Abstract
HIV-1 spreads through contacts between infected and target cells. Polarized viral budding at the contact site forms the virological synapse. Additional cellular processes, such as nanotubes, filopodia, virus accumulation in endocytic or phagocytic compartments promote efficient viral propagation. Cell-to-cell transmission allows immune evasion and likely contributes to HIV-1 spread in vivo. Anti-HIV-1 broadly neutralizing antibodies (bNAbs) defeat the majority of circulating viral strains by binding to the viral envelope glycoprotein (Env). Several bNAbs have entered clinical evaluation during the last years. It is thus important to understand their mechanism of action and to determine how they interact with infected cells. In experimental models, HIV-1 cell-to-cell transmission is sensitive to neutralization, but the effect of antibodies is often less marked than during cell-free infection. This may be due to differences in the conformation or accessibility of Env at the surface of virions and cells. In this review, we summarize the current knowledge on HIV-1 cell-to-cell transmission and discuss the role of bNAbs during this process.
Keywords: HIV-1, bNAbs, Cell-to-cell transmission, Neutralization
Background
Human Immunodeficiency Virus (HIV-1) is the etiological agent of AIDS [1]. Identification of molecular mechanisms governing the replication of HIV-1 allowed the design of potent antiretroviral treatment (ART). Combined ART restored the life expectancy of patients, transforming a fatal infection into a manageable chronic disease. However, limited access to therapy in many regions of the world and the existence of a viral reservoir insensitive to treatment urge the need for novel antiviral strategies.
HIV-1 infects cells by multiple mechanisms, either as cell-free or cell-associated particles [2, 3]. HIV-1 infection is more efficient when the virus is transmitted through direct cell contacts. HIV-1 follows different routes of cell-to-cell transmission [4]. One main mechanism involves a structure called the Virological Synapse (VS). It allows the polarized delivery of newly formed viral particles [5, 6]. Its organization requires both cellular and viral proteins. The virus also hijacks other cellular pathways to spread, such as nanotubes, filopodia, phagocytic or endocytic compartments.
As part of the immune response, infected individuals rapidly develop anti-HIV-1 antibodies, as soon as one week following initial viral exposure [7]. These early-produced antibodies do not neutralize the virus [7]. The first neutralizing antibodies are detected two to three months later [8]. These antibodies are inefficient against heterologous viral strains and are rapidly escaped by mutation of the autologous virus [9, 10]. Some patients called elite neutralizers develop antibodies with broad neutralization potency [11]. Deconvolution of their polyclonal response enabled the identification of several monoclonal bNAbs (reviewed in [12]). Potent bNAbs present peculiar molecular features, such as intensive hypermutation and often long CDRH3 regions (reviewed in [13]). bNAbs target conserved regions on the viral Env spike, called sites of vulnerability [13]. These include the CD4 binding site (CD4bs), the N-glycans of V1/V2 and V3 loops, the gp41 membrane proximal external region (MPER), and the gp120/gp41 interface, which comprises a recently described epitope composed of the fusion peptide at the N-terminus of gp41 and the N88 glycan on gp120 [14–16]. bNAbs are often screened and selected with assays that use cell-free virus. The capacity of bNAbs to suppress cell-to-cell transmission has been thus often under-evaluated. In vitro, bNAbs neutralize cell-free infection by many viral strains and trigger Fc-mediated effector mechanisms, including antibody-dependent cellular cytotoxicity (ADCC) [17]. In animal models, bNAbs display both prophylactic [18] and therapeutic efficacy (reviewed in [19]). They clear HIV-infected cells and modulate host immune responses [20, 21]. These findings suggest that bNAbs could target the latent HIV reservoir and contribute to long-term remission of HIV-1 infection in humans.
Phase 1 studies of bNAbs targeting the CD4bs (3BNC117 and VRC01) and the V3 loop (10-1074) demonstrated their safety and efficacy (reviewed in [22]). Infusion of single bNAbs induced a transient decline in viremia of approximately 1.5 log10 copies/ml, followed by selection of escape variants [23–25]. Of note, the half-life of 10-1074 (24 days) was higher than that of 3BNC117 and VRC01 (around 15 days). In ART-treated patients pre-screened for their susceptibility to 3BNC117, infusion of this antibody delayed viral rebound after ART cessation by an average of 8 weeks [26]. Moreover, 3BNC117 potentiated subsequent anti-HIV-1 host antibody responses, demonstrating an immunomodulatory potential that is not fully understood [27]. Thus, understanding the molecular and cellular bases of bNAbs antiviral activity is critical to optimize their in vivo efficacy.
In this review, we first summarize the current knowledge on HIV cell-to-cell transmission. We discuss the mechanisms that may account for the differences observed in neutralization of cell-free and cell-associated HIV-1. We then detail how bNAbs bound at the cell surface neutralize viral propagation but also destroy infected cells by ADCC and other mechanisms.
HIV-1 cell-to-cell transmission
The virological synapse between infected an uninfected T cells
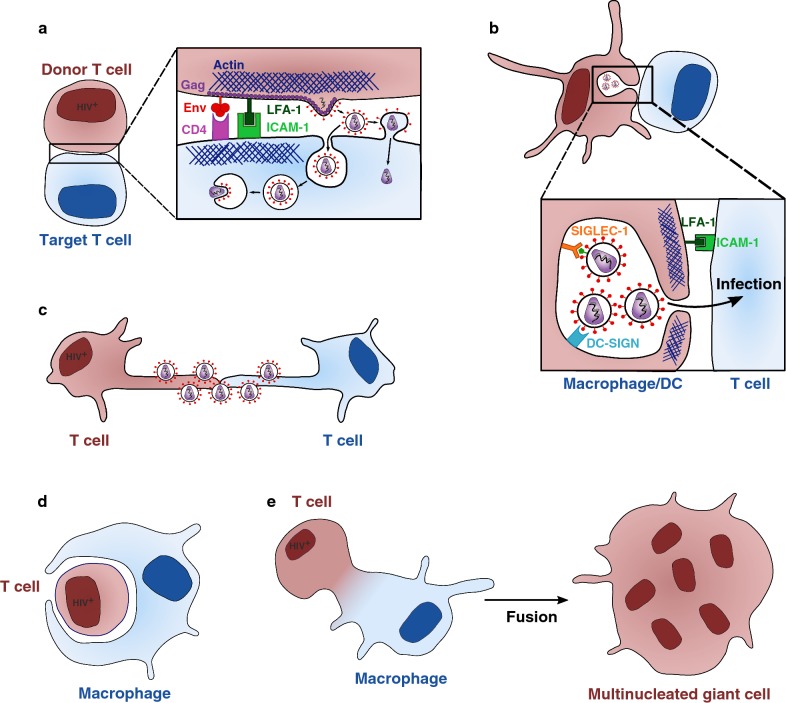
Early studies reported secretion of HIV-1 particles and relocalization of adhesion molecules at the contact zone between infected and uninfected T cells [28–30]. The precise mechanisms of viral cell-to-cell transmission were initially described with another retrovirus, Human T cell Leukemia Virus type 1 (HTLV-I) [31]. Upon cell–cell contacts, HTLV-I Env, Gag and the viral genome accumulate at cell–cell junctions, allowing polarized budding of viral particles and their transfer to the target cells in a confined area. Igakura and colleagues named this structure the “virological synapse” (VS) due to its similarities with the immunological synapse that forms between T lymphocytes and Dendritic Cells (DCs) during antigen presentation [32]. The VS was then observed during HIV-1 spread in T cells [5]. The HIV-1 VS displayed similar features: recruitment of Env and Gag at the interface on the producer cell side and of the cytoskeleton on the target side [5] (Fig. 1a). An infected cell can generate more than one VS, allowing simultaneous transfer of HIV-1 to multiple targets [33].
Fig. 1.
