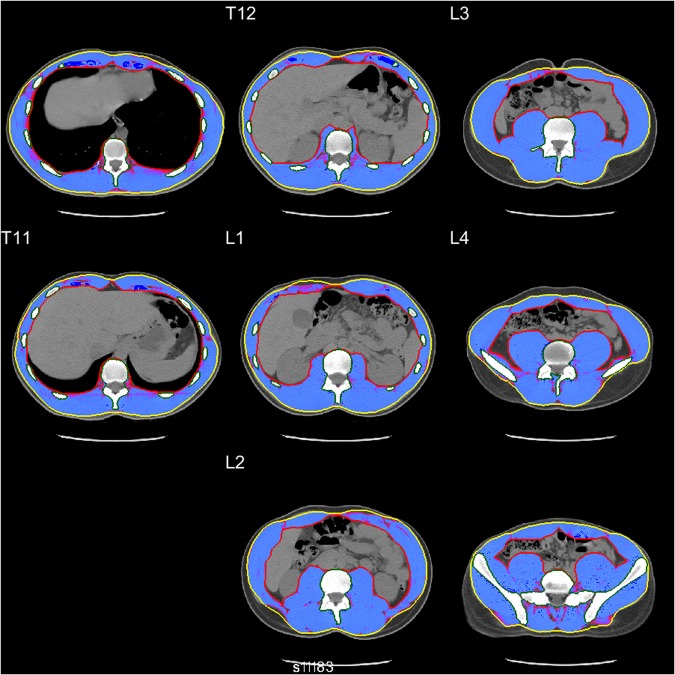
After being transferred into a spatial database, CT images were processed using Analytic Morphomics, a semi-automated image analysis method that has been previously described23,36. A combination of automated and user-guided algorithms written in Matlab (The Mathworks Inc, Natick, MA) identified the vertebral bodies to serve as an anatomical coordinate reference system. Next, the outer abdominal fascia and inner muscle wall were identified at all available vertebral levels to create enclosed regions of interest, which were confirmed by multiple trained researchers (Fig. 3).
Figure 3.
Example of healthy 20 y/o male T10-L5 axial CT slices showing SMA (blue-shaded area) between outer abdominal fascia (yellow line) and inner muscle wall (red line).
Sample size at each vertebral level varied due to differences in anatomy included in each scan. Measurements at T8 and T9 were excluded due to statistically significant differences in mean weight compared to those at T10 through L5. For T10-L5, there there were no significant differences in mean age, weight, or height within the male and female cohorts.
SMA was measured at the axial slice nearest the inferior aspect of each vertebral body as the area of pixels within −29 to +150 Hounsfield Units (HU) as previously validated21,23,27. Skeletal muscle index (SMI)–a heuristic that normalizes muscle area for height–was computed as SMA divided by height-squared37. Skeletal muscle radiation attenuation (SMRA) was computed as the mean Hounsfield Unit (HU) value of all pixels included in SMA27,38,39.
Statistical Methods
Male and female demographics, CT parameters, and skeletal muscle measurements are shown separately as mean +/− s.d. for continuous variables and proportion for categorical variables. Means were compared using two-tailed t-tests assuming unequal variance and donor proportion was compared using the Chi-squared test.
The sex-specific mean and standard deviation of each skeletal muscle measure were calculated independently for vertebral levels from T10 to L5. EWGSOP sarcopenia cutoffs were computed as the mean minus two standard deviations. Simultaneous differences in means for each pair of vertebral levels were tested and 95% confidence intervals generated using Tukey’s ‘Honest Significant Difference’ test40. Pearson correlations were used to test the linear association between measurements at each pair of vertebral levels. Agreement between sarcopenia classification for each variable and vertebra level was measured using overall accuracy, sensitivity, and specificity treating the L3 cutoffs as the reference standard.
An alpha level of 0.01 was used to determine statistical significance. All statistical tests were performed in R version 3.4.241, using the package ‘ggplot2’42 for data visualization.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
This study was approved by the Institutional Review Board of the University of Michigan. All methods were performed in accordance with the relevant guidelines and regulations of the United States. Because existing CT scans were used retrospectively, the requirement for informed consent was waived.
