Abstract
Background
Age-related cognitive impairment is rising in prevalence but is not yet fully characterized in terms of its epidemiology. Here, we aimed to elucidate the role of obesity, diabetes and hypertension as candidate risk factors.
Methods
Original baseline data from 3 studies (OCTOPUS, DECS, SuDoCo) were obtained for secondary analysis of cross-sectional associations of diabetes, hypertension, blood pressure, obesity (body mass index [BMI] ≥30 kg/m2) and BMI with presence of cognitive impairment in log-binomial regression analyses. Cognitive impairment was defined as scoring more than 2 standard deviations below controls on at least one of 5–11 cognitive tests. Underweight participants (BMI<18.5 kg/m2) were excluded. Results were pooled across studies in fixed-effects inverse variance models.
Results
Analyses totaled 1545 participants with a mean age of 61 years (OCTOPUS) to 70 years (SuDoCo). Cognitive impairment was found in 29.0% of participants in DECS, 8.2% in SuDoCo and 45.6% in OCTOPUS. In pooled analyses, after adjustment for age, sex, diabetes and hypertension, obesity was associated with a 1.29-fold increased prevalence of cognitive impairment (risk ratio [RR] 1.29; 95% CI 0.98, 1.72). Each 1 kg/m2 increment in BMI was associated with 3% increased prevalence (RR 1.03; 95% CI 1.00, 1.06). None of the remaining risk factors were associated with impairment.
Conclusion
Our results show that older people who are obese have higher prevalence of cognitive impairment compared with normal weight and overweight individuals, and independently of co-morbid hypertension or diabetes. Prospective studies are needed to investigate the temporal relationship of the association.
Keywords: obesity, body mass index, diabetes, hypertension, cognitive impairment, aging, cognitive epidemiology
Introduction
The metabolic syndrome and its complications threaten global health. In most countries, prevalence is high,1 tends to increase over time2,3 and generates huge economic costs.4 Prevalence is largest among older age groups,5 adding to the relevance of the syndrome as a candidate predictor of and potentially causal contributor to age-related disease including cognitive impairment, which itself is rising in prevalence due to globally ageing societies.6 It has been estimated that 22% of people aged over 70 years in the USA are currently cognitively impaired,7 and epidemiological studies have frequently demonstrated associations with the metabolic syndrome.8–13 Diabetes, hypertension and obesity together contribute to the diagnostic criteria of the metabolic syndrome14 and have each been assessed in detail for their relationship with cognitive outcome. Links of diabetes to presence and risk of future cognitive impairment are well established,15,16 while the evidence is less clear for obesity and hypertension. Here, the direction of associations appears to be dependent on the point of measurement during the lifespan. Whereas in prospective investigations spanning decades, midlife obesity and midlife hypertension increase the risk of later impairment,17,18 cross-sectional and prospective investigations with shorter follow-up periods have produced mixed results: late-life obesity and hypertension have each been associated with an increased13,19–22 but also with a reduced risk of impairment17,23–26 in those types of studies. For obesity, the analysis is further complicated by measurement issues of commonly assessed parameters such as body weight or body mass index (BMI) that do not capture body composition well. The roles of obesity and hypertension in particular thus warrant clarification. Importantly, many previous epidemiological investigations have also failed to consider that diabetes, obesity and hypertension tend to cluster in individuals and are highly correlated.14,27 Each could therefore confound the other’s relationship with cognitive risk.
Here, we used data from 3 large clinical trials with detailed baseline cognitive and metabolic characterization to investigate the relationships of obesity, hypertension and diabetes with presence of cognitive impairment in cross-sectional analyses that additionally considered potential mutual confounding among the metabolic risk factors. Results were pooled across the 3 studies for combined estimates.
Methods
Study design
We analyzed baseline data from 3 randomized controlled trials with primary/secondary outcome post-operative cognitive dysfunction (POCD) in an effectively observational, cross-sectional study design. All clinical and cognitive data were measured at pre-surgery assessment.
Study populations and designs of included studies
Data from the Surgery Depth of Anaesthesia Cognitive Outcome (SuDoCo),28 Dexamethasone for Cardiac Surgery (DECS)29,30 and OCTOPUS studies31,32 were used. Access to original study data resulted from a cross-institutional collaboration. Study designs, inclusion criteria and recruitment procedures have been described in detail previously.28,30,31 In brief, any patients with neurological deficits that did not allow cognitive testing were excluded in all the 3 studies. In SuDoCo, patients with Mini-Mental State Examination (MMSE) <24 were also excluded; those with diagnosed mental illness were additionally excluded in DECS. Each trial assessed the effect of an intervention (SuDoCo: monitoring depth of anesthesia during non-cardiac surgery; DECS: dexamethasone administration versus placebo during cardiac surgery; OCTOPUS: on-pump versus off-pump methods for cardiac surgery) on POCD risk. Hence, each study administered neurocognitive assessment before and after surgery. For the purpose of the present cross-sectional analysis, only data collected at pre-surgery baseline assessment were used. Data from participants who completed pre-surgery cognitive testing were included. A total of 19 underweight patients, who could obscure linear associations of obesity with cognitive impairment, and patients with missing data on diabetes, hypertension and obesity were excluded from our analyses.