Mechanisms of HIV-1 cell-to-cell transmission. a Infected and uninfected T cells come in contact to form a virological synapse. HIV-1 gains access to the cytoplasm of the target cell by direct fusion at the plasma membrane or eventually after endocytosis. This structure is dependent on Env/CD4 interaction, adhesion molecules (LFA-1/ICAM-1) interaction, and the cytoskeleton. b Uninfected macrophages or dendritic cells (DC) store HIV-1 particles in intracellular compartments after capture via DC-SIGN or SIGLEC-1. These particles can be released and transferred to CD4+ T cells through the infectious synapse. c HIV-1 surfs along nanotubes between uninfected and infected T cells. d Macrophages can be infected after phagocytosis of infected CD4+ T cells. e Macrophages can fuse with infected CD4+ T cells and with surrounding uninfected macrophages to form multinucleated giant cells. Donor cells are in brown and uninfected cells in blue
HIV-1 drives the organization of the VS. The VS is initiated by interactions between Env on the donor and CD4 on the target cell [5]. Env-mediated fusion seems to be regulated at the VS to decrease or slow down the formation of syncytia. The interaction between the Env cytoplasmic domain and the underlying immature Gag (p55) lattice reduces Env fusogenicity [34]. Fusion is also impaired by cellular proteins, such as tetraspanins or ezrin that accumulate at the VS [35, 36]. Co-receptor (CCR5 or CXCR4) engagement is not necessary for VS formation and transfer of virions [37]. However, co-receptors are required for subsequent productive infection [38].
After initial CD4/Env interactions, cellular adhesion molecules such as LFA-1, ICAM-1 and ICAM-3 are recruited to stabilize the VS [33, 39]. These adhesins are not mandatory, as blocking ICAM-1/3 and LFA-1 by antibodies does not inhibit the creation of cell conjugates and viral transfer [40]. Whether the recruitment of adhesion molecules to the VS is involved in its stabilization or has other functions is not fully understood. The cytoskeleton plays a predominant role during HIV-1 cell-to-cell transmission. The formation of the VS depends on actin and tubulin [5, 41, 42], and is associated with a relocalization of the MTOC towards the site of cell–cell contact, which contributes to the trafficking of viral and cellular proteins to the VS [31, 43, 44]. However, viral transfer can occur simultaneously to multiple targets, even if the MTOC is localized towards a single recipient cell [43]. Lipid rafts also promote Gag and Env clustering at the synapse [45].
Various viral and cellular proteins modulate positively or negatively HIV-1 cell-to-cell transfer. The viral protein Nef promotes the accumulation of Gag below the cellular membrane, increasing the transfer of mature HIV-1 virions and productive infection of target cells [46]. BST2/Tetherin, an interferon-induced gene that restricts HIV-1, accumulates with Gag and actin at the VS in infected donor cells and limits viral cell-to-cell spread [47, 48]. However, the inhibitory effect of tetherin is debated [49]. IFITM3, another interferon-stimulated gene with antiviral activities, also impairs cell-to-cell transfer of HIV-1 when expressed on either donor and target cells [50] and may act by infiltrating budding viral particles [50, 51].
Following VS formation, depending on the cell types used, newly produced viral particles can either fuse directly at the target cell plasma membrane [5] or be endocytosed by the acceptor T cell in a clathrin- and dynamin-dependent manner [38, 52, 53] (Fig. 1a). It has been proposed that HIV-1 viral particles transferred through the VS may undergo maturation after endocytosis [54]. However, this route of entry has not been observed during cell-free infection [55]. Whatever the entry route, polarized HIV-1 budding leads to a massive release of viral particles into the cytoplasm of the target cell. This high multiplicity of infection (MOI) leads to a two to three log increase in the efficiency of transmission for cell-associated HIV-1 compared to cell-free virus [37, 56, 57]. It also enhances the number of integrated proviruses [58, 59], and accelerates viral gene expression and spread [60].
VS formation has been observed in vivo by intravital imaging of mice infected with the Friend murine leukemia virus [61]. This study confirmed the role of Env for VS formation and the polarization of Gag at the sites of cell–cell contact in vivo. HIV-1 spread has also been studied in humanized mouse models. HIV-1-infected T cells migrate to lymph nodes, where they bind to target cells, transfer the virus, and also form syncytia [62, 63]. Tomographic analyses identified HIV-1 budding at sites of close cell–cell contact through LFA-1- and ICAM-1-positive structures [64]. Furthermore, the observation of Env-dependent stable contacts between infected and uninfected CD4+ T cells, co-transmission of multiple viral genotypes, and foci of viral replication suggests that cell-to-cell transmission occurs in lymphoid organs of humanized mice [65].
Thus, T cell-to-T cell transmission of HIV-1 is highly efficient in vitro and likely contributes to viral dissemination in vivo.
The infectious synapse between DCs/macrophages and T cells
DCs and macrophages transmit HIV-1 to T cells through different routes, namely cis- and trans-infections. During cis-infection, DCs and macrophages are productively infected and transmit HIV-1 to CD4+ T cells through a VS-like structure [66–68]. However, DCs are relatively resistant to productive infection [69]. They express SAMHD1, which inhibits reverse transcription [70–72] and regulates immune sensing and host responses [73–75]. These cells express low levels of HIV-1 receptor and co-receptors [76, 77]. Macrophages can be productively infected by HIV-1, which buds and accumulates into intracellular tetraspanin-rich compartments termed Virus Containing Compartments (VCCs) [78–80]. VCCs are connected to the cell membrane and release virus to neighboring cells [80].
DCs or macrophages that have captured viral particles but are not productively infected also transmit HIV-1 to CD4+ T cells [81]. This trans-infection mechanism is thought to play a role in vivo (reviewed in [82]). DCs and macrophages may capture HIV-1 in a CD4-independent manner. Different cellular proteins bind HIV-1 particles. Env interacts with the C-type lectin DC-SIGN prior to internalization into VCCs [83–86]. In mature DCs, Siglec-1 capture virions in an Env-independent manner by binding to gangliosides present on the viral membrane, also leading to internalization into VCCs [87–90]. After capture, HIV-1 is transferred to T cells through a structure reminiscent of the VS: the Infectious Synapse (IS) [91] (Fig. 1b). In contrast to the VS, CD4 and Env are dispensable for the formation of the IS, but are necessary for viral fusion and productive infection of T cells [92]. IS formation and subsequent viral transfer require the cortical actin cytoskeleton, which is stabilized by tetraspanin-7 and dynamin-2 in DCs [93]. Interactions between LFA-1 and ICAM-1, and between MHC and TCR modulate DC-to-T cell trans-infection [91]. Exosomes released by DCs may also facilitate viral transfer [94]. Recent multidimensional techniques have revealed that the myeloid compartment is more complex than initially thought and comprises at least four monocyte and six DC subsets, including novel pre-DC and plasmacytoid DC (pDC) populations [95, 96]. It will be of interest to determine the sensitivity to HIV-1 infection and the ability to transfer the virus across the spectrum of DC subsets [97].
Other modes of cell-to-cell transmission of HIV-1
Various additional modes of cell-associated HIV-1 transfer have been reported. HIV-1 can use close-ended membrane protrusions called tunneling nanotubes (TNTs) that form between infected and uninfected T cells to spread in a receptor-dependent manner [98] (Fig. 1c). A similar usage of TNTs was observed in macrophages and it has been proposed that Nef induces these TNTs [99, 100]. HIV-1 is contained within endosomes during TNT-mediated transfer in macrophages [101, 102]. Actin-rich membrane protrusions called filopodia are also induced in DCs after interaction between HIV-1 and DC-SIGN, in a Cdc42- [103] and Diaph2-dependent manner [93, 104], facilitating HIV-1 transfer to CD4+ T cells.
Macrophages also engulf living or dying HIV-1-infected T cells allowing their productive infection [105] (Fig. 1d). The impact of the most potent bNAbs on this mode of transmission has not been assessed yet. A two-step process for transfer of HIV-1 from infected CD4+ T cells to macrophages has been described [106]. First, CD4+ T cells establish a contact with macrophages and fuse. This macrophage-T cell hybrid will then fuse with surrounding uninfected macrophages, spreading the infection via multinucleated giant cells (Fig. 1e). CD4+ T cells can also form a VS-like structure with epithelial cells from the genital mucosa, which leads to transcytosis of HIV-1 through the epithelium and subsequent infection of stromal macrophages [107, 108].
Overall, HIV-1 hijacks various pathways to spread across cells that contact each other. This likely contributes to inter- and intra-individual viral propagation. Thus, efficacious antiviral agents must block both cell-free and cell-to-cell infection.
bNAbs and cell-to-cell transmission of HIV-1
Inhibition of HIV-1 transmission through the virological synapse
Before the discovery of bNAbs, several studies investigated the capacity of antibodies to block HIV-1 cell-to-cell transmission. Some sera from infected patients lost their neutralizing activity when the source of HIV-1 was cell-associated [57, 109, 110]. The ability of patients’ sera to maintain activity against cell-associated HIV-1 was patient-dependent and correlated with the neutralization breadth [111]. First generation neutralizing monoclonal antibodies, such as the anti-MPER 2F5 and 4E10, the anti-V3 antibody 257-D, and the anti-CD4bs b12 were also tested in cell–cell assays, but results were conflicting, and no clear pattern could be determined [112–114].
