Abstract
High-fat (HF) diets are thought to disrupt the profile of the gut microbiota in a manner that may contribute to the neuroinflammation and neurobehavioral changes observed in obesity. Accordingly, we hypothesize that by preventing HF-diet induced dysbiosis it is possible to prevent neuroinflammation and the consequent neurological disorders. Anthocyanins are flavonoids found in berries that exhibit anti-neuroinflammatory properties in the context of obesity. Here, we demonstrate that the blackberry anthocyanin-rich extract (BE) can modulate gut microbiota composition and counteract some of the features of HF-diet induced dysbiosis. In addition, we show that the modifications in gut microbial environment are partially linked with the anti-neuroinflammatory properties of BE. Through fecal metabolome analysis, we unravel the mechanism by which BE participates in the bilateral communication between the gut and the brain. BE alters host tryptophan metabolism, increasing the production of the neuroprotective metabolite kynurenic acid. These findings strongly suggest that dietary manipulation of the gut microbiota with anthocyanins can attenuate the neurologic complications of obesity, thus expanding the classification of psychobiotics to anthocyanins.
Introduction
The incidence of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases has been increasing as global population gets older. The World Health Organization (WHO) predicts that by 2040, neurodegenerative diseases will overtake cancer to become the second leading cause of death after cardiovascular disease1. Accumulating evidence suggests that neurodegeneration occurs, in part, because the neuronal environment is disturbed in a cascade of processes collectively called neuroinflammation2. Neuroinflammation is also central in other psychiatry disorders such as anxiety and depression which are often associated with obesity3,4. Neuroinflammatory processes are profoundly modulated by peripheral inflammatory stimuli, especially those coming from the gut microbiota5–7. In this regard, both central nervous system (CNS) and gut microbiota offer legitimate targets for novel therapeutic strategies aiming to treat the rising burden of neurodegenerative and neuropsychiatry disorders. Indeed, live microorganisms that produce a health benefit in patients suffering from psychiatric illness (psychobiotics) have been proposed as psychotropic drugs8. Recently, the definition of psychobiotics have been extended to also encompass prebiotics9.
Flavonoids are a class of polyphenolic compounds that have been proposed as novel agents capable of attenuating neurodegenerative pathology10. Data suggest that anthocyanins (often consumed in higher amounts than other flavonoids11) interact with neurons and microglia, facilitating synaptic connectivity under both normal and pathologic conditions12. In addition, as recently demonstrated, anthocyanins are able to attenuate the negative impact of high-fat (HF) diets on neuroinflammation13, although their precise mechanism of action is unclear. One possibility is that they may exert their effects through modulation of the gut microbiota14.
The aim of the present study was to test the hypothesis that anthocyanins modulate gut microbiota composition and prevent HF diet-induced dysbiosis. After confirming these assumptions and given the bilateral connectivity that exists between gut and brain (the gut-brain axis)15,16, further investigations were carried out to study whether the anti-neuroinflammatory properties of anthocyanins were related to the gut microbiota changes that these compounds bring about.
Results
Blackberry anthocyanin-rich extract (BE) modulate gut-microbiota composition and counteract some of the features of HF diet-induced dysbiosis
We compared the gut microbial community of Wistar rats fed either with standard (C) or high-fat (HF) diet and supplemented with BE by sequencing the V3-V4 regions of the 16S rRNA gene.
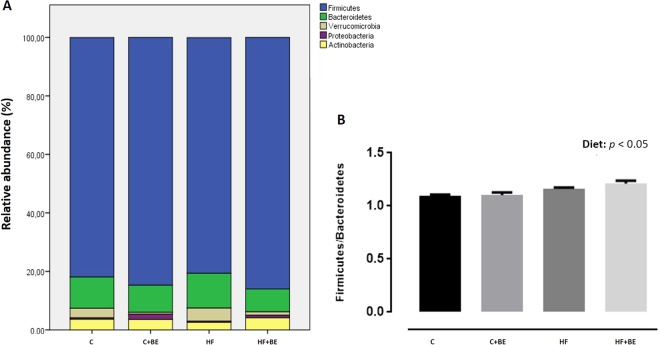
The gut microbiota of the animals was dominantly constituted by five phyla: Firmicutes, Bacteroidetes, Actinobacteria, Verrucomicrobia and Proteobacteria (Fig. 1A). The Firmicutes to Bacteroidetes ratio was significantly increased in HF diet groups (p > 0.05) (Fig. 1B).