Physical examination and education
In each study, detailed physical examination and self-reported medical history were used to identify participants with any type of diabetes and those with a history of hypertension. BMI was calculated from participants’ height and weight. “Obesity” was defined as BMI of at least 30 kg/m2. “Underweight” was defined as BMI <18.5 kg/m2. In OCTOPUS and SuDoCo, blood pressure was measured at pre-anesthetic assessment during the days prior to surgery. Blood pressure data were not available for DECS. Participants self-reported on their level of education in OCTOPUS and DECS; data on education were not available for SuDoCo.
Cognitive examination
Trained staff preoperatively administered 11 neuropsychological tests in OCTOPUS, 5 neuropsychological tests in DECS and 6 neuropsychological tests in SuDoCo. In each of the 3 studies, all tests were additionally completed by non-surgical control groups to provide normative data. The respective control groups were matched for age (OCTOPUS), age and sex (DECS), or age and cognitive function (SuDoCo) and had been recruited at a cardiology outpatient clinic (DECS30), or in nursing homes and senior citizen clubs (SuDoCo28). For OCTOPUS, healthy volunteers served as controls.33 All neuropsychological tests were age sensitive and covered a range of neurocognitive domains including working memory, attention, processing speed, manual dexterity, executive function and mental flexibility. In OCTOPUS, paper-pencil versions of the Rey Auditory Verbal Learning Test, Grooved Pegboard Test, Subjective Ordering Task, Sternberg Letter Cancellation Task, Trail-Making Test B, Stroop-Color-Word-Test and Symbol Digit Modalities Task were applied. For DECS, paper-pencil versions of the Rey Auditory Verbal Learning Test, Grooved Pegboard Test, Corsi Blocks, Wechsler Adult Intelligence Scale-Digit Span, Trail-Making Test A and B were used. The SuDoCo trial covered the Motor Screening Test, Pattern Recognition Memory, Spatial Recognition Memory and Choice Reaction Time tests from the CANTAB computerized test battery as well as the paper-pencil based Stroop Color and Word Test and visual Verbal Learning Test.
We first excluded patients with missing cognitive data and performed an outlier correction for extreme values in individual test parameters. Using the respective interquartile range of test scores, 8 out of 2176 single test scores in OCTOPUS and 92 out of 15015 single test scores in SuDoCo were excluded, but no single patient had to be excluded in total due to this outlier correction. There were no outliers to be removed in DECS. Presence of cognitive impairment was then defined as scores of more than 2 SDs below the respective control group on ≥1 test.34
Statistical analysis
Multiple log-binomial regression analyses determined associations of each of the parameters of metabolic function (diabetes, obesity, BMI, hypertension, systolic and diastolic blood pressure) with presence of cognitive impairment. The first model estimated unadjusted risk (prevalence) ratios (RRs) (model 0). Age and sex were entered as covariables in model 1. Model 2 additionally controlled for the respective remaining potential metabolic risk factors (e.g., analyses of obesity, hypertension and diabetes were controlled for) in order to evaluate independence of any associations from comorbidity with other components of the metabolic syndrome versus mutual confounding. For 2 of the studies (OCTOPUS; DECS), data on educational level of participants were available; thus, education was additionally adjusted for in a final step (model 3). Estimated risk ratios corresponded to 1-point increments in BMI and 10-point increments in blood pressure values to aid clinical interpretability.
Analyses were performed separately for each of the 3 studies and were then pooled in fixed-effects inverse variance analyses for each of the metabolic parameters. Model estimates of risk ratios and corresponding p-values were entered with precision up to the third decimal, and 95% CIs were entered with precision up to the first decimal point. Fixed-effects models were selected on the basis that the same effect was assumed to underlie estimates in all the 3 studies.35 Fully adjusted models (model 2) were repeated using random-effects models to show the mean distribution of effects (Table S1). The I2 index determined the proportion of variance between the 3 studies that would remain had we removed sampling error. These pooled analyses were necessary to combine risk estimates across all 1545 participants of the 3 studies and so should not be understood as a meta-analysis of previous research. The statistical analysis plan was approved by an internal committee before the analyses were performed in IBM© SPSS© Statistics (version 24), The R Project for Statistical Computing (version 3.3.3) and Review Manager (version 5.3).
Table S1.
Results for model 2 in fixed-effects model (as described in main manuscript) and random-effects models
| Exposure associations with cognitive impairment | Model 2 as fixed-effects model RR (95% CI) | Model 2 as random-effects model RR (95% CI) |
|---|---|---|
| Diabetes and cognitive impairment | 1.09 (0.80, 1.49) | 1.09 (0.79, 1.51) |
| Hypertension and cognitive impairment | 1.00 (0.78, 1.28) | 1.00 (0.78, 1.28) |
| Obesity and cognitive impairment | 1.29 (0.98, 1.72) | 1.29 (0.98, 1.72) |
| BMI and cognitive impairment | 1.03 (1.00, 1.06) | 1.03 (1.00, 1.06) |
| Systolic blood pressure and cognitive impairment | 0.96 (0.89, 1.03) | 0.96 (0.89, 1.03) |
| Diastolic blood pressure and cognitive impairment | 0.93 (0.81, 1.07) | 0.93 (0.81, 1.07) |
Abbreviations: BMI, body mass index; CI, confidence interval; RR, risk ratio.