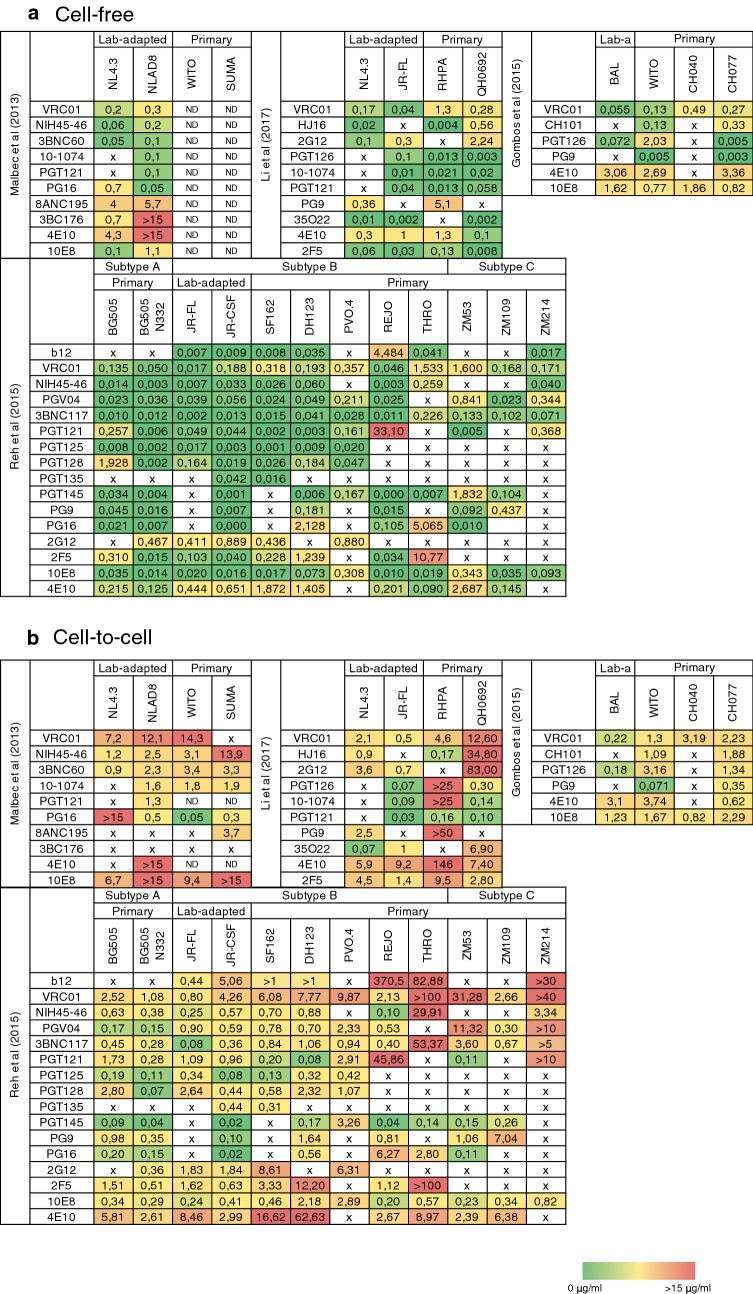
The development of second generation bNAbs allowed a more comprehensive examination of the role of antibodies during T cell-to-T cell transfer of HIV-1 (Fig. 2). The epitope targeted may influence the efficacy of a given antibody [115]. It has been shown that some CD4bs-directed antibodies were less potent neutralizers during cell-to-cell transmission than during cell-free transfer, with IC50s that were 10 times higher in intercellular systems [115]. Our laboratory tested the ability of 15 bNAbs targeting different Env epitopes to inhibit cell-to-cell transmission of both lab-adapted and Transmitted/Founder (T/F) HIV-1 strains [116]. We confirmed the relative neutralization resistance of cell-associated HIV-1. However, we identified bNAbs that were potent neutralizers of cell-associated virus in primary CD4+ T cells and pDCs. The most active bNAbs were targeting the CD4bs (NIH45-46 and 3BNC60) or the glycan/V3 loop (10-1074 and PGT121). They significantly decreased the formation of clusters and syncytia between uninfected and infected T cells, and the transfer of viral material through the VS. The efficacy of bNAbs against cell-associated HIV-1 was also dependent on the viral strain studied, indicating that the antibody breadth may be different against virions and infected cells. Another study analyzed the activity of 16 bNAbs during cell-free and cell-to-cell transmission of 11 viral strains [117]. Again, the neutralizing activity of bNAbs was generally decreased in cell-to-cell assays. Some bNAbs maintained a high level of inhibition against various viral strains, but no single bNAb was potent for all strains tested [117]. Combinating bNAbs may overcome this problem. For instance, a combination of PG9 and VRC01 demonstrated improved ability to neutralize cell-associated HIV-1 compared to individual antibodies [118]. Recently, a study focused on the maximum neutralization capacity of bNAbs during cell-to-cell transmission rather than on the IC50 [119]. During cell-to-cell transmission of two T/F strains, most of the tested bNAbs failed to reach 100% of neutralization, even at high concentrations. This phenomenon was not observed with two lab-adapted strains. This residual replication may allow the virus to keep spreading and may lead to the apparition of escape mutations. Whether the ability of primary HIV-1 isolates to spread by cell-to-cell transmission differs from lab-adapted strains, and how this may impair neutralization efficacy of bNAbs are still unresolved questions.
Fig. 2.
Neutralization potency of bNAbs against cell-free and cell-to-cell transmission of various viral strains. Cell-free (a) and cell-to-cell (b) neutralization IC50s of different bNAbs against several viral strains were compiled from the indicated studies (Malbec et al. [116]; Reh et al. [117], Gombos et al. [118], and Li et al. [119]). IC50s are color-coded with a heat map ranging from 0 (green) to 15 µg/ml and more (red). x not effective, no IC50 could be determined; ND not done; Lab-a lab-adapted
Antibodies interfere with HIV-1 cell-to-cell transmission through different mechanisms. For instance, b12 (a first generation anti-CD4bs antibody) inhibits the formation of the VS while 2F5 or 4E10 (anti-MPER) rather act later, by inhibiting viral fusion [114, 120]. Other bNAbs targeting the gp120, such as NIH45-46, 3BNC60, VRC01, 10-1074, or PGT121 also inhibit the formation of conjugates between infected and target CD4+ T cells [116]. Antibody efficacy varies depending on their time of addition in the co-culture [120]. For instance, b12 impairs VS formation, but does not disrupt an existing one [120]. Therefore, depending on the epitopes, bNAbs may either impair formation of cell conjugates and VS, transfer of viral material to target cells, or fusion.
Inhibition of HIV-1 transfer from DCs and macrophages
HIV-1 transiting through a macrophage/T cell VS is inhibited by anti-gp120 bNAbs, but less sensitive to some anti-gp41 antibodies [68]. Early studies showed that neutralizing antibodies 2F5, 2G12 and b12 inhibited HIV-1 transfer from infected DCs to T cells without impairing the formation of the IS [121, 122]. The role of bNAbs on trans-infection is debated. 2F5-, 4E10- and 2G12-opsonized HIV-1 particles are captured more efficiently by DCs in a DC-SIGN-dependent manner, probably because DC-SIGN also binds IgG [123]. The particles recover their infectivity after internalization, probably due to antigen–antibody dissociation, leading to enhanced trans-infection. However, some bNAbs were also shown to inhibit infection or trans-infection from monocyte-derived or plasmacytoid dendritic cells to CD4+ T cells and vice versa [116, 124, 125]. In another study, gp120-targeting antibodies (b12, VRC01, PG16 and 2G12) had a higher IC50 against DC-associated virus, whereas anti-MPER 4E10 and 2F5 maintained their potency during DC-to-T cell transmission [126].
Therefore, some bNAbs inhibit trans-infection and transmission from DCs or macrophages to lymphocytes. Discrepancies have been reported for the same antibodies in different studies. These discrepant results likely depend on the DC subtype used, which may express different levels of molecules such as DC-SIGN, Siglec-1, or Env, at the surface or within intracellular compartments.