Figure 1.
(A) Major bacterial phyla in the gut microbiota of rats after 17 weeks of high-fat (HF) feeding and anthocyanin-rich blackberry extract (BE) supplementation. Bars represent the average of each phylum relative abundance in the 4 different diet groups. Each phylum is represented by a different color (n = 5–6 rats per group). (B) Firmicutes to Bacteroidetes ratio among groups. This ratio was calculated by dividing the number of copies of Firmicutes by the number of copies of Bacteroidetes quantified by real-time PCR. Values are expressed as mean ± SEM (n = 5–6 rats per group).
Despite no differences observed at phylum level, at genus level, major differences were noted (Fig. 2A).
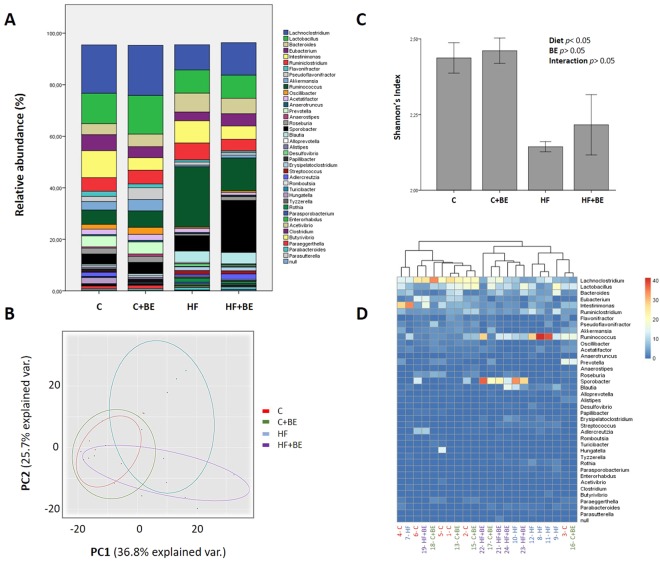
Figure 2.
Gut microbiota composition at the genus level among groups (n = 5–6 per group). (A) Relative abundance of gut bacterial genera. Bars represent the average of each genus relative abundance in the 4 different diet groups. Each genus is represented by a different color. (B) Shannon’s diversity index among groups. Values are expressed as mean ± SEM. (C) Gut bacterial genera were clustered using principal component analysis (PCoA). Results are plotted according to the first two principle components, which explain 36.8% (PC1) and 25.7% (PC2) of the variation in gut microbial composition (at genus level) between samples. Each point represents one sample and each diet group is denoted by a different color. Circles combine samples from the same diet group by their respective 95% confidence interval ellipse. (D) Heatmap and hierarchical clustering of the relative abundance of gut bacterial genera. Rows correspond to operational taxonomic units (OTUs) and columns represent the animals of the 4 different diet groups.
Results illustrated in Fig. 2A indicate the genus level distribution for the 4 diet groups. After 17 weeks of HF feeding, the profile of the gut microbiota of HF fed animals was significantly altered in comparison to the gut microbiota of control fed animals. Notably, bacteria belonging to the Firmicutes phylum such as Rumminococcus, Blautia, Erysipelatoclostridium, Streptococcus and Parasporobacterium were more abundant in HF fed animals (p < 0.05), whereas Lachnoclostridium, Pseudoflavonifractor, Oscillibacter and Anaerostipes (also Firmicutes) were more abundant in the animals of C group (p < 0.05) (Fig. 2A). In addition, HF diet decreased the abundance of Akkermansia, Prevotella and Paraeggerthella (p < 0.05), whereas it increased the abundance of Desulfovibrio, Rothia and Enterohabdus (p < 0.05) (Fig. 2A).
BE feeding induced several modifications in the gut microbiota composition of animals. Specifically, BE increased Pseudoflavonifractor when the animals were fed with C diet and increased Oscillobacter independently of the diet fat content (p < 0.05) (Fig. 2A). Moreover, BE struggled to recover the gut microbiota’s diversity when the animals were challenged with HF diet, as indicated by Shannon’s richness index (Fig. 2C).
