Abstract
Background
Rural areas and refugee camps are characterized by poor access of patients to needed noncommunicable disease (NCD)–related health services, including diabetes and hypertension. Employing low-cost innovative eHealth interventions, such as mobile health (mHealth), may help improve NCDs prevention and control among disadvantaged populations.
Objective
The aim of this study was to assess the effect of employing low-cost mHealth tools on the accessibility to health services and improvement of health indicators of individuals with NCDs in rural areas and refugee camps in Lebanon.
Methods
This is a randomized controlled trial study in which centers were allocated randomly into control and intervention sites. The effect of an employed mHealth intervention is assessed through selected quality indicators examined in both control and intervention groups. Sixteen primary health care centers (eight controls, eight interventions) located in rural areas and Palestinian refugee camps across Lebanon were included in this study. Data on diabetic and hypertensive patients—1433 in the intervention group and 926 in the control group—was extracted from patient files in the pre and postintervention periods. The intervention entailed weekly short message service messages, including medical information, importance of compliance, and reminders of appointments or regular physician follow-up. Internationally established care indicators were utilized in this study. Descriptive analysis of baseline characteristics of participants, bivariate analysis, logistic and linear regression were conducted using SPSS (IBM Corp).
Results
Bivariate analysis of quality indicators indicated that the intervention group had a significant increase in blood pressure control (P=.03), as well as a significant decrease in the mean systolic blood pressure (P=.02), mean glycated hemoglobin (HbA1c; P<.01), and in the proportion of HbA1c poor control (P=.02). Separate regression models controlling for age, gender, and setting showed a 28% increase in the odds of blood pressure control (P=.05) and a 38% decrease in the odds of HbA1c poor control (P=.04) among the intervention group in the posttest period. Females were at lower odds of HbA1c poor control (P=.01), and age was statistically associated with annual HbA1c testing (P<.01). Regression models for mean systolic blood pressure, mean diastolic blood pressure, and mean HbA1c showed that a mean decrease in HbA1c of 0.87% (P<.01) pretest to posttest period was observed among the intervention group. Patients in rural areas belonging to the intervention group had a lower HbA1c score as compared with those in refugee camps (P<.01).
Conclusions
This study underlines the importance of employing integrative approaches of diseases prevention and control in which existing NCD programs in underserved communities (ie, rural and refugee camps settings) are coupled with innovative, low-cost approaches such as mHealth to provide an effective and amplified effect of traditional NCD-targeted care that can be reflected by improved NCD-related health indicators among the population.
Trial Registration
ClinicalTrials.gov NCT03580330; https://clinicaltrials.gov/ct2/show/NCT03580330 (Archived by WebCite at http://www.webcitation.org/70mhVEUwQ)
Keywords: noncommunicable diseases, hypertension, diabetes mellitus, telemedicine, mobile health, rural health, refugees
Introduction
Prevalence of Noncommunicable Diseases
Noncommunicable diseases (NCDs) and its associated risk factors, including diabetes mellitus and hypertension (HTN), have become the leading cause of death and disability globally [1]. In 2015, NCDs accounted for 70% of the estimated 56.4 million deaths in the world [2]. Around 80% of deaths associated with NCDs occur in low- and middle-income countries (LMIC), with disproportional effects on underprivileged populations [3-5].
In the Eastern Mediterranean Region (EMR), the burden of NCDs is growing at an alarming rate, with approximately 57% of deaths in the region attributed to NCDs, an equivalent of 2.2 million death per year [6]. Around 51% of these deaths are premature (ie, below the age of 70 years) [6-8]. Unhealthy diets, physical inactivity, hyperglycemia, hyperlipidemia, elevated blood pressure (BP), and obesity are among the most prevalent underlying risk factors; linked to 65% of NCDs-related deaths [7,9,10].
Limited Access to Health Care in Rural Settings and Refugee Camps
Despite the rising burden of NCDs, health care systems of the majority of LMIC remain focused on treatment with minimal unsustainable investment in primary health care (PHC) [11]. Different economic, sociocultural, and geographic factors were also found to limit access of patients to NCDs preventive health care services in these settings [12,13]. Lack of essential knowledge and awareness on NCDs prevention, especially among underserved populations, often results in poor management of their disease, manifested in substandard health-related indicators manifested in poor glycemic or BP control and other preventable morbidities [14-22]. For refugees in specific, the situation is further aggravated by their restricted access to health care services because of a range of factors, including financial barriers, geographic attainability, safety, as well as cultural and language impediments [14]. NCD-specific care requires a systematic approach ranging from case finding and early detection, to identification of unhealthy behaviors, and compliance to regular long-term follow-up [23]. Such an approach is constrained by the limited health facility-based resources in rural and refugee settings, which hinders the ability to implement proper NCD preventive measures in those settings. Further compounding the situation is the ongoing displacement of Palestinian and Syrian refugees and the protracted crises in the EMR, adding burden to already fragmented health systems of refugee-hosting countries regionally [7,18].
Use of Mobile Health as an Effective Add-On to Traditional Care
Despite the fact that remarkable efforts have been invested to decrease the burden of NCDs in EMR [21,22], a comprehensive change in the approach to NCDs in these settings remains necessary to meet the health needs of the displaced populations and host communities [14,16,20]. A systematic review of primary care models for NCDs interventions in LMIC recommends a programmatic structure focused on monitoring and evaluation of indicators, standardized care, and compliance to follow-up [24].
With the spread of mobile technology, mobile Health (mHealth) is regarded as a promising approach that is being increasingly explored to improve community health outcomes. As a subset of electronic health (eHealth), mHealth is defined as the use of mobile devices in health care delivery, mainly through short message service (SMS) messaging, voice calls, mobile phone apps, tablets, or wearable devices’ apps [25]. SMS is an mHealth tool that holds great promise in addressing NCDs through health education and self-management, improving prevention and treatment strategies, and providing appointment reminders to improve compliance and the attendance of appointments in PHC centers (PHCCs). SMSs are also attractive because of their potential in overcoming financial and geographic barriers facing hard-to-reach populations [26-33]. A number of academic studies showed that mHealth helps improve prevention and control of diseases, including HTN and diabetes, by providing targeted interventions to disadvantaged populations living in remote areas where health services are often limited [26,34-43].
Lebanese Context and Relevance of the Study
According to recent reports, Lebanon faces an elevated NCDs-related mortality reaching as high as 85% [44]. Furthermore, the country hosts a large proportion of the world’s Palestinian refugees, accounting around 10% of the country’s population [45]. The burden of NCDs, namely diabetes and HTN, is expanding among underprivileged populations residing in rural areas and refugee camps primarily because of poor screening and low early detection rates [46]. Suffering from limited availability of resources and fragmented infrastructure, PHCCs are to a large extent the sole convenient health facilities serving people in Lebanese rural areas where most refugees are residing [47]. The 2011 assessments of the United Nations Relief and Works Agency (UNRWA) revealed that one-third of Palestinian refugees in Lebanon residing in camps face hardships related to NCDs [48]. The refugees’ struggle is aggravated by the unsatisfactory health services in such contexts [49]. Exploring innovative and effective strategies that can complement existing traditional care remains necessary in such settings to appropriately tackle the growing trend of NCDs in the country.
Mobile phone use is very common in Lebanon, reaching as high as 92.16% in 2015 [50]. A situational assessment study on Syrian refugees and digital health in Lebanon reported refugees as frequent users of mobile communications, including SMS, with each household having at least one mobile phone, suggesting the likely reach that an mHealth intervention could have in this context [51].
Despite the proven abundance of mobile phones in underserved settings, the potential role that mHealth could have in enhancing access of disadvantaged populations to adequate NCD care has never been investigated in Lebanon.
This study aims to assess the effect of employing a low-cost mHealth intervention on access to health services and improvement of health indicators of individuals suffering from NCDs in rural areas and refugee camps in Lebanon. The mHealth intervention is of dual components addressing both patients and health providers through informative health SMSs targeting the former and online training modules and support forums targeting the latter. This study, implemented in collaboration with the Lebanese Ministry of Public Health (MOPH) and UNRWA, is the first to employ a mHealth intervention in Lebanon in rural and refugee settings. The results will provide evidence that could help in restructuring existing NCDs action plan at the systems level to optimally respond to the needs of displaced refugees and host communities and enhance compliance to adequate care while containing cost through adopting an innovative preventive approach.
Methods
Study Design
This study reports on a community trial in which PHC centers, along their respective catchment areas, were randomly allocated into control and intervention sites with the aim of assessing the change in selected NCD care quality indicators (QIs) among community individuals and patients. Patients in the intervention sites received a 1-year mHealth intervention, and their pre- and postintervention outcomes were assessed through measurement of QIs. The study was conducted over a period of 2 years, covering 1 year of preintervention collection of QIs and 1 year of delivery of the mHealth intervention, followed by a postintervention period of 1 month of postintervention QIs collection (Figure 1).