Potential explanations for the increased resistance of cell-associated HIV-1 to neutralization by bNAbs
Different non-mutually exclusive mechanisms may account for the increased resistance of cell-to-cell HIV-1 transmission to bNAbs. They include steric hindrance at the VS, the MOI associated to this mode of viral propagation, the accessibility and conformation of Env at the cell surface, and the stability of Env-Ab complexes at the cell surface.
Steric hindrance at the VS and in other cellular compartments
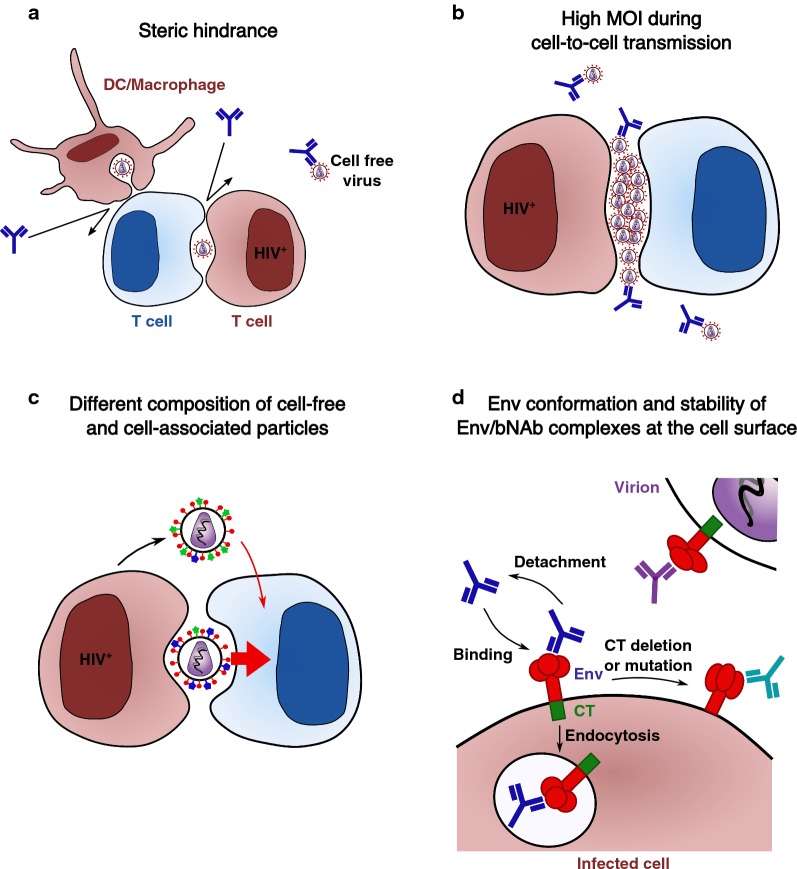
The VS involves a physical proximity of the membranes of donor and target T cells and may imply a low accessibility of bNAbs to the VS (Fig. 3a). However, some bNAbs like b12, NIH45-46 or 3BNC60 successfully accumulate at the VS between T cells [116, 120]. It will be of interest to determine whether access to the VS correlates with the inhibitory activity of each antibody. It is also possible that some antibodies bind to Env outside of the synapse, and will then be transported to the VS as a complex with their antigens. The virus may also be endocytosed after transmission through the VS [54], limiting the time frame of access of bNAbs. A llama antibody termed J3 is a potent neutralizer of cell-to-cell HIV-1 transmission [127]. The small size of the llama VHH compared to the human Fc may enable a better access to the VS. However, recombinant J3 with a human Fc display the same potency of neutralization against HIV-1 cell-to-cell transmission [127]. Thus, the size of the antibody does not seem to be a limiting factor in that case. The situation may be different in DCs or macrophages. A full-size 10E8 was less potent in these cells but 10E8 Fab, smaller in size, had more comparable neutralization IC50s during cell-free and cell-associated transmission [68]. This is consistent with the observation that bNAbs do not easily access virus contained within VCCs in macrophages [128]. This is also the case in DCs, where HIV-1 virions present in VCCs are protected from recognition by bNAbs, even if these compartments are connected to the extracellular milieu [89].
Fig. 3.
Potential mechanisms explaining the increased resistance of cell-associated HIV-1 to bNAbs-mediated neutralization. a bNAbs may poorly access virions present at the VS because of the physical proximity of donor and target cell membranes. b VS-mediated HIV-1 is associated with high MOIs. c Viruses budding at the VS may incorporate cellular proteins differently than cell-free virions, possibly leading to different susceptibilities to bNAbs. d Env conformation and stability of Env-bNAb complexes at the cell surface. Env conformation may be different at the surface of cell-free virus and at the plasma membrane. The stability of Env-bNAb complexes at the cell surface depends on the antibody and the viral strain. Donor cells are in brown and uninfected cells in blue
Thus, steric hindrance may impact neutralization of cell–cell transmission by some bNAbs and depends on the cell type and the antibody used. The most potent antibodies gain access to the VS and impair its function.
Cell-to-cell HIV-1 transmission is associated with higher MOIs
The VS leads to an elevated concentration of viral particles in the synaptic cleft, which most likely increases the MOI during cell-to-cell transmission [37, 56–60] (Fig. 3b). Increased amounts of virus would then require more antibody, thus increasing IC50s. However, with some antibodies, differences in IC50s are still observed when cell-free and cell-associated viral inputs are normalized [126]. With the most potent bNAbs, such as 10-1074 and 3BNC117, the IC50s remain low in coculture systems [116, 117, 119]. Thus, differences between cell-to-cell and cell-free modes of transmission are not only a matter of quantity of transferred virus.
Composition of cell-free particles and virions produced at the VS
Cell-free neutralization assays mostly use virus produced by transfection of 293T cells. Cell-to-cell assays generally rely on CD4 T cell lines or primary cells as the source of virus. Some HIV-1 strains are more susceptible to bNAbs when produced in 293T compared to primary cells [129]. This might be due to the content of cellular molecules in viral particles, as HIV-1 incorporates host membrane when budding. Thus, comparison between cell-free and cell-associated neutralization may be biased by the cell types in which the virus was produced. In addition, the composition of viral particles may vary at the VS (Fig. 3c). For example, HIV-1 virions can incorporate ICAM-1 that will increase infectivity, especially if the target cell expresses LFA-1 [130, 131]. ICAM-1-bearing virions are more resistant to neutralization by HIV-1-infected patients’ sera or neutralizing anti-gp120 antibodies [132]. Given that adhesion molecules accumulate at the VS, they could be more incorporated in viral particles budding at this site. The lipid component of VS-budding virions may be also different, since synapses are known to be enriched in rafts, and this may also impact sensitivity to neutralizing antibodies. Even though technically challenging, a characterization of the cellular composition of virions produced at the VS will give insights into the mechanisms underlying the resistance of cell-to-cell transmission to some bNAbs.
Conformation and amount of Env at the cell surface and at the VS
The conformation and oligomerization states of Env are probably more heterogeneous at the plasma membrane than at the surface of virions, that contain a very limited number of Env trimers [133]. At the plasma membrane, a high amount of Env monomers and trimers, at different stages of maturation and glycosylation, are present. Local variations at membrane subdomains, depending on the subcellular environment or the presence of lipids and cellular proteins, may also modify Env epitope exposure. These different dynamic parameters impact the accessibility of cell-surface Env to bNAbs. For example, the engagement of Env by CD4 exposes epitopes targeted by non-neutralizing antibodies (nnAbs) [134, 135]. Since the creation of the VS involves interaction of Env with CD4, the conformation of Env at the synapse may be different from that at other regions of the membrane. We also reported that some antibodies, such as 8ANC195, which recognizes a gp120/41 bridging epitope and neutralizes cell-free virions, does not efficiently bind to infected cells [17], confirming the existence of different conformations of Env on virions and cells.
Viral proteins may also modify epitope accessibility. Nef and Vpu modify the levels of Env at the cell surface [136, 137] and Nef decreases Env susceptibility to anti-MPER antibodies [138]. The Env cytoplasmic tail (CT) also regulates the exposure of Env epitopes, through mechanisms that deserve further characterization [139, 140]. A CT truncation increases sensitivity to neutralization during cell-to-cell transmission with little effect on cell-free infection [110]. Some mutations in the CT inhibit cell-free infection more strongly than cell-to-cell transmission [141]. Mutations in the tyrosine-based sorting signal (YXXL) in the CT of two T/F strains modulate neutralization efficacy of b12, 10-1074 and PGT126 in cell-to-cell neutralization assays [119]. This YXXL motif regulates Env recycling from the plasma membrane. The engagement of Env in recycling pathways not only modulates the amount and stability of the viral protein at the surface, but may also impact epitope exposure (Fig. 3d).