To reduce the number of variables, a principal coordinate analysis (PCoA) was performed. The gut microbiota communities from the four different groups (C, BE, HF and HFBE) were grouped into four different clusters (Fig. 2C). HF fed rats had a distinct gut microbiota that clustered separately from C and BE rats. Nevertheless, the gut microbiota communities of the HF + BE animals were also differentiated from those of HF rats (Fig. 2C). The reduction of Rumminococcus and the prevalence of Sporobacter were the main features that justified the segregation of HF + BE from HF group (Fig. 2D).
In summary, our data indicate that HF feeding strongly affected the composition of the gut microbiota and that BE can prevent some of the features of HF-diet induced dysbiosis (e.g. the increase of Rumminococcus and the decrease of Oscillobacter).
Changes in gut bacterial genera induced by BE are correlated with anti-neuroinflammatory properties
After characterizing the gut microbiota of the animals supplemented with BE, we sought to investigate if the protection against neuroinflammation, previously evaluated by our group, was associated with the changes observed in the gut bacterial genera of these animals.
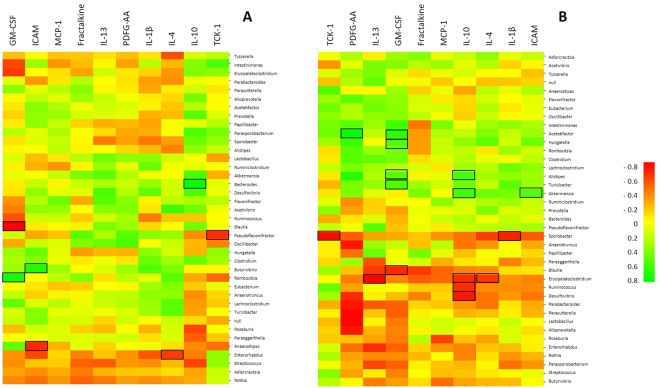
In a previous work, we showed that BE consumption decreased TCK-1 expression in rat’s hippocampus whereas fractalkine expression increased13. Here, we found that TCK-1 was negatively correlated with Pseudoflavonifractor and Sporobacter when the animals were fed with C and HF diet, respectively (p < 0.05) (Fig. 3A,B). Interestingly, these bacterial genera were those increased by BE in C and HF diet groups, respectively (Fig. 2A). However, fractalkine, a chemokine particularly important in the crosstalk between neurons and microglia, was not correlated with any bacterial genus despite being upregulated in BE supplemented rat’s hippocampus (Fig. 3A,B).
Figure 3.
Heatmap of Spearman’s correlation test between gut bacterial genera and neuroinflammatory markers measured in hippocampus, (A) in the animals fed with standard diet (C and BE groups) and (B) in high-fat fed animals (HF and HFBE groups). Green color indicates a positive correlation while red color indicates a negative correlation. Squared cells represent correlations with statistical significance (p < 0.05).
BE modulate CNS inflammation via the microbial metabolites of tryptophan
As a component of the gram-negative bacteria and a trigger of the inflammatory response, lipopolysaccharide (LPS) was measured in the fecal samples of all animals. Although fecal LPS levels were not statistically different between groups (p > 0.05), a noticeable increase in LPS levels was observed in the fecal samples of the animals of HF diet group (Fig. 4). In contrast, in HF + BE animals, fecal LPS was similar to that measured in control fed animals, probably as a reflection of the gut microbiota modifications induced by BE (Fig. 4).
Figure 4.
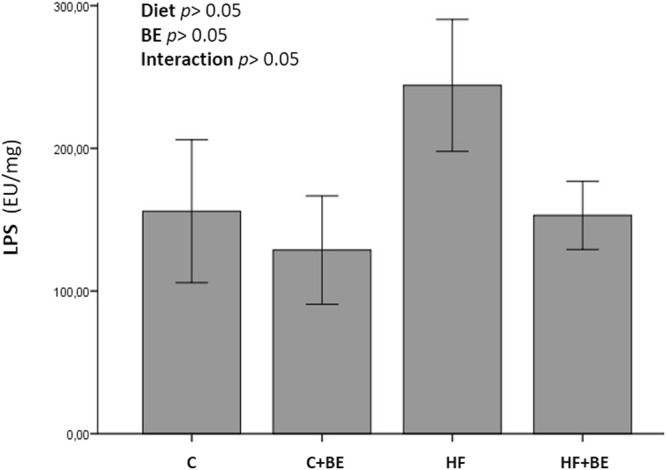
Fecal LPS concentrations. Values are expressed as mean ± SEM (n = 5–6 rats per group).