Ethics
Participants of all the studies gave written informed consent upon enrollment. Ethical approval was obtained for each of the studies and assessments complied with the Declaration of Helsinki. For the present secondary analysis, additional ethical approval was obtained (Ethikkommission der Charité – Universitätsmedizin Berlin, EA1/242/08).
Results
Metabolic and cognitive characterization of study samples
Analyses were based on N=272 patients from DECS, N=272 patients from OCTOPUS and N=1001 patients from SuDoCo (Figure S1). Participant characteristics for each of the 3 studies are summarized in Table 1. Mean sample age ranged from 61 years (OCTOPUS) to 70 years (SuDoCo). Reasons for surgery were severe cardiac disease in DECS and OCTOPUS; patients in SuDoCo underwent any non-cardiac surgery mainly of general surgery, orthopedic or gynecological/urological type. Mean BMI was in the overweight category in each of the 3 studies (BMI ≥25 kg/m2), with prevalence of obesity (BMI ≥30 kg/m2) ranging between 14.7% (OCTOPUS) and 24.0% (SuDoCo). Cognitive impairment was identified in 8.2% (SuDoCo) to 45.6% (OCTOPUS) of patients. Across all the 3 studies, 285 (18.4%) of 1545 patients had cognitive impairment.
Table 1.
Sample characteristics of the 3 studies
| Sample characteristics | OCTOPUS | DECS | SuDoCo |
|---|---|---|---|
| Country | The Netherlands | The Netherlands | Germany |
| N | 272 | 272 | 1001 |
| Age, years, mean ± SD | 61.4 ± 9.1 | 64.1 ± 11.9 | 69.9 ± 6.5 |
| Male, n (%) | 189 (69.5%) | 210 (77.2%) | 556 (55.5%) |
| Education, mean ± SD years, or n (%) | 9.4 ± 2.6 | Primary: n=119 (43.8%) Secondary: n=70 (25.7%) Further/higher: n=83 (30.5%) |
– |
| Systolic blood pressure, mmHg, mean ± SD | 138.9 ± 19.6 | – | 136.3 ± 19.3 |
| Diastolic blood pressure, mmHg, mean ± SD | 79.2 ± 10.0 | – | 73.9 ± 11.6 |
| Diabetes, n (%) | 35 (12.9%) | 44 (16.2%) | 215 (21.5%) |
| Hypertension, n (%) | 112 (41.2%) | 150 (55.1%) | 683 (68.2%) |
| Body mass index (kg/m2) mean ± SD | 26.6 ± 3.1 | 27.2 ± 4.5 | 27.4 ± 5.0 |
| Normal weight (BMI 18.5 to 24.9) n (%) | 94 (34.6%) | 99 (36.4%) | 326 (32.6%) |
| Overweight (BMI 25.0 to 29.9) n (%) | 138 (50.7%) | 114 (41.9%) | 435 (43.5%) |
| Class I obesity (BMI 30 to 34.9) n (%) | 40 (14.7%) | 45 (16.5%) | 166 (16.6%) |
| Class II obesity (BMI 35.0 to 39.9) n (%) | 9 (3.3%) | 49 (4.9%) | |
| Class III obesity (BMI ≥40) n (%) | 5 (1.8%) | 25 (2.5%) | |
| Cognitive impairment, n (%) | 124 (45.6%) | 79 (29.0%) | 82 (8.2%) |
Note: Data on systolic and diastolic blood pressure available for N=270 in OCTOPUS and N=949 in SuDoCo. % shown of total sample. Surgical procedures were cardiac surgery (OCTOPUS, DECS) or general surgery (SuDoCo). BMI ≥30 kg/m2 was used as cutoff for subgroup analyses on obesity. Different sets of cognitive tests were used in each of the studies (see Methods).
Abbreviations: BMI, body mass index; DECS, Dexamethasone for Cardiac Surgery; SD, standard deviation; SuDoCo, Surgery Depth of Anaesthesia Cognitive Outcome.
Associations of metabolic syndrome parameters with cognitive impairment
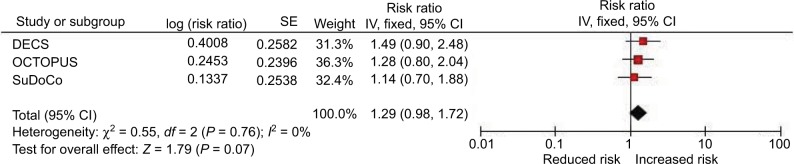
Associations of diabetes, hypertension and obesity with cognitive impairment are shown in Table 2. In pooled analyses, obesity was associated with presence of cognitive impairment and independently of age, sex, diabetes or hypertension. Obese participants were overall 1.29-fold more likely to present with cognitive impairment compared with normal weight and overweight individuals (RR 1.29; 95% CI 0.98, 1.72) with no evidence of statistical heterogeneity among the studies (Chi2=0.55; I2=0%; Table 2; Figure 1). Similar findings were observed with further adjustment for educational level (RR 1.33; 95% CI 0.94, 1.87). Diabetes and hypertension were not associated with cognitive impairment in any of the studies or in pooled analyses (Table 2).
Table 2.