Stability of Env-bNAb complexes at the cell surface
The stability of Env-bNAb complexes at the cell surface most likely regulates the neutralization activity of bNAbs against donor cells. The half-life of Env-bNAb surface complexes depends on the antibody and the viral strain [17]. It varies from less than 30 min to more than 6 h [17]. These variations are likely due to the affinity of the antibody (association and dissociation rates), to antibody-induced Env internalization or shedding, or to other parameters that deserve further investigation. The natural recycling of Env at the plasma membrane or at the VS may also impact antibody efficacy during cell-to-cell transmission.
Elimination of HIV-1-infected cells by bNAbs
Infected cells covered with potent bNAbs may be neutralized in their ability to transmit the virus, but may also become susceptible to antibody-mediated effector functions.
Antibodies are composed of a Fab region, responsible for antigen binding, and a Fc domain, recognized by Fc receptors expressed on immune cells. FcR engagement subsequently triggers various immune effector mechanisms (for a review, see [142]). For example, NK cells recruited by bNAbs kill HIV-1-infected cells through Antibody-Dependent Cellular Cytotoxicity (ADCC) [17, 143, 144]. Other Fc-dependent mechanisms include antibody–dependent cellular phagocytosis (ADCP) and activation of the complement pathway (reviewed in [142]). ADCC is mediated by bNAbs and nnAbs, depending on Env epitope accessibility at the cell surface [135, 145]. bNAbs require Fc-mediated immunity for optimal efficacy in vivo [146–148]. In humanized mice, nnAbs clear HIV-infected cells and impose selective pressure on the virus, as observed by mutation in Env [149]. However, primary strains are often poorly susceptible to nnAbs-mediated ADCC in vitro [135, 150]. HIV-1 propagation in vivo is the result of a balance between the rate of viral transmission and the clearance of infected cells. Thus, even if bNAbs do not totally neutralize viral cell-to-cell spread, Fc-mediated functions represent an additional mechanism of action of the antibodies against infected T cells. Whether these additional functions also impact DC/Macrophages-mediated cis- or trans-infection of CD4+ T cells remains poorly characterized.
In vivo implications of the increased resistance of cell-to-cell transmission to bNAbs
Infectious body fluids such as blood, semen or breast milk contain both cell-free and cell-associated HIV-1 [151, 152]. In humans, comparing cell-free and cell-associated genetic signatures of the infecting partner’s virus to those of the founder virus in the recipient partner suggests that some infections are initiated by cell-associated virus [153]. Moreover, cell-associated Simian Immunodeficiency Virus (SIV) initiates infection in macaques [154, 155]. However, even though bNAbs combinations are efficient in murine and simian models, they were mostly tested in animals challenged with cell-free HIV-1 (reviewed in [19]). Recently, the effect of the anti-V3 antibody PGT121 was compared after cell-free or cell-associated Simian-Human Immunodeficiency Virus (SHIV) challenge in macaques [156]. PGT121 is efficient against cell-associated HIV-1 in vitro, requiring higher concentrations than during cell-free infection [116, 119]. PGT121 infusion protected all 6 animals challenged with cell-free SHIV. However, the antibody only protected 3 out the 6 animals challenged with infected cells. The 3 non-protected animals displayed 1- to 7-week delays in the onset of viremia. This delay correlated with PGT121 serum concentrations. Thus, PGT121 was only partially effective against cell-associated SHIV challenge in macaques. This may be due to an “occult” infection which triggered viral spread when bNAbs levels waned, or to the transfer of latently infected cell that reactivated late after the challenge. These results highlight the need for high and sustained concentrations of antibodies to confer resistance to challenge with infected cells. In this macaque model, a high dose of cell-associated SHIV was used as a challenge. Humans probably receive a lower level of infectious challenge during natural contamination. Future trials of bNAbs will be of great interest to assess their prophylactic efficacy in humans.
Noteworthy, the main issue of using single bNAbs in vivo is the rapid occurrence of escape mutations [23–26]. Mathematical modelling suggested that escape mutations to bNAbs are more likely to happen during cell-to-cell transmission than during cell-free infection [117]. Again, current and future clinical trials using combination of bNAbs will be instrumental in determining whether this immunotherapy is counteracting the different modes of HIV-1 spread in humans.
Conclusion
HIV-1 cell-to-cell transfer has been extensively characterized in cell culture systems. In vivo experiments confirmed the contribution of this mechanism during viral spread. Conventional antiretroviral drugs efficiently inhibit cell-free and cell-associated viral transmission [157] but the impact of bNAbs on intercellular viral spread may be less marked. There are important mechanistic differences depending on antibodies, viral strains, and the nature of donor and recipient cells. The most potent bNAbs, that stably bind to infected cells and impair CD4/Env interaction or viral fusion, efficiently inhibit cell-to-cell transfer. These bNAbs display transient therapeutic efficacy in humans. In addition to neutralization, bNAbs trigger the destruction of infected cells. Future basic and clinical studies will help determining whether the targeting of infected cells by combinations of bNAbs with long half-lives and increased potency are a promising approach to the prevention, treatment, and possibly cure of HIV-1 infection.
Authors’ contributions
All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the members of the Virus and Immunity Unit for helpful discussions.
Competing interests
The authors declare they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Work in the Virus & Immunity unit is funded by Institut Pasteur, ANRS, Sidaction, the Vaccine Research Institute (ANR-10-LABX-77), the Labex IBEID (ANR-10-IHUB-0002, the “TIMTAMDEN” ANR-14-CE14-0029, the “CHIKV-Viro-Immuno” ANR-14-CE14-0015-01, L’Oréal Sponsorhip and the Gilead HIV cure program.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jérémy Dufloo, Email: jeremy.dufloo@pasteur.fr.
Timothée Bruel, Email: timothee.bruel@pasteur.fr.
Olivier Schwartz, Email: schwartz@pasteur.fr.
References
- 1.Barre-Sinoussi F, Chermann J, Rey F, Nugeyre M, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Schiffner T, Sattentau QJ, Duncan CJA. Cell-to-cell spread of HIV-1 and evasion of neutralizing antibodies. Vaccine. 2013;31:5789–5797. doi: 10.1016/j.vaccine.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Casartelli N. HIV-1 cell-to-cell transmission and antiviral strategies: an overview. Curr Drug Targets. 2016;17:65–75. doi: 10.2174/138945011701151217105638. [DOI] [PubMed] [Google Scholar]
- 4.Bracq L, Xie M, Benichou S, Bouchet J. Mechanisms for cell-to-cell transmission of HIV-1. Front Immunol. 2018;9:260. doi: 10.3389/fimmu.2018.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monel B, Beaumont E, Vendrame D, Schwartz O, Brand D, Mammano F. HIV cell-to-cell transmission requires the production of infectious virus particles and does not proceed through Env-mediated fusion pores. J Virol. 2012;86:3924–3933. doi: 10.1128/JVI.06478-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frost SDW, Wrin T, Smith DM, Pond SLK, Liu Y, Paxinos E, et al. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci. 2005;102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 10.Deeks SG, Schweighardt B, Wrin T, Galovich J, Hoh R, Sinclair E, et al. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J Virol. 2006;80:6155–6164. doi: 10.1128/JVI.00093-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCoy LE, Burton DR. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev. 2017;275:11–20. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouquet H. Antibody B cell responses in HIV-1 infection. Trends Immunol. 2014;35:549–561. doi: 10.1016/j.it.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Kong R, Xu K, Zhou T, Acharya P, Lemmin T, Liu K, et al. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science. 2016;352:828–833. doi: 10.1126/science.aae0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gils MJ, van den Kerkhof TLGM, Ozorowski G, Cottrell CA, Sok D, Pauthner M, et al. An HIV-1 antibody from an elite neutralizer implicates the fusion peptide as a site of vulnerability. Nat Microbiol. 2016;2:16199. doi: 10.1038/nmicrobiol.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K, Acharya P, Kong R, Cheng C, Chuang G-Y, Liu K, et al. Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat Med. 2018;24:857–867. doi: 10.1038/s41591-018-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura Y, Martin MA. Of mice, macaques, and men: broadly neutralizing antibody immunotherapy for HIV-1. Cell Host Microbe. 2017;22:207–216. doi: 10.1016/j.chom.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu C-L, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura Y, Gautam R, Chun T-W, Sadjadpour R, Foulds KE, Shingai M, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017;543:559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen YZ, Caskey M. Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr Opin HIV AIDS. 2018;13:366–737. doi: 10.1097/COH.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 23.Caskey M, Klein F, Lorenzi JCC, Seaman MS, West AP, Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;2015:319ra206–319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, et al. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23:185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu C-L, Lorenzi JCC, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535:556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, Nogueira L, et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science. 2016;352:997–1001. doi: 10.1126/science.aaf0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce-Pratt R, Phillips DM. Studies of adhesion of lymphocytic cells: implications for sexual transmission of human immunodeficiency virus1. Biol Reprod. 1993;48:431–445. doi: 10.1095/biolreprod48.3.431. [DOI] [PubMed] [Google Scholar]