Figure 1.
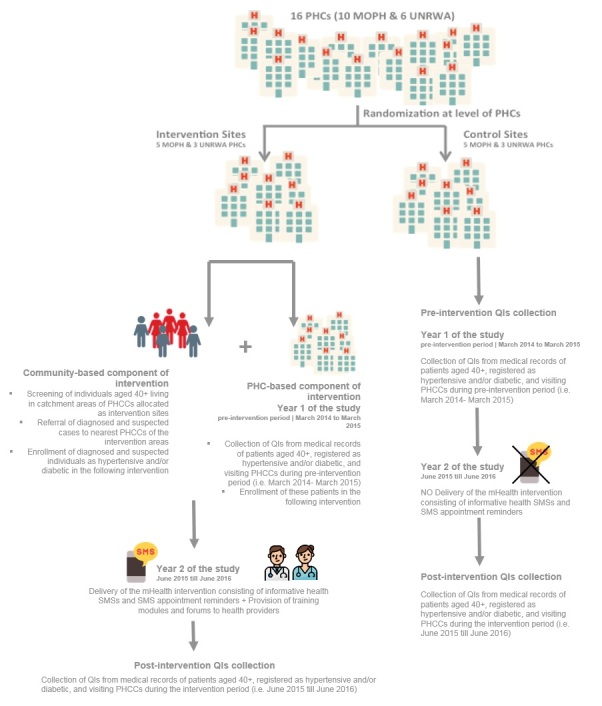
Summary of the methodology of the study. MOPH: Ministry of Public Health; PHC: primary health care; PHCC: primary health care center; QI: quality indicator; SMS: short message service; UNRWA: United Nations Relief and Works Agency.
Participants Selection and Data Collection
The study population comprised sixteen PHCCs in Lebanon: ten located in rural areas and belonging to the Lebanese MOPH PHC National Network [52] and six are UNRWA centers chosen from Palestinian refugee camps in Lebanon. These centers were randomly assigned into intervention and control groups. Five MOPH and three UNRWA centers were allocated to each of the intervention and control groups for a total of eight sites in each of the groups.
One QI collector was hired at each of the sixteen PHCCs included in the study (both intervention and control) to collect relevant QIs from patients’ records at two points in time: (1) at baseline period, also noted as the preintervention period, where QIs were collected from records of all patients visiting included PHCCs for the 1-year preintervention period from March 2014 to March 2015 and (2) after delivery of intervention, also noted as the postintervention period, where QIs were collected from records of patients visiting the PHCCs for the 1-year intervention period from June 2015 to June 2016.
The inclusion criteria of the records of patients during the QIs collection periods was based on the health status and age of the patients. To be included in this study, patients had to be registered at the PHCCs as diabetics or hypertensive and aged 40 years or more. Only Lebanese patients registered at the included MOPH PHCCs in rural areas and Palestinian refugee patients registered at the included UNRWA health centers were eligible for inclusion if the aforementioned criteria were met. Records of patients whose nationality was not Lebanese nor Palestinian and whose age was less than 40 years were not eligible for inclusion. No exclusion based on gender, educational and literacy level, disability, or presence of other medical conditions took place. Data were extracted from patients’ medical records for all patients meeting the inclusion criteria. Demographics including gender, age, and telephone number, in addition to medical information related to the QIs such as last result of BP, last result of glycated hemoglobin (HbA1c), smoking status, and dates of last visit for HbA1c testing, for eye check-up, or foot exam, were collected. Numerical values and dates were reported whenever available within the corresponding data collection period; otherwise, not reported was entered for missing values.
Intervention
The eSahha project is a two-pronged mHealth interventional project targeting catchment areas of PHCCs located in Lebanese rural areas and Palestinian refugee camps, where access to health knowledge and health services is known to be limited.
The overall eSahha intervention consisted of two related components: one that is community-based and another that is PHC center-based. The community-based component included community screening for HTN and diabetes by trained community health workers among individuals falling within the age group at higher risk of developing NCDs—40 years or older—in the catchment areas of the eight intervention centers.
Individuals already diagnosed with or suspected of being diabetic, hypertensive, or both were referred to the nearest intervention PHCC for NCD-specific clinical care and were targeted by SMS messages originating from a preexisting mobile communication platform used for mass messaging hosted by a Lebanese telecommunication company and scheduled for delivery by a research assistant of the research team. The SMSs were developed by a family physician based on the MOPH guidelines for prevention and management of HTN and diabetes. A weekly educational health SMS was sent every Monday afternoon for the intervention period of 1 year. SMS content covered different health themes providing health information on lifestyle, dietary habits, body weight, smoking, medications, importance of compliance, as well as symptoms and self-management of HTN and diabetes. Community individuals who were diagnosed and were receiving necessary care previous to our intervention were sent weekly informative health SMS, as well as customized SMSs reminders to follow up on their scheduled medical appointments (eg, to check their HbA1c levels and have their annual foot or eye exams). The messages were sent to mobile phones of the targeted individuals suffering from HTN or diabetes included in the intervention or, in cases where the targeted individuals did not own a mobile phone, messages were sent with their consent to the cell phones of their respective closest relatives (eg, son, daughter, or husband). Individuals in the control group (ie, living in catchment areas of control PHCCs) did not receive SMS messages and were thus receiving the usual care. The messages were initially formulated in English and then translated to Arabic and sent using simplified Arabic terms to match the different levels of health literacy of the target lay population. The length of each message was restricted to a maximum of 70 characters. On the other hand, the PHC center-based component of the intervention consisted of sending the same weekly informative health SMS, as well as appointment reminders customized to the respective time for check-ups to patients registered as diabetic or hypertensive at baseline at the PHCCs belonging to the intervention group. Patients receiving the SMSs were not required to reply to the SMSs at any point in time.
The PHC center-based component of the intervention also included training of health care providers, namely physicians and nurses, working in the intervention PHCCs, using eHealth tools consisting of (1) Online modules focusing on clinical guidelines for treating diabetes and HTN and others on provider-patient communication strategies (ie, increasing compliance) and (2) Online forums and frequently asked questions mainly dedicated to peer-to-peer knowledge sharing of treatment and communication techniques.
Measurements of Quality Indicators
A set of internationally recognized QIs for diabetes and HTN were employed to monitor the effectiveness of the intervention (Table 1). The choice of indicators was based on their wide acceptance and use in evaluating care effectiveness on one hand and on the ability of the health information system available in the included MOPH and UNRWA PHCCs to extract the needed data on the other hand.
Table 1.
Selected quality indicators to monitor the effectiveness of the intervention.
| Domain and Measure | Measure description | |
| Hypertension | ||
|
|
Mean blood pressure | Means SBPa and DBPb were assessed after collecting the most recent result of each patient’s blood pressure in terms of SBP or DBP |
|
|
Blood pressure control | Percentage of patients with most recent blood pressure <140/90 mmHg |
|
|
Annual eye check-up | Percentage of patients receiving at least one eye check-up annually as per recommended guideline (date within the data collection period, while a 30-day grace period was allowed) |
| Diabetes | ||
|
|
Annual HbA1cc testing | Percentage of patients with one or more HbA1c tests annually as per recommended guideline (date within the data collection period, while a 30-day grace period was allowed) |
|
|
Mean HbA1c | Mean HbA1c was assessed after collecting each patient’s most recent result of HbA1c |
|
|
HbA1c poor control | Percentage of patients with most recent HbA1c level >9.0% (poor control); Mean HbA1c is also assessed |
|
|
Annual smoking status check | Percentage of patients whose smoking status was ascertained and documented annually |
|
|
Annual foot exam | Percentage of patients receiving at least one foot exam annually as per recommended guideline (date within the data collection period, while a 30-day grace period was allowed) |
|
|
Annual eye check-up | Percentage of patients receiving at least one eye check-up annually as per recommended guideline (date within the data collection period, while a 30-day grace period was allowed) |
aSBP: systolic blood pressure.
bDBP: diastolic blood pressure.
cHbA1c: glycated hemoglobin.
Statistical Analysis
Data on BP (systolic blood pressure, SBP or diastolic blood pressure, DBP), HbA1c, smoking status, as well as dates of eye and foot check-ups were obtained and analyzed using statistical software package SPSS (version 24.0). Sample baseline characteristics were summarized for the intervention and control groups using mean and SD for numerical variables and frequency and percentage for categorical variables. Annual check-ups, including eye and foot exams, as well as HbA1c testing were considered acceptable if done according to recommended guideline periods, while a 30-days grace period was allowed. Pearson chi-square test (χ2) and independent t test were used to assess the difference in quality indicators before and after the intervention. Logistic regression was used to evaluate the impact of the intervention on HbA1c poor control, BP control, and annual HbA1c testing, while controlling for age, gender, and setting. Similarly, linear regression was used to assess the impact on mean SBP, DBP, and HbA1c, while controlling for age, gender, and setting. All analyses were carried at a .05 significance level.