Electronic supplementary material
Author Contributions
B.D., S.H., G.S. and S.W. designed the study. N.W. and S.H. developed the Analytic Morphomics processing code. B.D. and B.R. performed CT processing. B.D. collected and interpreted the data, performed the data analyses, and wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
Brian A. Derstine, Brian E. Ross, Nicholas C. Wang, and Grace L. Su declare that they have no conflict of interest. Sven A. Holcombe and Stewart C. Wang are listed as inventors on a US Patent for Analytic Morphomics (#US 20140064583 A1).
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29825-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the academy of nutrition and dietetics/american society for parenteral and enteral nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) Journal of the Academy of Nutrition and Dietetics. 2012;112:730–738. doi: 10.1016/j.jand.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 2. Prado, C. M. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol9, 629–35 https://www.ncbi.nlm.nih.gov/pubmed/18539529, 10.1016/s1470-2045(08)70153-0 (2008). [DOI] [PubMed]
- 3. Prado, C. M. et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res15, 2920–6 http://clincancerres.aacrjournals.org/content/clincanres/15/8/2920.full.pdf, 10.1158/1078-0432.ccr-08-2242 (2009). [DOI] [PubMed]
- 4.Martin L, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 5.Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38:940–53. doi: 10.1177/0148607114550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reisinger KW, et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Annals of surgical oncology. 2015;22:4445–4452. doi: 10.1245/s10434-015-4558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vugt J, et al. Systematic review and meta-analysis of the impact of computed tomography–assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. American Journal of Transplantation. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 8.Shachar SS, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clinical Cancer Research. 2017;23:658–665. doi: 10.1158/1078-0432.CCR-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamaguchi Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in asian adults. Nutrition. 2016;32:1200–1205. doi: 10.1016/j.nut.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Werf, A. et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy caucasian population. European journal of clinical nutrition 1 (2017). [DOI] [PMC free article] [PubMed]
- 11. Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing39, 412–23 http://ageing.oxfordjournals.org/content/39/4/412.full.pdf, 10.1093/ageing/afq034 (2010). [DOI] [PMC free article] [PubMed]
- 12. Studenski, S. A. et al. The fnih sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci69, 547–58 https://www.ncbi.nlm.nih.gov/pubmed/24737557, 10.1093/gerona/glu010 (2014). [DOI] [PMC free article] [PubMed]
- 13. Chen, L. K. et al. Sarcopenia in asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc15, 95–101 https://www.ncbi.nlm.nih.gov/pubmed/24461239, 10.1016/j.jamda.2013.11.025 (2014). [DOI] [PubMed]
- 14. Fielding, R. A. et al. Sarcopenia: an undiagnosed condition in older adults. current consensus definition: prevalence, etiology, and consequences. international working group on sarcopenia. J Am Med Dir Assoc12, 249–56 https://www.ncbi.nlm.nih.gov/pubmed/21527165, 10.1016/j.jamda.2011.01.003 (2011). [DOI] [PMC free article] [PubMed]
- 15.Rosenberg, I. H. Sarcopenia: origins and clinical relevance. J Nutr127, 990S–991S https://www.ncbi.nlm.nih.gov/pubmed/9164280 (1997). [DOI] [PubMed]
- 16.Mitsiopoulos, N. et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985)85, 115–22 http://jap.physiology.org/content/jap/85/1/115.full.pdf (1998). [DOI] [PubMed]
- 17.Shen, W. et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985)97, 2333–8 https://www.ncbi.nlm.nih.gov/pubmed/15310748, 10.1152/japplphysiol.00744.2004 (2004). [DOI] [PubMed]
- 18.Heymsfield, S. B., Adamek, M., Gonzalez, M. C., Jia, G. & Thomas, D. M. Assessing skeletal muscle mass: historical overview and state of the art. J Cachexia Sarcopenia Muscle 5, 9–18 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3953319/pdf/13539_2014_Article_130.pdf, 10.1007/s13539-014-0130-5 (2014). [DOI] [PMC free article] [PubMed]