- 29.Pearce-Pratt R, Malamud D, Phillips DM. Role of the cytoskeleton in cell-to-cell transmission of human immunodeficiency virus. J Virol. 1994;68:2898–2905. doi: 10.1128/jvi.68.5.2898-2905.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fais S, Capobianchi MR, Abbate I, Castilletti C, Gentile M, Cordiali Fei P, et al. Unidirectional budding of HIV-1 at the site of cell-to-cell contact is associated with co-polarization of intercellular adhesion molecules and HIV-1 viral matrix protein. AIDS. 1995;9:329–335. doi: 10.1097/00002030-199509040-00003. [DOI] [PubMed] [Google Scholar]
- 31.Igakura T, Stinchcombe JC, Goon PKC, Taylor GP, Weber JN, Griffiths GM, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 32.Vasiliver-Shamis G, Dustin ML, Hioe CE. HIV-1 virological synapse is not simply a copycat of the immunological synapse. Viruses. 2010;2:1239–1260. doi: 10.3390/v2051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost M-C, et al. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol. 2009;83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy NH, Chan J, Lambele M, Thali M. Clustering and mobility of HIV-1 Env at viral assembly sites predict its propensity to induce cell-cell fusion. J Virol. 2013;87:7516–7525. doi: 10.1128/JVI.00790-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng J, Krementsov DN, Khurana S, Roy NH, Thali M. Formation of syncytia is repressed by tetraspanins in human immunodeficiency virus type 1-producing cells. J Virol. 2009;83:7467–7474. doi: 10.1128/JVI.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy NH, Lambele M, Chan J, Symeonides M, Thali M. Ezrin is a component of the HIV-1 virological presynapse and contributes to the inhibition of cell-cell fusion. J Virol. 2014;88:7645–7658. doi: 10.1128/JVI.00550-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco J, Bosch B, Fernández-Figueras MT, Barretina J, Clotet B, Esté JA. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J Biol Chem. 2004;279:51305–51314. doi: 10.1074/jbc.M408547200. [DOI] [PubMed] [Google Scholar]
- 38.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FYS, et al. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jolly C, Mitar I, Sattentau QJ. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol. 2007;81:13916–13921. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puigdomenech I, Massanella M, Izquierdo-Useros N, Ruiz-Hernandez R, Curriu M, Bofill M, et al. HIV transfer between CD4 T cells does not require LFA-1 binding to ICAM-1 and is governed by the interaction of HIV envelope glycoprotein with CD4. Retrovirology. 2008;5:32. doi: 10.1186/1742-4690-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jolly C, Mitar I, Sattentau QJ. Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J Virol. 2007;81:5547–5560. doi: 10.1128/JVI.01469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haller C, Tibroni N, Rudolph JM, Grosse R, Fackler OT. Nef does not inhibit F-actin remodelling and HIV-1 cell–cell transmission at the T lymphocyte virological synapse. Eur J Cell Biol. 2011;90:913–921. doi: 10.1016/j.ejcb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Sol-Foulon N, Sourisseau M, Porrot F, Thoulouze M-I, Trouillet C, Nobile C, et al. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. EMBO J. 2007;26:516–526. doi: 10.1038/sj.emboj.7601509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jolly C, Welsch S, Michor S, Sattentau QJ. The regulated secretory pathway in CD4 + T cells contributes to human immunodeficiency virus type-1 cell-to-cell spread at the virological synapse. PLoS Pathog. 2011;7:e1002226. doi: 10.1371/journal.ppat.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jolly C, Sattentau QJ. Human immunodeficiency virus type 1 virological synapse formation in T cells requires lipid raft integrity. J Virol. 2005;79:12088–12094. doi: 10.1128/JVI.79.18.12088-12094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malbec M, Sourisseau M, Guivel-Benhassine F, Porrot F, Blanchet F, Schwartz O, et al. HIV-1 Nef promotes the localization of Gag to the cell membrane and facilitates viral cell-to-cell transfer. Retrovirology. 2013;10:80. doi: 10.1186/1742-4690-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casartelli N, Sourisseau M, Feldmann J, Guivel-Benhassine F, Mallet A, Marcelin A-G, et al. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010;6:e1000955. doi: 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhl BD, Sloan RD, Donahue DA, Bar-Magen T, Liang C, Wainberg MA. Tetherin restricts direct cell-to-cell infection of HIV-1. Retrovirology. 2010;7:115. doi: 10.1186/1742-4690-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jolly C, Booth NJ, Neil SJD. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J Virol. 2010;84:12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Compton AA, Bruel T, Porrot F, Mallet A, Sachse M, Euvrard M, et al. IFITM proteins incorporated into HIV-1 virions impair viral fusion and spread. Cell Host Microbe. 2014;16:736–747. doi: 10.1016/j.chom.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tartour K, Appourchaux R, Gaillard J, Nguyen X-N, Durand S, Turpin J, et al. IFITM proteins are incorporated onto HIV-1 virion particles and negatively imprint their infectivity. Retrovirology. 2014;11:103. doi: 10.1186/s12977-014-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosch B, Grigorov B, Senserrich J, Clotet B, Darlix J, Muriaux D, et al. A clathrin–dynamin-dependent endocytic pathway for the uptake of HIV-1 by direct T cell–T cell transmission. Antiviral Res. 2008;80:185–193. doi: 10.1016/j.antiviral.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Sloan RD, Kuhl BD, Mesplede T, Munch J, Donahue DA, Wainberg MA. Productive entry of HIV-1 during cell-to-cell transmission via dynamin-dependent endocytosis. J Virol. 2013;87:8110–8123. doi: 10.1128/JVI.00815-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dale BM, McNerney GP, Thompson DL, Hubner W, de los Reyes K, Chuang FYS, et al. Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. Cell Host Microbe. 2011;10:551–562. doi: 10.1016/j.chom.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herold N, Anders-Osswein M, Glass B, Eckhardt M, Muller B, Krausslich H-G. HIV-1 entry in SupT1-R5, CEM-ss, and primary CD4 + T cells occurs at the plasma membrane and does not require endocytosis. J Virol. 2014;88:13956–13970. doi: 10.1128/JVI.01543-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007;81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007;81:12582–12595. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Del Portillo A, Tripodi J, Najfeld V, Wodarz D, Levy DN, Chen BK. Multiploid inheritance of HIV-1 during cell-to-cell infection. J Virol. 2011;85:7169–7176. doi: 10.1128/JVI.00231-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell RA, Martin N, Mitar I, Jones E, Sattentau QJ. Multiple proviral integration events after virological synapse-mediated HIV-1 spread. Virology. 2013;443:143–149. doi: 10.1016/j.virol.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Boullé M, Müller TG, Dähling S, Ganga Y, Jackson L, Mahamed D, et al. HIV cell-to-cell spread results in earlier onset of viral gene expression by multiple infections per cell. PLoS Pathog. 2016;12:e1005964. doi: 10.1371/journal.ppat.1005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sewald X, Gonzalez DG, Haberman AM, Mothes W. In vivo imaging of virological synapses. Nat Commun. 2012;3:1320. doi: 10.1038/ncomms2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, et al. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012;490:283–287. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Compton AA, Schwartz O. They might be giants: does syncytium formation sink or spread HIV infection? PLoS Pathog. 2017;13:e1006099. doi: 10.1371/journal.ppat.1006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ladinsky MS, Kieffer C, Olson G, Deruaz M, Vrbanac V, Tager AM, et al. Electron tomography of HIV-1 infection in gut-associated lymphoid tissue. PLoS Pathog. 