Ethical Considerations
Ethical approval was obtained from the American University of Beirut Institutional Review Board before conducting the study. As per the approved protocol, approval of participation and informed consent were obtained from PHC centers’ directors. Directors and QI collectors recruited from the centers in the two groups were informed about the purpose of the study and its expected outcomes. Confidential handling of all data collected was ensured throughout the study period. In addition, SMS messages were sent to patients in the intervention group after obtaining their consent. In case the targeted patients did not own a mobile phone, their consent was received to send the messages to the phone of their selected closest relatives (eg, son, daughter, or husband). Those relatives in turn had to also consent to receiving those messages and passing them to the patients.
Results
Participants’ Demographic Characteristics
Table 2 presents the baseline demographic and clinical characteristics of patients in the intervention and control groups. The data of 1433 patients in the intervention groups and 926 patients in the control groups were included in the analysis. At baseline, more than half of patients were females, 56.3% (454/807) in the intervention group and 56.2% (292/520) in the control group. As for age distribution, a higher proportion was in the age range of 56 to 70 years, representing 44.1% (353/800) of those in the intervention group and 40.1% (200/499) of those in the control. Majority of patients in both groups were living in Lebanese rural areas (61.97%, 888/1433 and 60.8%, 563/926 respectively). In the intervention group, 64.27% (921/1433) of patients were hypertensive, and 35.73% (512/1433) were diabetic. On the other hand, 67.6% (626/926) of patients in the control group were hypertensive, and 32.4% (300/926) were diabetic. There was no statistical difference between the two groups except for age (P=.003; Table 2).
Table 2.
Baseline demographic and clinical characteristics of participants by study group. No significant differences between gender, setting, and disease category across the two groups were identified using chi-square test; the difference in age groups between intervention and control at baseline is statistically significant (P=.003). Bonferroni post-hoc test reveals that the difference is in the age group ≥71 years.
| Variable | Intervention, n (%) | Control, n (%) | |
| Total number of participantsa | 1433 (100.0) | 926 (100.0) | |
| Gender |
|
|
|
|
|
Male | 353 (43.7) | 228 (43.8) |
|
|
Female | 454 (56.3) | 292 (56.2) |
| Age group (years) |
|
|
|
|
|
40-55 | 252 (31.5) | 134 (26.9) |
|
|
56-70 | 353 (44.1) | 200 (40.1) |
|
|
≥71 | 195 (24.4) | 165 (33.1) |
| Setting |
|
|
|
|
|
Rural areas | 888 (62.0) | 563 (60.8) |
|
|
Palestinian refugee camps | 545 (38.0) | 363 (39.2) |
| Disease category |
|
|
|
|
|
Diabetes | 512 (35.7) | 300 (32.4) |
|
|
Hypertension | 921 (64.3) | 626 (67.6) |
aSome numbers under some categories may not add up to the total because of missing values.
Hypertension Quality Indicators
Bivariate analyses of quality indicators are shown in Table 3. All dates are considered acceptable if done within the recommended guideline periods, while a 30-day grace period was allowed (refer to Table 1). A significant increase in BP control was observed in the intervention group between the pre- and the posttest periods (58.2%, 530/911 to 63.6%, 426/670, P=.03), which was not replicated in the control group (P=.37). Similarly, a significant drop in the mean SBP was observed among the intervention group (133.7 mmHg, SD 16.1 pretest and 131.8 mmHg, SD 15.8 posttest, P=.02) but not in the control group (P=.65). In both study groups, a lower proportion of annual eye check-up was observed in the post test as compared with the pretest (48.4%, 446/921 to 38.9%, 268/689 in intervention group and 27.8%, 174/626 to 21.1%, 133/630 in the control group, P<.01 for both). No statistical significance was found in the pre- or posttest difference in the mean DBP in either groups (P=.14 and P=.81, respectively).
Table 3.
Bivariate analysis of hypertension (HTN) and diabetes mellitus quality indicators by study group.
| Type of quality indicators | Intervention | Control | |||||
|
|
Pretest | Posttest | P value | Pretest | Posttest | P value | |
| HTN quality indicators |
|
|
|
|
|
|
|
|
|
BPa controlled, n (%) | 530 (58.2) | 426 (63.6) | .03b | 316 (60.9) | 362 (58.4) | .37 |
|
|
SBPc, mean mmHg (SD) | 133.69 (16.10) | 131.80 (15.79) | .02b | 133.24 (17.08) | 133.68 (16.91) | .65 |
|
|
DBPd, mean mmHg (SD) | 79.16 (9.13) | 78.47 (9.09) | .14 | 78.24 (9.41) | 78.09 (8.97) | .81 |
|
|
Annual eye check-up, n (%) | 446 (48.4) | 268 (38.9) | <.01b | 174 (27.8) | 133 (21.1) | <.01b |
| Diabetes mellitus quality indicators |
|
|
|
|
|
|
|
|
|
Annual HbA1ce testing, n (%) | 264 (51.6) | 397 (74.1) | <.01b | 100 (33.3) | 208 (71.2) | <.01b |
|
|
HbA1c poor control, n (%) | 75 (28.2) | 82 (20.3) | .02b | 22 (21.8) | 46 (22.2) | .93 |
|
|
HbA1c, mean % (SD) | 8.00 (1.89) | 7.20 (2.06) | <.01b | 7.73 (1.72) | 7.69 (1.84) | .88 |
|
|
Proportion of smokers, n (%) | 159 (35.0) | 169 (31.8) | .29 | 60 (36.1) | 64 (31.4) | .33 |
|
|
Annual foot exam, n (%) | 224 (43.8) | 227 (42.4) | .65 | 118 (39.3) | 105 (36.0) | .40 |
|
|
Annual eye check-up, n (%) | 266 (52.0) | 183 (34.1) | <.01b | 111 (37.0) | 68 (23.3) | <.01b |
aBP: blood pressure.
bIndicates to statistical significance at .05 CI.
cSBP: systolic blood pressure.
dDBP: diastolic blood pressure.
eHbA1c: glycated hemoglobin.
Diabetes Quality Indicators
In the intervention group, glycemic control results improved in the intervention group but not in the control group. The proportion of HbA1c poor control decreased significantly in the intervention group from 28.2% to 20.3% (P=.02), whereas it remained unchanged in the control group (21.8% vs 22.2%, P=.93). Likewise, a significant reduction in the mean HbA1c was noted in the intervention group (8.00%, SD 1.9 to 7.2%, SD 2.1, P<.01) but not in the control group (P=.88). Furthermore, the proportion of annual HbA1c testing done within recommended guideline period increased for both groups (51.6%-74.1% in the intervention and 33.3%-71.2% in the control, P<.01 for both). However, the proportion of annual eye check-up done within recommended guideline period decreased significantly in both groups (52.0% down to 34.1% in intervention and 37.0% down to 23.3% in control, P<.01 for both). Differences in proportions of smoking and annual foot exam within recommended guideline period were not statistically significant in either group.
Regression Models
Separate regression models were run to gauge the intervention impact on the study groups while controlling for baseline characteristics: age, gender, and setting. Table 4 shows the results of the logistic regressions, where BP control, HbA1c poor control, and annual HbA1c testing act as the dependent variables (consecutively). Annual HbA1c testing is within recommended guideline: dates are considered acceptable if done within the recommended guideline period, while a 30-day grace period was allowed (refer to Table 1). When considering BP control, Table 4 indicates that for the intervention group, there was a 28% increase in the odds of BP control in the posttest period as compared with the pretest period (odds ratio, OR 1.28, 95% CI 1.00-1.64, P=.05), independent of age, gender, and setting. The OR for study period was not statistically significant in the control group (P=.11). Rural areas reported lower odds of BP control as compared with refugee camps in both study groups (OR 0.31, 95% CI 0.24-0.40 intervention group; OR 0.22, 95% CI 0.15-0.30 control group; P<.01 for both). A 38% decrease in the odds of HbA1c poor control among the intervention group from the pretest to the posttest study periods was observed (OR 0.62, 95% CI 0.39-0.97; P=.04), independent of age, gender, and setting. The study period OR was not statistically significant among the control group (P=.26). Females were at lower odds of HbA1c poor control among the intervention group (OR 0.59, 95% CI 0.39-0.89; P=.01), whereas age ORs were statistically significant for both study groups (OR 0.97 for both, P<.01 and P=.03, respectively). With regard to annual HbA1c testing, the results reveal that both groups had an increase in the odds of doing the test with recommended guideline period (OR 2.52, 95% CI 1.81-3.49 for intervention and OR 4.26, 95% CI 2.79-6.49 for control; P<.01 for both). Age was statistically associated with annual HbA1c testing (OR 0.98, P<.01) for the intervention group, and rural settings were at higher odds of conforming with HbA1c testing guidelines in both study groups as compared with refugee camps participants (OR 4.43 for intervention and OR 2.22 for control; P<.01 for both).