Association of diabetes, hypertension, and obesity with cognitive impairment in each study, and pooled estimates of prevalence ratios
| Exposure associations with cognitive impairment | OCTOPUS
|
DECS
|
SuDoCo
|
Pooled estimates
|
|||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | Weight | Estimate (95% CI) | Weight | Estimate (95% CI) | Weight | Estimate (95% CI) | |
| Diabetes and cognitive impairment | |||||||
| Model 0: no adjustment | 0.93 (0.59, 1.34) | 38.1% | 1.53 (0.97, 2.25) | 34.8% | 1.18 (0.71, 1.87) | 27.1% | 1.18 (0.92, 1.52) |
| Model 1: age, sex | 0.82 (0.46, 1.37) | 25.1% | 1.46 (0.93, 2.16) | 42.5% | 1.21 (0.73, 1.91) | 32.4% | 1.19 (0.91, 1.56) |
| Model 2: +hypertension, obesity | 0.77 (0.43, 1.31) | 30.5% | 1.35 (0.76, 2.30) | 31.6% | 1.20 (0.71, 1.95) | 37.9% | 1.09 (0.80, 1.49) |
| Model 3: +education | 0.92 (0.50, 1.57) | 47.9% | 1.39 (0.79, 2.35) | 52.1% | – | – | 1.14 (0.77, 1.69) |
| Hypertension and cognitive impairment | |||||||
| Model 0: no adjustment | 1.22 (0.94, 1.57) | 56.0% | 1.13 (0.78, 1.67) | 26.0% | 1.06 (0.69, 1.70) | 18.1% | 1.16 (0.96, 1.41) |
| Model 1: age, sex | 1.08 (0.75, 1.55) | 39.5% | 1.06 (0.73, 1.58) | 35.4% | 0.98 (0.63, 1.57) | 25.1% | 1.05 (0.83, 1.32) |
| Model 2: +diabetes, obesity | 1.10 (0.76, 1.59) | 44.8% | 0.95 (0.60, 1.53) | 27.9% | 0.91 (0.57, 1.49) | 27.3% | 1.00 (0.78, 1.28) |
| Model 3: +education | 1.07 (0.74, 1.56) | 60.6% | 1.01 (0.64, 1.62) | 39.4% | – | – | 1.05 (0.78, 1.40) |
| Obesity and cognitive impairment | |||||||
| Model 0: no adjustment | 1.25 (0.88, 1.67) | 47.2% | 1.58 (1.05, 2.28) | 32.1% | 1.09 (0.66, 1.72) | 20.8% | 1.31 (1.05, 1.63) |
| Model 1: age, sex | 1.26 (0.77, 1.96) | 29.4% | 1.56 (1.04, 2.26) | 42.5% | 1.16 (0.70, 1.83) | 28.1% | 1.35 (1.05, 1.73) |
| Model 2: +diabetes, hypertension | 1.28 (0.78, 2.00) | 36.3% | 1.49 (0.89, 2.45) | 31.3% | 1.14 (0.68, 1.85) | 32.4% | 1.29 (0.98, 1.72) |
| Model 3: +education | 1.29 (0.79, 2.02) | 53.4% | 1.38 (0.82, 2.25) | 46.6% | – | – | 1.33 (0.94, 1.87) |
Note: Results from log-binomial regression analyses. For each study, results for Model 2 and Model 3 are based on a single model respectively.
Abbreviations: CI, confidence interval; DECS, Dexamethasone for Cardiac Surgery; RR, risk ratio; SuDoCo, Surgery Depth of Anaesthesia Cognitive Outcome.
Figure 1.
Pooled association of obesity with cognitive impairment (model 2).
Abbreviations: CI, confidence interval; DECS, Dexamethasone for Cardiac Surgery; SE, standard error; SuDoCo, Surgery Depth of Anaesthesia Cognitive Outcome.
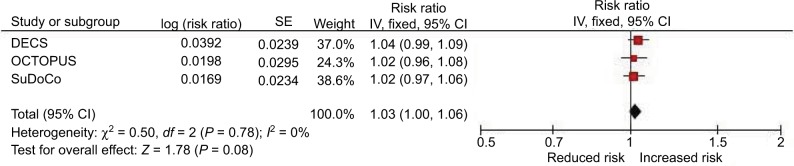
Associations of BMI and blood pressure with cognitive impairment are shown in Table 3. A higher BMI was associated with an increased prevalence of impairment across studies. Independently of age, sex, diabetes and hypertension, each one unit increment in BMI was associated with a 3% increased prevalence of cognitive impairment (RR 1.03; 95% CI 1.00, 1.06). There was no evidence of statistical heterogeneity among the studies (Chi2=0.50; I2=0%; Table 3; Figure 2), and the finding remained similar following additional adjustment for education (RR 1.03; 95% CI 0.99, 1.07). In a post hoc analysis to further elucidate the relationship of BMI and cognitive impairment, model 2 (controlling for age, sex, diabetes, hypertension) was repeated for the “obese” category (BMI ≥30 kg/m2) rather than the total sample. When effects were pooled across 339 obese participants in this subgroup, each one unit increment in BMI was associated with an 8% increased prevalence of cognitive impairment (RR 1.08; 95% CI 1.01, 1.16).
Table 3.