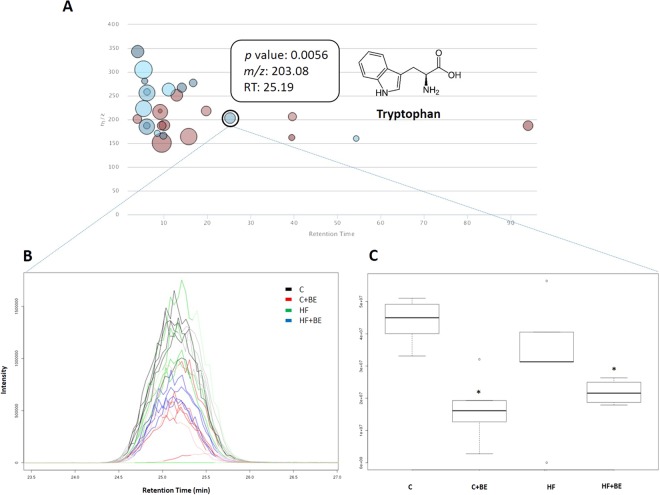
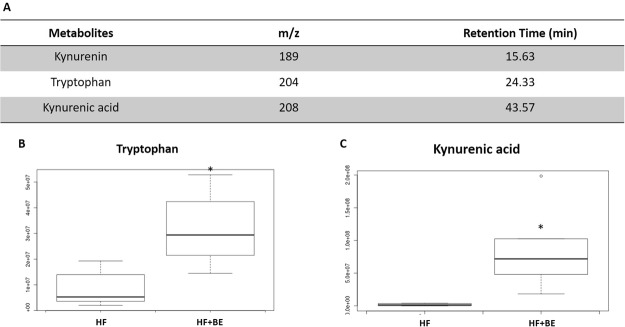
The liquid chromatography-mass spectrometry (LC-MS) analysis revealed the existence of additional compounds in the animals’ fecal samples. Twenty-six compounds (with a maximum intensity above 1,000,000) were observed to be significantly altered between samples (p < 0.01) (Fig. 5). Tryptophan (one of the identified metabolites) was decreased in the groups supplemented with BE (p < 0.05, Fig. 5). Interestingly, fecal concentrations of tryptophan were positively correlated with TCK-1 expression in the hippocampus (r = 0.733, p < 0.05), indicating that changes in the gut bacterial genera prompted by BE could have altered host tryptophan metabolism. To verify this assumption, tryptophan and tryptophan metabolites (kynurenine and kynurenic acid) were searched in the urine of all animals. Tryptophan and kynurenic acid (Fig. 6) were increased in the urine of the animals fed with HF diet and supplemented with BE (p < 0.05), while kynurenine levels remained unchanged. On the contrary, no changes were observed in the animals of C + BE group.
Figure 5.
(A) Metabolite features whose level varies significantly (p < 0.01) across groups are projected on the cloud plot depending on their retention time (x-axis) and m/z (y-axis). Statistical significance (p-value) is represented by the bubble’s color intensity. The size of the bubble denotes feature intensity (only features with maximum intensity above 1 000 000 are displayed). Feature assignments (p-value, m/z, RT) are displayed in a pop-up window for the identified metabolite tryptophan. (B) Extracted ion chromatogram (EIC) and (C) Boxplot of tryptophan. *p < 0.05 vs respective control.
Figure 6.
(A) Tryptophan and tryptophan metabolites searched in the urine of the animals of all groups. (B) Boxplot of tryptophan and (C) Boxplot of kynurenic acid. *p < 0.05 vs respective control.
Discussion
The present findings demonstrate for the first time that blackberry anthocyanins are able to prevent some of the features of HF diet-induced dysbiosis. In addition, we demonstrate that anthocyanin-induced changes in the gut microbiota composition are related with their anti-neuroinflammatory properties. Finally, we propose that anthocyanins are able to counteract diet-induced neuroinflammation through the stimulation of tryptophan metabolism along the kynurenine pathway.
A number of studies have shown that mental illness may have origins in the gut17–19. Indeed, compelled by the study of Bruce-Keller et al., we have already proposed that HF diet-induced dysbiosis could be the trigger of neuroinflammation, a common hallmark of neuropsychiatry disorders7.