Table 4.
Logistic regression model of blood pressure (BP) control, glycated hemoglobin (HbA1c) poor control, and annual HbA1c testing by study group.
| Indicator | Intervention |
|
Control |
|
||
| ORa (95% CI) | P value | OR (95% CI) | P value | |||
| BP control |
|
|
|
|
||
|
|
Study period |
|
|
|
|
|
|
|
|
Posttest | 1.28 (1.00-1.64) | .05b | 1.28 (0.95-1.72) | .11 |
|
|
|
Pretest | —c | — | — | — |
|
|
Gender |
|
|
|
|
|
|
|
|
Female | 1.12 (0.88-1.44) | .36 | 0.97 (0.74-1.29) | .85 |
|
|
|
Male | — | — | — | — |
|
|
Age (continuous) | 0.99 (0.98-1.00) | .06 | 0.99 (0.98-1.00) | .03b | |
|
|
Setting |
|
|
|
|
|
|
|
|
Rural areas | 0.31 (0.24-0.40) | <.01b | 0.22 (0.15-0.30) | <.01b |
|
|
|
Palestinian refugee camps | — | — | — | — |
| HbA1c poor control |
|
|
|
|
||
|
|
Study period |
|
|
|
|
|
|
|
|
Posttest | 0.62 (0.39-0.97) | .04b | 0.68 (0.35-1.33) | .26 |
|
|
|
Pretest | — | — | — | — |
|
|
Gender |
|
|
|
|
|
|
|
|
Female | 0.59 (0.39-0.89) | .01b | 0.84 (0.47-1.49) | .56 |
|
|
|
Male | — | — | — | — |
|
|
Age (continuous) | 0.97 (0.96-0.99) | <.01b | 0.97 (0.95-1.00) | .03b | |
|
|
Setting |
|
|
|
|
|
|
|
|
Rural areas | 0.71 (0.45-1.11) | .13 | 0.81 (0.43-1.51) | .51 |
|
|
|
Palestinian refugee camps | — | — | — | — |
| Annual HbA1c testing |
|
|
|
|
||
|
|
Study period |
|
|
|
|
|
|
|
|
Posttest | 2.52 (1.82-3.49) | <.01b | 4.26 (2.79-6.49) | <.01b |
|
|
|
Pretest | — | — | — | — |
|
|
Gender |
|
|
|
|
|
|
|
|
Female | 1.17 (0.85-1.61) | .34 | 1.04 (0.69-1.56) | .87 |
|
|
|
Male | — | — | — | — |
|
|
Age (continuous) | 0.98 (0.97-0.99) | <.01b | 1.01 (0.99-1.03) | .37 | |
|
|
Setting |
|
|
|
|
|
|
|
|
Rural areas | 4.43 (3.20-6.13) | <.01b | 2.22 (1.46-3.39) | <.01b |
|
|
|
Palestinian refugee camps | — | — | — | — |
aOR: odds ratio.
cIndicates to statistical significance of .05 CI.
cReference category.
Table 5 summarizes the regression models for mean SBP, mean DBP, and mean HbA1c. After controlling for age, gender, and setting, the mean changes in SBP and DBP across the study period were not statistically significant (beta=−1.12 mmHg, SD 0.90, P=.21 for mean SBP intervention group; beta=−1.83 mmHg, SD 1.18, P=.12 for mean SBP control group; beta=−.49 mmHg, SD 0.53, P=.36 for mean for DBP intervention group; and beta=−.90 mmHg, SD 0.65, P=.16 for mean DBP control group). Only mean HbA1c retained statistical significance in the multivariate analysis. A mean decrease in HbA1c of 0.87 pretest to posttest period was observed among the intervention group (beta=−.87, SD 0.19, P<.01) but not in the control group (P=.41). This change was independent of age, gender, and setting. On average, females in the intervention group had a lower HbA1c score compared with their male counterparts (beta=−.42, SD 0.17, P=.01). Age was associated with a decrease in HbA1c, which was seen in the control group only (beta=−.03, SD 0.01, P<.01), and patients in rural areas belonging to the intervention group had a lower HbA1c score as compared with refugee camps (beta=−.65, SD 0.19, P<.01).
Table 5.
Linear regression model of the means systolic blood pressure (SBP), diastolic blood pressure (DBP), and glycated hemoglobin (HbA1c) by study group.
| Indicator | Intervention | Control | ||||||
|
|
Beta | SE | P value | Beta | SE | P value | ||
| Mean SBP |
|
|
|
|
|
|
||
|
|
Study period |
|
|
|
|
|
|
|
|
|
|
Posttest | −1.12 | 0.90 | .21 | −1.83 | 1.18 | .12 |
|
|
|
Pretest | —a | — | — | — | — | — |
|
|
Gender |
|
|
|
|
|
|
|
|
|
|
Female | −.59 | 0.90 | .51 | .30 | 1.11 | .79 |
|
|
|
Male | — | — | — | — | — | — |
|
|
Age (continuous) | .08 | 0.04 | .03b | .12 | 0.04 | .01b | |
|
|
Setting |
|
|
|
|
|
|
|
|
|
|
Rural areas | 7.98 | 0.92 | <.01b | 9.07 | 1.21 | <.01b |
|
|
|
Palestinian refugee camps | — | — | — | — | — | — |
| Mean DBP |
|
|
|
|
|
|
||
|
|
Study period |
|
|
|
|
|
|
|
|
|
|
Posttest | −.49 | 0.53 | .36 | −.90 | 0.65 | .16 |
|
|
|
Pretest | — | — | — | — | — | — |
|
|
Gender |
|
|
|
|
|
|
|
|
|
|
Female | −1.89 | 0.53 | <.01b | −.73 | 0.61 | .23 |
|
|
|
Male | — | — | — | — | — | — |
|
|
Age (continuous) | −.02 | 0.02 | .26 | −.07 | 0.02 | <.01b | |
|
|
Setting |
|
|
|
|
|
|
|
|
|
|
Rural areas | 1.66 | 0.54 | <.01b | 2.17 | 0.67 | <.01b |
|
|
|
Palestinian refugee camps | — | — | — | — | — | — |
| Mean HbA1c |
|
|
|
|
|
|
||
|
|
Study period |
|
|
|
|
|
|
|
|
|
|
Posttest | −.87 | 0.19 | <.01b | −.22 | 0.27 | .41 |
|
|
|
Pretest | — | — | — | — | — | — |
|
|
Gender |
|
|
|
|
|
|
|
|
|
|
Female | −.42 | 0.17 | .01b | −.21 | 0.22 | .35 |
|
|
|
Male | — | — | — | — | — | — |
|
|
Age (continuous) | −.01 | 0.01 | .12 | −.03 | 0.01 | <.01b | |
|
|
Setting |
|
|
|
|
|
|
|
|
|
|
Rural areas | −.65 | 0.19 | <.01b | .07 | 0.25 | .76 |
|
|
|
Palestinian refugee camps | — | — | — | — | — | — |
aRefers to reference category.
bRefers to statistical significance at .05 CI.
Discussion
Principal Findings
First of its kind in the country, the study revealed promising findings in regard to the use of mobile phone SMS technology to improve the management of NCDs among individuals living in rural areas and Palestinian refugee camps in Lebanon. Postintervention measurements of quality health indicators in the intervention group showed remarkable improvement in comparison with preintervention assessments through enhanced BP control, reduced mean SBP, as well as decrease in HbA1c levels and HbA1c poor control. Comparison across the two settings (ie, rural areas vs refugee camps) showed differential improvements in diabetes and HTN quality indicators where patients living in refugee camps exhibited improved BP control and lower means of SBP and DBP compared with patients from rural areas. Patients in rural areas had comparatively lower HbA1c scores and were at higher odds of conforming to HbA1c testing. No clear direct effect of the implemented mHealth intervention on change in smoking habits, patients’ utilization of PHC services, and compliance with visits for HbA1c testing, eye check-ups, and foot exams were noted.
Our findings of a considerable increase in BP control only in patients receiving the mHealth intervention are in agreement with those of similar studies conducted in Spain, South Korea, and Russia [40,53,54]. In contrast, Wald et al and Orsama et al had findings showing no statistically significant patterns for amelioration neither in BP control nor in means SBP and DBP in the SMS intervention group [55,56]. After controlling for age, gender, and setting, only BP control retained statistical significance in the regression model, with a 28% increase in the odds of BP control in the posttest period as compared with the pretest period. No significant difference for BP control was found across age and gender. There is a paucity of data in the literature with regard to differences in intervention outcomes among hypertensive patients based on age, gender, or setting [40,53,54].