Association of BMI, systolic and diastolic blood pressure with cognitive impairment in each study, and pooled estimates of prevalence ratios
| OCTOPUS
|
DECS
|
SuDoCo
|
Pooled estimates
|
||||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | Weight | Estimate (95% CI) | Weight | Estimate (95% CI) | Weight | Estimate (95% CI) | |
| BMI and cognitive impairment | |||||||
| Model 0: no adjustment | 1.02 (0.97, 1.06) | 35.5% | 1.04 (1.00, 1.09) | 31.2% | 1.01 (0.97, 1.05) | 33.3% | 1.02 (1.00, 1.05) |
| Model 1: age, sex | 1.02 (0.96, 1.08) | 22.6% | 1.04 (1.00, 1.09) | 38.0% | 1.02 (0.97, 1.06) | 39.4% | 1.03 (1.00, 1.06) |
| Model 2: +diabetes, hypertension | 1.02 (0.96, 1.08) | 24.3% | 1.04 (0.99, 1.09) | 37.0% | 1.02 (0.97, 1.06) | 38.6% | 1.03 (1.00, 1.06) |
| Model 3: +education | 1.02 (0.97, 1.09) | 38.7% | 1.03 (0.98, 1.08) | 61.3% | – | – | 1.03 (0.99, 1.07) |
| Systolic blood pressure and cognitive impairment | |||||||
| Model 0: no adjustment | 0.98 (0.89, 1.07) | 61.4% | – | – | 1.03 (0.91, 1.14) | 38.6% | 0.99 (0.93, 1.07) |
| Model 1: age, sex | 0.94 (0.85, 1.03) | 64.9% | – | – | 1.01 (0.89, 1.12) | 35.1% | 0.96 (0.89, 1.03) |
| Model 2: +diabetes, obesity | 0.94 (0.85, 1.03) | 60.7% | – | – | 1.00 (0.89, 1.12) | 39.3% | 0.96 (0.89, 1.03) |
| Model 3: +education | 0.95 (0.86, 1.04) | – | – | – | – | – | – |
| Diastolic blood pressure and cognitive impairment | |||||||
| Model 0: no adjustment | 0.86 (0.72, 1.03) | 54.1% | – | – | 0.96 (0.79, 1.17) | 45.9% | 0.90 (0.79, 1.03) |
| Model 1: age, sex | 0.89 (0.74, 1.07) | 52.1% | – | – | 0.98 (0.81, 1.19) | 47.9% | 0.93 (0.81, 1.06) |
| Model 2: +diabetes, obesity | 0.89 (0.74, 1.07) | 53.2% | – | – | 0.98 (0.81, 1.18) | 46.8% | 0.93 (0.81, 1.07) |
| Model 3: +education | 0.90 (0.75, 1.08) | – | – | – | – | – | – |
Note: Results from log-binomial regression analyses. Estimates correspond to 1 kg/m2 increment in BMI and 10 mmHg increment in blood pressure. Data on systolic/diastolic blood pressure available for N=270 participants in OCTOPUS and for N=949 participants in SuDoCo. Data on blood pressure not available for DECS. For each study, results for Model 2 and Model 3 are based on a single model respectively.
Abbreviations: BMI, body mass index; CI, confidence interval; DECS, Dexamethasone for Cardiac Surgery; RR, risk ratios; SuDoCo, Surgery Depth of Anaesthesia Cognitive Outcome.
Figure 2.
Pooled association of BMI with cognitive impairment (model 2).
Abbreviations: BMI, body mass index; CI, confidence interval; DECS, Dexamethasone for Cardiac Surgery; SE, standard error; SuDoCo, Surgery Depth of Anaesthesia Cognitive Outcome.
Systolic and diastolic blood pressures were not associated with cognitive impairment (Table 3).
Of note, there were no associations of sex with cognitive impairment in any of our analyses (data not shown); thus, sex was not further explored as a modifier of the association of obesity or BMI with cognitive impairment.
Discussion
In this secondary analysis of cross-sectional data from 3 studies, prevalence of cognitive impairment as defined by a lower performance compared with controls was relatively high compared with some previous investigations.36 Overall, 18.4% of patients had cognitive impairment. Though there was substantial heterogeneity in prevalence between the 3 studies that ranged from 8.2% (SuDoCo) to 45.6% (OCTOPUS). When results were pooled across the 3 studies to assess metabolic predictors of cognitive impairment, we found a 29% increased prevalence of cognitive impairment in participants who are obese (BMI ≥30 kg/m2) compared with normal weight to overweight individuals. Each 1 kg/m2 increment of BMI was associated with 3% increased prevalence. That estimate even increased to 8% increased prevalence of impairment for each 1 kg/m2 increment of BMI when analyses of BMI were restricted to participants in the “obese” category. Overall this is suggestive of a non-linear dose–response relationship of BMI with impairment.
Previous epidemiological studies identified diabetes15,16 and, although less consistently, hypertension and obesity measured in later life13,17,19,21,22 as risk factors for cognitive impairment. However, many of these studies assessed each of these candidate predictors in isolation or with consideration of few other metabolic factors. Because all correlate strongly with one another,14,27 the individual contribution of each to cognitive outcome may have been obscured in those analyses. Even in cases where some of these factors have been controlled for, residual confounding is a real possibility.