Besides changing the gut microbiota composition (HF-diet induced dysbiosis), HF diets can also increase intestinal permeability, facilitating the passage of LPS into the circulation20. In addition, LPS transport from the intestine towards target tissues can also be enabled by the chylomicrons synthesized in response to fat feeding21. Once in circulation, LPS may activate CD14/TLR4 signaling in target tissues, including the brain and can also disrupt the blood-brain barrier and induce neuroinflammation22. In our study, plasma LPS levels in animals fed with HF diet were not altered (data not shown), probably because the animals were fasted before sacrifice23. However, the propensity for increased fecal LPS levels may reflect the alterations in the gut microbiota composition that HF diet brought about and might be indicative of higher amounts of LPS in plasma. On the other hand, increased fecal LPS levels may activate dendritic cells within the intestinal barrier, increasing the production of cytokines and, thus contributing to peripheral and systemic inflammation24. In the present study, we evaluated whether anthocyanins were able to counteract these effects of HF diet.
The bioavailability of anthocyanins is considered to be low, therefore, it is expected that high amounts of these compounds reach the colon after consumption of anthocyanins-rich foods14. In fact, anthocyanins are extensively metabolized by the gut bacteria and these compounds can predominate during several days in the organism given the enterohepatic recirculation14,25. Concurrently, they are utilized by these microorganisms, and anthocyanins have been shown to selectively stimulate the growth of some bacterial groups14. Hidalgo et al. analyzed the effects of anthocyanins upon gut microbiota composition by real-time PCR, using batch culture fermentations. The authors showed that anthocyanins significantly enhanced the growth of Lactobacillus-Enterococcus spp and Bifidobacterium spp26. Here, we show for the first time a complete picture of the modulation of gut microbial genera by blackberry anthocyanins, in a rodent model.
BE was able to prevent some of the features of HF-diet induced dysbiosis, including fecal LPS levels. In the context of a standard diet, BE increased Pseudoflavonifractor. Previously, Pseudoflavonifractor was found to be decreased in long-term users of proton pump inhibitors which have been associated with increased risk of Clostridium difficile infection27. In addition, it has been suggested that increases in Pseudoflavonifractor may increase the success of obese patients in losing weight consistently28. In the context of HF diet, BE increased Sporobacter similarly to Goji berries that ameliorated chronic colitis through the promotion of beneficial gut bacteria29.
After showing that BE modulates the gut microbiota composition and prevent some of the features of HF-diet induced dysbiosis, we demonstrated that BE-induced changes in gut microbiota profile were correlated with BE anti-neuroinflammatory properties, previously assessed on the hippocampus of the same animals. BE consumption decreased TCK-1 expression in rat’s hippocampus whereas increased Pseudoflavonifractor and Sporobacter, in the context of C and HF diet, respectively. Although correlations do not always point towards causation, this reinforces the idea that BE protection against neuroinflammation can be due to gut microbiota modifications.
Besides LPS, other pathways may be involved in the bilateral communication between the gut and the brain30. Since tryptophan was decreased in the fecal samples of the animals supplemented with BE, we decided to explore the metabolic pathway of tryptophan. As a precursor of serotonin and kynurenine, changes in the supply and availability of this essential amino acid can have many implications for CNS functioning31. The dietary protein intake was the same in the groups supplemented with BE compared to the respective controls. Therefore, if tryptophan was decreased in the fecal samples of the animals supplemented with BE, it could be because BE was stimulating either the microbial catabolism of tryptophan or the host tryptophan metabolism. Around 90% of tryptophan is metabolized by the kynurenine pathway32. In this regard, we looked for kynurenine and kynurenic acid (tryptophan metabolites produced along this pathway) in the urine of all animals. Interestingly, we found that tryptophan and kynurenic acid were increased in the HF + BE group. This result suggests that tryptophan is being converted in kynurenine and, in turn, kynurenine is being converted into kynurenic acid which may act as an antagonist at excitatory amino acid receptors and has been implicated in major psychiatric diseases33. Alterations in the gut microbial composition might result in changes in serum and urine kynurenic acid levels and could thus modify CNS excitation and behavior30. Some studies have also reported the anti-inflammatory properties of kynurenic acid34,35.
The increased production in kynurenic acid could explain, at least in part, the anti-neuroinflammatory properties of anthocyanins, especially in the context of HF diet.