The group that received SMS messages had a clinically important and statistically significant change in glycemic control. More specifically, the intervention group showed a significant decrease both in HbA1c levels (from 8.00%, SD 1.89 to 7.2%, SD 2.06) and in HbA1c poor control (from 28.2%-20.3%) after a 1-year SMS intervention. The control group, on the other hand, showed a slight increase in HbA1c poor control within the same period. The 38% decrease in the odds of HbA1c poor control among the intervention group from the pretest to the posttest study periods was independent of age, gender, and setting in the multivariate analysis. The same applied for the reported change in HbA1c (drop by 0.8%) in the intervention group. This finding concurs with those of a study from Bangladesh, where HbA1c levels dropped by a mean of 0.85% after the SMS intervention [38]. Furthermore, this reported change is higher than those from previous studies, such as a 0.7% reduction in HbA1c posttest in Iraq [57], 0.4% in Saudi Arabia [58], 0.53% in a meta-analysis by Arambepola et al [26], and 0.39% in a meta-analysis by Liu and Ogwu [59]. Other studies have shown a higher reductions in HbA1c posttest ranging from 0.89% to 2.76% [60-63]. In this study, it was shown that HbA1c levels and HbA1c poor control differed significantly across gender, with females in the intervention group having a lower HbA1c score and a lower odds of HbA1c poor control compared with their male counterparts. One explanation relates to females being more attentive to SMS messages [38]. However, such explanation requires confirmation in future studies. On the other hand, in an Iraqi study [57] and a systematic review of 17 articles [64], changes in HbA1c levels following SMS intervention were not related to any of the demographic factors including age, gender, and nationality. Similarly, age was not associated with a decrease in HbA1c in our study’s intervention group, yet the odds of having HbA1c poor control decreased with age for both study groups.
Difference in Quality Indicators Across Setting
The logistic regression model revealed that participants living in rural areas reported lower odds of BP control compared with patients living in refugee camps in both study groups. This was also apparent in the linear regression model, where the means SBP and DBP were higher among participants in rural areas as compared with their refugee camps counterparts. In contrast, patients living in rural areas had a lower HbA1c score in intervention group and were also at higher odds of conforming to HbA1c testing guidelines in both study groups as compared with Palestinian refugee camps participants. The fact that the improvement in diabetes and HTN quality indicators do not align across the two settings flags an improvement opportunity in the mHealth program design and in the NCD care configurations across the two settings. It also highlights an opportunity for learning across the two settings. The fact that BP control was better among patients living in refugee camps in both study groups might reflect the role of the UNRWA’s NCD program integrated in the intervention and control centers and entailing primary, secondary, and tertiary NCDs’ prevention [10]. The presence of such a program at UNRWA may have enhanced the effectiveness of the mHealth interventions and facilitated improved access to services. However, as our study reveals, further efforts are needed to improve diabetes prevention and care for Palestinian refugees in regards to improving their glycemic control and treatment compliance [65]. Furthermore, the finding that patients’ conforming to HbA1c testing guidelines was better among patients from rural areas in both study groups echoes that the MOPH NCD program was more effective in diabetes management and control as compared with HTN [66]. The presence of an advanced diabetes control program may have enhanced the effectiveness of the mHealth interventions employed in this study. Our study reveals that the success and effectiveness of mHealth interventions is contingent on the service configurations and care programs employed at PHCCs. Despite the best efforts of the research team to ensure comparable service configuration at both rural PHCCs and those at UNRWA refugee camps, it appears that the maturity of the existing programs and the service configurations at each setting played an important role in the care outcomes at the end of the study. This underlines the importance of employing integrative approaches of diseases prevention and control in which existing NCD programs in underserved communities (ie, rural and refugee camps settings) are coupled with innovative approaches such as mHealth to provide an amplified effect of traditional NCD-targeted care.
Changes in Smoking Habits
Our study revealed an unremarkable and insignificant reduction in the proportion of smokers in intervention and control groups after a 1-year SMS intervention. This is in agreement with a study that showed that lifestyle behaviors such as smoking were not modified throughout the self-care education intervention whether via SMS, pamphlets or face-to-face meetings [36]. However, our finding is in contrast with other studies carried out in Iran, United Kingdom, and New Zealand [63,67,68], the three of which revealed a significant positive modification in smoking cessation in the intervention group based on SMSs. mHealth has the potential to support smoking cessation, especially when considered as an add-on to other smoking cessation services [67,69]. This again reveals that a successful mHealth program is contingent on the presence of other programs to support the creation of successful and sustainable change in behavior. Yet, it is worth mentioning that achieving actual changes in smoking habits and attaining smoking cessation necessitate employing integrated theoretical models of health behavioral change to design effective health behavior interventions. Such models recognize that behavioral change is dependent on a number of factors that are necessary for it to take place [70]. These factors include the individual’s strong intention to perform this behavior; having necessary information, skills, and capabilities required to actually perform it; and is not faced by any environmental constraints that may hinder the behavioral performance [70]. Although our mHealth intervention may have supported one of these factors toward the achievement of changes in smoking habits, it may have not interfered with other factors that are necessary to address to witness a significant and sustainable change.
Changes in Patients’ Utilization of Primary Care Services
In this study, targeted SMSs for reminders did not generate a clear intervention effect on patients’ utilization of primary care services, as well as their compliance with visits for HbA1c testing, eye check-ups, and foot exams. Both bivariate and multivariate analyses revealed that access for annual HbA1c testing increased significantly in both the intervention and control groups. This can be explained by the initiatives of the MOPH and UNRWA’s NCD programs aiming at the early detection of NCDs, proper management of these diseases, and the promotion of health awareness in all MOPH and UNRWA centers. However, the results of this study’s QIs reveal that these efforts were translated into measurement but not into outcomes, except for the intervention group where patients were receiving SMS messages. Furthermore, intervention bias could have taken place because QI collectors at PHCCs were aware of data collection post intervention. As a matter of fact, the increased percentage of recorded dates of visits to PHCCs for HbA1c testing in both the control and intervention groups may be the result of improved documentation rather than an actual enhanced access to PHC services. Nonetheless, clinical measurements of QIs, such as HbA1c levels, as well as SBP and DBP levels, reflect the general centers’ performance as they represent actual results rather than recorded dates. In addition, age was found to be statistically associated with annual HbA1c testing for the intervention group, reflecting a better compliance. Similarly, in New Jersey, older age seemed to have contributed to enhanced access to health services as older individuals may have conditions which necessitate a closer follow-up in PHCCs [71].
Studies have shown that SMS reminders considerably ameliorate the prospect of attending clinical appointments in general [33,72,73]. However, there is a notable gap in the literature with regard to whether SMS reminders sent to patients’ mobiles are successful and effective in decreasing nonattendance and increasing compliance to eye check-ups and foot exams among diabetic and hypertensive patients. In this study, there was a decrease in the access for annual eye check-up and foot exam among patients in both groups, in the posttest as compared with the pretest. This decrease was significant only for annual eye check-up among both hypertensive and diabetic patients. Intervention and control PHCCs assured that the same ophthalmologists are still providing eye care in these centers, and none of them quitted. Yet, the decrease in the access for annual eye check-up and foot exam can be explained by the considerable strain that the Syrian refugee influx in Lebanon is putting on host communities and on the resources in PHCCs. Consequently, the demand for chronic diseases’ care has increased dramatically leading to long waiting times in PHCCs and causing providers to practice in more than one center. Hence, Lebanese patients are adversely affected by an increased competition for accessing services. Although this was less evident in the general care for chronic diseases because of the presence of a good number of providers, competition for services was more pronounced in referral to specialist care as in the case of ophthalmologists as their numbers in rural and refugee camps is quite limited.
Additional efforts should be made from the providers’ side to underscore the importance of improved patients’ compliance with visits for HbA1c testing, eye check-ups, and foot exams. Providers are encouraged to utilize the integrated online modules, one of this study’s provider-side eHealth tools, especially the provider-patient communication strategies to focus on the importance of increasing compliance. Conveying face-to-face healthy information has a beneficial effect on adherence. Therefore, using SMSs as a reminder can come after face-to-face education to support it [36,42].
Limitations
A number of limitations in this study are worth mentioning. Data on age and gender of certain participants in both the intervention and control groups were missing. In addition, the study is characterized by its large sample size, which may have led to statistical significance without necessarily a parallel clinical or practical significance. Our results cannot be solely attributed to our intervention; the presence of advanced NCD programs at both the MOPH and UNRWA PHCC networks may have biased the findings, especially in the cases where a control site showed a significant change. Given that in some cases the owners of the phone numbers to which the SMSs were sent were not the patients themselves but rather family members, the interventional SMS messages may have not been transmitted to their final recipients (ie, patients) who are the target population of our study.