In one of the first studies to investigate cognitive impairment in later life to consider such confounding, we established that the cross-sectional associations of obesity and a higher BMI with presence of cognitive impairment were independent of comorbid diabetes and hypertension. As we adjusted for 3 of 4 components of the metabolic syndrome (all except dyslipidemia), it follows that obesity might be one driving force behind the cognitive impairment seen in people with the metabolic syndrome.8–13 Mediation of the obesity-cognition association by presence of diabetes or hypertension is unlikely, as controlling for mediating factors would have led to a profound reduction in effect size. However, the possibility of an influence of subclinical insulin resistance or subclinical elevated blood pressure remains.
Our cross-sectional data suggest that diabetes and hypertension themselves are not at all or only weakly associated with cognitive impairment. Reasons for disparity from previous epidemiological research that had implicated hypertension and (even more strongly) diabetes in cognitive risk16,20 are unclear but may stem from the fact that 2 of our studies were of a high-risk (rather than general) population. Further, our definition of “cognitive impairment” may be less sensitive to pathological changes associated with hypertension or diabetes, and none of the 3 studies had set out to determine associations of metabolic risk factors with cognitive impairment, so that data on diabetes and hypertension, in contrast to measurement of participants’ cognitive status, height and weight, may not have been collected with sufficient rigor. This could have led to the lack of a finding on diabetes and hypertension.
Obesity – though both preventable and modifiable – is threatening global health through increasing risk of poor health outcomes. Four million deaths per year are currently attributed to a high BMI globally.37 In our study, we found that older people who were obese were more likely to be cognitively impaired, which highlights the relevance of cognitive impairment as an obesity-related organ dysfunction that is equal in importance to others such as coronary heart or kidney disease, for instance. With BMI as a crude reflection of actual body composition particularly in older people38,39 effect sizes could have been even larger than reported here had we used more detailed assessments such as body fat. Importantly, we found evidence for a non-linear dose–response relationship that suggests that cognitive risk increases exponentially with increasing BMI among people with normal weight, overweight and obesity. Our study lacked data on BMI change across the life span. This reflects one aspect that complicates research of obesity and cognitive outcome: unless participants are followed up over the course of decades,40 even studies with prospective designs provide only “snapshots” of adiposity status. Exposure to weight change due to aging and/or disease remains obscure despite evidence from rare long-term prospective investigations of a potential role of weight change in cognitive risk prediction.24
The pathophysiology linking obesity with cognitive impairment is poorly understood but may be causal. Obesity constitutes a pro-inflammatory state,41 which itself has been associated with cognitive impairment,42 and animal models suggest that elevated triglyceride levels which are common in obese individuals, impair brain function.43,44 Relatedly, obesity-induced systemic damage of the vasculature could cause cerebral white matter lesions.45 The apparent non-linear relationship of BMI with cognitive impairment in our analysis may indicate cumulative effects of these mechanisms. Because the effect size of the association of obesity with cognitive impairment was unchanged after adjustment for education, it is unlikely that it was due to confounding by this factor that could have led to exposure of people of low socioeconomic status to an increased risk of both late-life obesity46,47 and late-life cognitive impairment.48,49 Reverse causality underlying our findings is also possible, however, due to the cross-sectional study design. Obesity following increased food intake50 or reduced physical activity51 might also be the result of beginning cognitive impairment.
We investigated several parameters of metabolic derangement for their cross-sectional association with cognitive impairment. This enabled us to tease out the contribution of each to cognitive risk. We took advantage of comprehensive neuropsychological test batteries that tapped a range of cognitive domains, and combined results across the 3 studies to obtain more reliable parameter estimates.
Limitations
Our study has several limitations. First, analyses were of patients scheduled to undergo surgery within the next few days. Cognitive performance could therefore have been influenced by surgery-related factors such as psychological distress, anxiety and pain, and patients will have been less healthy compared with community-dwelling samples. This is likely reflected in the relatively high prevalence of cognitive impairment. At the same time, self-selection bias for healthier patients to enroll compared with all approached individuals is also likely. These factors all limit the external validity of our findings. Second, we pooled results across 3 studies that were heterogeneous in terms of design and sample characteristics, which complicates the interpretation of our findings. For instance, 2 of the studies included rather sick individuals undergoing cardiac surgery, whereas another focused on less severe (e.g., orthopedic) procedures, and different cognitive test batteries each with a different number of tests were used in each of the 3 studies. This may have influenced prevalence of cognitive impairment. Also, readers should note that the clinical significance of our findings is unclear due to the definition of “cognitive impairment” that may have captured mild forms of impairment. Third, the metabolic parameters were determined by single-time assessment; none of the studies prospectively investigated their development or change over time, and so we cannot draw conclusions on fluctuations in the severity of hypertension, diabetes or obesity and associated cognitive risk. Fourth, obesity was defined by BMI despite the fact that BMI does not capture body fat and body fat distribution which are likely driving forces behind obesity links to negative health outcomes.52 The use of BMI in older people for this purpose appears to be particularly limited.38,39 Fifth, we had no data on dyslipidemia to allow adjustment for the final component of the metabolic syndrome. Sixth, our results are limited by relatively large CIs of estimates due to small sample size. Finally, due to the cross-sectional study design our finding may well reflect reverse causality.