Furthermore, tryptophan could be also be utilized in serotonin synthesis in the gut36 or for indole production by gut bacteria which has been shown to increase intestinal barrier integrity37.
Nevertheless, a direct role of anthocyanins in the brain should not be ruled out. Anthocyanin metabolites can accumulate in the brain and exert their effects directly, including the stimulation of fractalkine secretion12,38.
In conclusion, anthocyanins alter host tryptophan metabolism, generating metabolites responsible for the control of CNS inflammation. This might be a possible mechanism behind their anti-neuroinflammatory properties. Anthocyanins may act, therefore, as mediators of the microbiota-gut-brain axis, allowing the control of neuroinflammation by gut microbiota modulation.
These results strongly suggest that dietary manipulation of the gut microbiota by anthocyanins could attenuate the neurologic complications of obesity. Anthocyanins emerge, therefore, as a new class of psychobiotics.
Lastly, these preclinical studies have prompted interest in whether targeting the gut microbiota with anthocyanins might be a viable strategy to influence tryptophan availability for kynurenine metabolism and neuroinflammatory control within CNS.
Experimental Procedures
Animals
Twenty-four male Wistar rats were randomly divided into four groups (n = 6 per group), as previously described: (C) standard diet; (C + BE) standard diet + blackberry anthocyanin rich extract; (HF) high-fat diet; (HF + BE) high-fat diet + blackberry anthocyanin rich extract13,39. Animals were fed ad libitum with “standard” (Teklad 2014, Harlan Laboratories, Santiga, Spain) or “high-fat” diets (D1245 Research Diets, New Brunswick, USA) for 17 weeks. Blackberry anthocyanin rich extract (BE, 25 mg/kg body weight/day) was obtained as previously described13. Briefly, blackberry anthocyanins (Rubus fruticosus) were extracted with 50% aqueous ethanol (pH 1.5, acidified with HCl) for 24 h at 22 °C. The solution obtained was filtered (50-μmnylon membrane) and concentrated using a rotary evaporator under at 30 °C. The concentrated extract was added to a polyamide gel column (mesh 100–120) to remove sugars. The sugar-free anthocyanin extract was freeze-dried and stored at −20 °C. BE was dissolved daily in sterile water and embedded in food pellets that animals had daily access to. Animal handling and housing protocols followed European Union guidelines (86/609/EEC) for the use of experimental animals. The study obtained ethical approval from the Ethical Committee of the Faculty of Medicine of University of Porto.
DNA extraction from stool and 16S rRNA sequence analysis
Fresh fecal samples were collected directly from the colon of all animals, snap-frozen in liquid nitrogen and stored at −80 °C until further analysis. Genomic DNA was extracted and purified from stool samples using NZY Tissue gDNA Isolation Kit (NZYTech, Lisbon, Portugal) as previously described by Marques et al.23. Firmicutes and Bacteroidetes were quantified by real-time PCR as previously described by Marques et al.23. Libraries were prepared following the 16S Metagenomic Sequencing Library Preparation protocol from illumina (illumina; San Diego, CA, USA). The region of interest was captured using the Klidnworth et al. set of primers that covered the hypervariable region V3–V4 of the bacterial 16s rRNA40. Samples were pooled and loaded into the illumina MiSeq System and, then, sequenced using a 300PE combination according to manufacturer’s specifications.
Raw sequencing reads were merged with PEAR v0.9.6. Amplification primers were trimmed from the sequences obtained using the default program settings of cutadapt v1.9.141. Sequencing quality filtering was subsequently applied to isolate the sequences having more than 300 nts with a mean quality score ≥20. Sequences were excluded from all downstream analyses. Sequences were also inspected for PCR chimera constructs. The resulting sequence reads were clustered into operational taxonomic units (OTU) at 98% similarity, using cd-hit program. NCBI database was used to assign a taxonomic classification to each read in the representative set. Reads with no hits in the reference sequence collection were classified as “null”. OTUs with a relative abundance <1% in all samples were considered non-significant and are not presented. Shannon’s richness index was calculated using the formula described in42.
Neuroinflammation assessment
As previously described, neuroinflammatory markers were measured in the hippocampus of all animals using a predefined cytokine glass-based array (Quantibody Rat Cytokine Array; RayBiotech), according to manufacturer’s instructions13.