Other limitations may be embedded in the design of the intervention itself. For example, patients of low literacy level may have not benefitted optimally from the intervention because of a decreased capacity of understanding its content. Thus, it is worth bringing to attention for future research the need for pilot testing the SMS text messages interventions with individuals of low literacy level, using further simplified content of messages, and more importantly coupling SMS text messages with voice messages to enhance equitable access of illiterate patients to the information shared [74].
Policy and Practice Recommendations
Findings from this study should be considered by decision makers at the MOPH and the UNRWA, as the employed eHealth strategies, especially SMS messages, could be easily implemented within the PHC context and adapted to suit all diabetic and hypertensive patients across all centers. Given that the proposed eHealth interventions stem from the needs of the communities served, decision makers are advised to scale-up and use mHealth as a strategy for improving access to PHC, especially with the ongoing influx of Syrian refugees. However, decision makers are reminded to ensure the presence of adequate NCD curative and preventive services to enhance the effectiveness of mHealth programs. The study can also serve as a guidance in the formulation of the national eHealth policy in Lebanon. The findings should also be of interest to primary care providers, who should be adequately trained on accessing the proposed eHealth tools, while emphasizing to patients the importance of SMSs as a method to increase awareness about diseases and compliance to treatment. As mHealth is promising in delivering messages to hard-to-reach populations, the findings of this study may be applicable to similar contexts in the region.
Conclusions
Given the potential benefits of mHealth, more specifically SMS-based health interventions for the management and control of chronic conditions, its implementation in the EMR, and specifically Lebanon, is crucial. In this study, the statistically significant improvements in clinical measurements of NCD-related QIs among diabetic and hypertensive patients in Lebanese rural areas and Palestinian refugee camps reveal that the employment of SMSs may make a difference in NCDs care. The most pronounced effect was observed in improved BP control, mean SBP, HbA1c poor control, and mean HbA1c among patients who received weekly SMSs for 1 year. Further studies are needed to provide concrete evidence with regard to the effectiveness and usefulness of reminder SMSs for improving compliance and access to services in PHC centers. Future research is also advisable on patients’ perceptions and views on the acceptability and utility of the SMS service, as well as providers’ attitudes and barriers toward the full implementation of clinical guidelines. A separate analysis of different activities using this project’s eHealth interventions is currently underway with promising results. mHealth is a simple and a socially acceptable technique that can be integrated into routine care at a low cost. As mobile phones are widely accessible across the globe, including hard to reach and underserved populations, the need is underscored for additional studies to provide evidence on the utility of SMS-based health interventions and their impact on the care of underserved individuals and communities.
Acknowledgments
The research team wishes to acknowledge the valuable support and contributions of Dr. Walid Ammar, the Director General of the Ministry of Public Health (MOPH) and Dr. Randa Hamadeh, the Head of Social Health Service & PHC Department at MOPH. The authors also acknowledge the technical support was provided by the MOPH and the United Nations Relief and Works Agency (UNRWA) to ensure the commitment of participating centers. The study authors would like to acknowledge all the Community Health Workers and all data collectors for their efforts.
Abbreviations
- BP
blood pressure
- DBP
diastolic blood pressure
- eHealth
electronic health
- EMR
Eastern Mediterranean Region
- HbA 1c
glycated hemoglobin
- HTN
hypertension
- mHealth
mobile health
- MOPH
Ministry of Public Health
- LMIC
low- and middle-income countries
- NCD
noncommunicable disease
- OR
odds ratio
- PHC
primary health care
- PHCC
primary health care center
- QI
quality indicator
- SBP
systolic blood pressure
- SMS
short message service
- UNRWA
United Nations Relief and Works Agency
CONSORT‐EHEALTH checklist (V 1.6.1).
Footnotes
Conflicts of Interest: None declared.
References
- 1.World Health Organization. 2012. The top 10 causes of death http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death .
- 2.World Health Organization. 2015. NCD mortality and morbidity, Global Health Observatory (GHO) data http://www.who.int/gho/ncd/mortality_morbidity/en/
- 3.Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg. 2006 Mar;100(3):191–9. doi: 10.1016/j.trstmh.2005.07.021.S0035-9203(05)00317-2 [DOI] [PubMed] [Google Scholar]
- 4.The PLoS Medicine Editors Addressing global disparities in the burden of noncommunicable diseases: call for papers. PLoS Med. 2012 Dec 27;9(12):e1001360. doi: 10.1371/journal.pmed.1001360. [DOI] [Google Scholar]
- 5.Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics, and directions for public health action. Public Health Nutr. 2002 Feb;5(1A):231–7. doi: 10.1079/phn2001298.S1368980002000320 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2016. Regional Office of the Eastern Mediterranean: Non-Communicable diseases http://www.emro.who.int/entity/ncds/index.html .
- 7.Rahim HF, Sibai A, Khader Y, Hwalla N, Fadhil I, Alsiyabi H, Mataria A, Mendis S, Mokdad AH, Husseini A. Non-communicable diseases in the Arab world. Lancet. 2014 Jan 25;383(9914):356–67. doi: 10.1016/S0140-6736(13)62383-1.S0140-6736(13)62383-1 [DOI] [PubMed] [Google Scholar]
- 8.The world health report 2002 - reducing risks, promoting healthy life. Geneva: World Health Organization (WHO); 2002. Oct, [DOI] [PubMed] [Google Scholar]
- 9.Islam SM, Purnat TD, Phuong NT, Mwingira U, Schacht K, Fröschl G. Non-communicable diseases (NCDs) in developing countries: a symposium report. Global Health. 2014 Dec 11;10:81. doi: 10.1186/s12992-014-0081-9. https://globalizationandhealth.biomedcentral.com/articles/10.1186/s12992-014-0081-9 .s12992-014-0081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahin Y. World Health Organization. 2016. UNRWA's NCD Programme http://www.who.int/global-coordination-mechanism/working-groups/STKH-1PHC-UNRWA-shahin.pdf?ua=1 .
- 11.Arokiasamy P, Uttamacharya. Kowal P, Capistrant BD, Gildner TE, Thiele E, Biritwum RB, Yawson AE, Mensah G, Maximova T, Wu F, Guo Y, Zheng Y, Kalula SZ, Salinas RA, Manrique EB, Liebert MA, Eick G, Sterner KN, Barrett TM, Duedu K, Gonzales E, Ng N, Negin J, Jiang Y, Byles J, Madurai SL, Minicuci N, Snodgrass JJ, Naidoo N, Chatterji S. Chronic noncommunicable diseases in 6 low-and middle-income countries: findings from wave 1 of the World Health Organization's study on global ageing and adult health (SAGE) Am J Epidemiol. 2017 Dec 15;185(6):414–428. doi: 10.1093/aje/kww125.3003178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig J, Patterson V. Introduction to the practice of telemedicine. J Telemed Telecare. 2005;11(1):3–9. doi: 10.1177/1357633X0501100102. [DOI] [PubMed] [Google Scholar]
- 13.Mechael P, Batavia H, Kaonga N, Searle S, Kwan A, Goldberger A, Fu L, Ossman J. Barriers and gaps affecting mHealth in low and middle income countries: Policy white paper. New York, NY: Center for Global Health and Economic Development Earth Institute, Columbia University; 2010. http://www.globalproblems-globalsolutions-files.org/pdfs/mHealth_Barriers_White_Paper.pdf . [Google Scholar]
- 14.Amara AM, Aljunid SM. Noncommunicable diseases among urban refugees and asylum-seekers in developing countries: a neglected health care need. Global Health. 2014 Apr 03;10:24. doi: 10.1186/1744-8603-10-24. https://globalizationandhealth.biomedcentral.com/articles/10.1186/1744-8603-10-24 .1744-8603-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Jardali F, Lavis JN, Ataya N, Jamal D, Ammar W, Raouf S. Use of health systems evidence by policymakers in eastern Mediterranean countries: views, practices, and contextual influences. BMC Health Serv Res. 2012 Jul 16;12:200. doi: 10.1186/1472-6963-12-200. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-12-200 .1472-6963-12-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher D, Ford N, Unwin N. Priorities for developing countries in the global response to non-communicable diseases. Global Health. 2012 Jun 11;8:14. doi: 10.1186/1744-8603-8-14. https://globalizationandhealth.biomedcentral.com/articles/10.1186/1744-8603-8-14 .1744-8603-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maher D, Harries AD, Zachariah R, Enarson D. A global framework for action to improve the primary care response to chronic non-communicable diseases: a solution to a neglected problem. BMC Public Health. 2009 Sep 22;9:355. doi: 10.1186/1471-2458-9-355. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-9-355 .1471-2458-9-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norris SL, Chowdhury FM, Van Le K, Horsley T, Brownstein JN, Zhang X, Jack L, Satterfield DW. Effectiveness of community health workers in the care of persons with diabetes. Diabet Med. 2006 May;23(5):544–56. doi: 10.1111/j.1464-5491.2006.01845.x.DME1845 [DOI] [PubMed] [Google Scholar]