Further studies are needed to evaluate the external validity of our findings through replication in community-dwelling samples, and should examine the underlying pathophysiological mechanisms as well as the influence of body weight trajectories over the life-course on late-life cognition. Comparison among various cognitive domains could determine any domain-specific effects of obesity. Trials modeled on the Action for Health in Diabetes study53 could further determine the influence of weight loss on cognitive outcome in different weight categories to determine whether weight loss effects on cognition, too, may be non-linear. Once the role of obesity in cognitive impairment is better understood, preventive pharmacological strategies or health programs could reduce cognitive risk in people who are at risk of developing obesity, such as overweight and physically inactive individuals.
Conclusion
Our cross-sectional analysis suggests that among high-risk older people who are scheduled to undergo surgery, those who are obese have a higher likelihood of cognitive impairment compared to normal weight or overweight persons. Among normal weight, overweight and obese persons, a higher BMI is associated with a higher prevalence of cognitive impairment. The association appears to increase in strength with increasing BMI. Further studies are needed to prospectively investigate the temporal relationship of body weight and cognitive risk.
Supplementary materials
Enrollment into the 3 studies.
Footnotes
Disclosure
Insa Feinkohl and Gunnar Lachmann were supported by funding from the European Union, Seventh Framework Programme [FP7/2007–2013], under grant agreement no. HEALTH-F2-2014-602461 BioCog (Biomarker Development for Postoperative Cognitive Impairment in the Elderly): www.biocog.eu. Gunnar Lachmann was supported by the Clinician Scientist Program granted by the Berlin Institute of Health (BIH). We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin. The authors report no other conflicts of interest in this work.
References
- 1.Ellulu M, Abed Y, Rahmat A, Ranneh Y, Ali F. Epidemiology of obesity in developing countries: challenges and prevention. Global Epidemic Obesity. 2014;2(1):2. [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2(8):634–647. doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedner T, Kjeldsen SE, Narkiewicz K. Health economy of the metabolic syndrome pandemic. Blood Press. 2005;14(3):131–132. doi: 10.1080/08037051510034310. [DOI] [PubMed] [Google Scholar]
- 5.Denys K, Cankurtaran M, Janssens W, Petrovic M. Metabolic syndrome in the elderly: an overview of the evidence. Acta Clin Belg. 2009;64(1):23–34. doi: 10.1179/acb.2009.006. [DOI] [PubMed] [Google Scholar]
- 6.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahana-Amitay D, Spiro A, 3rd, Cohen JA, et al. Effects of metabolic syndrome on language functions in aging. J Int Neuropsychol Soc. 2015;21(2):116–125. doi: 10.1017/S1355617715000028. [DOI] [PubMed] [Google Scholar]
- 9.Chen B, Jin X, Guo R, et al. Metabolic syndrome and cognitive performance among Chinese ≥50 years: a cross-sectional study with 3988 participants. Metab Syndr Relat Disord. 2016;14(4):222–227. doi: 10.1089/met.2015.0094. [DOI] [PubMed] [Google Scholar]
- 10.Siervo M, Harrison SL, Jagger C, Robinson L, Stephan BC. Metabolic syndrome and longitudinal changes in cognitive function: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41(1):151–161. doi: 10.3233/JAD-132279. [DOI] [PubMed] [Google Scholar]
- 11.Exalto LG, van der Flier WM, van Boheemen CJM, et al. The metabolic syndrome in a memory clinic population: relation with clinical profile and prognosis. J Neurol Sci. 2015;351(1–2):18–23. doi: 10.1016/j.jns.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Frisardi V, Solfrizzi V, Seripa D, et al. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev. 2010;9(4):399–417. doi: 10.1016/j.arr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Ng TP, Feng L, Nyunt MS, et al. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: follow-up of the Singapore longitudinal ageing study cohort. JAMA Neurol. 2016;73(4):456–463. doi: 10.1001/jamaneurol.2015.4899. [DOI] [PubMed] [Google Scholar]
- 14.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 15.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 16.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 17.Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in midlife and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45(1):14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- 18.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19(3):24. doi: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fergenbaum JH, Bruce S, Lou W, Hanley AJ, Greenwood C, Young TK. Obesity and lowered cognitive performance in a Canadian First Nations population. Obesity (Silver Spring) 2009;17(10):1957–1963. doi: 10.1038/oby.2009.161. [DOI] [PubMed] [Google Scholar]
- 20.Israeli-Korn SD, Masarwa M, Schechtman E, et al. Hypertension increases the probability of Alzheimer’s disease and of mild cognitive impairment in an Arab community in northern Israel. Neuroepidemiology. 2010;34(2):99–105. doi: 10.1159/000264828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbatecola AM, Lattanzio F, Spazzafumo L, et al. Adiposity predicts cognitive decline in older persons with diabetes: a 2-year follow-up. PLoS One. 2010;5(4):e10333. doi: 10.1371/journal.pone.0010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benito-León J, Mitchell AJ, Hernández-Gallego J, Bermejo-Pareja F. Obesity and impaired cognitive functioning in the elderly: a population-based cross-sectional study (NEDICES) Eur J Neurol. 2013;20(6):899–906. e76–e77. doi: 10.1111/ene.12083. [DOI] [PubMed] [Google Scholar]