Fecal LPS quantification
Quantification of LPS was performed using the Chromo-Limulus Amebocyte Lysate (Chromo-LAL) reagent (Associates of Cape Cod, Inc., Falmouth, MA, USA). Briefly, 1 mL of sterile saline solution (NaCl 0.9%) was added to 100 mg feces, vortexed and centrifuged (10 min, 10000 g, 4 °C) twice. Total supernatant (fecal water) was filtered with 0.45 μm filter and then with 0.22 μm filter. Fecal water and Chromo-LAL (1:1) were incubated at 37 °C for 20 min and absorbance was read every 10 s at 405 nm.
Fecal and urine metabolome analysis by HPLC/Orbitrap
The fecal water obtained for LPS quantification was also used for metabolomics experiments. Urine samples were prepared according to the procedure described by Marques et al.43. Samples were analyzed by HPLC/Orbitrap according to the method described by Fernandes et al.44. Mass spectrometry (MS) data was uploaded into XCMS Online and was processed as a multi-group experiment using the default HPLC/Orbitrap parameters in negative mode (fecal samples) or positive mode (urine samples)45. Isotopes and adducts were annotated using CAMERA and arranged into feature groups. Metabolite features were selected to assess the differences between the fecal and urine samples of C, C + BE, HF and HF + BE groups. Tryptophan, kynurenine and kynurenic acid (MilliporeSigma, St. Louis, MO, USA) were used as standards.
Statistical analysis
Two-way ANOVA was used to determine the main effects of diet (CDe vs HF diet), BE supplementation (No BE vs BE) and their interaction. In XCMS online, one-way ANOVA followed by a post hoc multi comparison test was used in multi group experiment. To compare the differences between two groups, t-test was used. Correlation between variables was established using Spearman’s correlation test. Statistical analyses were performed using SPSS Statistics 23 (IBM, USA) software. Differences were considered statistically significant when p < 0.05. Principal component analysis (PCoA) was performed in R 3.0.2 (The R Foundation, New Zealand) with the RStudio 0.97.310 package. Heatmaps were elaborated using CIMminer platform (https://discover.nci.nih.gov/cimminer/home.do).
Acknowledgements
This article was supported by FEDER through the operation POCI-01-0145-ERDF-007746 funded by the Programa Operacional Competitividade e Internacionalização – COMPETE2020 and by National Funds through FCT - Fundação para a Ciência e a Tecnologia within CINTESIS, R&D Unit (reference UID/IC/4255/2013). SFRH/BD/93073/2013; SFRH/BPD/86173/2012; UID/QUI/50006/2013.
Author Contributions
C.M., M.M. and A.F. conducted the animal experiments. I.F. performed the LC-MS/MS analysis. C.M. analyzed data, performed statistical analysis and wrote the manuscript. C.C., N.M. and J.E.S. contributed to the critical review of the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gammon K. Neurodegenerative disease: brain windfall. Nature. 2014;515:299–300. doi: 10.1038/nj7526-299a. [DOI] [PubMed] [Google Scholar]
- 2.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 3.Schachter, J. et al. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav Immun, 10.1016/j.bbi.2017.08.026 (2017). [DOI] [PubMed]
- 4.Castanon N, Luheshi G, Laye S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front Neurosci. 2015;9:229. doi: 10.3389/fnins.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosher KI, Wyss-Coray T. Go with your gut: microbiota meet microglia. Nat Neurosci. 2015;18:930–931. doi: 10.1038/nn.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marques C, Meireles M, Faria A, Calhau C. High-Fat Diet-Induced Dysbiosis as a Cause of Neuroinflammation. Biol Psychiatry. 2016;80:e3–4. doi: 10.1016/j.biopsych.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar A, et al. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solanki I, Parihar P, Mansuri ML, Parihar MS. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv Nutr. 2015;6:64–72. doi: 10.3945/an.114.007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 12.Meireles M, et al. Anthocyanin effects on microglia M1/M2 phenotype: Consequence on neuronal fractalkine expression. Behav Brain Res. 2016;305:223–228. doi: 10.1016/j.bbr.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Meireles M, et al. The impact of chronic blackberry intake on the neuroinflammatory status of rats fed a standard or high-fat diet. J Nutr Biochem. 2015;26:1166–1173. doi: 10.1016/j.jnutbio.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Faria A, Fernandes I, Norberto S, Mateus N, Calhau C. Interplay between anthocyanins and gut microbiota. J Agric Food Chem. 2014;62:6898–6902. doi: 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- 15.Ho P, Ross DA. More Than a Gut Feeling: The Implications of the Gut Microbiota in Psychiatry. Biol Psychiatry. 