2014;10:e1003899. doi: 10.1371/journal.ppat.1003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Law KM, Komarova NL, Yewdall AW, Lee RK, Herrera OL, Wodarz D, et al. In vivo HIV-1 cell-to-cell transmission promotes multicopy micro-compartmentalized infection. Cell Rep. 2016;15:2771–2783. doi: 10.1016/j.celrep.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 66.Gousset K, Ablan SD, Coren LV, Ono A, Soheilian F, Nagashima K, et al. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 2008;4:e1000015. doi: 10.1371/journal.ppat.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 68.Duncan CJA, Williams JP, Schiffner T, Gartner K, Ochsenbauer C, Kappes J, et al. High-multiplicity HIV-1 infection and neutralizing antibody evasion mediated by the macrophage-T cell virological synapse. J Virol. 2014;88:2025–2034. doi: 10.1128/JVI.03245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Ségéral E, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HCT, Rice GI, Christodoulou E, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480:379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 72.St Gelais C, de Silva S, Amie SM, Coleman CM, Hoy H, Hollenbaugh JA, et al. SAMHD1 restricts HIV-1 infection in dendritic cells (DCs) by dNTP depletion, but its expression in DCs and primary CD4 + T-lymphocytes cannot be upregulated by interferons. Retrovirology. 2012;9:105. doi: 10.1186/1742-4690-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luban J. Innate immune sensing of HIV-1 by dendritic cells. Cell Host Microbe. 2012;12:408–418. doi: 10.1016/j.chom.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ayinde D, Bruel T, Cardinaud S, Porrot F, Prado JG, Moris A, et al. SAMHD1 limits HIV-1 antigen presentation by monocyte-derived dendritic cells. J Virol. 2015;89:6994–7006. doi: 10.1128/JVI.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chauveau L, Donahue DA, Monel B, Porrot F, Bruel T, Richard L, et al. HIV fusion in dendritic cells occurs mainly at the surface and is limited by low CD4 levels. J Virol. 2017;91:e01248–17. doi: 10.1128/JVI.01248-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pelchen-Matthews A, Kramer B, Marsh M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol. 2003;162:443–455. doi: 10.1083/jcb.200304008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007;177:329–341. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaudin R, Berre S, Cunha de Alencar B, Decalf J, Schindler M, Gobert F-X, et al. Dynamics of HIV-containing compartments in macrophages reveal sequestration of virions and transient surface connections. PLoS ONE. 2013;8:e69450. doi: 10.1371/journal.pone.0069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4 + T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 82.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 84.Arrighi J-F, Pion M, Garcia E, Escola J-M, van Kooyk Y, Geijtenbeek TB, et al. DC-SIGN–mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia E, Pion M, Pelchen-Matthews A, Collinson L, Arrighi J-F, Blot G, et al. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse: HIV-1 localization in human dendritic cells. Traffic. 2005;6:488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 86.Nobile C, Petit C, Moris A, Skrabal K, Abastado J-P, Mammano F, et al. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J Virol. 2005;79:5386–5399. doi: 10.1128/JVI.79.9.5386-5399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, et al. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on siglec-1/CD169. PLoS Pathog. 2013;9:e1003291. doi: 10.1371/journal.ppat.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akiyama H, Ramirez N-GP, Gudheti MV, Gummuluru S. CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies. PLoS Pathog. 2015;11:e1004751. doi: 10.1371/journal.ppat.1004751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hammonds JE, Beeman N, Ding L, Takushi S, Francis AC, Wang J-J, et al. Siglec-1 initiates formation of the virus-containing compartment and enhances macrophage-to-T cell transmission of HIV-1. PLoS Pathog. 2017;13:e1006181. doi: 10.1371/journal.ppat.1006181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodriguez-Plata MT, Puigdomènech I, Izquierdo-Useros N, Puertas MC, Carrillo J, Erkizia I, et al. The infectious synapse formed between mature dendritic cells and CD4 + T cells is independent of the presence of the HIV-1 envelope glycoprotein. Retrovirology. 2013;10:42. doi: 10.1186/1742-4690-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 93.Ménager MM, Littman DR. Actin Dynamics regulates dendritic cell-mediated transfer of HIV-1 to T cells. Cell. 2016;164:695–709. doi: 10.1016/j.cell.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Villani A-C, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356:eaah4573. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.See P, Dutertre C-A, Chen J, Günther P, McGovern N, Irac SE, et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science. 2017;356:eaag3009. doi: 10.1126/science.aag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silvin A, Yu CI, Lahaye X, Imperatore F, Brault J-B, Cardinaud S, et al. Constitutive resistance to viral infection in human CD141+ dendritic cells. Sci Immunol. 2017;2:eaai8071. doi: 10.1126/sciimmunol.aai8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 99.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142–148. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hashimoto M, Bhuyan F, Hiyoshi M, Noyori O, Nasser H, Miyazaki M, et al. Potential role of the formation of tunneling nanotubes in HIV-1 spread in macrophages. J Immunol. 2016;196:1832–1841. doi: 10.4049/jimmunol.1500845. [DOI] [PubMed] [Google Scholar]
- 101.Kadiu I, Gendelman HE. Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. J Proteome Res. 2011;10:3225–3238. doi: 10.1021/pr200262q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kadiu I, Gendelman HE. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J Neuroimmune Pharmacol. 2011;6:658–675. doi: 10.1007/s11481-011-9298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, Subramaniam S, et al. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood. 2011;118:4841–4852. doi: 10.1182/blood-2010-09-305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aggarwal A, Iemma TL, Shih I, Newsome TP, McAllery S, Cunningham AL, et al. Mobilization of HIV spread by diaphanous 2 dependent filopodia in infected dendritic cells. PLoS Pathog. 2012;8:e1002762. doi: 10.1371/journal.ppat.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baxter AE, Russell RA, Duncan CJA, Moore MD, Willberg CB, Pablos JL, et al. Macrophage infection via selective capture of HIV-1-infected CD4 + T cells. Cell Host Microbe. 2014;16:711–721. doi: 10.1016/j.chom.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bracq L, Xie M, Lambelé M, Vu L-T, Matz J, Schmitt A, et al. T cell-macrophage fusion triggers multinucleated giant cell formation for HIV-1 spreading. J Virol. 2017;91:e01237–17. doi: 10.1128/JVI.01237-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alfsen A, Yu H, Magérus-Chatinet A, Schmitt A, Bomsel M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. MBoC. 2005;16:4267–4279. doi: 10.1091/mbc.e05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Real F, Sennepin A, Ganor Y, Schmitt A, Bomsel M. Live imaging of HIV-1 transfer across T cell virological synapse to epithelial cells that promotes stromal macrophage infection. Cell Rep. 2018;23:1794–1805. doi: 10.1016/j.celrep.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 109.Gupta P, Balachandran R, Ho M, Enrico A, Rinaldo C. Cell-to-cell transmission of human immunodeficiency virus type 1 in the presence of azidothymidine and neutralizing antibody. J Virol. 1989;63:2361–2365. doi: 10.1128/jvi.63.5.2361-2365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Durham ND, Yewdall AW, Chen P, Lee R, Zony C, Robinson JE, et al. Neutralization resistance of virological synapse-mediated HIV-1 infection is regulated by the gp41 cytoplasmic tail. J Virol. 2012;86:7484–7495. doi: 10.1128/JVI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sánchez-Palomino S, Massanella M, Carrillo J, García A, García F, González N, et al. A cell-to-cell HIV transfer assay identifies humoral responses with broad neutralization activity. Vaccine. 2011;29:5250–5259. doi: 10.1016/j.vaccine.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 112.Purtscher M, Trkola A, Gruber G, Buchacher A, Predl R, Steindl F, et al. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994;10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 113.Pantaleo G, Demarest JF, Vaccarezza M, Graziosi C, Bansal GP, Koenig S, et al. Effect of anti-V3 antibodies on cell-free and cell-to-cell human immunodeficiency virus transmission. Eur J Immunol. 1995;25:226–231. doi: 10.1002/eji.1830250137. [DOI] [PubMed] [Google Scholar]
- 114.Massanella M, Puigdomènech I, Cabrera C, Fernandez-Figueras MT, Aucher A, Gaibelet G, et al. Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. AIDS. 2009;23:183–188. doi: 10.1097/QAD.0b013e32831ef1a3. [DOI] [PubMed] [Google Scholar]