- 19.Mourtzakis M, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. Journal of applied physiology. 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 21.Aubrey J, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta physiologica. 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams GR, et al. Skeletal muscle measures and physical function in older adults with cancer: sarcopenia or myopenia? Oncotarget. 2017;8:33658. doi: 10.18632/oncotarget.16866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derstine, B. A. et al. Quantifying sarcopenia reference values using lumbar and thoracic muscle areas in a healthy population. The journal of nutrition, health & aging 1–6 (2017). [DOI] [PubMed]
- 24.Broughman JR, et al. Prevalence of sarcopenia in older patients with colorectal cancer. Journal of geriatric oncology. 2015;6:442–445. doi: 10.1016/j.jgo.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bring J. How to standardize regression coefficients. The American Statistician. 1994;48:209–213. [Google Scholar]
- 26.Schuit S, et al. Fracture incidence and association with bone mineral density in elderly men and women: the rotterdam study. Bone. 2004;34:195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 27. Werf, A. et al. Skeletal muscle analyses: agreement between non-contrast and contrast ct scan measurements of skeletal muscle area and mean muscle attenuation. Clinical Physiology and Functional Imaging (2017). [DOI] [PubMed]
- 28. Fuchs, G. et al. Quantifying the effect of slice thickness, intravenous contrast and tube current on muscle segmentation: Implications for body composition analysis. European radiology 1–9 (2018). [DOI] [PubMed]
- 29. Hanauer, D. A., Mei, Q., Law, J., Khanna, R. & Zheng, K. Supporting information retrieval from electronic health records: A report of university of michigan’s nine-year experience in developing and using the electronic medical record search engine (emerse). J Biomed Inform55, 290–300 https://www.ncbi.nlm.nih.gov/pubmed/25979153, 10.1016/j.jbi.2015.05.003 (2015). [DOI] [PMC free article] [PubMed]
- 30.Organization, W. H. Obesity: preventing and managing the global epidemic. 894 (World Health Organization, 2000). [PubMed]
- 31.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 32.Hingson RW, Heeren T, Edwards EM, Saitz R. Young adults at risk for excess alcohol consumption are often not asked or counseled about drinking alcohol. Journal of General Internal Medicine. 2012;27:179–184. doi: 10.1007/s11606-011-1851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamler J, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 34.Ogden CL, et al. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 35.Geiger AM, Castellino SM. Delineating the age ranges used to define adolescents and young adults. Journal of Clinical Oncology. 2011;29:e492–e493. doi: 10.1200/JCO.2011.35.5602. [DOI] [PubMed] [Google Scholar]
- 36. Krishnamurthy, V. et al. Use of analytic morphomics of liver, spleen, and body composition to identify patients at risk for cirrhosis. Clin Gastroenterol Hepatol13, 360–368 e5 https://www.ncbi.nlm.nih.gov/pubmed/25083565, 10.1016/j.cgh.2014.07.042 (2015). [DOI] [PMC free article] [PubMed]
- 37. Heymsfield, S. B., Heo, M., Thomas, D. & Pietrobelli, A. Scaling of body composition to height: relevance to height-normalized indexes. Am J Clin Nutr93, 736–40 https://www.ncbi.nlm.nih.gov/pubmed/21248190, 10.3945/ajcn.110.007161 (2011). [DOI] [PubMed]
- 38.Goodpaster BH, et al. Attenuation of skeletal muscle and strength in the elderly: The health abc study. Journal of applied physiology. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 39.Boutin RD, Kaptuch JM, Bateni CP, Chalfant JS, Yao L. Influence of iv contrast administration on ct measures of muscle and bone attenuation: Implications for sarcopenia and osteoporosis evaluation. American Journal of Roentgenology. 2016;207:1046–1054. doi: 10.2214/AJR.16.16387. [DOI] [PubMed] [Google Scholar]
- 40.Rupert, G. Jr. et al. Simultaneous statistical inference (Springer Science & Business Media, 2012).
- 41.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/ (2016).
- 42.Wickham, H. ggplot2: Elegant Graphics for Data Analysishttp://ggplot2.org (Springer-Verlag New York, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.