- 23.Cova I, Clerici F, Maggiore L, et al. Body mass index predicts progression of mild cognitive impairment to dementia. Dement Geriatr Cogn Disord. 2016;41(3–4):172–180. doi: 10.1159/000444216. [DOI] [PubMed] [Google Scholar]
- 24.Ye BS, Jang EY, Kim SY, et al. Unstable body mass index and progression to probable Alzheimer’s disease dementia in patients with amnestic mild cognitive impairment. J Alzheimers Dis. 2016;49(2):483–491. doi: 10.3233/JAD-150556. [DOI] [PubMed] [Google Scholar]
- 25.Sibbett RA, Russ TC, Deary IJ, Starr JM. Risk factors for dementia in the ninth decade of life and beyond: a study of the Lothian birth cohort 1921. BMC Psychiatry. 2017;17(1):205. doi: 10.1186/s12888-017-1366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doruk H, Naharci MI, Bozoglu E, Isik AT, Kilic S. The relationship between body mass index and incidental mild cognitive impairment, Alzheimer’s disease and vascular dementia in elderly. J Nutr Health Aging. 2010;14(10):834–838. doi: 10.1007/s12603-010-0113-y. [DOI] [PubMed] [Google Scholar]
- 27.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 28.Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(Suppl 1):i98–i105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 29.Dieleman JM, Nierich AP, Rosseel PM, et al. Dexamethasone for Cardiac Surgery (DECS) Study Group Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. 2012;308(17):1761–1767. doi: 10.1001/jama.2012.14144. [DOI] [PubMed] [Google Scholar]
- 30.Ottens TH, Dieleman JM, Sauër AM, et al. DExamethasone for Cardiac Surgery (DECS) Study Group Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121(3):492–500. doi: 10.1097/ALN.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 31.Van Dijk D, Jansen EW, Hijman R, et al. Octopus Study Group Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial. JAMA. 2002;287(11):1405–1412. doi: 10.1001/jama.287.11.1405. [DOI] [PubMed] [Google Scholar]
- 32.van Dijk D, Nierich AP, Eefting FD, et al. The Octopus Study: rationale and design of two randomized trials on medical effectiveness, safety, and cost-effectiveness of bypass surgery on the beating heart. Control Clin Trials. 2000;21(6):595–609. doi: 10.1016/s0197-2456(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 33.van Dijk D. Outcomes After Off-Pump Coronary Bypass Surgery. Utrecht: Universiteit Utrecht; 2002. [Google Scholar]
- 34.Silbert B, Evered L, Scott DA, et al. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology. 2015;122(6):1224–1234. doi: 10.1097/ALN.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 35.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 36.Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8(1):14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 37.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond) 2016;40(5):761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Yi Y, Roebothan B, et al. Body mass index trajectories among middle-aged and elderly Canadians and associated health outcomes. J Environ Public Health. 2016;2016:7014857. doi: 10.1155/2016/7014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease--the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull. 2012;89(3–4):144–149. doi: 10.1016/j.brainresbull.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Chen JM, Cui GH, Jiang GX, et al. Cognitive impairment among elderly individuals in Shanghai suburb, China: association of C-reactive protein and its interactions with other relevant factors. Am J Alzheimers Dis Other Demen. 2014;29(8):712–717. doi: 10.1177/1533317514534758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farr SA, Yamada KA, Butterfield DA, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149(5):2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karimi SA, Salehi I, Komaki A, Sarihi A, Zarei M, Shahidi S. Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: an in vivo study. Brain Res. 2013;1539:1–6. doi: 10.1016/j.brainres.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 45.Gustafson DR, Steen B, Skoog I. Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. Int Psychogeriatr. 2004;16(3):327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- 46.Cameron AJ, Spence AC, Laws R, Hesketh KD, Lioret S, Campbell KJ. A review of the relationship between socioeconomic position and the early-life predictors of obesity. Curr Obes Rep. 2015;4(3):350–362. doi: 10.1007/s13679-015-0168-5. [DOI] [PubMed] [Google Scholar]
- 47.Tamayo T, Christian H, Rathmann W. Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: a systematic review. BMC Public Health. 2010;10:525. doi: 10.1186/1471-2458-10-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caamaño-Isorna F, Corral M, Montes-Martínez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology. 2006;26(4):226–232. doi: 10.1159/000093378. [DOI] [PubMed] [Google Scholar]
- 49.Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7(6):e38268. doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geda YE, Ragossnig M, Roberts LA, et al. Caloric intake, aging, and mild cognitive impairment: a population-based study. J Alzheimers Dis. 2013;34(2):501–507. doi: 10.3233/JAD-121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed RM, Landin-Romero R, Collet TH, et al. Energy expenditure in frontotemporal dementia: a behavioural and imaging study. Brain. 2017;140(1):171–183. doi: 10.1093/brain/aww263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu J, Hofker M, Wijmenga C. Apple or pear: size and shape matter. Cell Metab. 2015;21(4):507–508. doi: 10.1016/j.cmet.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Espeland MA, Luchsinger JA, Baker LD, et al. Look AHEAD Study Group Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. 2017;88(21):2026–2035. doi: 10.1212/WNL.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Enrollment into the 3 studies.