- 19.Ouma S, Herselman ME. E-health in rural areas: case of developing countries. Int J Humanit Soc Sci. 2008;2(4):194–200. [Google Scholar]
- 20.Samb B, Desai N, Nishtar S, Mendis S, Bekedam H, Wright A, Hsu J, Martiniuk A, Celletti F, Patel K, Adshead F, McKee M, Evans T, Alwan A, Etienne C. Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. Lancet. 2010 Nov 20;376(9754):1785–97. doi: 10.1016/S0140-6736(10)61353-0. [DOI] [PubMed] [Google Scholar]
- 21.Wakerman J, Humphreys JS. Sustainable primary health care services in rural and remote areas: innovation and evidence. Aust J Rural Health. 2011 Jun;19(3):118–24. doi: 10.1111/j.1440-1584.2010.01180.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilson NW, Couper ID, De Vries E, Reid S, Fish T, Marais BJ. A critical review of interventions to redress the inequitable distribution of healthcare professionals to rural and remote areas. Rural Remote Health. 2009;9(2):1060. http://www.rrh.org.au/articles/subviewnew.asp?ArticleID=1060 .1060 [PubMed] [Google Scholar]
- 23.Beaglehole R, Epping-Jordan J, Patel V, Chopra M, Ebrahim S, Kidd M, Haines A. Improving the prevention and management of chronic disease in low-income and middle-income countries: a priority for primary health care. Lancet. 2008 Sep 13;372(9642):940–9. doi: 10.1016/S0140-6736(08)61404-X. [DOI] [PubMed] [Google Scholar]
- 24.Kane J, Landes M, Carroll C, Nolen A, Sodhi S. A systematic review of primary care models for non-communicable disease interventions in Sub-Saharan Africa. BMC Fam Pract. 2017 Mar 23;18(1):46. doi: 10.1186/s12875-017-0613-5. https://bmcfampract.biomedcentral.com/articles/10.1186/s12875-017-0613-5 .10.1186/s12875-017-0613-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyder AA, Wosu AC, Gibson DG, Labrique AB, Ali J, Pariyo GW. Noncommunicable disease risk factors and mobile phones: a proposed research agenda. J Med Internet Res. 2017 May 05;19(5):e133. doi: 10.2196/jmir.7246. http://www.jmir.org/2017/5/e133/ v19i5e133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arambepola C, Ricci-Cabello I, Manikavasagam P, Roberts N, French DP, Farmer A. The impact of automated brief messages promoting lifestyle changes delivered via mobile devices to people with type 2 diabetes: a systematic literature review and meta-analysis of controlled trials. J Med Internet Res. 2016 Apr 19;18(4):e86. doi: 10.2196/jmir.5425. http://www.jmir.org/2016/4/e86/ v18i4e86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016 Dec 10;18(6):e135. doi: 10.2196/jmir.5066. http://www.jmir.org/2016/6/e135/ v18i6e135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobrow K, Brennan T, Springer D, Levitt N, Rayner B, Namane M, Yu LM, Tarassenko L, Farmer A. Efficacy of a text messaging (SMS) based intervention for adults with hypertension: protocol for the StAR (SMS Text-message Adherence suppoRt trial) randomised controlled trial. BMC Public Health. 2014 Jan 11;14:28. doi: 10.1186/1471-2458-14-28. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-14-28 .1471-2458-14-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burner ER, Menchine MD, Kubicek K, Robles M, Arora S. Perceptions of successful cues to action and opportunities to augment behavioral triggers in diabetes self-management: qualitative analysis of a mobile intervention for low-income Latinos with diabetes. J Med Internet Res. 2014 Jan 29;16(1):e25. doi: 10.2196/jmir.2881. http://www.jmir.org/2014/1/e25/ v16i1e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caburnay CA, Graff K, Harris JK, McQueen A, Smith M, Fairchild M, Kreuter MW. Evaluating diabetes mobile applications for health literate designs and functionality, 2014. Prev Chronic Dis. 2015 May 07;12:E61. doi: 10.5888/pcd12.140433. https://www.cdc.gov/pcd/issues/2015/14_0433.htm .E61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010 Mar 30;32(1):56–69. doi: 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müssener U, Bendtsen M, McCambridge J, Bendtsen P. User satisfaction with the structure and content of the NEXit intervention, a text messaging-based smoking cessation programme. BMC Public Health. 2016 Dec 22;16(1):1179. doi: 10.1186/s12889-016-3848-5. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-016-3848-5 .10.1186/s12889-016-3848-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youssef A. Use of short message service reminders to improve attendance at an internal medicine outpatient clinic in Saudi Arabia: a randomized controlled trial/Utilisation de rappels par minimessages afin d'améliorer l'assiduité des patients dans un service de consultations externes en médecine interne en Arabie saoudite: essai contrôlé randomisé. East Mediterr Health J. 2014 Jun 09;20(5):317–23. [PubMed] [Google Scholar]
- 34.Axler RE, Strong KA, Jordens C, Ankeny RA, Barlow-Stewart K, Kerridge IH. What's in a name? searching the web for information about ethically contentious and emerging healthcare technologies. J Commun Healthc. 2013 Jul 18;2(2):173–183. doi: 10.1179/cih.2009.2.2.173. [DOI] [Google Scholar]
- 35.Capozza K, Woolsey S, Georgsson M, Black J, Bello N, Lence C, Oostema S, North C. Going mobile with Diabetes support: a randomized study of a text message-based personalized behavioral intervention for type 2 diabetes self-care. Diabetes Spectr. 2015 May;28(2):83–91. doi: 10.2337/diaspect.28.2.83. http://europepmc.org/abstract/MED/25987806 .83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golshahi J, Ahmadzadeh H, Sadeghi M, Mohammadifard N, Pourmoghaddas A. Effect of self-care education on lifestyle modification, medication adherence and blood pressure in hypertensive adults: randomized controlled clinical trial. Adv Biomed Res. 2015 Sep 28;4:204. doi: 10.4103/2277-9175.166140. http://www.advbiores.net/article.asp?issn=2277-9175;year=2015;volume=4;issue=1;spage=204;epage=204;aulast=Golshahi .ABR-4-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Heerden A, Tomlinson M, Swartz L. Point of care in your pocket: a research agenda for the field of m-health. Bull World Health Organ. 2012 May 01;90(5):393–4. doi: 10.2471/BLT.11.099788. http://www.scielosp.org/scielo.php?script=sci_arttext&pid=BLT.11.099788&lng=en&nrm=iso&tlng=en .BLT.11.099788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shariful Islam SM, Niessen L, Ferrari U, Ali L, Seissler J, Lechner A. Effects of mobile phone SMS to improve glycemic control among patients with type 2 diabetes in Bangladesh: a prospective, parallel-group, randomized controlled trial. Diabetes Care. 2015 Aug;38(8):e112–3. doi: 10.2337/dc15-0505.38/8/e112 [DOI] [PubMed] [Google Scholar]
- 39.Kasparian NA, Lieu N, Winlaw DS, Cole A, Kirk E, Sholler GF. eHealth literacy and preferences for eHealth resources in parents of children with complex CHD. Cardiol Young. 2017 May;27(4):722–730. doi: 10.1017/S1047951116001177.S1047951116001177 [DOI] [PubMed] [Google Scholar]
- 40.Kiselev AR, Gridnev VI, Shvartz VA, Posnenkova OM, Dovgalevsky PY. Active ambulatory care management supported by short message services and mobile phone technology in patients with arterial hypertension. J Am Soc Hypertens. 2012 Sep;6(5):346–55. doi: 10.1016/j.jash.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Krishna S, Boren SA. Diabetes self-management care via cell phone: a systematic review. J Diabetes Sci Technol. 2008 May;2(3):509–17. doi: 10.1177/193229680800200324. http://europepmc.org/abstract/MED/19885219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuboyejo S, Eyesan O. mHealth: using mobile technology to support healthcare. Online J Public Health Inform. 2014;5(3):233. doi: 10.5210/ojphi.v5i3.4865. http://europepmc.org/abstract/MED/24678384 .ojphi-05-e233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011 Mar;1(1):53–71. doi: 10.1007/s13142-011-0021-7. http://europepmc.org/abstract/MED/21796270 .21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. 2014. Noncommunicable diseases and mental health- Lebanon http://www.who.int/nmh/countries/lbn_en.pdf .
- 45.UNRWA. Where we work https://www.unrwa.org/where-we-work/lebanon .