2017;81:e35–e37. doi: 10.1016/j.biopsych.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogbonnaya ES, et al. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry. 2015;78:e7–9. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Bruce-Keller AJ, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77:607–615. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bercik P, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(599–609):609 e591–593. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 19.Rogers GB, et al. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brun P, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 21.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 22.Banks WA, et al. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation. 2015;12:223. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques C, et al. High-fat diet-induced obesity Rat model: a comparison between Wistar and Sprague-Dawley Rat. Adipocyte. 2016;5:11–21. doi: 10.1080/21623945.2015.1061723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut. 2003;52:1522–1529. doi: 10.1136/gut.52.10.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kay CD, Pereira-Caro G, Ludwig IA, Clifford MN, Crozier A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu Rev Food Sci Technol. 2017;8:155–180. doi: 10.1146/annurev-food-030216-025636. [DOI] [PubMed] [Google Scholar]
- 26.Hidalgo M, et al. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J Agric Food Chem. 2012;60:3882–3890. doi: 10.1021/jf3002153. [DOI] [PubMed] [Google Scholar]
- 27.Clooney AG, et al. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment Pharmacol Ther. 2016;43:974–984. doi: 10.1111/apt.13568. [DOI] [PubMed] [Google Scholar]
- 28.Louis S, Tappu RM, Damms-Machado A, Huson DH, Bischoff SC. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. Plos One. 2016;11:e0149564. doi: 10.1371/journal.pone.0149564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang, Y. F., Du, M., Yang, G. & Zhu, M. J. Goji berry improves colitis in IL10−/− mice through modulating gut microbiota. Faseb J29 (2015).
- 30.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112:399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 32.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 33.Wu HQ, et al. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salimi Elizei S, Poormasjedi-Meibod MS, Wang X, Kheirandish M, Ghahary A. Kynurenic acid downregulates IL-17/1L-23 axis in vitro. Mol Cell Biochem. 2017;431:55–65. doi: 10.1007/s11010-017-2975-3. [DOI] [PubMed] [Google Scholar]
- 35.Steiner L, Gold M, Mengel D, Dodel R, Bach JP. The endogenous alpha7 nicotinic acetylcholine receptor antagonist kynurenic acid modulates amyloid-beta-induced inflammation in BV-2 microglial cells. J Neurol Sci. 2014;344:94–99. doi: 10.1016/j.jns.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 36.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield-Cargile CM, et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7:246–261. doi: 10.1080/19490976.2016.1156827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease beta-amyloid oligomerization. Mol Nutr Food Res. 2015;59:1025–1040. doi: 10.1002/mnfr.201400544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meireles M, et al. Effect of chronic consumption of blackberry extract on high-fat induced obesity in rats and its correlation with metabolic and brain outcomes. Food Funct. 2016;7:127–139. doi: 10.1039/C5FO00925A. [DOI] [PubMed] [Google Scholar]
- 40.Klindworth A, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. 2011 17, 10.14806/ej.17.1.200 (2011).
- 42.Hill MO. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology. 1973;54:427–432. doi: 10.2307/1934352. [DOI] [Google Scholar]
- 43.Marques C, et al. Pharmacokinetics of blackberry anthocyanins consumed with or without ethanol: A randomized and crossover trial. Mol Nutr Food Res. 2016;60:2319–2330. doi: 10.1002/mnfr.201600143. [DOI] [PubMed] [Google Scholar]
- 44.Fernandes A, et al. Antioxidant and Biological Properties of Bioactive Phenolic Compounds from Quercus suber L. Journal of Agricultural and Food Chemistry. 2009;57:11154–11160. doi: 10.1021/jf902093m. [DOI] [PubMed] [Google Scholar]
- 45.Gowda H, et al. Interactive XCMS Online: simplifying advanced metabolomic data processing and subsequent statistical analyses. Anal Chem. 2014;86:6931–6939. doi: 10.1021/ac500734c. [DOI] [PMC free article] [PubMed] [Google Scholar]