- 115.Abela IA, Berlinger L, Schanz M, Reynell L, Günthard HF, Rusert P, et al. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog. 2012;8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med. 2013;210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reh L, Magnus C, Schanz M, Weber J, Uhr T, Rusert P, et al. Capacity of broadly neutralizing antibodies to inhibit HIV-1 cell-cell transmission is strain- and epitope-dependent. PLoS Pathog. 2015;11:e1004966. doi: 10.1371/journal.ppat.1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gombos RB, Kolodkin-Gal D, Eslamizar L, Owuor JO, Mazzola E, Gonzalez AM, et al. Inhibitory effect of individual or combinations of broadly neutralizing antibodies and antiviral reagents against cell-free and cell-to-cell HIV-1 transmission. J Virol. 2015;89:7813–7828. doi: 10.1128/JVI.00783-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H, Zony C, Chen P, Chen BK. Reduced potency and incomplete neutralization of broadly neutralizing antibodies against cell-to-cell transmission of HIV-1 with transmitted founder Envs. J Virol. 2017;91:e02425–16. doi: 10.1128/JVI.02425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martin N, Welsch S, Jolly C, Briggs JAG, Vaux D, Sattentau QJ. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J Virol. 2010;84:3516–3527. doi: 10.1128/JVI.02651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frankel SS, Steinman RM, Michael NL, Kim SR, Bhardwaj N, Pope M, et al. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J Virol. 1998;72:9788–9794. doi: 10.1128/jvi.72.12.9788-9794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ganesh L, Leung K, Lore K, Levin R, Panet A, Schwartz O, et al. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J Virol. 2004;78:11980–11987. doi: 10.1128/JVI.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Montfort T, Nabatov AA, Geijtenbeek TBH, Pollakis G, Paxton WA. Efficient capture of antibody neutralized HIV-1 by cells expressing DC-SIGN and transfer to CD4 + T lymphocytes. J Immunol. 2007;178:3177–3185. doi: 10.4049/jimmunol.178.5.3177. [DOI] [PubMed] [Google Scholar]
- 124.Su B, Xu K, Lederle A, Peressin M, Biedma ME, Laumond G, et al. Neutralizing antibodies inhibit HIV-1 transfer from primary dendritic cells to autologous CD4 T lymphocytes. Blood. 2012;120:3708–3717. doi: 10.1182/blood-2012-03-418913. [DOI] [PubMed] [Google Scholar]
- 125.Su B, Lederle A, Laumond G, Ducloy C, Schmidt S, Decoville T, et al. Broadly neutralizing antibody VRC01 prevents HIV-1 transmission from plasmacytoid dendritic cells to CD4 T lymphocytes. J Virol. 2014;88:10975–10981. doi: 10.1128/JVI.01748-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I, Gummuluru S. Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing HIV-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-infection. J Infect Dis. 2012;205:1248–1257. doi: 10.1093/infdis/jis183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCoy LE, Groppelli E, Blanchetot C, de Haard H, Verrips T, Rutten L, et al. Neutralisation of HIV-1 cell-cell spread by human and llama antibodies. Retrovirology. 2014;11:83. doi: 10.1186/s12977-014-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koppensteiner H, Banning C, Schneider C, Hohenberg H, Schindler M. Macrophage internal HIV-1 Is protected from neutralizing antibodies. J Virol. 2012;86:2826–2836. doi: 10.1128/JVI.05915-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cohen YZ, Lorenzi JCC, Seaman MS, Nogueira L, Schoofs T, Krassnig L, et al. Neutralizing activity of broadly neutralizing anti-HIV-1 antibodies against clade B clinical isolates produced in peripheral blood mononuclear cells. J Virol. 2017;92:e01883–17. doi: 10.1128/JVI.01883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fortin JF, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fortin J-F, Cantin R, Tremblay MJ. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72:2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fortin J-F, Cantin R, Bergeron MG, Tremblay MJ. Interaction between virion-bound host intercellular adhesion molecule-1 and the high-affinity state of lymphocyte function-associated antigen-1 on target cells renders R5 and X4 isolates of human immunodeficiency virus type 1 more refractory to neutralization. Virology. 2000;268:493–503. doi: 10.1006/viro.2000.0190. [DOI] [PubMed] [Google Scholar]
- 133.Chojnacki J, Staudt T, Glass B, Bingen P, Engelhardt J, Anders M, et al. Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy. Science. 2012;338:524–528. doi: 10.1126/science.1226359. [DOI] [PubMed] [Google Scholar]
- 134.Veillette M, Desormeaux A, Medjahed H, Gharsallah N-E, Coutu M, Baalwa J, et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol. 2014;88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bruel T, Guivel-Benhassine F, Lorin V, Lortat-Jacob H, Baleux F, Bourdic K, et al. Lack of ADCC breadth of human nonneutralizing anti-HIV-1 antibodies. J Virol. 2017;91:e02440–16. doi: 10.1128/JVI.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schwartz O, Maréchal V, Danos O, Heard JM. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neil SJD, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 138.Lai RPJ, Yan J, Heeney J, McClure MO, Göttlinger H, Luban J, et al. Nef decreases HIV-1 sensitivity to neutralizing antibodies that target the membrane-proximal external region of TMgp41. PLoS Pathog. 2011;7:e1002442. doi: 10.1371/journal.ppat.1002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Joyner AS, Willis JR, Crowe JE, Aiken C. Maturation-induced cloaking of neutralization epitopes on HIV-1 particles. PLoS Pathog. 2011;7:e1002234. doi: 10.1371/journal.ppat.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen J, Kovacs JM, Peng H, Rits-Volloch S, Lu J, Park D, et al. Effect of the cytoplasmic domain on antigenic characteristics of HIV-1 envelope glycoprotein. Science. 2015;349:191–195. doi: 10.1126/science.aaa9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Durham ND, Chen BK. HIV-1 cell-free and cell-to-cell infections are differentially regulated by distinct determinants in the Env gp41 cytoplasmic tail. J Virol. 2015;89:9324–9337. doi: 10.1128/JVI.00655-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2017;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.von Bredow B, Arias JF, Heyer LN, Moldt B, Le K, Robinson JE, et al. Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J Virol. 2016;90:6127–6139. doi: 10.1128/JVI.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee WS, Kent SJ. Anti-HIV-1 antibody-dependent cellular cytotoxicity: is there more to antibodies than neutralization? Curr Opin HIV AIDS. 2018;13:160–166. doi: 10.1097/COH.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 145.Richard J, Prévost J, Alsahafi N, Ding S, Finzi A. Impact of HIV-1 envelope conformation on ADCC responses. Trends Microbiol. 2018;26(4):253–265. doi: 10.1016/j.tim.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 146.Hessell AJ, Hangartner L, Hunter M, Havenith CEG, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 147.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Horwitz JA, Bar-On Y, Lu C-L, Fera D, Lockhart AAK, Lorenzi JCC, et al. Non-neutralizing antibodies alter the course of HIV-1 infection in vivo. Cell. 2017;170(637–648):e10. doi: 10.1016/j.cell.2017.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prévost J, Richard J, Ding S, Pacheco B, Charlebois R, Hahn BH, et al. Envelope glycoproteins sampling states 2/3 are susceptible to ADCC by sera from HIV-1-infected individuals. Virology. 2018;515:38–45. doi: 10.1016/j.virol.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ndirangu J, Viljoen J, Bland RM, Danaviah S, Thorne C, Van de Perre P, et al. Cell-free (RNA) and cell-associated (DNA) HIV-1 and postnatal transmission through breastfeeding. PLoS ONE. 2012;7:e51493. doi: 10.1371/journal.pone.0051493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N, et al. Semen CD4 + T cells and macrophages are productively infected at all stages of SIV infection in Macaques. PLoS Pathog. 2013;9:e1003810. doi: 10.1371/journal.ppat.1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu T, Wang N, Carr A, Nam DS, Moor-Jankowski R, Cooper DA, et al. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sallé B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, et al. Infection of Macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis. 2010;202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 155.Kolodkin-Gal D, Hulot SL, Korioth-Schmitz B, Gombos RB, Zheng Y, Owuor J, et al. Efficiency of cell-free and cell-associated virus in mucosal transmission of human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 2013;87:13589–13597. doi: 10.1128/JVI.03108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parsons MS, Lloyd SB, Lee WS, Kristensen AB, Amarasena T, Center RJ, et al. Partial efficacy of a broadly neutralizing antibody against cell-associated SHIV infection. Sci Transl Med. 2017;9:eaaf1483. doi: 10.1126/scitranslmed.aaf1483. [DOI] [PubMed] [Google Scholar]
- 157.Titanji BK, Pillay D, Jolly C. Combination antiretroviral therapy and cell–cell spread of wild-type and drug-resistant human immunodeficiency virus-1. J Gen Virol. 2017;98:821–834. doi: 10.1099/jgv.0.000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.