- 46.Yamout R, Adib SM, Hamadeh R, Freidi A, Ammar W. Screening for cardiovascular risk in asymptomatic users of the primary health care network in Lebanon, 2012-2013. Prev Chronic Dis. 2014 Jul 17;11:e120. doi: 10.5888/pcd11.140089. https://www.cdc.gov/pcd/issues/2014/14_0089.htm .E120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen B, Cammett M. Informal politics and inequity of access to health care in Lebanon. Int J Equity Health. 2012 May 09;11:23. doi: 10.1186/1475-9276-11-23. https://equityhealthj.biomedcentral.com/articles/10.1186/1475-9276-11-23 .1475-9276-11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.UNRWA. 2011. Palestine refugees in Lebanon: a special case https://www.unrwa.org/userfiles/20111002306.pdf .
- 49.Habib RR, Seyfert K, Hojeij S. Health and living conditions of Palestinian refugees residing in camps and gatherings in Lebanon: a cross-sectional survey. Lancet. 2012 Oct;380:S3. doi: 10.1016/S0140-6736(13)60189-0. [DOI] [Google Scholar]
- 50.Union I. Union IT. 2016. Time series by country (until 2015): mobile-cellular subscriptions https://www.itu.int/en/ITU-D/Statistics/Pages/stat/default.aspx .
- 51.Talhouk R, Mesmar S, Thieme A, Balaam M, Olivier P, Akik C, Ghattas H. Syrian refugees and digital health in Lebanon: opportunities for improving antenatal health. CHI '16 Proceedings of the 2016; CHI Conference on Human Factors in Computing Systems; 07-12 May, 2016; San Jose, California, USA. 2016. pp. 331–42. [DOI] [Google Scholar]
- 52.MOPH. 2016. Strategic plan for the medium term (2016 to 2020) https://www.moph.gov.lb/en/Pages/0/11665/strategic-plan-2016-2020-
- 53.Márquez Contreras E, de la Figuera von Wichmann M, Gil Guillén V, Ylla-Catalá A, Figueras M, Balaña M, Naval J. [Effectiveness of an intervention to provide information to patients with hypertension as short text messages and reminders sent to their mobile phone (HTA-Alert)] Aten Primaria. 2004 Nov 15;34(8):399–405. doi: 10.1016/S0212-6567(04)78922-2. https://linkinghub.elsevier.com/retrieve/pii/13068215 .13068215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park LG, Beatty A, Stafford Z, Whooley MA. Mobile phone interventions for the secondary prevention of cardiovascular disease. Prog Cardiovasc Dis. 2016;58(6):639–50. doi: 10.1016/j.pcad.2016.03.002. http://europepmc.org/abstract/MED/27001245 .S0033-0620(16)30020-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wald DS, Bestwick JP, Raiman L, Brendell R, Wald NJ. Randomised trial of text messaging on adherence to cardiovascular preventive treatment (INTERACT trial) PLoS One. 2014 Dec 05;9(12):e114268. doi: 10.1371/journal.pone.0114268. http://dx.plos.org/10.1371/journal.pone.0114268 .PONE-D-14-35596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orsama AL, Lähteenmäki J, Harno K, Kulju M, Wintergerst E, Schachner H, Stenger P, Leppänen J, Kaijanranta H, Salaspuro V, Fisher WA. Active assistance technology reduces glycosylated hemoglobin and weight in individuals with type 2 diabetes: results of a theory-based randomized trial. Diabetes Technol Ther. 2013 Aug;15(8):662–9. doi: 10.1089/dia.2013.0056. [DOI] [PubMed] [Google Scholar]
- 57.Haddad NS, Istepanian R, Philip N, Khazaal FA, Hamdan TA, Pickles T, Amso N, Gregory JW. A feasibility study of mobile phone text messaging to support education and management of type 2 diabetes in Iraq. Diabetes Technol Ther. 2014 Jul;16(7):454–9. doi: 10.1089/dia.2013.0272. [DOI] [PubMed] [Google Scholar]
- 58.Bin Abbas B, Al-Fares A, Jabbari M, El Dali A, Al-Orifi F. Effect of mobile phone short text messages on glycemic control in type 2 diabetes. Int J Endocrinol Metab. 2015 Jan 01;13(1):e18791. doi: 10.5812/ijem.18791. http://europepmc.org/abstract/MED/25745493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu L, Ogwu S. A meta-analysis of mobile health and risk reduction in patients with diabetes mellitus: challenge and opportunity. J Mob Technol Med. 2012;1(3):17–24. doi: 10.7309/jmtm.18. [DOI] [Google Scholar]
- 60.Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform. 2008 Jun;77(6):399–404. doi: 10.1016/j.ijmedinf.2007.07.006.S1386-5056(07)00139-6 [DOI] [PubMed] [Google Scholar]
- 61.Goodarzi M, Ebrahimzadeh I, Rabi A, Saedipoor B, Jafarabadi MA. Impact of distance education via mobile phone text messaging on knowledge, attitude, practice, and self efficacy of patients with type 2 diabetes mellitus in Iran. J Diabetes Metab Disord. 2012 Aug 31;11(1):10. doi: 10.1186/2251-6581-11-10. https://jdmdonline.biomedcentral.com/articles/10.1186/2251-6581-11-10 .2251-6581-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arora S, Peters AL, Burner E, Lam CN, Menchine M. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med. 2014 Jun;63(6):745–54.e6. doi: 10.1016/j.annemergmed.2013.10.012.S0196-0644(13)01486-8 [DOI] [PubMed] [Google Scholar]
- 63.Hussein WI, Hasan K, Jaradat AA. Effectiveness of mobile phone short message service on diabetes mellitus management; the SMS-DM study. Diabetes Res Clin Pract. 2011 Oct;94(1):e24–6. doi: 10.1016/j.diabres.2011.07.025.S0168-8227(11)00401-3 [DOI] [PubMed] [Google Scholar]
- 64.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32:56–69. doi: 10.1093/epirev/mxq004. http://europepmc.org/abstract/MED/20354039 .mxq004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.UNRWA. 2017. International conference explores how to improve diabetes prevention and care for refugees https://www.unrwa.org/newsroom/press-releases/international-conference-explores-how-improve-diabetes-prevention-and-care .
- 66.MOPH. 2015. Integration of non-communicable disease services within primary health care http://www.moph.gov.lb/en/Pages/6/776/non-communicable-disease-program-primary-health-care .
- 67.Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, Rodgers A, Cairns J, Kenward Mg, Roberts I. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011 Jul 02;378(9785):49–55. doi: 10.1016/S0140-6736(11)60701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodgers A, Corbett T, Bramley D, Riddell T, Wills M, Lin RB, Jones M. Do u smoke after txt? results of a randomised trial of smoking cessation using mobile phone text messaging. Tob Control. 2005 Aug;14(4):255–61. doi: 10.1136/tc.2005.011577. http://tobaccocontrol.bmj.com/cgi/pmidlookup?view=long&pmid=16046689 .14/4/255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McIvor A, Kayser J, Assaad JM, Brosky G, Demarest P, Desmarais P, Hampson C, Khara M, Pathammavong R, Weinberg R. Best practices for smoking cessation interventions in primary care. Can Respir J. 2009 Jul;16(4):129–34. doi: 10.1155/2009/412385. http://europepmc.org/abstract/MED/19707607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fishbein M, Yzer MC. Using theory to design effective health behavior interventions. Commun Theory. 2003 May 01;13(2):164–183. doi: 10.1111/j.1468-2885.2003.tb00287.x. [DOI] [Google Scholar]
- 71.Parikh A, Gupta K, Wilson AC, Fields K, Cosgrove NM, Kostis JB. The effectiveness of outpatient appointment reminder systems in reducing no-show rates. Am J Med. 2010 Jun;123(6):542–8. doi: 10.1016/j.amjmed.2009.11.022.S0002-9343(10)00108-7 [DOI] [PubMed] [Google Scholar]
- 72.Downer SR, Meara JG, Da Costa AC. Use of SMS text messaging to improve outpatient attendance. Med J Aust. 2005 Oct 03;183(7):366–8. doi: 10.5694/j.1326-5377.2005.tb07085.x.dow10816_fm [DOI] [PubMed] [Google Scholar]
- 73.Guy R, Hocking J, Wand H, Stott S, Ali H, Kaldor J. How effective are short message service reminders at increasing clinic attendance? A meta-analysis and systematic review. Health Serv Res. 2012 Apr;47(2):614–32. doi: 10.1111/j.1475-6773.2011.01342.x. http://europepmc.org/abstract/MED/22091980 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaman SB, Hossain N, Ahammed S, Ahmed Z. Contexts and opportunities of e-health technology in medical care. J Med Res Innov. 2017 May 01;1(2):AV1–AV4. doi: 10.15419/jmri.62. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT‐EHEALTH checklist (V 1.6.1).


